Mmr vaccine during pregnancy
Vaccine Safety for Moms-to-Be | CDC
Pregnant women may safely receive inactivated vaccines (Tdap and flu), mRNA (Moderna and Pfizer), and viral vector vaccines (J&J).
Vaccines help protect pregnant people and babies against serious diseases
Pregnant people share everything with their babies. That means when a pregnant person gets vaccines, she isn’t just protecting herself— they are giving the baby some early protection too.
CDC has recommendations for the vaccines needed before, during, and after pregnancy. Currently, CDC routinely recommends Tdap and flu shots during pregnancy.
- Get the Tdap vaccine (to help protect against whooping cough), during pregnancy.
- The flu shot can be given before or during pregnancy, depending on whether or not it is flu season during a pregnancy.
- It is safe for pregnant people to receive vaccines right after giving birth, even while breastfeeding.
- Some vaccines, such as the measles, mumps, rubella (MMR) vaccine, should be given a month or more before pregnancy if a pregnant person didn’t get the vaccine as a child.
Live virus vaccines, such as the MMR and chickenpox, should not be given to pregnant people, but should be given to them before or after pregnancy, if indicated. Talk to your doctor about the MMR, Tdap, and flu vaccines before getting vaccinated. The COVID-19 vaccine is also recommended for pregnant people. The authorized and recommended COVID-19 vaccines for pregnant people are the mRNA Moderna and Pfizer-BioNTech vaccines, which contain no live virus, and the J&J/Janssen viral vector vaccine, meaning it uses a modified version of a different virus (the vector) to deliver important instructions to our cells. If you have any questions about these vaccines, talk to your doctor.
Vaccine safety before, during, and after pregnancy
It’s important to know that the Tdap and flu vaccines are safe for a pregnant person and their baby. Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
- The Tdap and flu vaccines are inactivated vaccines, which means they are made by inactivating or killing the germ during the process of making the vaccine.
- Studies done on the Tdap vaccine have concluded that it is safe and effective for pregnant people and babies.
- Similarly, results from multiple studies on the flu shot continue to support the safety and effectiveness of the vaccine during pregnancy.
- There is limited information available about the safety of the COVID-19 vaccines for people who are pregnant; however, based on how these vaccines work in the body, experts believe they are unlikely to pose risk for pregnant people.
It is important to get MMR before becoming pregnant to reduce the risk of becoming infected with rubella which can pass on to the unborn child, causing Congenital Rubella Syndrome (CRS). CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
- Live vaccines are generally not recommended during pregnancy.
- If a pregnant person did not get MMR as a child, she should get the vaccine before pregnancy.
All vaccines are held to the highest standards of safety—meaning they are carefully studied and monitored for side effects. Vaccines are like any medicine, which means they can have some side effects. However, most people who get vaccinated have no side effects or only mild side effects. CDC continually monitors vaccine safety, and the most common side effects seen are mild and go away quickly on their own (redness, swelling, and tenderness at the site where the shot was given. Other possible side effects associated with the COVID-19 vaccine are tiredness, headache, muscle pain, chills, fever, and nausea.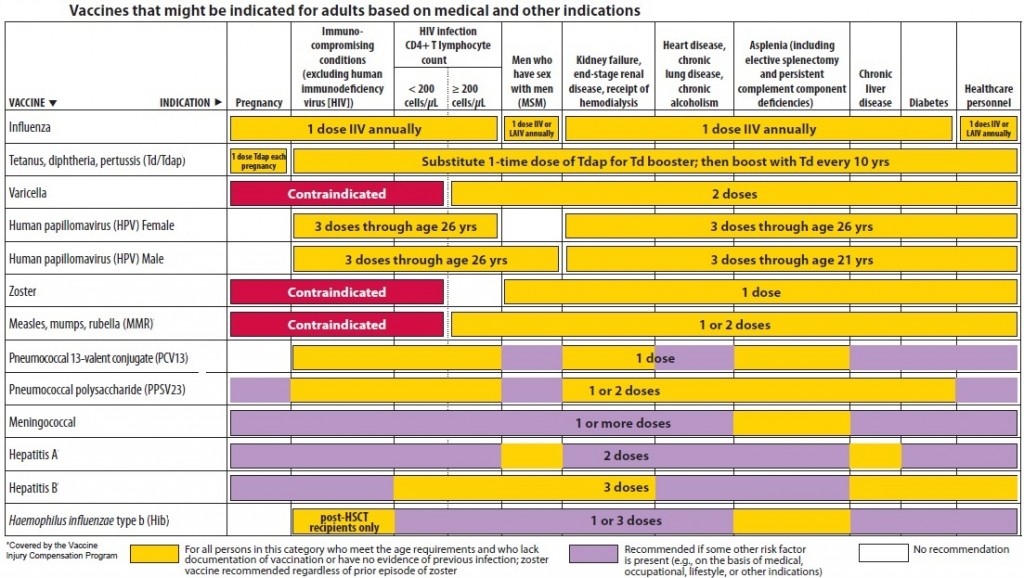 ).
).
For more studies, the FDA also has a pregnancy exposure registry,external icon which is a study that collects health information from pregnant persons who take medicines or vaccines when they are pregnant.
Vaccination during pregnancy - PMC
1. Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-15):1–48. Errata in: MMWR Morb Mortal Wkly Rep 2006;55(48):1303, Pediatrics 2007;119(5):1008, MMWR Morb Mortal Wkly Rep 2007;56(11):256. [PubMed] [Google Scholar]
2. Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1998;47(RR-8):1–57. [PubMed] [Google Scholar]
3. Centers for Disease Control and Prevention Rubella vaccination during pregnancy—United States, 1971–1988. MMWR Morb Mortal Wkly Rep. 1989;38(17):289–93. [PubMed] [Google Scholar]
Centers for Disease Control and Prevention Rubella vaccination during pregnancy—United States, 1971–1988. MMWR Morb Mortal Wkly Rep. 1989;38(17):289–93. [PubMed] [Google Scholar]
4. Bar-Oz B, Levichek Z, Moretti ME, Mah C, Andreou S, Koren G. Pregnancy outcome following rubella vaccination: a prospective controlled study. Am J Med Genet A. 2004;130A(1):52–4. [PubMed] [Google Scholar]
5. Minussi L, Mohrdieck R, Bercini M, Ranieri T, Sanseverino MT, Momino W, et al. Prospective evaluation of pregnant women vaccinated against rubella in southern Brazil. Reprod Toxicol. 2008;25(1):120–3. Epub 2007 Sep 16. [PubMed] [Google Scholar]
6. Namaei MH, Ziaee M, Naseh N. Congenital rubella syndrome in infants of women vaccinated during or just before pregnancy with measles-rubella vaccine. Indian J Med Res. 2008;127(6):551–4. [PubMed] [Google Scholar]
7. Badilla X, Morice A, Avila-Aguero ML, Saenz E, Cerda I, Reef S, et al. Fetal risk associated with rubella vaccination during pregnancy. Pediatr Infect Dis J. 2007;26(9):830–5. [PubMed] [Google Scholar]
Pediatr Infect Dis J. 2007;26(9):830–5. [PubMed] [Google Scholar]
8. Nasiri R, Yoseffi J, Khajedaloe M, Sarafraz Yazdi M, Delgoshaei F. Congenital rubella syndrome after rubella vaccination in 1–4 weeks periconceptional period. Indian J Pediatr. 2009;76(3):279–82. [PubMed] [Google Scholar]
9. Birthistle K, Carrington D. Fetal varicella syndrome—a reappraisal of the literature. A review prepared for the UK Advisory Group on Chickenpox on behalf of the British Society for the Study of Infection. J Infect. 1998;36(Suppl 1):25–9. [PubMed] [Google Scholar]
10. Merck, Centers for Disease Control and Prevention . Pregnancy registry for Varivax. The 14th annual report: 2009. Covering the period from approval (March 17, 1995) through March 16, 2009. Whitehouse Station, NJ: Merck & Co Inc; 2009. [Google Scholar]
11. Singh SP. Hepatitis B immunization: FAQs. Hepatitis B Annual. 2004;1(1):240–8. [Google Scholar]
12. Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al.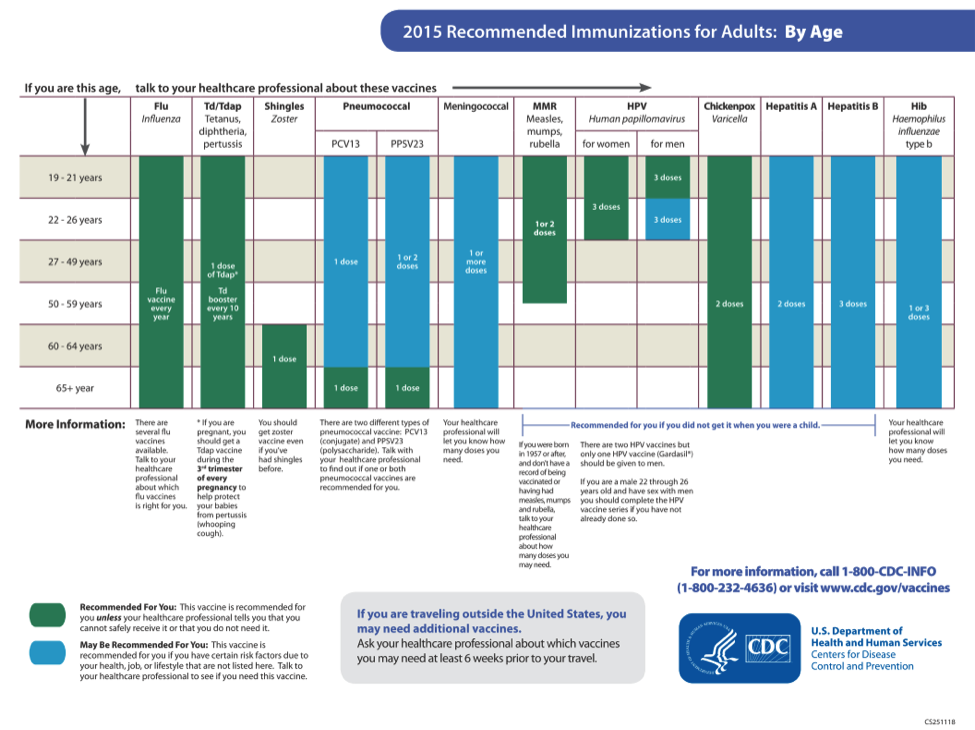 A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR-16):1–23. Errata in: MMWR Morb Mortal Wkly Rep 2006;55(6):158–9, MMWR Morb Mortal Wkly Rep 2007;56(48):1267. [PubMed] [Google Scholar]
A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54(RR-16):1–23. Errata in: MMWR Morb Mortal Wkly Rep 2006;55(6):158–9, MMWR Morb Mortal Wkly Rep 2007;56(48):1267. [PubMed] [Google Scholar]
13. Reddy PA, Gupta I, Ganguly NK. Hepatitis-B vaccination in pregnancy: safety and immunogenic response in mothers and antibody transfer to neonates. Asia Oceania J Obstet Gynaecol. 1994;20(4):361–5. [PubMed] [Google Scholar]
14. Ayoola EA, Johnson AO. Hepatitis B vaccine in pregnancy: immunogenicity, safety and transfer of antibodies to infants. Int J Gynaecol Obstet. 1987;25(4):297–301. [PubMed] [Google Scholar]
15. Levy M, Koren G. Hepatitis B vaccine in pregnancy: maternal and fetal safety. Am J Perinatol. 1991;8(3):227–32. [PubMed] [Google Scholar]
16. Grosheide PM, Schalm SW, van Os HC, Fetter WP, Heijtink RA. Immune response to hepatitis B vaccine in pregnant women receiving post-exposure prophylaxis. Eur J Obstet Gynecol Reprod Biol. 1993;50(1):53–8. [PubMed] [Google Scholar]
Grosheide PM, Schalm SW, van Os HC, Fetter WP, Heijtink RA. Immune response to hepatitis B vaccine in pregnant women receiving post-exposure prophylaxis. Eur J Obstet Gynecol Reprod Biol. 1993;50(1):53–8. [PubMed] [Google Scholar]
17. Public Health Agency of Canada [website] Canadian immunization guide. 7th edition. Ottawa, ON: Public Health Agency of Canada; 2006. Available from: www.phac-aspc.gc.ca/publicat/cig-gci/index-eng.php. Accessed 2010 Nov 4. [Google Scholar]
18. Silveira CM, Cáceres VM, Dutra MG, Lopes-Camelo J, Castilla EE. Safety of tetanus toxoid in pregnant women: a hospital-based case-control study of congenital anomalies. Bull World Health Organ. 1995;73(5):605–8. [PMC free article] [PubMed] [Google Scholar]
19. Immunization during pregnancy ACOG technical bulletin number 160—October 1991. Int J Gynaecol Obstet. 1993;40(1):69–79. [PubMed] [Google Scholar]
20. Dastur FD, Awatramani VP, Chitre SK, D’Sa JA. A single dose vaccine to prevent neonatal tetanus.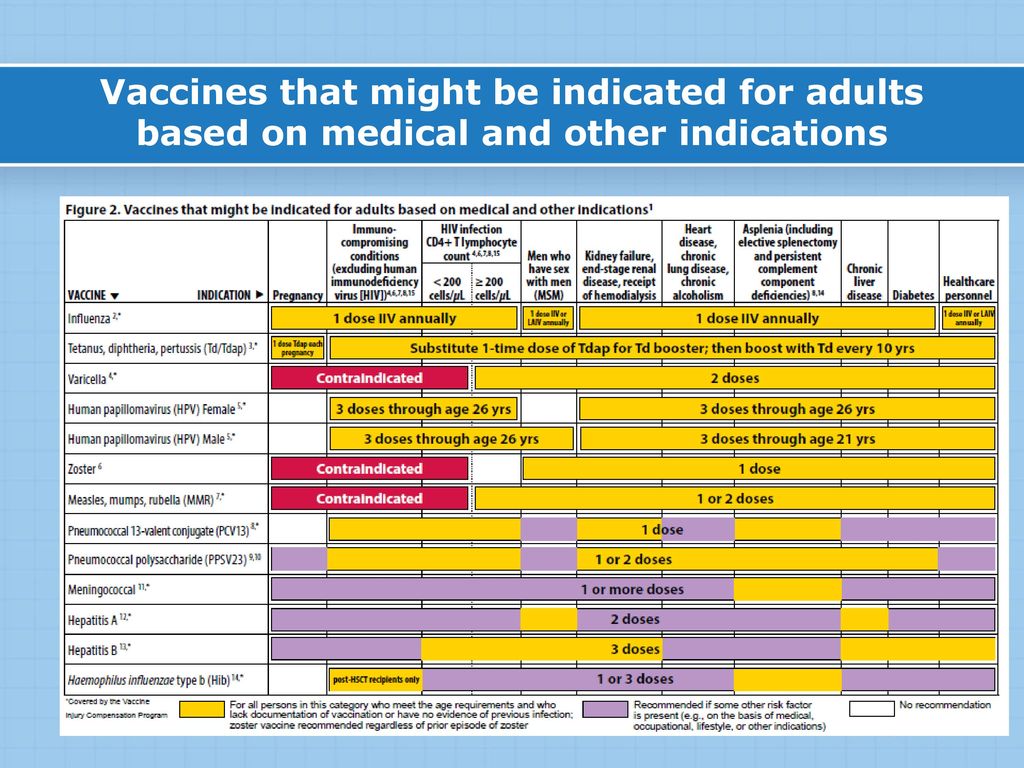 J Assoc Physicians India. 1993;41(2):97–9. [PubMed] [Google Scholar]
J Assoc Physicians India. 1993;41(2):97–9. [PubMed] [Google Scholar]
21. Czeizel AE, Rockenbauer M. Tetanus toxoid and congenital abnormalities. Int J Gynaecol Obstet. 1999;64(3):253–8. [PubMed] [Google Scholar]
22. Murphy TV, Slade BA, Broder KR, Kretsinger K, Tiwari T, Joyce PM, et al. Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-4):1–51. [PubMed] [Google Scholar]
23. Cohen P, Scadron SJ. The effects of active immunization of the mother upon the offspring. J Pediatr. 1946;29(5):609–19. [PubMed] [Google Scholar]
MMR vaccine II: measles, rubella, mumps
MMR II vaccine available! We vaccinate children against measles, rubella and mumps.
We stand for safe, responsible vaccination! All vaccination rules are strictly observed. We have anti-stress vaccinations in our clinic: interactive toys, soap bubbles, a special Buzzy Ladybug, virtual reality glasses with 3D cartoons!
Make an appointment via WhatsApp
Video Prices Doctors
The first children's clinic of evidence-based medicine in Moscow
No unnecessary examinations and drugs! We will prescribe only what has proven effective and will help your child.
Treatment according to world standards
We treat children with the same quality as in the best medical centers in the world.
The best team of doctors in Fantasy!
Pediatricians and subspecialists Fantasy - highly experienced doctors, members of professional societies. Doctors constantly improve their qualifications, undergo internships abroad.
Ultimate safety of treatment
We have made children's medicine safe! All our staff work according to the strictest international standards JCI
We have fun, like visiting best friends
Game room, cheerful animator, gifts after the reception. We try to make friends with the child and do everything to make the little patient feel comfortable with us.
You can make an appointment by calling or by filling out the form on the site
Other vaccinations
- Imported vaccines in stock!
- Adasel - vaccination against diphtheria, tetanus and whooping cough
- BiVac polio polio vaccine
- Vactrivir Measles, rubella, mumps vaccine
- DPT 9 vaccine0042 Whooping cough, diphtheria, tetanus vaccine
- Vaccine Infanrix Whooping cough, diphtheria, tetanus
- Vaccine Infanrix Hexa
- Pentaxim vaccine Poliomyelitis, diphtheria, tetanus, whooping cough, Haemophilus influenzae type b (Hib) vaccine
- Rabies vaccination
- Varilrix - chickenpox vaccine
- Polimilex polio vaccine
- Chickenpox vaccine Varivax
- HPV (human papillomavirus) vaccine
- Hepatitis A vaccination
- Hepatitis B vaccination
- Tick-borne encephalitis vaccine
- Measles, rubella and mumps vaccine
- Vaccination against meningococcal infection
- Vaccination against pneumococcal infection
- Vaccination against rotavirus infection
- Ultrix Quadri or Influvac.
 Flu shot for the whole family
Flu shot for the whole family - Hiberix: Haemophilus influenzae type b (Hib) vaccine
Online payment
Documents online
Online services
Vaccine "MMP-II" - Virilis
"MMP 2" - vaccination against measles, mumps and rubella
Live vaccine (sterile lyophilized preparation, attenuated) for the prevention of measles, rubella, mumps
Manufacturer: Merck Sharp & Dohme Corp (USA)
Protects against viral diseases: measles, mumps and rubella.
Applies to: children aged 12 months and over.
Included in the National Immunization Schedule.
Benefits of MMR-II vaccine
- Low reactogenicity of the vaccine.

- Can be used for emergency immunization.
Indications for vaccination "MMR-II"
The vaccine contains attenuated measles, mumps, and rubella viruses and is indicated for simultaneous vaccination against these infections in children 12 months of age and older. Vaccination is carried out at the age of 12 to 15 months, revaccination - at the age of 4 to 6 years.
Vaccination of persons in contact with a patient with measles may provide some protection if the vaccine is given within the first 72 hours of exposure.
Compatibility with other vaccines
MMR-II should be given one month before or after other live virus vaccines. Co-administration of measles, mumps, and rubella vaccine is not recommended with DTP or oral poliovirus vaccine.
Immunoglobulin should not be given concomitantly with MMP-II.
If a tuberculin test (Mantoux test) is required, it must be done either before or at the same time as vaccination with MMP-II
Warnings: Do not use in pregnant or lactating women
The use of the vaccine during pregnancy and breastfeeding is strictly contraindicated. If women are vaccinated, pregnancy should be avoided for 3 months after vaccination.
If women are vaccinated, pregnancy should be avoided for 3 months after vaccination.
Contraindications
Only a doctor can decide if the MMR-II vaccine is suitable for vaccination
"MMR-II" vaccine is contraindicated in the presence of a history of allergic reaction to any component of the vaccine.
MMR-II vaccination is contraindicated in the following cases:
- Allergic reactions to vaccine components, including gelatin, neomycin.
- Acute infectious and non-communicable diseases, exacerbation of chronic diseases.
- Leukemias, lymphomas of any type, or other malignant neoplasms affecting the bone marrow or lymphatic system.
- Primary or acquired immunodeficiency states.
- Family history of congenital or hereditary immunodeficiency.
- Immunosuppressive therapy.
Special care should be taken when using MMR-II for persons with:
- history of traumatic brain injury,
- individual or family history of seizures, including fever,
- for egg allergy;
- for thrombocytopenia.