Throwing up water during pregnancy
Throwing Up Yellow and Pregnant: Causes and Treatments
If you’re pregnant, you may be paying way more attention to everyday aches, pains, discomforts, and bodily fluids than usual.
Since many common pregnancy symptoms fall into two categories — annoying but totally harmless and warning signs of a serious problem — you may find yourself focusing intensely on each and every burp, fart, cramp, twinge, and craving, wondering whether you should ignore it or call your doctor.
And while you were expecting to do some puking during pregnancy, you might not have expected it to be yellow — and now you don’t know what to do about it.
That’s OK! We do know, and we’re happy to tell you.
Yup, it certainly can be!
Yellow vomit is just stomach acid. When you don’t have any food in your stomach but you’re still throwing up, it’s inevitable that you’ll start vomiting the only thing left in there: bile.
Bile is acid produced by your stomach to help break down your food.
If you’re throwing up first thing in the morning when you haven’t eaten anything yet — or if you’re persistently throwing up — it’s normal to eventually see yellow bile instead of whatever your last meal was.
The most likely reason you’re throwing up yellow liquid during pregnancy is the same reason you’re throwing up anything at all during pregnancy: hormones.
Particularly in the first trimester, pregnancy hormones like estrogen and progesterone are skyrocketing.
You’re also developing higher levels of hCG, or human chorionic gonadotropin, as your body accepts the fact that it’s now home to a growing human being (and needs to prepare itself, stat).
So, the puking is normal, but again, because you might be doing it more often than usual and way more often first thing in the morning with an empty stomach, it’s likely to be yellow at least once in a while.
Depending on whether you’ve had any liquids recently and how vigorously you’re throwing up, the consistency of your yellow vomit might vary.
It could be super liquidy and clear, foamy, or even thick and mucousy (yup, nasty). This all falls into the “normal” category.
In addition to your sunshine-colored puke, you may also have the usual morning sickness symptoms:
- a queasy, carsick feeling
- stomach cramps
- loss of appetite
- dehydration
- a bitter or metallic taste in your mouth
Morning sickness usually fires up around the 6th or 7th week of pregnancy, peaks around 9 to 12 weeks, and then tapers off by 12 to 20 weeks. So, if you’re having a lot of yellow vomiting, you can assume it will probably follow that trajectory.
But here’s where we share the bad news: Some stay sick for longer, and some truly unfortunate souls get stuck with morning sickness until they give birth (WHY?!).
It’s not how things usually go, thankfully, so don’t stress about this too much. We just have to put it out there as a possibility.
It helps to settle your stomach after you’ve vomited, so you can maybe catch a few hours of relief.
Sipping nausea-friendly drinks, like peppermint tea and ginger ale, can work wonders. So can nibbling on carbs: Think crackers, toast, dry cereal, pretzels, or plain bagels.
It might be hard to drink water on a queasy stomach, but try to avoid getting dehydrated. If you can’t handle all that liquid going down into your stomach, suck on ice cubes or popsicles, or take small sips from a straw.
Basically, just don’t brush your teeth, as tempting as it is.
Yes, your mouth tastes gross and your breath smells bad. But when stomach acid comes into contact with your teeth (especially if you’re vomiting frequently), it can soften your tooth enamel.
Brushing your teeth when your enamel is softened can actually strip away some enamel, which isn’t great for your teeth in the long run.
If you can’t stand the taste in your mouth, try swishing some water around and then spitting it out again to give your teeth and tongue a good rinse without all the damage.
You can’t necessarily prevent morning sickness — it’s triggered by hormones, which are totally out of your control. (Get used to that, BTW.)
(Get used to that, BTW.)
But you can often find a couple of tricks that stave off the worst of it. And you might be able to avoid vomiting of the yellow sort, in particular.
Try the following:
- Eat a small snack right before going to bed or before getting out of bed in the morning. Having something in your stomach might prevent some morning episodes of vomiting and, even if it doesn’t, you won’t be throwing up stomach bile, at least. Protein, like almonds, and carbs are a good choice.
- Don’t skip meals. Consider having something in your stomach at all times, however small. Lots of folks notice their nausea is worse when they haven’t eaten for 2 or 3 hours, so you may want to adopt a constantly grazing approach to food for the time being.
- Avoid excessively spicy foods, which can worsen nausea.
- Get plenty of rest. Some find their morning sickness is worse when they’re tired or running on empty.
- Talk with your doctor about managing your nausea.
 There may be medications you can use during the worst weeks of morning sickness to avoid throwing up from morning ’til night.
There may be medications you can use during the worst weeks of morning sickness to avoid throwing up from morning ’til night.
While few get away with zero vomiting during pregnancy, there’s definitely a point when you surpass a “normal” amount of puking and veer into medical condition territory.
This is called hyperemesis gravidarum (HG). You can’t just ignore it; left untreated, HG can lead to:
- dehydration
- malnutrition
- weight loss
- fainting
- mental confusion
Typical red flags suggesting you might have HG and not run-of-the-mill morning sickness include:
- vomiting constantly
- not being able to keep down any food
- fainting or dizzy spells
- weight loss of more than 5 percent of your body weight
If you think you might have HG, call your doctor. Also call your doctor if you have any of the following symptoms:
- dark urine or inability to pee
- severe headache
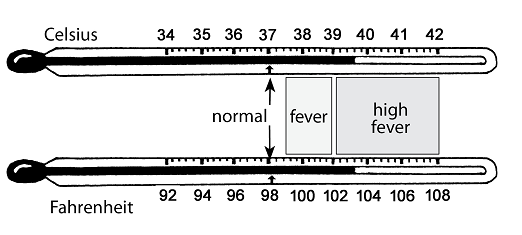
- fever
- shortness of breath
- dizziness or confusion
- severe pain or cramping in your abdomen
- bloody vomit
- muscular weakness
- vision changes
- vaginal bleeding
- sudden swelling of extremities
- abdominal pain
This could mean your morning sickness isn’t under control, or that you have an infection or other serious medical condition.
Not all nausea is necessarily due to pregnancy. Other things like appendicitis, gallbladder inflammation, and gallstones can still occur in pregnancy.
Throwing up yellow might be alarming, but it isn’t a cause for concern in the majority of cases. It means you’re throwing up on an empty stomach — the yellow stuff is your stomach acid.
You might be able to prevent it by having some food in your stomach first thing in the morning, before you get up, but you won’t be able to stop morning sickness entirely if you’re someone who gets it in the first place.
Thankfully, for most people, morning sickness wraps up by the end of the first trimester (if you’ve been throwing up yellow, that should go away then, too!).
YOU MUST DO THIS IF YOU ARE VOMITING DURING PREGNANCY – GOODONYA®
Hydrate and replenish your electrolytes!
Pregnancy is such a happy occasion until you start vomiting. Unfortunately, some women experience a severe form of morning sickness known as hyperemesis gravidarum, which can be serious. Whether you are vomiting now and then or continually vomiting, you need to be aware of fluid intake so that you don't become dehydrated.
Whether you are vomiting now and then or continually vomiting, you need to be aware of fluid intake so that you don't become dehydrated.
Vomiting is a major cause of fluid loss. Dehydration during pregnancy can become dangerous for you and your baby. You may find it hard to consume six to eight glasses of water per day when you are nauseated; however, keep in mind that dehydration can increase nausea. Increased nausea is the second to the last thing you need during pregnancy morning sickness. The last thing you need is dehydration.
Let's take a look at the best way to hydrate during pregnancy, tips to restore electrolytes with food, and some morning sickness remedies that might help.
HOW DO YOU HYDRATE WITH MORNING SICKNESS?
As naturally as possible. Obviously, water is a great way to hydrate. The problem with water alone is that it doesn't replace the electrolytes that you lose when vomiting. Electrolytes are electrically charged compounds that your body requires to perform vital functions like controlling blood volume, blood clotting, and muscle contractions, to name a few.
Health care providers often recommend sports drinks like Gatorade or oral rehydrating solutions such as Pedialyte. At first glance, these seem like good options until you read the labels. They are full of sugar, carbohydrates, and artificial ingredients. You may think when you're pregnant, there is no need to be concerned with your sugar and carbohydrate intake. However, pregnancy-induced diabetes or gestational diabetes is prevalent and can lead to complications with your pregnancy.
More concerning than the sugar content, however, is the artificial ingredients and processed electrolytes. The last thing you want to put in your body and the body of your developing baby are chemicals that could have long-term effects. Fortunately, there is a more natural alternative. It's called HYDRATE.
HYDRATE is an all-natural, organic way to prevent dehydration. and restore electrolytes. It has only six ingredients with 7g of carbohydrates and 1g of sugar from dehydrated lemon juice. HYDRATE is made of organic lemon juice, organic coconut water, organic stevia, Himalayan pink salt, magnesium sea minerals, and ascorbic acid. These are all-natural ingredients that come from the earth and not from a laboratory.
HYDRATE is made of organic lemon juice, organic coconut water, organic stevia, Himalayan pink salt, magnesium sea minerals, and ascorbic acid. These are all-natural ingredients that come from the earth and not from a laboratory.
Just fill your water bottle with HYDRATE when you get up in the morning and drink it throughout the day with confidence. The light homemade lemonade taste is clean, crisp, and will provide healthy hydration for you and your baby.
TIPS FOR REPLACING ELECTROLYTES WITH FOOD
Drinking fluids is not the only way to rehydrate and replace electrolytes. You can also get fluids and electrolytes in the foods that you eat. Juicy fruits like watermelon, berries, and citrus might also help curb nausea. Some foods that help restore hydration and electrolytes include:
- Milk
- Kale
- Beans
- Yogurt
- Turkey
- Chicken
- Spinach
- Oranges
- Broccoli
- Bananas
- Almonds
- Potatoes
- Avocados
- Tomatoes
- Buttermilk
- Watermelon
- Strawberries
TIPS FOR MANAGING MORNING SICKNESS
Fortunately for most women, morning sickness only lasts until the second trimester of pregnancy, and then it starts to subside. Only about one in five women find that their morning sickness lasts into the third trimester.
Only about one in five women find that their morning sickness lasts into the third trimester.
Although you may not feel like eating, you must try to eat regular meals even if they are small. Here are some tips for managing nausea and vomiting during pregnancy:
- Suck on hard candy.
- Avoid spicy and fatty foods.
- Avoid smells that upset your stomach.
- Acupressure wristbands can sometimes ease nausea.
- Don't take your prenatal vitamin on an empty stomach.
- Get outdoors for some fresh air or open a window and let some in.
- Eat bland foods like rice, bananas, chicken broth, gelatin, or ice pops.
- Take in plenty of fluids: water, weak tea, or clear sodas like ginger ale.
- Some women find relief using essential oils in calming scents like lavender or peppermint.
- Eat saltine crackers, dry toast, or dry cereal before you get out of bed to calm your stomach.
- Herbal ginger supplements can relieve nausea but talk to your doctor before taking any supplements.

WRAPPING UP
If you feel like your vomiting is excessive, you cannot keep even water down, or you are losing weight due to morning sickness, make sure that you contact your doctor. Otherwise, morning sickness is a relatively common occurrence during pregnancy, and it will pass.
In the meantime, try the tips provided here to control nausea and vomiting, stay hydrated by drinking HYDRATE and eating hydrating foods that replace electrolytes. When you are nauseated, it may take some work to keep up with your fluid intake. Just make sure to stay mindful of it throughout the day, especially if you cannot eat a whole lot.
Better days are coming, and before you know it, you'll be bringing your new baby home.
Preventive measures against regurgitation in children
08. 03.2017
03.2017
Regurgitation is the spontaneous reflux of gastric contents into the esophagus and mouth. This condition is not uncommon in infants and is often a cause for concern for parents. The frequency of regurgitation syndrome in children of the first year of life is 18-50%: up to 4 months - 67%, up to 6 months 24%, up to 1 year 5%. In most cases, regurgitation is "benign" and disappears on its own after 12-18 months. At the same time, “benign” or physiological regurgitation characterizes:
-
the age of the child is up to 12 months;
-
spitting up 2 or more times a day for 3 or more weeks;
-
sufficient weight gain;
The child has no signs of metabolic disorders, diseases of the gastrointestinal tract or the central nervous system. The child does not experience difficulty in swallowing or feeding, there is no forced position of the body.
Do not confuse regurgitation with vomiting. When a child burps, the abdominal muscles do not tense up. With vomiting, on the contrary, muscle tension occurs and food is ejected by pressure not only through the mouth, but also through the nose. In some cases, there may be general anxiety, pallor, cold extremities. Often with vomiting, the temperature rises, loose stools appear, which is a sign of an infectious disease. Vomit may contain unchanged milk, mucus, blood or bile.
When a child burps, the abdominal muscles do not tense up. With vomiting, on the contrary, muscle tension occurs and food is ejected by pressure not only through the mouth, but also through the nose. In some cases, there may be general anxiety, pallor, cold extremities. Often with vomiting, the temperature rises, loose stools appear, which is a sign of an infectious disease. Vomit may contain unchanged milk, mucus, blood or bile.
What explains physiological regurgitation
What is the tendency of babies to spit up? This phenomenon is explained by the peculiarity of the structure of the gastrointestinal tract of young children. At the age of one year, the esophagus is shorter and wider, physiological narrowing is weakly expressed. The stomach is located horizontally, its capacity is small, and the muscles that close the entrance to the stomach and prevent the contents from being thrown back into the esophagus are poorly developed. As the child begins to walk, the axis of the stomach becomes more vertical. The capacity of the stomach increases by the year from 30-35 ml to 250-300 ml. The secretory apparatus matures, the work of the closing muscles (sphincters) improves, which leads to a gradual decrease in the frequency and disappearance of regurgitation. These features explain the predisposition of young children to regurgitation and even the inevitability of this condition. However, there are measures to help reduce the frequency of regurgitation.
The capacity of the stomach increases by the year from 30-35 ml to 250-300 ml. The secretory apparatus matures, the work of the closing muscles (sphincters) improves, which leads to a gradual decrease in the frequency and disappearance of regurgitation. These features explain the predisposition of young children to regurgitation and even the inevitability of this condition. However, there are measures to help reduce the frequency of regurgitation.
Factors contributing to physiological regurgitation include:
-
Overfeeding. As a rule, actively sucking babies begin to suffer from overfeeding, with abundant milk secretion, as well as when switching to artificial or mixed feeding with an incorrect calculation of the required amount of milk formula. Regurgitation appears immediately or some time after feeding in the amount of 5-10 ml. Milk can flow out unchanged or curdled.
-
Swallowing air during feeding (aerophagia). A similar situation arises if the child suckles greedily at the breast, and the mother's milk is not very plentiful; due to the retracted, flat nipple of the mother's breast, since the child fails to fully capture the nipple and areola; with artificial feeding, if the hole at the nipple of the bottle is large enough or the nipple is not completely filled with milk.
 Babies with aerophagia often experience anxiety after feeding, bulging of the abdominal wall (belly inflates). After 10-15 minutes, the swallowed milk flows out unchanged, which is accompanied by a loud sound of air eructation.
Babies with aerophagia often experience anxiety after feeding, bulging of the abdominal wall (belly inflates). After 10-15 minutes, the swallowed milk flows out unchanged, which is accompanied by a loud sound of air eructation. -
Intestinal colic or constipation. These conditions lead to an increase in pressure in the abdominal cavity and a violation of the movement of food through the gastrointestinal tract, causing regurgitation.
Until the child is four months old, spitting up up to two teaspoons of milk after feeding, or one spitting up of more than three spoons during the day, is considered the norm. You can check the amount of spitting up in the following way: take a diaper, pour one teaspoon of water on its surface, and then compare this spot with the spot formed after the next spitting up.
Pathological regurgitation may be due to:
-
surgical diseases and malformations of the digestive system;
-
diaphragmatic hernia;
-
pathology of the central nervous system, trauma of the cervical spine during childbirth;
-
food intolerance, lactase deficiency;
-
increased intracranial pressure.

Such regurgitation is characterized by intensity, systematicity, the child spits up a large amount of milk. At the same time, there is a violation of the general condition of the baby - the child is whiny, loses or does not gain weight, cannot eat the amount of food necessary for his age. In such a situation, a pediatrician, gastroenterologist, surgeon, allergist, neurologist should be examined. It also requires examination and exclusion of anomalies in the structure of the upper gastrointestinal tract, the preservation of regurgitation for more than 1 year.
Scale for assessing the intensity of regurgitation:
-
Less than 5 regurgitations per day with a volume of not more than 3 ml - 1 point.
-
More than 5 regurgitations per day with a volume of more than 3 ml - 2 points.
-
More than 5 regurgitations per day up to half the amount of formula or breast milk, not more often than in half of the feedings - 3 points.
-
Spitting up a small amount of milk for 30 minutes or more after each feeding - 4 points.
-
Regurgitation from half to full volume of formula or breast milk in at least half of the feedings - 5 points.
Regurgitation with an intensity of 3 or more points requires a visit to a doctor.
Preventive measures against regurgitation in children
If regurgitation is physiological in nature, then it is not worth treating or correcting in this case. It is necessary to deal with the elimination of the cause, if possible, and carry out prevention.
Prevention of regurgitation in children includes the following measures:
-
Postural therapy: when feeding, it is necessary to hold the baby at an angle of 45 °, make sure that he completely grasps the nipple with the areola; after feeding, hold the baby in an upright position ("column") for 20 minutes - to drain the swallowed air.
 Due to this, the air that has entered the stomach will be able to go out. If nothing happened, then put the baby down and after a minute or two, lift him upright again.
Due to this, the air that has entered the stomach will be able to go out. If nothing happened, then put the baby down and after a minute or two, lift him upright again. -
Make sure that the opening in the bottle is not too large and that the nipple is filled with milk. Experiment with nipples - perhaps the other will be better. Milk should come out in drops, not a trickle.
-
Before you start feeding your baby, lay him belly down on a solid base.
-
After feeding, try to minimize the baby's physical activity, do not disturb him unnecessarily, and change clothes only if there is an emergency.
-
Avoid squeezing diapers or clothes on the abdomen of the child.
-
If the baby's appetite is good, then it is better to feed him often, but in small portions, otherwise, due to the large amount of food, the stomach may overflow, and this, as a result, leads to regurgitation of excess food.

-
The surface in the bed on which the baby lies should rise 10 cm at the head.
-
In addition, it is possible to use special "thickeners" of milk or anti-reflux mixtures, which the doctor will help you choose.
In the event that regurgitation begins to become more frequent or becomes abundant, or first began after six months of the baby's life, or does not subside by one and a half to two years of life, the child should be consulted by a pediatrician. With a high probability, additional help from a gastroenterologist will be needed.
In our Family Medical Center you can always find highly professional help.
← Back to the list of articles
Intrauterine infection of the fetus: a modern view of the problem
The progressive increase in the number of cases of intrauterine infection of the fetus is one of the most urgent problems of modern obstetrics and perinatology. This is facilitated by the polyetiology of this pathology, the lack of a clear relationship between the severity of the clinical manifestations of infection in the mother and the degree of fetal damage, the multifactorial effect of the infectious agent on the fetus.
Despite increased attention to the problem of intrauterine infection (IUI), many questions remain unresolved. Issues of diagnosis, treatment and prevention of IUI need further research and development. Until now, there are no clear criteria for treatment tactics, data on the effectiveness of complex therapy have not been summarized.
Epidemiology, etiology and pathogenesis of intrauterine infection
In the development of the infectious process in the fetus, the type of pathogen, its virulence, the ways of infection from mother to fetus, the protective reserves of the mother's body and the ability of the fetus to immune response are important [11].
According to modern data [10, 20], the number of IUI cases varies widely from 6 to 70%. Recently, the structure of the infectious morbidity of pregnant women, parturient women and puerperas, as well as the fetus and newborn has changed [5, 8, 14]. It has been proven that the causative agents of IUI are more than 27 types of bacteria, many viruses, parasites, 6 types of fungi, 4 types of protozoa and rickettsia. So, according to a number of researchers [8, 14, 30], chlamydia (17-50%) and viruses (herpes simplex virus, HSV - 7-47%, cytomegalovirus, CMV - 28-9%) are considered to be the predominant pathogens of antenatal infections.1.6%, enteroviruses - 8-17%). The causative agents of intranatal infections are group B streptococcus (3-12%), staphylococci (1-9%), fungi of the genus Candida (3-7%). Associations of pathogens occupy a leading position (75-95%).
So, according to a number of researchers [8, 14, 30], chlamydia (17-50%) and viruses (herpes simplex virus, HSV - 7-47%, cytomegalovirus, CMV - 28-9%) are considered to be the predominant pathogens of antenatal infections.1.6%, enteroviruses - 8-17%). The causative agents of intranatal infections are group B streptococcus (3-12%), staphylococci (1-9%), fungi of the genus Candida (3-7%). Associations of pathogens occupy a leading position (75-95%).
It is known that most bacteria exist in nature in the form of specifically organized biofilms (biofilms) [2, 25, 27]. This form of existence creates a lot of advantages for bacteria. Bacteria in biofilms have an increased survival rate in the presence of aggressive substances, immune defense factors, and antibiotics [27]. In this regard, one of the main problems of practical medicine is the problem of treating diseases of microbial origin.
In our study [15, 17], based on the results of bacteriological analysis of the species composition of the vaginal biotope, the strongest influence of Streptococcus faecalis (p=0. 00171), E. coli (p=0.01424) and Staphylococcus epidermidis (p=0.02714) for intrauterine infection of the fetus. When assessing the pathogens identified in the cervical canal of pregnant women by polymerase chain reaction (PCR) and enzyme immunoassay (ELISA), the following was established: in the group without the implementation of IUI, mycoplasma, chlamydia and ureaplasma accounted for 8%, CMV - 20%, HSV - 36%, candida - 3%, associations - 60%. When analyzing a group of newborns with the implementation of IUI, the most common pathogens were identified. Thus, mycoplasmas, chlamydia and HSV were found in 50%, CMV infection was detected in 45% of cases, ureaplasmas (20%) and candida (15%) were less common, associations were observed in 95%. The frequency of detection of pathogenic pathogens in newborns with signs of IUI was higher than in patients without infection.
00171), E. coli (p=0.01424) and Staphylococcus epidermidis (p=0.02714) for intrauterine infection of the fetus. When assessing the pathogens identified in the cervical canal of pregnant women by polymerase chain reaction (PCR) and enzyme immunoassay (ELISA), the following was established: in the group without the implementation of IUI, mycoplasma, chlamydia and ureaplasma accounted for 8%, CMV - 20%, HSV - 36%, candida - 3%, associations - 60%. When analyzing a group of newborns with the implementation of IUI, the most common pathogens were identified. Thus, mycoplasmas, chlamydia and HSV were found in 50%, CMV infection was detected in 45% of cases, ureaplasmas (20%) and candida (15%) were less common, associations were observed in 95%. The frequency of detection of pathogenic pathogens in newborns with signs of IUI was higher than in patients without infection.
In the pathogenesis of IUI, there are "maternal", "afterbirth", "fetal" stages of development [23]:
"Maternal" stage reflects the beginning of the infectious process within the lower parts of the urogenital tract.
The "following" stage occurs with hematogenous spread of the inflammatory process, occurs with bacteremia and viremia.
At the "fetal" stage, the infectious process spreads to the organs and tissues of the fetus. This occurs when the uteroplacental and placental-fetal antimicrobial barrier, the boundary of which is the layer of chorionic epithelium, is inconsistent.
The main source of infection in IUI is the mother of the child, from whose body the pathogen enters the fetus (vertical transmission mechanism). In this case, infection occurs both by ascending, transplacental and transovarial routes, as well as by contact and aspiration (directly during childbirth) routes. Moreover, for antenatal infections, the hematogenous route is most typical, and for intranatal infections, the ascending route of infection [4, 10, 20, 21].
The effect of IUI on the embryo and fetus is the effect of a complex of the following factors [22]:
1. Pathological effect of microorganisms and their toxins (infectious disease, fetal hypoxia, fetal growth retardation).
2. Violation of the process of implantation and placentation (low placentation, placenta previa).
3. Decreased metabolic processes and immune defense of the fetus.
The duration of pregnancy is of particular importance in the pathogenesis of the onset and development of IUI [13, 22]. The fetus does not react to infectious antigens until the 14th week of pregnancy, since it lacks immunocompetent cells, immunoglobulins and does not show immune reactions. With the beginning of the second trimester of pregnancy, the mechanism of action of ascending infection changes due to the merger of decidua vera and decidua capsularis into a single complex decidua parietalis. At this time, ascending infection may enter the fetus from the vagina or cervical canal. From this period of pregnancy, the internal pharynx of the cervical canal comes into contact with the water membranes of the fetus and, in the presence of infection, microorganisms penetrate into the amniotic fluid. Amniotic fluid acquires antimicrobial properties only after the 20th week of pregnancy, when, in response to the action of an infectious agent, an inflammatory proliferative reaction develops, which limits further penetration of the infection due to the appearance of lysozyme, complement, interferons, immunoglobulins [1, 22]. In the III trimester of pregnancy, the antibacterial protection of amniotic fluid increases. During this period, the role of the exudative component prevails in the inflammatory reaction of the fetal tissues, when inflammatory leukocyte reactions develop in the fetus (encephalitis, hepatitis, pneumonia, interstitial nephritis) in response to infection [22].
Amniotic fluid acquires antimicrobial properties only after the 20th week of pregnancy, when, in response to the action of an infectious agent, an inflammatory proliferative reaction develops, which limits further penetration of the infection due to the appearance of lysozyme, complement, interferons, immunoglobulins [1, 22]. In the III trimester of pregnancy, the antibacterial protection of amniotic fluid increases. During this period, the role of the exudative component prevails in the inflammatory reaction of the fetal tissues, when inflammatory leukocyte reactions develop in the fetus (encephalitis, hepatitis, pneumonia, interstitial nephritis) in response to infection [22].
Especially dangerous in IUI in the II and III trimesters of pregnancy is damage to the fetal brain, which can lead to mental retardation, delayed psychomotor development of children [3, 7]. Intrauterine infection of the CNS structures by pathogens in the fetus is accompanied by various severe disorders in the formation of the brain (hydrocephalus, subependymal cysts, cystic degeneration of the brain substance, anomalies in the development of the cortex, microcephaly). It is also possible to develop ventriculitis (deformation of the choroid plexus, heterogeneity or doubling of the reflection from the ependyma of the ventricles) [11].
It is also possible to develop ventriculitis (deformation of the choroid plexus, heterogeneity or doubling of the reflection from the ependyma of the ventricles) [11].
Thus, infection of the fetus in the later stages of pregnancy does not, as a rule, lead to the formation of gross malformations, but may disrupt the functional mechanisms of cell and tissue differentiation [10, 11, 13].
Changes in the state of the fetus and the functioning of the fetoplacental system caused by intrauterine infection of the fetus affect the composition and properties of the amniotic fluid [1, 9, 26, 30]. When an infectious agent enters the amniotic fluid, it reproduces unhindered, followed by the development of chorioamnionitis [1, 9]. The fetus is in an infected environment, which creates favorable conditions for infection of the fetus by contact, i.e. through the skin, mucous membranes, respiratory and gastrointestinal tracts.
The syndrome of "amniotic fluid infection" develops, the mechanism of occurrence of which is presented as follows [20]:
1. Ingestion and aspiration of infected water in a newborn shows signs of intrauterine infection (pneumonia, enterocolitis, vesiculosis, omphalitis, conjunctivitis, etc.).
Ingestion and aspiration of infected water in a newborn shows signs of intrauterine infection (pneumonia, enterocolitis, vesiculosis, omphalitis, conjunctivitis, etc.).
2. At the same time, microorganisms, spreading through the membranes or between them, reach the basal plate of the placenta (deciduitis). Further spread of the inflammatory reaction leads to the development of chorionitis (placentitis), manifested by leukocyte infiltration of the intravillous space and endovasculitis in the chorionic plate. Vasculitis in the decidua, stem and terminal villi leads to vascular obliteration, the appearance of infarcts, calcifications, massive fibrinoid deposits, which can manifest as "premature maturation of the placenta."
3. Polyhydramnios in intrauterine infection is usually secondary and is a manifestation of damage to the kidneys or urinary tract of the fetus. The reason for its development is also a change in the ratio of the processes of production and resorption of amniotic fluid by the cells of the amniotic epithelium against the background of amnionitis.
4. In the genesis of the symptom complex of placental insufficiency in IUI, the main role belongs to vascular disorders.
5. Typical manifestations of intrauterine infection are miscarriage and premature birth [24]. Premature development of labor activity and untimely rupture of the membranes are due to the action of bacterial phospholipases that trigger the prostaglandin cascade and the damaging effect of inflammatory toxins on the membranes.
6. Due to the fact that phospholipases of gram-negative bacteria contribute to the destruction of surfactant in the lungs of the fetus, the newborn develops respiratory disorders.
In modern literature [4, 9, 29] there are many works devoted to the study of the relationship between immunological parameters and the severity of the infectious-inflammatory process during pregnancy. Increasingly, in foreign and domestic literature, data appear on the relationship between bacterial invasion and cytokine synthesis by amnion, chorion, decidua, and fetal tissues [5, 29]. The multiplication of microorganisms in the amniotic fluid leads to an increase in the level of lipopolysaccharides, which activate the synthesis of cytokines by fetal trophoblast cells. It seems promising in IUI to study changes in the cytokine system, which provides the processes of intercellular cooperation, growth and differentiation of lymphoid cells, hematopoiesis, and neuroimmunoendocrine interactions. Cytokines in the pre-implantation period and during the development of pregnancy are actively produced by many maternal and embryonic cells, in particular, decidual cells of the uterus and trophoblast cells. It was found that cell cultures have different ability to synthesize cytokines under the influence of lipopolysaccharides. Thus, tumor necrosis factor (TNF) is produced by amnion cells, interleukins (IL)-6 and IL-8 are produced by amnion and chorion, and IL-1 is produced only by chorion [9, 11, 28]. According to N.V. Ordzhonikidze [14], from a variety of pro-inflammatory (IL-1, IL-2, IL-6, IL-8, IL-15, TNF, etc.
The multiplication of microorganisms in the amniotic fluid leads to an increase in the level of lipopolysaccharides, which activate the synthesis of cytokines by fetal trophoblast cells. It seems promising in IUI to study changes in the cytokine system, which provides the processes of intercellular cooperation, growth and differentiation of lymphoid cells, hematopoiesis, and neuroimmunoendocrine interactions. Cytokines in the pre-implantation period and during the development of pregnancy are actively produced by many maternal and embryonic cells, in particular, decidual cells of the uterus and trophoblast cells. It was found that cell cultures have different ability to synthesize cytokines under the influence of lipopolysaccharides. Thus, tumor necrosis factor (TNF) is produced by amnion cells, interleukins (IL)-6 and IL-8 are produced by amnion and chorion, and IL-1 is produced only by chorion [9, 11, 28]. According to N.V. Ordzhonikidze [14], from a variety of pro-inflammatory (IL-1, IL-2, IL-6, IL-8, IL-15, TNF, etc. ) and anti-inflammatory (IL-4, IL-10, IL-13, transforming growth factor, etc.) of cytokines, IL-1, IL-6, IL-10, and TNF are considered to be the main markers of the inflammatory process in human tissues and organs [9, 16, 28].
) and anti-inflammatory (IL-4, IL-10, IL-13, transforming growth factor, etc.) of cytokines, IL-1, IL-6, IL-10, and TNF are considered to be the main markers of the inflammatory process in human tissues and organs [9, 16, 28].
Clinical characteristics of intrauterine infection
In the pre-implantation period, under the influence of an infectious agent, the embryo dies (alternative inflammation) or continues to develop.
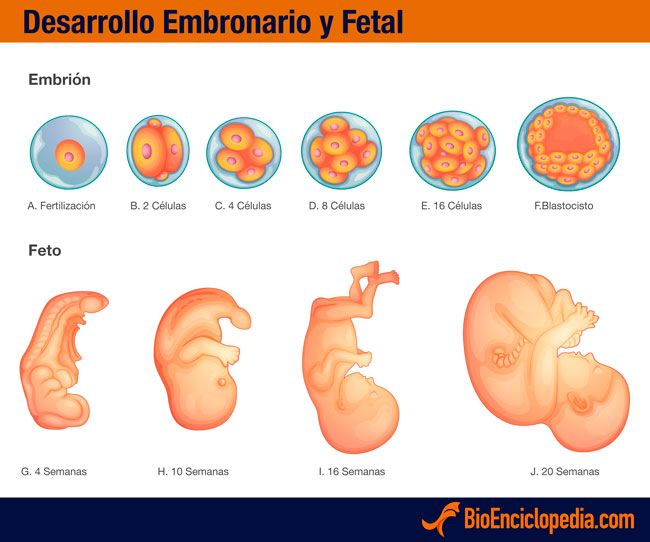
Infectious damage to the embryo at 3-12 weeks is usually associated with a viral infection that freely penetrates the chorion. The fetus does not yet have anti-infective protection systems, and during the period of organogenesis, IUI placentation leads to the formation of malformations (teratogenic) or death of the embryo (embryotoxic effect) [5, 23].
Infectious fetopathies occur from the 16-27th week of gestation, when the infection in the fetus generalizes, the formation of pseudomalformations (myocardial fibroelastosis, polycystic lung disease, hydrocephalus, hydronephrosis) occurs. When infected after 28 weeks, the fetus acquires the ability to a specific local reaction to the introduction of the pathogen, as a result, IUI (encephalitis, pneumonia, hepatitis, interstitial nephritis), miscarriage, intrauterine growth retardation, and fetal death are possible [2, 3, 10].
When infected after 28 weeks, the fetus acquires the ability to a specific local reaction to the introduction of the pathogen, as a result, IUI (encephalitis, pneumonia, hepatitis, interstitial nephritis), miscarriage, intrauterine growth retardation, and fetal death are possible [2, 3, 10].
Currently, the following types of intrauterine lesions in IUI are distinguished [20, 21]:
- blastopathy - with a gestation period of 0-14 days; possible death of the embryo, spontaneous miscarriage or the formation of a systemic pathology similar to genetic diseases;
- embryopathy - with a period of 15-75 days; characteristic malformations at the organ or cellular levels (true malformations), spontaneous miscarriage;
- early fetopathy - with a period of 76-180 days; characteristic is the development of a generalized inflammatory reaction with a predominance of alterative and exudative components and an outcome in fibrosclerotic deformities of organs (false defects), abortion;
- late fetopathy - with a period of 181 days before delivery; it is possible to develop a manifest inflammatory reaction with damage to various organs and systems (hepatitis, encephalitis, thrombocytopenia, pneumonia).
IUI often does not have clear clinical manifestations [4, 10, 15, 17]. Rarely, the first signs in a newborn are present immediately after birth, more often they appear during the first 3 days of life. When infected in the postnatal period, the symptoms of the infectious process are detected at a later date. Clinical manifestations of congenital bacterial or mycotic skin lesions in a newborn may have the character of vesiculo-pustulosis [4]. Conjunctivitis, rhinitis and otitis media that appeared on the 1st-3rd day of life can also be manifestations of IUI. Congenital aspiration pneumonia can also appear on the 2-3rd day of life. From the moment of birth, children have signs of respiratory failure: shortness of breath, cyanosis, often dullness of percussion sound and small bubbling moist rales. The course of intrauterine pneumonia is severe, because as a result of aspiration, large areas of the lung (lower and middle lobes) are turned off from breathing due to bronchial obstruction with infected amniotic fluid containing an admixture of meconium, fetal skin scales.:strip_icc():format(jpeg)/kly-media-production/medias/2785562/original/028627600_1556001360-shutterstock_1019963743.jpg) Enterocolitis in newborns occurs as a result of the penetration of the pathogen along with the amniotic fluid into the gastrointestinal tract. Dyspeptic symptoms usually develop on the 2-3rd day of life. Characterized by sluggish sucking, regurgitation, bloating, hepatosplenomegaly, expansion of the venous network of the anterior abdominal wall, frequent loose stools. In the microbiological study of intestinal contents, the predominance of Klebsiella, Proteus and Pseudomonas aeruginosa. Damage to the central nervous system during IUI in newborns can be both primary (meningitis, encephalitis) and secondary, due to intoxication. With damage to the choroid plexuses of the lateral ventricles of the brain, congenital hydrocephalus develops. It is necessary to pay attention to such symptoms as lethargy, poor sucking, regurgitation, delayed recovery or secondary weight loss, delayed healing of the umbilical wound, development of omphalitis. Typical symptoms of infectious intoxication in a newborn are respiratory and tissue metabolism disorders.
Enterocolitis in newborns occurs as a result of the penetration of the pathogen along with the amniotic fluid into the gastrointestinal tract. Dyspeptic symptoms usually develop on the 2-3rd day of life. Characterized by sluggish sucking, regurgitation, bloating, hepatosplenomegaly, expansion of the venous network of the anterior abdominal wall, frequent loose stools. In the microbiological study of intestinal contents, the predominance of Klebsiella, Proteus and Pseudomonas aeruginosa. Damage to the central nervous system during IUI in newborns can be both primary (meningitis, encephalitis) and secondary, due to intoxication. With damage to the choroid plexuses of the lateral ventricles of the brain, congenital hydrocephalus develops. It is necessary to pay attention to such symptoms as lethargy, poor sucking, regurgitation, delayed recovery or secondary weight loss, delayed healing of the umbilical wound, development of omphalitis. Typical symptoms of infectious intoxication in a newborn are respiratory and tissue metabolism disorders. There is a pale cyanotic coloration of the skin with a pronounced vascular pattern. Intoxication is accompanied by a violation of the excretory function of the liver and kidneys, an increase in the spleen and peripheral lymph nodes.
There is a pale cyanotic coloration of the skin with a pronounced vascular pattern. Intoxication is accompanied by a violation of the excretory function of the liver and kidneys, an increase in the spleen and peripheral lymph nodes.
Modern methods of diagnosing intrauterine infections
The prevalence of IUI among the causes of adverse outcomes, the high level of infection in pregnant women and puerperas necessitate the search for reliable methods for its diagnosis. The nonspecificity of the clinical manifestations of IUI creates diagnostic difficulties, which dictates the need for the combined use of clinical and laboratory research methods. In the last decade, the main methods for diagnosing IUI are bacteriological and immunological [10, 11, 21].
There are 3 stages in the diagnosis of intrauterine infection: 1) diagnosis during pregnancy; 2) early diagnosis at the time of birth; 3) diagnosis in the development of clinical signs of infection in the early neonatal period [12].
Of the non-invasive methods of prenatal diagnosis of IUI, the most informative are ultrasound and dopplerography [6, 7, 15, 26]. Direct methods of laboratory diagnostics (cordocentesis, dark-field microscopy, PCR, ELISA, culture) can detect the pathogen in biological fluids or tissue biopsies of an infected child. Indirect methods for diagnosing IUI include clinical symptoms of the mother, ultrasound, and help to make only a presumptive diagnosis of IUI [10, 17, 21]. Screening tests for IUI in newborns include studies of smears of amniotic fluid, placenta, cultures of cord blood and stomach contents of the newborn, and sometimes blood culture [6, 7, 10]. The “gold standard” for post-diagnosis of IUI is the histological examination of the placenta, umbilical cord, and fetal membranes [4, 14, 23].
Any changes in homeostasis in the mother's body are reflected in the cellular and chemical parameters of the amniotic fluid, which very finely characterize the course of the pathological process, and therefore the amniotic fluid can serve as an important diagnostic material [1, 6, 16]. According to I.V. Bakhareva [1], the most significant in the diagnosis of IUI is the determination of the antimicrobial activity of the amniotic fluid, based on the migration of leukocytes in it when bacteria accumulate in the amniotic membrane, exceeding 103 CFU/ml. The appearance of a large number of leukocytes in the amniotic fluid, an increase in cytosis due to epithelial cells without detecting microflora may indicate IUI.
According to I.V. Bakhareva [1], the most significant in the diagnosis of IUI is the determination of the antimicrobial activity of the amniotic fluid, based on the migration of leukocytes in it when bacteria accumulate in the amniotic membrane, exceeding 103 CFU/ml. The appearance of a large number of leukocytes in the amniotic fluid, an increase in cytosis due to epithelial cells without detecting microflora may indicate IUI.
Currently, great importance is attached to ultrasound research methods, which can be used to determine indirect signs of IUI of the fetus (polyhydramnios, ventriculomegaly, microcephaly, hepatomegaly, an increase in the thickness of the placenta, a fine suspension in the amniotic fluid) and structural changes in various organs [4, 11, 18, 26].
We have developed the necessary list of diagnostic measures for the early detection of IUI [19].
The complex examination of pregnant women included:
1. General clinical and biochemical blood and urine tests with the definition of standard indicators.
2. Determination of TORCH-complex pathogens by PCR in vaginal swabs, amniotic fluid.
3. Determination of blood antibodies to chlamydia, mycoplasmas and ureaplasmas, CMV and HSV by ELISA.
4. Carrying out an amine test, pH-metry of vaginal contents.
5. Bacterioscopic examination of the contents of the vagina, cervical canal and urethra.
6. Bacteriological examination of the maternal surface of the placenta, amniotic fluid, intestinal contents.
7. Determination of the level of pro-inflammatory (IL-1β, TNF) and anti-inflammatory (IL-10) cytokines in amniotic fluid, maternal venous blood serum and fetal cord blood.
8. Ultrasound scanning of the fetus, amniotic fluid and placenta.
9. Histomorphological examination of the placenta.
Newborn examination complex included:
1. Apgar score, measurement of body weight and length at birth, dynamics of body weight gain until discharge from the maternity hospital.