Vaccines given during pregnancy
Vaccines During Pregnancy FAQs | Vaccine Safety
Moro PL, Zheteyeva Y, Lewis P, Shi J, Yue X, Museru OI, et al. Safety of quadrivalent human papillomavirus vaccine (Gardasil®) in pregnancy: Adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006-2013. Vaccine. 2015 Jan 15; 33(4):519-22. Epub 2014 Dec 8.
Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway AL, Klein NP, Cheetham TC, et al. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink sites. Prev Med. 2014 Oct; 67: 316-9. Epub 2014 Jun 18.
Naleway AL, Irving SA, Henninger ML, Li DK, Shifflett P, Ball S, Williams JL, Cragan J, Gee J, Thompson MG, Vaccine Safety Datalink and Pregnancy and Influenza Project. Safety of influenza vaccination during pregnancy: A review of subsequent maternal obstetric events and findings from two recent cohort studies. Vaccine. 2014 May 30; 32(26): 3122-3127. Epub 2014 Apr 14.
Naleway AL, Kurosky S, Henninger ML, Gold R, Nordin JD, Kharbanda EO, Irving S, Craig Cheetham T, Nakasato C, Glanz JM, Hambridge SJ, Davis RL, Klein NP, McCarthy NL, Weintraub E. Vaccinations given during pregnancy, 2002-2009: A descriptive study. Am J Prev Med. 2014 Feb; 46(2): 150-157.
Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014 Jun; 210(6): 561.e1-6. Epub 2013 Dec 27.
Moro PL, Museru OI, Broder K, Cragan J, Zheteyeva Y, Tepper N, Revzina N, Lewis P, Arana J, Barash F, Kissin D, Vellozzi C. Safety of influenza A (h2N1) 2009 live attenuated monovalent vaccine in pregnant women. Obstet Gynecol. 2013 Dec; 122(6): 1271-8.
Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD, Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013 Sep; 122(3): 659-667.
Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, Kharbanda EO, Irving S, Cheetham TC, McCarthy NL. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013 Jun 12; 31(27): 2898-2903. Epub 2013 Apr 30.
Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013 Jun 12; 31(27): 2898-2903. Epub 2013 Apr 30.
Henninger M, Naleway A, Crane B, Donahue J, Irving S. Predictors of seasonal influenza vaccination during pregnancy. Obstet Gynecol. 2013 Apr; 121(4): 741-749.
Zheteyeva Y, Moro PL, Yue X, Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: A review of the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2013 Jun; 208(6): 478.e1-6. Epub 2013 Feb 20.
Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, Cheetham TC, Jackson LA, Naleyway AL, GLanz JM, Nordin JD, Belongia EA, Vaccine Safety Datalink. Trivalent inactivated Influenza vaccine and spontaneous abortion. Obstet Gynecol. 2013 Jan; 121(1): 159-65.
Moro PL, Tepper NK, Grohskopf LA, Vellozzi C, Broder K. Safety of seasonal influenza and influenza A (h2N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines. 2012 Aug; 11(8): 911-21.
Expert Rev Vaccines. 2012 Aug; 11(8): 911-21.
Kharbanda EO, Vazquez-Benitez G, Shi WX, Lipkind H, Naleway A, Molitor B, Kuckler L, Olsen A, Nordin JD. Assessing the safety of influenza immunization during pregnancy: The Vaccine Safety Datalink. Am J Obstet Gynecol. 2012 Sep; 207(3 Suppl): S47-S51. Epub 2012 Jul 9.
Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, Kissin D, Lewis PW, Yue X, Haber P, Tokars JI, Vellozzi C, Broder KR. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012 Jul; 207(1):59.e1-7. Epub 2012 May 14.
Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, Barash F, Arana J, Brantley MD, Ding H, Singleton JA, Walton K, Haber P, Lewis P, Yue X, Destefano F, Vellozzi C. Adverse events following administration to pregnant women of influenza A (h2N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2011 Nov; 205(5): 473.e1-9. Epub 2011 Jun 21.
Am J Obstet Gynecol. 2011 Nov; 205(5): 473.e1-9. Epub 2011 Jun 21.
Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011 Feb; 204(2): 146.e1-7. Epub 2010 Oct 20.
France EK, Smith-Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, Shay D, Weintraub E, Fry AM, Black SB, Shinefield HR, Mullooly JP, Jackson LA. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med. 2006 Dec; 160(12): 1277-1283.
Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D, Vaccine Safety Datalink Workgroup. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004 Aug; 21(6):333-339.
Am J Perinatol. 2004 Aug; 21(6):333-339.
Vaccine Safety for Moms-to-Be | CDC
Pregnant women may safely receive inactivated vaccines (Tdap and flu), mRNA (Moderna and Pfizer), and viral vector vaccines (J&J).
Vaccines help protect pregnant people and babies against serious diseases
Pregnant people share everything with their babies. That means when a pregnant person gets vaccines, she isn’t just protecting herself— they are giving the baby some early protection too.
CDC has recommendations for the vaccines needed before, during, and after pregnancy. Currently, CDC routinely recommends Tdap and flu shots during pregnancy.
- Get the Tdap vaccine (to help protect against whooping cough), during pregnancy.
- The flu shot can be given before or during pregnancy, depending on whether or not it is flu season during a pregnancy.
- It is safe for pregnant people to receive vaccines right after giving birth, even while breastfeeding.

- Some vaccines, such as the measles, mumps, rubella (MMR) vaccine, should be given a month or more before pregnancy if a pregnant person didn’t get the vaccine as a child.
Live virus vaccines, such as the MMR and chickenpox, should not be given to pregnant people, but should be given to them before or after pregnancy, if indicated. Talk to your doctor about the MMR, Tdap, and flu vaccines before getting vaccinated. The COVID-19 vaccine is also recommended for pregnant people. The authorized and recommended COVID-19 vaccines for pregnant people are the mRNA Moderna and Pfizer-BioNTech vaccines, which contain no live virus, and the J&J/Janssen viral vector vaccine, meaning it uses a modified version of a different virus (the vector) to deliver important instructions to our cells. If you have any questions about these vaccines, talk to your doctor.
Vaccine safety before, during, and after pregnancy
It’s important to know that the Tdap and flu vaccines are safe for a pregnant person and their baby. Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
- The Tdap and flu vaccines are inactivated vaccines, which means they are made by inactivating or killing the germ during the process of making the vaccine.
- Studies done on the Tdap vaccine have concluded that it is safe and effective for pregnant people and babies.
- Similarly, results from multiple studies on the flu shot continue to support the safety and effectiveness of the vaccine during pregnancy.
- There is limited information available about the safety of the COVID-19 vaccines for people who are pregnant; however, based on how these vaccines work in the body, experts believe they are unlikely to pose risk for pregnant people.
It is important to get MMR before becoming pregnant to reduce the risk of becoming infected with rubella which can pass on to the unborn child, causing Congenital Rubella Syndrome (CRS). CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
- Live vaccines are generally not recommended during pregnancy.
- If a pregnant person did not get MMR as a child, she should get the vaccine before pregnancy.
All vaccines are held to the highest standards of safety—meaning they are carefully studied and monitored for side effects. Vaccines are like any medicine, which means they can have some side effects. However, most people who get vaccinated have no side effects or only mild side effects. CDC continually monitors vaccine safety, and the most common side effects seen are mild and go away quickly on their own (redness, swelling, and tenderness at the site where the shot was given. Other possible side effects associated with the COVID-19 vaccine are tiredness, headache, muscle pain, chills, fever, and nausea. ).
).
For more studies, the FDA also has a pregnancy exposure registry,external icon which is a study that collects health information from pregnant persons who take medicines or vaccines when they are pregnant.
Doctors explained when pregnant women need to be vaccinated against COVID-19 - RBC
adv.rbc.ru
adv.rbc.ru
adv.rbc.ru
Hide banners
What is your location ?
YesSelect other
Categories
Euro exchange rate as of November 30
EUR CB: 63.39 (+0.09) Investments, 16:04
Dollar exchange rate as of November 30
USD Central Bank: 61. 07 (+0.32) Investments, 16:04
07 (+0.32) Investments, 16:04
The coach of the national team with a quote from Utyosov's song answered Maigurov's criticism Sport, 20:28
Medvedev invited NATO to "repent before humanity" Politics, 20:24
The Central Bank announced the creation of a housing savings system for mortgage lenders Real estate, 20:19
adv.rbc.ru
adv.rbc.ru
Belarusian oppositionist Kolesnikova was taken to intensive care from a colony Politics, 20:18
Education of the future: technology, quality and new perspectives Special project, 20:18
Actress Svetlana Svetlichnaya hospitalized Society, 20:08
The Netherlands and Senegal made it to the 1/8 finals. What's happening in Qatar Sports, 20:05
What's happening in Qatar Sports, 20:05
Explaining what the news means
RBC Evening Newsletter
Subscribe
The Senegal national team for the first time in 20 years reached the playoffs of the World Cup Sport, 19:58
How the used car market works in 2022 RBC and Avto.ru, 19:55
Qatar team showed the worst result in history among the hosts of the World Cup Sports, 19:55
The Foreign Ministry announced the expectation from the United States to create conditions for a meeting on START Politics, 19:43
Visa-free paradise for snowboarders: how to choose a winter resort in Korea RBC and NOTK, 19:32
Arzamas Machine Plant denied the appointment of Galkin's brother as the head of the enterprise Politics, 19:32
Rosgosstrakh Life client from Astrakhan won ₽1 million Press release, 19:28
adv. rbc.ru
rbc.ru
adv.rbc.ru
adv.rbc.ru
Photo: AGN "Moscow"
Vaccination of pregnant women is usually carried out from the third trimester, when all the organs and systems of the child are fully formed, Zoya Skorpileva, an immunologist at the European Vaccination Center, told RBC.
Pregnant women should only be vaccinated if they are at risk and if the risk of getting COVID-19 and its complications outweighs all other risks, she said.
adv.rbc.ru
“The virus primarily affects the blood vessels, and in pregnant women the placenta suffers. Premature births and miscarriages can happen - we saw this both in the first and in the second wave. Children born with intrauterine pneumonia, no matter whether bacterial or viral, have a not very favorable course of the disease, ”explained Skorpileva.
adv.rbc.ru
So far, only Sputnik V can be grafted. Vaccines "Sputnik Light", "EpiVacCorona" and "KoviVac" are not recommended for vaccination of pregnant women, the doctor added.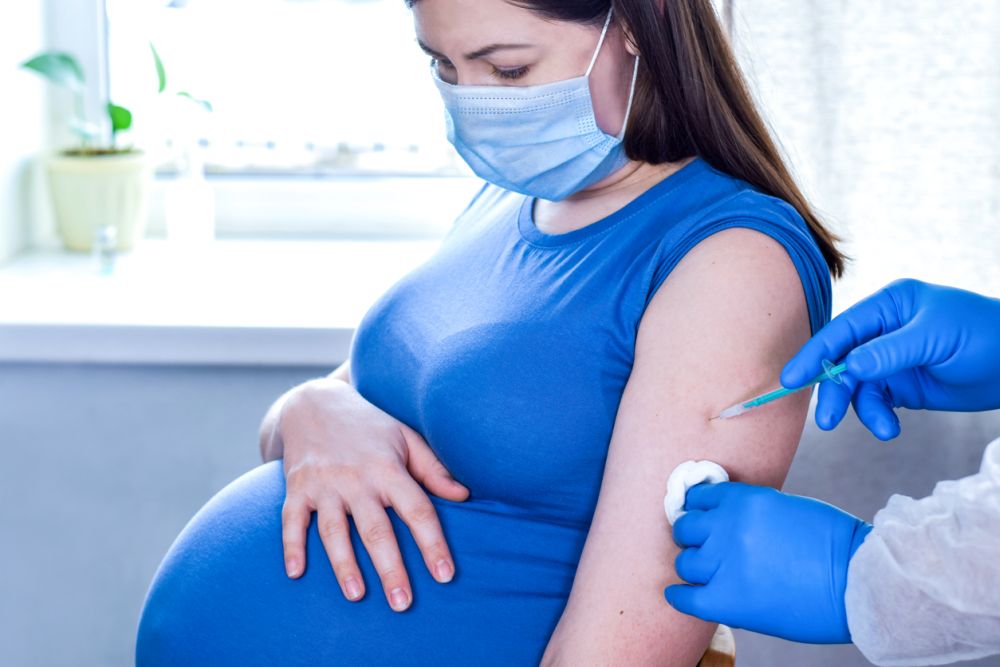
Obstetrician, Deputy Chief Physician of the Center for Motherhood and Childhood in Shchelkovo Arina Chak, in a conversation with RBC, recalled that, according to the recommendations of the Ministry of Health, only pregnant women from the risk group and from the 22nd week of pregnancy (second trimester) are recommended to be vaccinated against COVID-19 .
“Vaccination is recommended from the 22nd week because organogenesis is already completed, that is, the organs and systems of the fetus's organs have already been formed. The risk to the child becomes minimal. In the first trimester, until the 12th-14th week, no vaccination is recommended at all, as organogenesis is underway, ”the doctor explained.
Previously, Alexander Gorelov, deputy director for research at the Central Research Institute of Epidemiology of Rospotrebnadzor, on the air of the Moskva Speaks radio station, said that pregnant women can only be vaccinated in the third trimester of pregnancy, when all organs and systems of the child are already developed.
According to the recommendations of the Ministry of Health, it is advisable to vaccinate pregnant women at risk of severe COVID-19 and from the 22nd week of pregnancy. These are pregnant women with obesity, chronic lung disease, diabetes mellitus, cardiovascular disease, chronic kidney disease and liver disease. The department noted that at the moment there is no evidence that the Sputnik V vaccine poses a threat to pregnant women or to the fetus.
9Olo with virusData for Russia i
As far as vaccines against COVID-19safe for pregnant women and those planning to become pregnant? WHO responds
1. Can pregnant women be vaccinated against COVID-19?
Short answer: yes. Pregnant women can be vaccinated against COVID-19. Vaccines provide reliable protection against serious diseases caused by coronavirus. Pregnant women, if not already vaccinated, should have access to WHO-approved vaccines, as COVID-19 during pregnancy puts them at higher risk of serious illness and premature birth.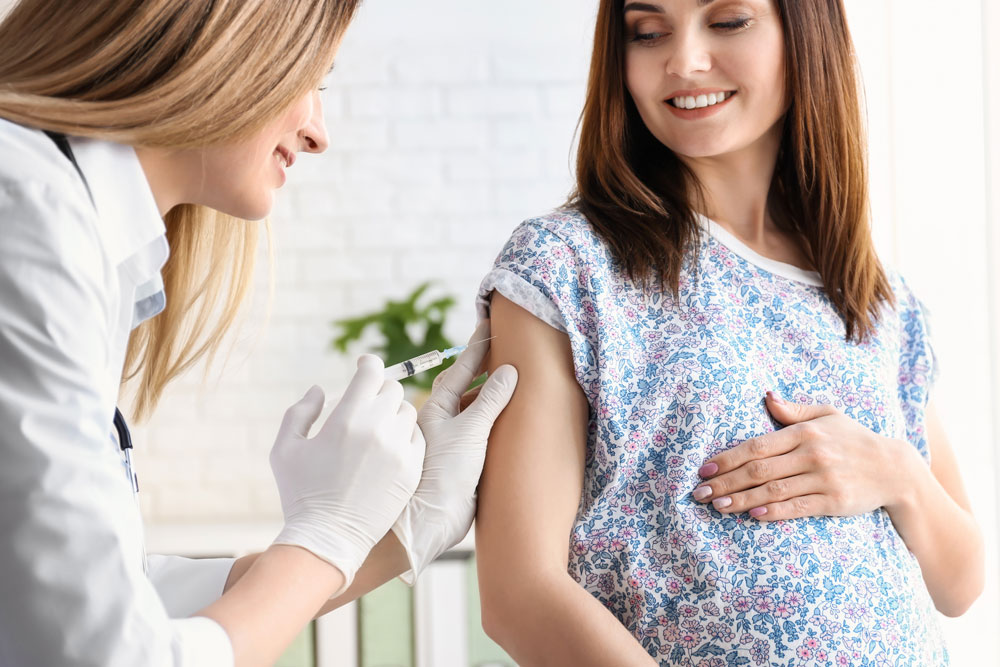
Growing evidence of the safety and efficacy of COVID-19 vaccination during pregnancy suggests that the benefits of vaccination during pregnancy outweigh the potential risks. Vaccination against COVID-19 before or during pregnancy is particularly important in settings with moderate or high risk of transmission in a particular community, and for women with an increased individual risk of infection or severe disease.
2. Like COVID-19affect pregnant women?
Multiple studies show that pregnant women with COVID-19 are more likely to develop severe illness than non-pregnant women. This means that pregnant women who are infected with the coronavirus are more likely to require hospitalization, intensive care, and invasive ventilation to help breathe easier. In addition, compared with healthy pregnant women, pregnant women with COVID-19 have an increased risk of preterm birth and babies requiring intensive care. They may also have an increased risk of stillbirth and maternal death.
Pregnant women of mature age (35 years and older), who are overweight, or who have conditions such as diabetes or hypertension may be at even higher risk of serious adverse health outcomes.
3. Are COVID-19 vaccines effective during pregnancy?
Research has found that COVID-19 vaccines are highly effective in preventing severe illness, hospitalizations, and deaths from COVID-19. Given experience with other vaccines during pregnancy, scientists expect all WHO-approved COVID-19 vaccines towill work equally effectively regardless of the presence or absence of pregnancy.
In addition, studies have shown that pregnant women who are vaccinated against COVID-19 develop antibodies that pass into the cord blood of babies. This suggests that children may also be protected as a result of mothers being vaccinated.
4. What is known about the safety of COVID-19 vaccines during pregnancy?
Although pregnant women were not included in initial clinical trials of COVID-19 vaccines, the database of evidence of the safety of vaccination during pregnancy is constantly expanding.
In several countries where COVID-19 vaccination is widely used during pregnancy, pregnant women are under constant surveillance. At the same time, they did not have any problems associated with the course of pregnancy.
Thus, as of February 2022, more than 198,000 pregnant women were under observation after vaccination in the USA. Most of them received Pfizer-BioNTech, BNT162b2 and Moderna mRNA-1273 vaccines. The study did not find any adverse outcomes associated with vaccination during pregnancy.
In the UK, as of February 2022, more than 100,000 pregnant women have been vaccinated against COVID-19. Most of them were vaccinated with mRNA vaccines; approximately 10 percent received the AstraZeneca AZD1222 vaccine. Data analysis revealed similar rates of birth outcome in both vaccinated and unvaccinated pregnant women.
All vaccines covered by WHO advance recommendations have been tested in animals. These studies did not demonstrate any harmful effects of vaccination in pregnant animals and their young.
None of the vaccines covered by the WHO interim recommendations contain the live virus that causes COVID-19. This means that vaccines are basically incapable of infecting pregnant women or their children.
5. Should women who are trying to conceive be vaccinated against COVID-19?
Yes. Pre-vaccination is an important tool to protect women and their children from COVID-19 during pregnancy. Women who are trying to get pregnant can get vaccinated against COVID-19. A growing body of data has not revealed any negative effect of vaccination on fertility or the ability to conceive. In vaccine clinical trials and in a large study of couples trying to conceive, pregnancy rates were similar for those who received COVID-19 vaccines and those who did not.
WHO does not recommend postponing or terminating pregnancy due to COVID-19 vaccination. According to experts, no pregnancy tests should be done before vaccination.
6. So, in summary, what do pregnant women and those who plan to get pregnant need to know about COVID-19 vaccinations?
Given the significant risks associated with COVID-19 during pregnancy, it is critical that pregnant women and those who are planning to become pregnant have access to WHO-approved COVID-19 vaccines as soon as possible to help protect their health and that of their children. Current evidence suggests that if pregnant women are not already vaccinated, the benefits of vaccination against COVID-19during pregnancy outweighs any potential risks.
Current evidence suggests that if pregnant women are not already vaccinated, the benefits of vaccination against COVID-19during pregnancy outweighs any potential risks.
Pregnant women and those who plan to become pregnant should be informed in a timely manner about the risks of contracting COVID-19 during pregnancy, the benefits of vaccination, and the factors that indicate the benefits of vaccination:
• Infection with COVID-19 during pregnancy can lead to consequences: Available evidence suggests that pregnant women with COVID-19 are at increased risk of severe illness, premature birth, and likely other adverse pregnancy outcomes such as stillbirth.
• COVID-19 vaccines are extremely effective, providing strong protection against serious illness and death due to coronavirus infection. Pregnant women appear to receive the same level of protection from vaccination as non-pregnant women.
• New data on the safety of vaccination during pregnancy are encouraging: to date, animal studies, observation of pregnant women who have received vaccines, and experience with vaccines with similar components have not revealed any safety problems during pregnancy.