Radiation exposure to fetus
Radiation and Pregnancy: Information for Clinicians
This overview provides physicians with information about prenatal radiation exposure as an aid in counseling pregnant women.
How to use this document
This information is for clinicians. If you are a patient, we strongly advise that you consult with your physician to interpret the information provided, as it may not apply to you. Information on radiation exposure during pregnancy for members of the public can be found on the Health Information for Specific Groups webpage.
CDC recognizes that providing information and advice about radiation to expectant mothers falls into the broader context of preventive healthcare counseling during prenatal care. In this setting, the purpose of the communication is always to promote health and long-term quality of life for the mother and child.
This page is also available as a PDF pdf icon[365 KB]
Radiation exposure to a fetus
Most of the ways a pregnant woman may be exposed to radiation, such as from a diagnostic medical exam or an occupational exposure within regulatory limits, are not likely to cause health effects for a fetus. However, accidental or intentional exposure above regulatory limits may be cause for concern.
Although radiation doses to a fetus tend to be lower than the dose to the mother, due to protection from the uterus and surrounding tissues, the human embryo and fetus are sensitive to ionizing radiation at doses greater than 0.1 gray (Gy). Depending on the stage of fetal development, the health consequences of exposure at doses greater than 0.5 Gy can be severe, even if such a dose is too low to cause an immediate effect for the mother. The health consequences can include growth restriction, malformations, impaired brain function, and cancer.
Estimating the Radiation Dose to the Embryo or Fetus
Health effects to a fetus from radiation exposure depend largely on the radiation dose. Estimating the radiation dose to the fetus requires consideration of all sources external and internal to the mother’s body, including the following:
- Dose from an external source of radiation to the mother’s abdomen.

- Dose from inhaling or ingesting a radioactive substance that enters the bloodstream and that may through the placenta.
- Dose from radioactive substances that may concentrate in maternal tissues surrounding the uterus, such as the bladder, and that could irradiate the fetus.
Most radioactive substances that reach the mother’s blood can be detected in the fetus’ blood. The concentration of the substance depends on its specific properties and the stage of fetal development. A few substances needed for fetal growth and development (such as iodine) can concentrate more in the fetus than in corresponding maternal tissue.
Consideration of the dose to specific fetal organs is important for substances that can localize in specific organs and tissues in the fetus, such as iodine-131 or iodine-123 in the thyroid, iron-59 in the liver, gallium-67 in the spleen, and strontium-90 and yttrium-90 in the skeleton.
Radiation experts can assist in estimating the radiation dose to the embryo or fetus
Hospital medical physicists and health physicists are good resources for expertise in estimating the radiation dose to the fetus.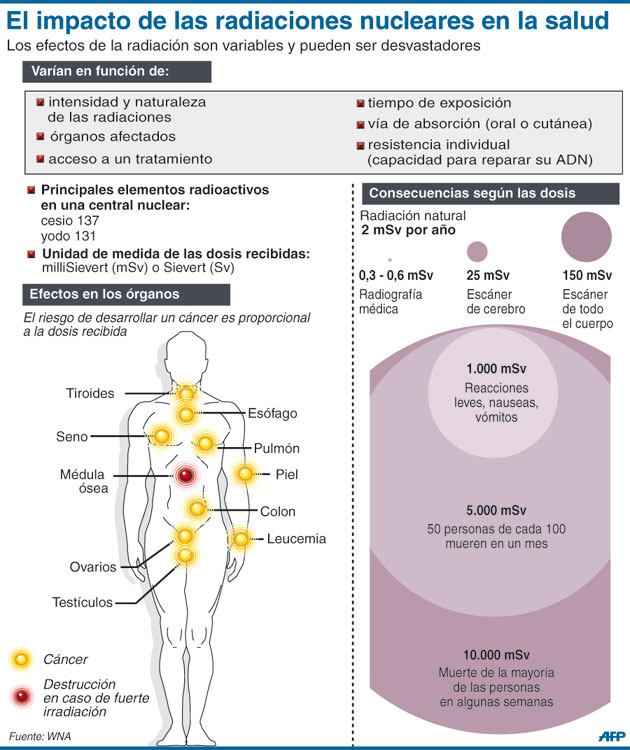 In addition to the hospital or clinic’s specialized staff, physicians may access resources from or contact the following organizations for assistance in estimating fetal radiation dose.
In addition to the hospital or clinic’s specialized staff, physicians may access resources from or contact the following organizations for assistance in estimating fetal radiation dose.
- The National Council on Radiation Protection and Measurementsexternal icon’ Report No. 174, “Preconception and Prenatal Radiation Exposure: Health Effects and Protective Guidance” [NCRP2013] provides detailed information for assessing fetal doses from internal uptakes.
- The International Commission on Radiological Protection’s “Publication 84: Pregnancy and Medical Radiation”external icon [ICRP2000] provides fetal dose estimations from medical exposures to pregnant women.
- The Conference of Radiation Control Program Directorsexternal icon maintains a list of state Radiation Control/Radiation Protection program contact information.
- The Health Physics Societyexternal icon maintains a list of active certified Health Physicists.
- The American Association of Physicists in Medicineexternal icon provides information resources.
Once the fetal radiation dose is estimated, potential health effects can be assessed.
Potential Health Effects of Prenatal Radiation Exposure (Other Than Cancer)
Table 1 summarizes the potential non-cancer health risks of concern. This table is intended to help physicians advise pregnant women who may have been exposed to radiation, not as a definitive recommendation. The indicated doses and times post-conception are approximations.
| Acute Radiation Dose* to the Embryo/Fetus | Time Post Conception (up to 2 weeks) | Time Post Conception (3rd to 5th weeks) | Time Post Conception (6th to 13th weeks) | Time Post Conception (14th to 23rd weeks) | Time Post Conception (24th week to term) |
|---|---|---|---|---|---|
<0. 10 Gy 10 Gy (10 rads) | Noncancer health effects NOT detectable | ||||
| 0.10–0.50 Gy (10–50 rads) | Failure to implant may increase slightly, but surviving embryos will probably have no significant (non-cancer) health effects. | Growth restriction possible | Growth restriction possible | Noncancer health effects unlikely | |
| > 0.50 Gy (50 rads) The expectant mother may be experiencing acute radiation syndrome in this range, depending on her whole-body dose. | Failure to implant will likely be high, depending on dose, but surviving embryos will probably have no significant (non- cancer) health effects. | Probability of miscarriage may increase, depending on dose. Probability of major malformations, such as neurological and motor deficiencies, increases. Growth restriction is likely | Probability of miscarriage may increase, depending on dose. Growth restriction is likely. | Probability of miscarriage may increase, depending on dose. Growth restriction is possible, depending on dose. (Less likely than during the 6th to 13th weeks post conception) Probability of major malformations may increase | Miscarriage and neonatal death may occur, depending on dose. § |
| 8th to 25th Weeks Post Conception: The most vulnerable period for intellectual disability is 8th to 15th weeks post conception Severe intellectual disability is possible during this period at doses > 0.5 Gy Prevalence of intellectual disability (IQ<70) is 40% after an exposure of 1 Gy from 8th to 15th week Prevalence of intellectual disability (IQ<70) is 15% after an exposure of 1 Gy from 16th to 25th week Table adapted from Table 1.1. of the National Council on Radiation Protection and Measurements’ Report No. | |||||
Gestational age and radiation dose are important determinants of potential non-cancer health effects. The following points are of particular note.
- During the first 2 weeks post-conception, the health effect of concern from an exposure of ≥ 0.1 Gy is the possibility of death of the embryo. Because the embryo is made up of only a few cells, damage to one cell, the progenitor of many other cells, may cause the death of the embryo, and the blastocyst may fail to implant in the uterus. Embryos that survive, however, are unlikely to exhibit congenital abnormalities or other non-cancer health effects, no matter what dose of radiation they received.
- In all stages post-conception, radiation-induced non-cancer health effects are not detectable for fetal doses below about 0.10 Gy.
Carcinogenic Effects of Prenatal Radiation Exposure
Radiation exposure to an embryo/fetus may increase the risk of cancer in the offspring, especially at radiation doses > 0. 1 Gy, which are well above typical doses received in diagnostic radiology. However, attempting to quantify cancer risks from prenatal radiation exposure presents many challenges. These challenges include the following:
1 Gy, which are well above typical doses received in diagnostic radiology. However, attempting to quantify cancer risks from prenatal radiation exposure presents many challenges. These challenges include the following:
- The primary data for the risk of developing cancer from prenatal exposure to radiation come from the lifespan study of the Japanese atomic bomb (A-bomb) survivors. [Preston et al. 2008]. The analysis of that cohort includes cancer incidence data only up to the age of 50 years. This precludes making lifespan risk estimates as a result of prenatal radiation exposure.
- From the Japanese lifespan study [Preston et al. 2008], it can be concluded that for those exposed
in early childhood (birth to age 5 years), the theoretical risk of an adult-onset cancer by age 50 is approximately ten-fold greater than the risk for those who received prenatal exposure. Therefore, the risk following prenatal exposure may be considerably lower than for radiation exposure in early childhood
[NCRP2013].
- No reliable epidemiological data are available from studies to determine which stage of pregnancy is the most sensitive for radiation-induced cancer in the offspring [NCRP2013].
The lifespan study of the Japanese A-bomb survivors is continuing as the cohort ages. Future analyses of the accumulating data should provide a better understanding of the lifetime risk of cancer from prenatal and early childhood radiation exposure.
References
ICRP2000] International Commission on Radiological Protection. 2000. Valentin, J. (2000). Pregnancy and medical radiation. Oxford: Published for the International Commission on Radiological Protection.
[NCRP2013] National Council on Radiation Protection and Measurements. 2013. Preconception and Prenatal Radiation Exposure Health Effects and Protective Guidance. (2013). Bethesda: National Council on Radiation Protection & Measurements.
Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K, Kasagi F, Shore RE. 2008. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst 100(6):428-436.
2008. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst 100(6):428-436.
[UNSCEAR2013] United Nations Scientific Committee on the Effects of Atomic Radiation. 2013. Sources, effects and risks of ionizing radiation. Vol. II, Scientific Annex B: Effects of radiation exposure of children.
For more information on medical management and other topics on radiation emergencies:
Radiation Emergency Assistance Center/Training Siteexternal icon (REAC/TS) is a program uniquely qualified to teach medical personnel, health physicists, first responders and occupational health professionals about radiation emergency medical response.
Radiation Emergency Medical Management (REMM)external icon provides guidance to health care providers (primarily physicians) about clinical diagnosis and treatment of radiation injury during radiological and nuclear emergencies.
Conference of Radiation Control Program Directorsexternal icon
Health Physics Societyexternal icon
International Commission on Radiological Protectionexternal icon
National Council on Radiation Protection and Measurementsexternal icon
American Association of Physicists in Medicineexternal icon
Radiation Exposure In Pregnancy - StatPearls
Ilsup Yoon; Todd L. Slesinger.
Slesinger.
Author Information
Last Update: May 8, 2022.
Introduction
Choosing the most appropriate imaging modality for pregnancy patients is a common clinical question encountered daily. The general principle for imaging during pregnancy is similar to imaging for the general population, with the goal of radiation exposure being as low as reasonably achievable (ALARA). What is unique during pregnancy is that fetus radiation exposure is an essential factor in deciding optimal imaging studies. Understanding of consequences of radiation exposure on a fetus, degrees of fetal radiation exposure by each imaging modality, and techniques on reducing fetal radiation exposure is vital in choosing the best diagnostic imaging modality. While it is crucial to minimize fetal radiation exposure as much as possible, it is essential to remember that diagnostic studies should not be avoided for fear of radiation exposure, especially when these studies can dramatically change patient management.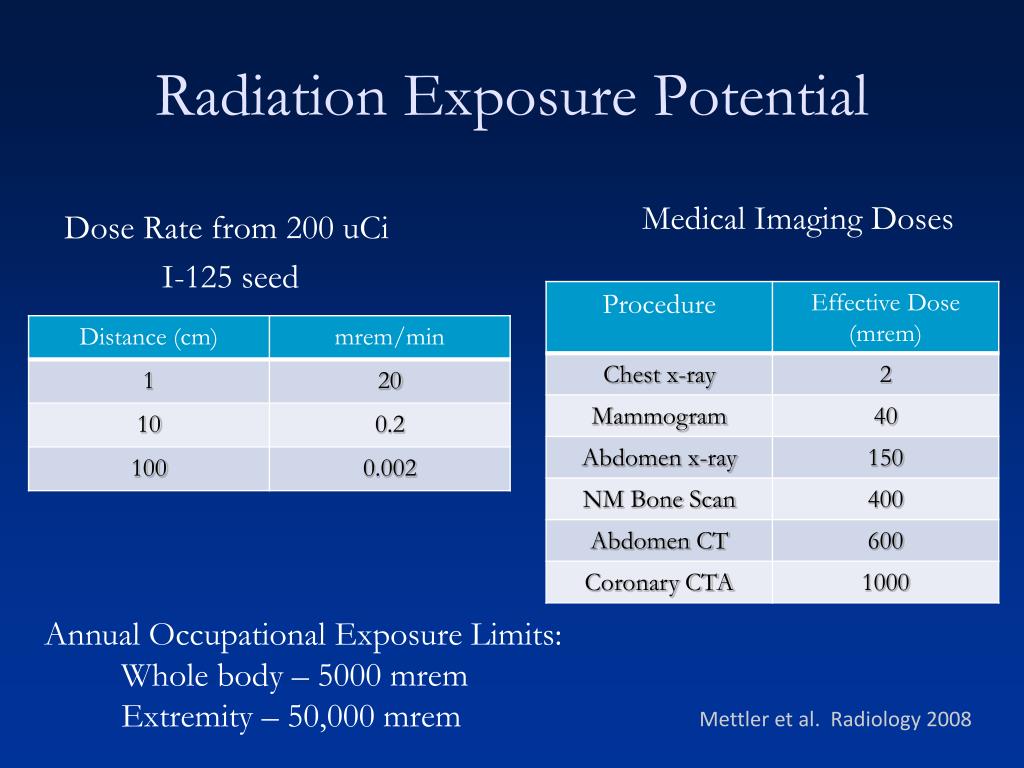 This activity will discuss the consequences of radiation exposure on a fetus, degrees of radiation exposure by each modality, and techniques of reducing fetal radiation exposure. Solid understanding of how each imaging modality contributes to fetal radiation dose will significantly help in choosing the most appropriate imaging study that provides the best diagnostic information at the lowest level of radiation exposure.
This activity will discuss the consequences of radiation exposure on a fetus, degrees of radiation exposure by each modality, and techniques of reducing fetal radiation exposure. Solid understanding of how each imaging modality contributes to fetal radiation dose will significantly help in choosing the most appropriate imaging study that provides the best diagnostic information at the lowest level of radiation exposure.
The consequence of radiation exposure in fetuses is mostly based on observations rather than based on scientific research. Ethical issues prohibit researching on the fetus. Therefore, most of the data on the impact of radiation on the fetus derives from observations of patients who suffered Japan’s Hiroshima bombing and the Chernobyl nuclear power plant disaster.[1][2] Based on the observations made from the victims of the high level of radiation exposure, the consequences of radiation exposure can categorize into four broad groups, including pregnancy loss, malformation, developmental delay or retardation, and carcinogenesis. [1] Pregnancy loss most often happens when radiation exposure happens during early gestation (less than two weeks).[2] Malformations of body parts and developmental delays occur during the organogenesis period (2 weeks to 8 weeks) and are dependent on the radiation dose.[2] Below the threshold level of radiation exposure, there is minimal disruption of organogenesis. Above the threshold, the degree of malformation is related to the dose of the radiation. Lastly, carcinogenesis is considered a stochastic effect. In other words, cancer can develop at any level of radiation exposure. However, the probability of developing cancer increases with the increase in the dose of radiation.
[1] Pregnancy loss most often happens when radiation exposure happens during early gestation (less than two weeks).[2] Malformations of body parts and developmental delays occur during the organogenesis period (2 weeks to 8 weeks) and are dependent on the radiation dose.[2] Below the threshold level of radiation exposure, there is minimal disruption of organogenesis. Above the threshold, the degree of malformation is related to the dose of the radiation. Lastly, carcinogenesis is considered a stochastic effect. In other words, cancer can develop at any level of radiation exposure. However, the probability of developing cancer increases with the increase in the dose of radiation.
In the United States, the background radiation exposure for the whole body per year is estimated to be 3.1 mSv (310 mrem). United States Nuclear Regulation Commission (USNRC) also recommends total fetus exposure during pregnancy to be less than 5.0 mSv (500 mrem). The fetus radiation dose below 50 mGy is considered safe and not cause any harm.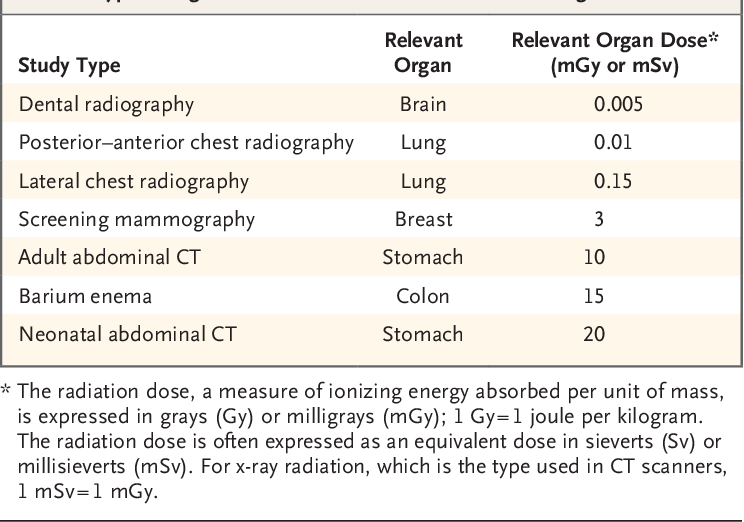 According to the Center for Disease Control (CDC), radiation dose between 50 mGy to 100 mGy is regarded inconclusive in terms of impact on the fetus. Doses above 100 mGy, especially doses above 150 mGy, are viewed as the minimum amount of dosage at which negative fetal consequences will occur, based on observation. The majority of the diagnostic studies performed during the pregnancy are below the threshold level.
According to the Center for Disease Control (CDC), radiation dose between 50 mGy to 100 mGy is regarded inconclusive in terms of impact on the fetus. Doses above 100 mGy, especially doses above 150 mGy, are viewed as the minimum amount of dosage at which negative fetal consequences will occur, based on observation. The majority of the diagnostic studies performed during the pregnancy are below the threshold level.
The effect of radiation exposure during pregnancy also depends on the gestational age of the fetus. The embryo/fetus is most susceptible to radiation during organogenesis (2 to 7 weeks gestational age) and in the first trimester. The fetus is more resistant to the radiation during the second and third trimester. Dose between 0.05 to 0.5 Gy is generally considered safe for the fetus during the second and third trimester while it is considered potentially harmful during the 1st-trimester fetus. Even though the fetus is more resistant to the radiation during the second and third trimester, a high dose of radiation (greater than 0. 5 Gy or 50 rad) may result in adverse effects including miscarriage, growth reduction, IQ reduction, and severe mental retardation. Therefore, clinicians and radiologists should counsel the pregnant patient regardless of the gestational age.[3]
5 Gy or 50 rad) may result in adverse effects including miscarriage, growth reduction, IQ reduction, and severe mental retardation. Therefore, clinicians and radiologists should counsel the pregnant patient regardless of the gestational age.[3]
Occupational radiation exposure for a pregnant employee should be monitored to make sure the total amount of radiation exposure is under the regulatory limit. According to the National Council of Radiation Protection and Measurement (NCRP), the total dose equivalent to the embryo/fetus should not exceed 500 mRem during the length of the pregnancy. It should not exceed 50mRem in any month during pregnancy.[4]
The practice decision on selecting the most appropriate imaging modality for pregnancy should have their basis in the expert opinions of the treating clinician. Nonetheless, the American College of Radiology does provide recommendations on the appropriateness of imaging modalities in accessing common clinical conditions. Each imaging modality is categories into usually appropriate, may be appropriate, and usually not appropriate. For instance, for a pregnant patient presenting with right lower abdominal pain concerning for appendicitis, ultrasound and MRI are usually appropriate imaging modality. CT abdomen and pelvis with or without contrast is categorized as may be appropriate. Abdominal radiograph, Tc-99m WBC scan, and fluoroscopy contrast enema are considered usually not appropriate. The American College of Radiology appropriateness criteria provides the current practice policies and guidelines in the US regarding imaging in pregnant patients.
For instance, for a pregnant patient presenting with right lower abdominal pain concerning for appendicitis, ultrasound and MRI are usually appropriate imaging modality. CT abdomen and pelvis with or without contrast is categorized as may be appropriate. Abdominal radiograph, Tc-99m WBC scan, and fluoroscopy contrast enema are considered usually not appropriate. The American College of Radiology appropriateness criteria provides the current practice policies and guidelines in the US regarding imaging in pregnant patients.
Anatomy
Understanding the anatomic location of the uterus is essential in understanding why a particular type of study contributes to a higher radiation dose. The uterus is located within the female pelvis. Therefore, studies that are further away from the pelvis contribute to less radiation dose than the studies that aim at the pelvis. Additionally, the uterus is located superior and anterior aspect of the pelvis during the pregnancy. X-ray beams that project in a posterior to anterior (PA) direction contribute to less radiation than the beam projected in anterior to posterior (AP) direction because, in PA projection, the X-ray gets attenuated before reaching anteriorly located uterus.
Plain Films
A single plain radiograph does not contribute to a significant radiation dose to the fetus. The estimated radiation dose to the fetus varies and ranges from 0.001 mGy to 10 mGy depending on the type of the study.[5] The highest radiation dose is the lumbar spine radiograph, which has a maximal fetal radiation dose of 10 mGy.[5] Nonetheless, even fetus radiation exposure for the lumbar spine radiograph is significantly lower than the threshold limit of the safe radiation exposure dose of 50 mGy, radiation level that is considered safe and without significant harm.
Regardless of the radiation dose, it is vital to reduce radiation exposure to the fetus as much as possible. Plain films are obtained more frequently than computed tomography (CT), and radiation doses from multiple plains films can easily accumulate. Therefore, it is essential to employee dose reducing techniques as often as possible. When performing plain film examination of non-pelvis structure, pelvic lead apron should always be utilized to reduced unnecessary fetus radiation. PA projection of the pelvis also contributes to the lower dose than the AP projection. Technologists should optimally position patients before taking a radiograph to reduce the number of repeat examinations from unsatisfactory views. Lastly, clinicians should obtain radiographs only when it is helping clinical management.
PA projection of the pelvis also contributes to the lower dose than the AP projection. Technologists should optimally position patients before taking a radiograph to reduce the number of repeat examinations from unsatisfactory views. Lastly, clinicians should obtain radiographs only when it is helping clinical management.
Computed Tomography
On the other hand, CT contributes to a significant amount of fetal radiation. The amount of fetus radiation exposure again varies by the type of study with CT pelvis contributing the highest amount of fetal radiation of 50 mGy.[5] This dose is right at the limit above which there is a documented negative impact to the fetus.
Because CT contributes to much higher fetal radiation, it is essential always to be considerate of other options when contemplating utilizing CT on pregnancy patients. Other imaging modalities, including MRI, plain radiograph, ultrasound, and nuclear medicine studies, should be considered first before performing CT. Common clinical presentations like appendicitis first require evaluation with MRI instead of CT.[6] Right upper quadrant ultrasound should be utilized if there is a clinical concern for cholecystitis.[7] Renal ultrasound should merit consideration before CT for nephrolithiasis and collecting system obstruction. If CT is the first indicate study of choice as in traumatic pregnancy patients to evaluate for intra-abdominal trauma, it is crucial to optimize CT setting to minimize the dose. Using wide pitch and narrow collimation can reduce the radiation dose. Besides, CT protocols should be optimized to minimize unnecessary radiation exposure. Clinicians should only perform additional delayed imaging when there is a clinical indication. Unnecessary multiphasic protocols should get simplified into a single-phase protocol.
Common clinical presentations like appendicitis first require evaluation with MRI instead of CT.[6] Right upper quadrant ultrasound should be utilized if there is a clinical concern for cholecystitis.[7] Renal ultrasound should merit consideration before CT for nephrolithiasis and collecting system obstruction. If CT is the first indicate study of choice as in traumatic pregnancy patients to evaluate for intra-abdominal trauma, it is crucial to optimize CT setting to minimize the dose. Using wide pitch and narrow collimation can reduce the radiation dose. Besides, CT protocols should be optimized to minimize unnecessary radiation exposure. Clinicians should only perform additional delayed imaging when there is a clinical indication. Unnecessary multiphasic protocols should get simplified into a single-phase protocol.
When obtaining CT images of body parts outside of the abdomen and pelvis, the scattered radiation exposure to the fetus is minimal. Therefore, a shield does not significantly reduce the radiation exposure on the fetus during the CT scan. A lead shield may be an unnecessary precaution. However, it does minimally reduce the dose from scatter radiation and may provide patients a sense of reassurance and protection. The use of a lead shield is up to the discretion of the institution and provider.[8]
A lead shield may be an unnecessary precaution. However, it does minimally reduce the dose from scatter radiation and may provide patients a sense of reassurance and protection. The use of a lead shield is up to the discretion of the institution and provider.[8]
Magnetic Resonance
MRI uses a magnetic field to generate diagnostic images and does not contribute to ionizing radiation.
Ultrasonography
Ultrasound uses sound waves to generate diagnostic images and does not contribute to ionizing radiation.
Nuclear Medicine
In nuclear medicine, radiopharmaceuticals are injected into patients. These radiopharmaceuticals are distributed throughout the body and emit radiation at the target location. Radiation energies from these radiotracers then convert into diagnostic images. The overall radiation exposure to the fetus depends on how much radiotracer gets delivered to or near the fetus. Fetal radiation exposure in nuclear medicine depends on multiple variables, including maternal excretion and uptake of the radiopharmaceutical, fetal distribution of the radiopharmaceutical, placental permeability of the radiopharmaceutical, tissue affinity of the radiopharmaceutical, the half-life of the radiotracer, the dose of the radiotracer, and type of radiation emitted from the radiotracer. Generally, for nuclear medicine studies utilizing a radiopharmaceutical that gets excreted through the kidneys, patients are encouraged to hydrate and urinate to maximize the urinary excretion of radiopharmaceuticals.
Generally, for nuclear medicine studies utilizing a radiopharmaceutical that gets excreted through the kidneys, patients are encouraged to hydrate and urinate to maximize the urinary excretion of radiopharmaceuticals.
Specific clinical scenarios worth mentioning for nuclear medicine study is pregnancy patient with concern for pulmonary embolism. The first imaging modality should be an ultrasound of lower extremity to look for deep venous thrombosis. If there is still clinical suspicion for pulmonary embolism, CT pulmonary angiogram (CTPA) is preferable to the ventilation-perfusion (VQ) scan. The fetal dose of VQ is much higher than CTPA, even though the maternal dose is much lower. Due to the lower fetal dose, CTPA is the preferred test of choice.[9]
The thyroid iodine scan is not an option during pregnancy. Iodine 121 and 131 are taken up by fetus thyroid and therefore contraindicated.[10]
Angiography
Fetal radiation exposure from angiography and fluoroscopy should only be for emergent clinical settings. A physician performing fluoroscopy should utilize essential dose reducing techniques including pulse fluoroscopy instead of continuous fluoroscopy, last image hold rather than full exposure, and colimitation to appropriate field of view. Magnification increases the radiation dose and should be used only if necessary.
A physician performing fluoroscopy should utilize essential dose reducing techniques including pulse fluoroscopy instead of continuous fluoroscopy, last image hold rather than full exposure, and colimitation to appropriate field of view. Magnification increases the radiation dose and should be used only if necessary.
Patient Positioning
Appropriate patient positioning is vital in producing a diagnostic quality of images and prevent repeat examination that increases fetal radiation. Technologists should optimally position patients before imaging to obtain appropriate views for the exam. Obtaining diagnostic imaging at the first attempt eliminates the need for a repeat examination and significantly reduces unnecessary radiation exposure to the fetus.
Clinical Significance
The plain film, CT, nuclear medicine studies, and fluoroscopy uses ionizing radiation to obtain diagnostic images. A high level of radiation has adverse effects on the fetus; therefore, referring clinicians should consider alternative imaging modalities for pregnancy patients.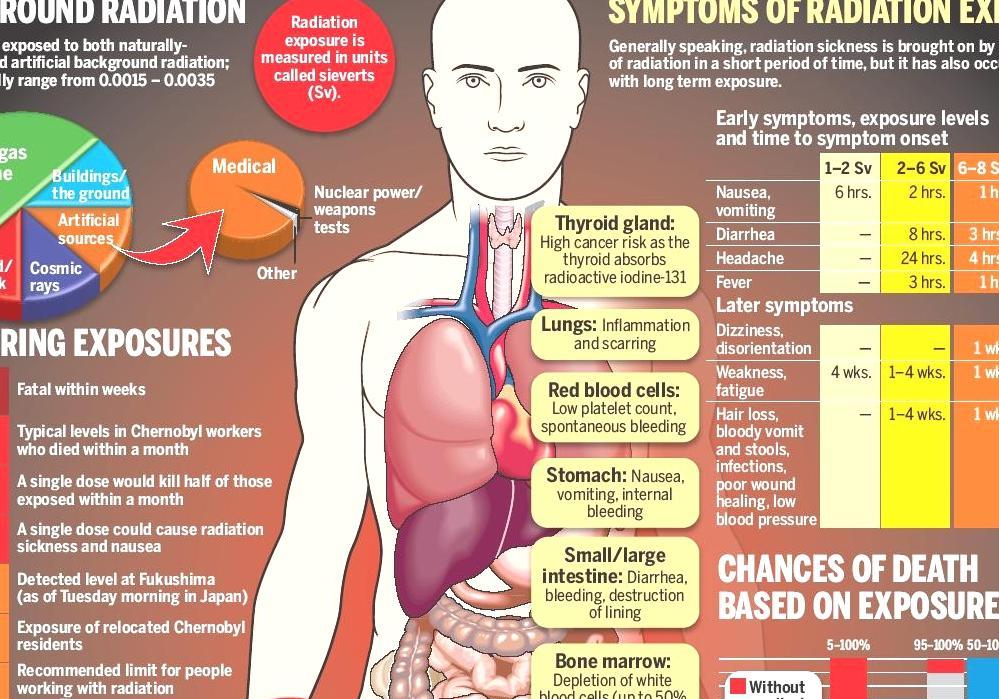 If diagnostic studies that expose radiation to the fetus are clinically required, it should be performed without delay but must take place in a way that minimizes radiation exposure to the fetus. Solid understanding of how each imaging modality contributes to fetal radiation exposure, techniques to reducing radiation exposure in the fetus, and adverse consequences of high radiation exposure is critical in providing the most appropriate imaging studies for pregnant patients.
If diagnostic studies that expose radiation to the fetus are clinically required, it should be performed without delay but must take place in a way that minimizes radiation exposure to the fetus. Solid understanding of how each imaging modality contributes to fetal radiation exposure, techniques to reducing radiation exposure in the fetus, and adverse consequences of high radiation exposure is critical in providing the most appropriate imaging studies for pregnant patients.
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
References
- 1.
Brent RL. Saving lives and changing family histories: appropriate counseling of pregnant women and men and women of reproductive age, concerning the risk of diagnostic radiation exposures during and before pregnancy. Am J Obstet Gynecol. 2009 Jan;200(1):4-24. [PubMed: 19121655]
- 2.
De Santis M, Cesari E, Nobili E, Straface G, Cavaliere AF, Caruso A.
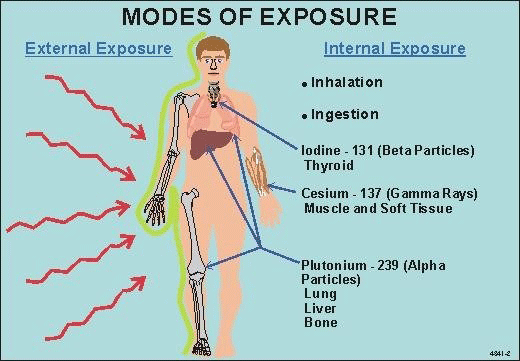 Radiation effects on development. Birth Defects Res C Embryo Today. 2007 Sep;81(3):177-82. [PubMed: 17963274]
Radiation effects on development. Birth Defects Res C Embryo Today. 2007 Sep;81(3):177-82. [PubMed: 17963274]- 3.
Williams PM, Fletcher S. Health effects of prenatal radiation exposure. Am Fam Physician. 2010 Sep 01;82(5):488-93. [PubMed: 20822083]
- 4.
McCollough CH, Schueler BA, Atwell TD, Braun NN, Regner DM, Brown DL, LeRoy AJ. Radiation exposure and pregnancy: when should we be concerned? Radiographics. 2007 Jul-Aug;27(4):909-17; discussion 917-8. [PubMed: 17620458]
- 5.
Tremblay E, Thérasse E, Thomassin-Naggara I, Trop I. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics. 2012 May-Jun;32(3):897-911. [PubMed: 22403117]
- 6.
Vu L, Ambrose D, Vos P, Tiwari P, Rosengarten M, Wiseman S. Evaluation of MRI for the diagnosis of appendicitis during pregnancy when ultrasound is inconclusive. J Surg Res. 2009 Sep;156(1):145-9. [PubMed: 19560166]
- 7.
Wallace GW, Davis MA, Semelka RC, Fielding JR. Imaging the pregnant patient with abdominal pain. Abdom Imaging. 2012 Oct;37(5):849-60. [PubMed: 22160283]
- 8.
Uzoigwe CE, Middleton RG. Occupational radiation exposure and pregnancy in orthopaedics. J Bone Joint Surg Br. 2012 Jan;94(1):23-7. [PubMed: 22219242]
- 9.
Pahade JK, Litmanovich D, Pedrosa I, Romero J, Bankier AA, Boiselle PM. Quality initiatives: imaging pregnant patients with suspected pulmonary embolism: what the radiologist needs to know. Radiographics. 2009 May-Jun;29(3):639-54. [PubMed: 19270072]
- 10.
Jain C. ACOG Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol. 2019 Jan;133(1):186. [PubMed: 30575654]
The effect of radiation on the embryo and fetus.
Radiosensitivity the fetus is high, and it is higher than the fetus younger. Malformations and deformities, arising from irradiation in utero, are grouped under the term teratogenic effects.
Teratogenic radiation effect — is the occurrence of malformations and deformities due to irradiation in utero ("in the womb", from the Latin "uterus" - the uterus).
There are three main period of intrauterine development of the organism, during which study the damaging effect of ionizing emissions: up to implantation , period of the main organogenesis , fetal period . Irradiation in the early stages (before implantation and at the beginning of organogenesis), as a rule, ends in fetal death or death newborn (for irradiation in the middle of the period organogenesis). Impact in period basic organogenesis causes deformities, and irradiation of the fetus - radiation disease newborn.
Organism embryos and fruit has extremely high radiosensitivity . Extremely high radiosensitivity organisms in the intrauterine period of development is easily explained, since at that time he is a conglomerate of dividing and differentiating cells , with the highest radiosensitivity.
Radiosensitivity embryo or fetus is determined by the most sensitive system located currently in a state active development.
In the same time The embryo has an important feature undetectable at other stages life cycle - expressed ability to recover, regeneration and restructuring.
The nature of developing long-term effects will depend on physical characteristics of the ionizing radiation (power, type of energy, character irradiation, prolongation during time) and the age of the fetus at the time irradiation. The stage is especially important intrauterine development, because differentiation of systems and organs occurs at certain times of development, and this will determine the type of damage.
When irradiated pregnant women are divided into four classic effects in offspring:
-
embryonic, neonatal and postnatal death fetus;
-
birth defects development;
-
growth disorders and physical development;
-
dysfunction central nervous system.
Embryonic, neonatal and postnatal death fetus. Most high risk of fetal death observed during irradiation preimplantation period. Received in animal embryos, the data indicate that radiation-induced events prenatal fetal death observed in doses less than 10 rad (0.1 Sv) during irradiation before implantation.
To avoid prenatal exposure in the early, unrecognized stages of pregnancy scheduled X-ray diagnostic procedures in women of childbearing age recommended only for the first 10 days after the start of menstruation (“rule of 10 days”).
Birth defects development (VPR). More often In total, the following VLOOKUPs occur:
-
Structural disturbance head: craniocerebral hernia, disorders skull shape structures, splitting upper palate and lips, structural disturbances ear;
-
CNS - anencephaly, microcephaly, hydrocephalus;
-
The organ of vision microophthalmia, anophthalmia;
-
Skeleton - polydactyly, reduction in fetal growth and weight.
In addition, at prenatally irradiated animals are noted CM of the heart and large vessels, urogenital systems, thoracic, inguinal and umbilical hernia. VPRs are observed mainly in irradiation during the period of basic organogenesis (9-60 days after fertilization). At present time, it is considered that exposure during the period basic organogenesis even at low absorbed doses per fetus (about 10 glad) is a risk for development microcephaly and birth defects development from the CNS.
Growth disorders and physical development. Along with weight and body size reduction a decrease in the mass of internal organs (especially the spleen and brain) brain), a decrease in head circumference.
Function violation central nervous system. Radiation effects can occur upon death glial or neural progenitor cells during mitosis or as a result of death post-mitotic but still immature neurons or the death of "guide cells" - migrating neurons. In addition, at high doses (1.8 -5.5 Gy) may occur red bone marrow damage decrease in erythropoiesis with a decrease oxygen transport to the brain fetus.
In addition, at high doses (1.8 -5.5 Gy) may occur red bone marrow damage decrease in erythropoiesis with a decrease oxygen transport to the brain fetus.
The effect of ionizing radiation on the embryo, fetus
Yu.A. Aleksandrov
Fundamentals of Radiation Ecology
Textbook. – Yoshkar-Ola: Mar. state un-t, 2007. - 268 p.
| Previous | Contents | Next |
Contents of the article:
- 1 Section 3. Biological effect of ionizing radiation
- 1.1 3.5. Effect of ionizing radiation on critical body systems
- 1.1.1 3.5.6. Effect of ionizing radiation on the embryo, fetus
- 1.1 3.5. Effect of ionizing radiation on critical body systems
3.5.6. The effect of ionizing radiation on the embryo, fetus
Data on the effect of ionizing radiation on the human embryo and fetus were obtained as a result of studying the consequences of radiation therapy (during irradiation of the abdomen of pregnant women) and studies of children exposed to intrauterine radiation in Hiroshima and Nagasaki. The general conclusion from these observations is unequivocal - the radiosensitivity of the fetus is high and it is the greater, the younger it is.
The general conclusion from these observations is unequivocal - the radiosensitivity of the fetus is high and it is the greater, the younger it is.
In surviving children, the damaging effect of radiation manifests itself in the form of various deformities, physical and mental retardation, or combinations thereof. The most common deformities are microcephaly, hydrocephalus, and anomalies in the development of the heart.
Malformations and deformities resulting from irradiation in utero , are grouped under the term teratogenic effects.
The extremely high radiosensitivity of the organism in the antenatal, intrauterine period of development is easily explained, since at this time it is a conglomerate of dividing and differentiating cells with the highest radiosensitivity.
Depending on the time of formation, formation and differentiation of certain tissues, organs or systems, any of them can be extremely radiosensitive, regardless of its radiosensitivity in adulthood.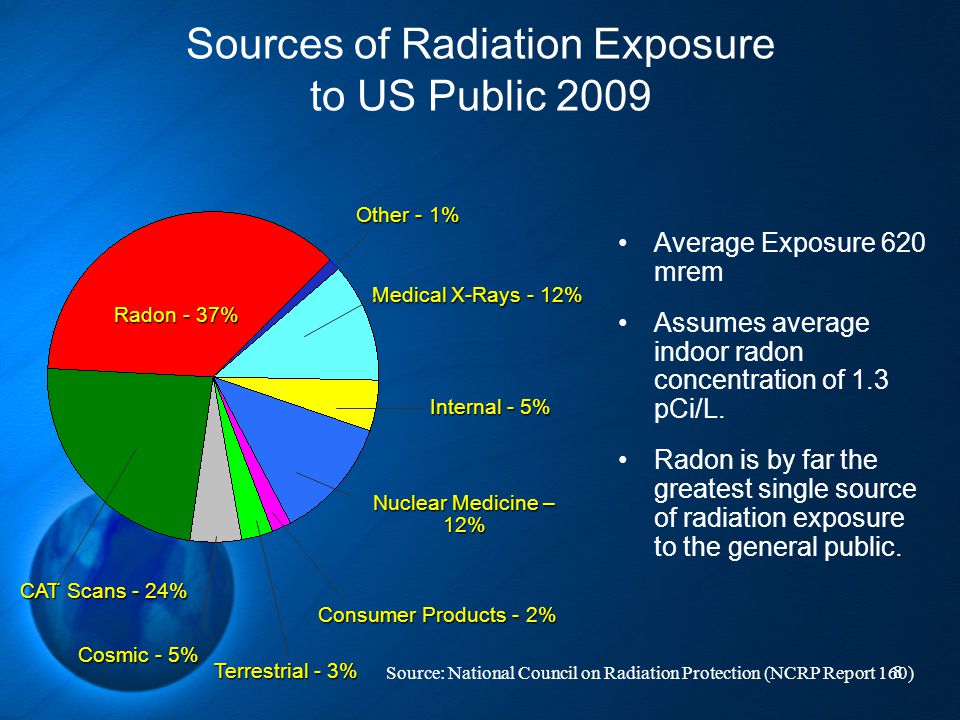
For example, a very common consequence of irradiation of an embryo is microcephaly or the absence of the central nervous system at all, which occurs when irradiated already at doses of 0.5-2 Gy, although this is not observed in an adult organism even at the highest doses. Therefore, it is quite fair to consider the embryo and fetus as the most radiosensitive stages in the development of any organism.
With a mosaic of developing centers, each of which is vital for the survival of the embryo, irradiation in doses that are lethal to any center will be fatal to the whole organism. In this case, the radiosensitivity of the embryo is determined by the most sensitive system that is currently in a state of active development.
At the same time, the embryo has an important feature that is not found at other stages of the life cycle - a pronounced ability to restore, regenerate and rebuild. Already at the earliest stages, the embryo contains active phagocytes capable of absorbing and eliminating cellular decay products and the remains of cells destroyed by irradiation.
There are three main periods of intrauterine development of the organism - pre-implantation (before the introduction of the embryo into the thickness of the uterine mucosa), the period of basic organogenesis, the fetal period .
Irradiation in the early stages (before implantation and at the beginning of organogenesis) usually ends in intrauterine death or death of the newborn (with irradiation in the middle of the period of organogenesis). Exposure during the period of the main organogenesis causes deformities, and irradiation of the fetus causes radiation sickness of the newborn.
Fig. 13. Intrauterine mortality of newborn mice and the number of animals with signs of abnormality by the time of delivery after exposure of females at various periods before fertilization at a dose of 4 Gy and after fertilization at a dose of 2 Gy
(according to L. Russell, W. Russell, 1954)
After their removal, the “organism as a whole” tries, as far as possible, to fill the resulting deficit with the remaining undifferentiated and undestroyed original cells.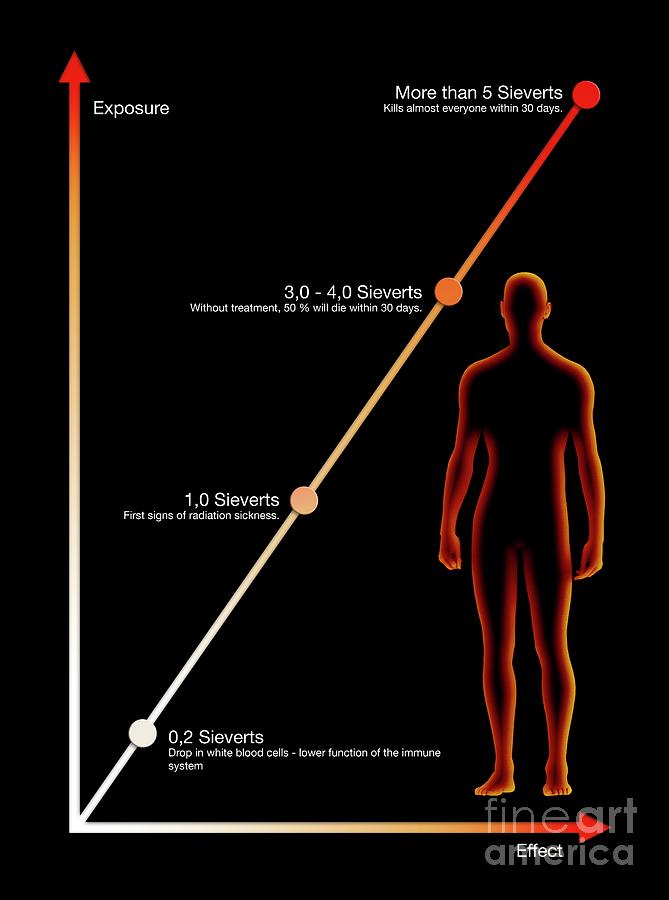
It has been convincingly shown that the embryogenic effect of radiation is predominantly direct, the possibility of remote influence on fetal development disorders is no more than 5% of the total damaging effect of radiation (Neifakh A.A., 1975). A striking proof of the validity of this conclusion can be, for example, the data of L. Russell that noticeable deviations of the embryo from the norm can be easily caused on the 7-8th day. pregnancy by irradiation in doses of 0.1-0.25 Gy, to which the mother's body essentially does not respond. At the same time, when a mother is irradiated before conception at obviously damaging doses (up to 4 Gy), no signs of damage to the developing fetus are found.
According to I.A. Piontkovsky (1969), the changes that occur in the neuroblasts of the embryo are detected as early as 2 hours after irradiation, i.e. much earlier than the development of radiation syndrome in the mother. Finally, the direct dependence of the teratogenic (induction of malformations, deformities) effect on the degree of radiosensitivity of the embryo, determined by the radiosensitivity of specific systems at different stages of development, testifies to the direct traumatic effect of radiation on the embryo.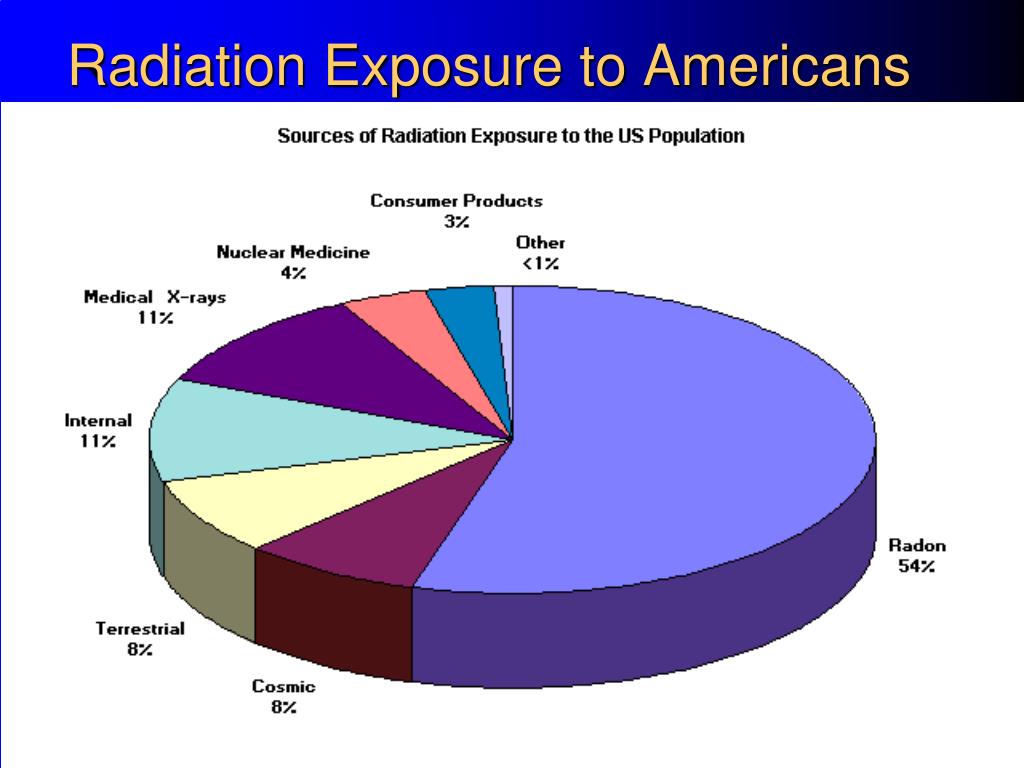 The overall picture, schematically presented in Figure 13, will be discussed in more detail below.
The overall picture, schematically presented in Figure 13, will be discussed in more detail below.
Embryos before implantation (up to 5 days) are the most radiosensitive to radiation - from 80 to 40% of them die before birth, and even during this period (from 1 to 5 days) radiosensitivity with age is noticeable goes down. Surviving embryos usually have no noticeable deformities. This is followed by a period of 6.5-12.5 days, when irradiation causes the highest frequency of deformities with minimal intrauterine mortality and the greatest death of newborns. At a dose of 2 Gy, death is highest if exposure occurs between 9.5 to 10.5 days. and does not differ from the control when irradiated up to 7.5 or after 11.5 days. Thus, the period of the main
organogenesis (6.5-12.5 days) should be considered as the most radiosensitive for most organs and systems of the body, the irradiation of which (depending on their vital significance) leads to the death of the fetus, newborn or the occurrence of deformities.
This is not something unexpected, as it once again confirms the general connection between the radiosensitivity of cells and such processes of their life as division and differentiation. At the same time, differentiating cells are the most radiosensitive; they determine the most radiosensitive stages in the development of a particular tissue, organ, or system. That is why ionizing radiation is an excellent tool in the hands of embryologists, with the help of which it is possible, with much greater accuracy than through the previously used extirpation, to determine the expected zones of formation of one or another organ in the embryo in the early stages of development, since it is known that differentiation of an organ may precede visible appearance of its rudiment. For clarity, below are the main developmental anomalies noted in the literature on the effect of radiation on the embryo of animals and humans.
Table 38 - Main abnormalities found in mammals
(including humans) after fetal exposure
| Brain | Eyes | Skeleton | Other anomalies |
| Missing brain Parencephaly Microcephaly Brain herniation Mongolism Medulla oblongata reduction Brain atrophy Mental retardation Idiot Neuroblastoma Narrowing of the sylvian water pipe Dropsy of the brain Rosettes in nerve tissue Expansion of the third and lateral ventricles of the brain Reduction or absence of some cranial nerves | Complete absence of anophthalmia Microphthalmia Microcornea Coloboma Iris deformity Absence of lens (alone or in combination with absence of retina) Open eyelids Strabismus Retinoblastoma Hyperopia Congenital glaucoma Partial albinism | Uniform reduction: stunting Skull reduction Vaulted skull Narrow head Skull Bladder Funnel chest Congenital dislocation of hips Tail reduction and deformity Overdevelopment and deformity of the legs Finger Reduction Heel Limb development disorder Syndactyly Brachydactyly Disruption of ontogeny Exostosis of the tibia Change of metaphysis Violation of enamel formation Sclerotomy necrosis | Reverse organ arrangement Dropsy of the kidney Hydrocele of the ureter Dropsy testis Absence of a kidney Gonadal degeneration Lower limb atrophy Depigmentation and hyperpigmentation of the skin Movement disorders of limbs Increased likelihood of leukemia Congenital heart disease Ear deformity Facial deformity Pituitary dysfunction Dermatomal myotomal necrosis |
14. Extrapolation curve showing the expected results of irradiation of a human embryo at different stages of development, built on the basis of animal experiments (according to R. Raf, 1962): 2 - change in the cranial vault; 3 - degeneration of cerebral vessels, reduction of the medulla oblongata; 4 - degeneration of the midbrain and cortical cells; 5 - degeneration of the spinal cord and medulla oblongata; 6 - violation of the development of the corpus callosum; 7 - impaired development of the cerebral hemispheres,
Extrapolation curve showing the expected results of irradiation of a human embryo at different stages of development, built on the basis of animal experiments (according to R. Raf, 1962): 2 - change in the cranial vault; 3 - degeneration of cerebral vessels, reduction of the medulla oblongata; 4 - degeneration of the midbrain and cortical cells; 5 - degeneration of the spinal cord and medulla oblongata; 6 - violation of the development of the corpus callosum; 7 - impaired development of the cerebral hemispheres,
ammon's horn; 8 – Striatal disorder
The period of greatest radiosensitivity of the human embryo is greatly extended in time. It probably begins at conception and ends approximately 38 days after implantation; during this period of development, the rudiments of all organs begin to form in the human embryo through rapid differentiation from primary cell types. Similar transformations in a human embryo between
18 and 38 days occur in almost every tissue. Since the transition of any cell from the embryonic state to the state of maturity is the most radiosensitive period of its formation and life (regardless of whether it is a neuro-, myo-, osteo- or erythroblast, etc.), all tissues at this time turn out to be highly radiosensitive .
Since the transition of any cell from the embryonic state to the state of maturity is the most radiosensitive period of its formation and life (regardless of whether it is a neuro-, myo-, osteo- or erythroblast, etc.), all tissues at this time turn out to be highly radiosensitive .
The mosaic nature of the process of embryo differentiation and the change in the number of the most radiosensitive cells associated with this process determine the degree of radiosensitivity of a particular system or organ and the likelihood of a specific anomaly at each moment of time. Therefore Fractionated irradiation of the fetus leads to more severe damage, since the effect captures a variety of germ cell types and their different distribution, which causes damage to a large number of organ buds that are at critical stages of development. During this period, the maximum damage can be provoked by very small doses of ionizing radiation; to obtain anomalies in the later period of embryonic development, exposure to large doses is required.
Approximately 40 days after conception, gross deformities are difficult to cause, and after birth it is impossible. However, it should be remembered that in each period of development, the human embryo and fetus contain a certain number of neuroblasts that are highly radiosensitive, as well as individual germ cells that can accumulate the effect of radiation.
As shown by the results of studying the consequences of exposure of pregnant women during the atomic bombing in Hiroshima and Nagasaki, the degree of manifestation of anomalies and their features basically corresponded to the expected ones. So, according to one of the surveys, in 30 women who were 2 km from the epicenter of the explosion and had serious symptoms of radiation exposure, about half of the cases had intrauterine fetal mortality, the death of newborns or infants, and four out of 16 surviving children had mental retardation. According to another observation, almost half (45%) of children born to mothers exposed to radiation at 7-15 weeks of gestation had signs of mental retardation.
In addition, microcephaly, growth retardation, mongolism and congenital heart defects were noted in the offspring of women who underwent radiation in the first half of pregnancy, the frequency and degree of anomalies were higher when the affected mothers were at a distance of less than 2 km from the epicenter of the explosion . But even in these cases, no such severe neurological disorders were observed as were obtained when mice were irradiated, which is probably due to the low survival rate of such children. These observations refer only to 6-7-year-old children, and at this age many disorders that can be detected only in adolescence and later age do not yet appear.
According to a 1975 survey, an annual survey in Hiroshima and Nagasaki of 1600 prenatal exposures showed that individuals whose mothers were ~2 km weight loss. At lower doses, there were no noticeable deviations in the physical development and growth of children.
After clarification of individual doses, it was found that in Hiroshima the teratogenic effect of radiation was already manifested in the dose range from 0. 1 to 0.2 Gy.
1 to 0.2 Gy.
The greatest risk of developing mental disorders occurs when the fetus is exposed to radiation between 8 and 15 weeks after conception.
It should be borne in mind that irradiation of an embryo in small doses can cause such functional changes in the cell that cannot be registered by modern research methods, but which contribute to the development of a disease process many years after irradiation. Consequently, all the long-term effects of irradiation of the embryo can be expressed to a greater extent than in the case of irradiation of an adult organism. For example, according to statistical analysis (Stuart A. et al., 1956), the incidence of leukemia in the offspring of mothers exposed to x-rays during pregnancy is approximately doubled.
Irradiation of an embryo in the first 26 weeks at a dose of > 0.1 Gy is fraught with the birth of handicapped children, and therefore such a situation may serve as a basis for medically indicated abortion.
Noteworthy are the effects of maternal exposure in the second half of pregnancy.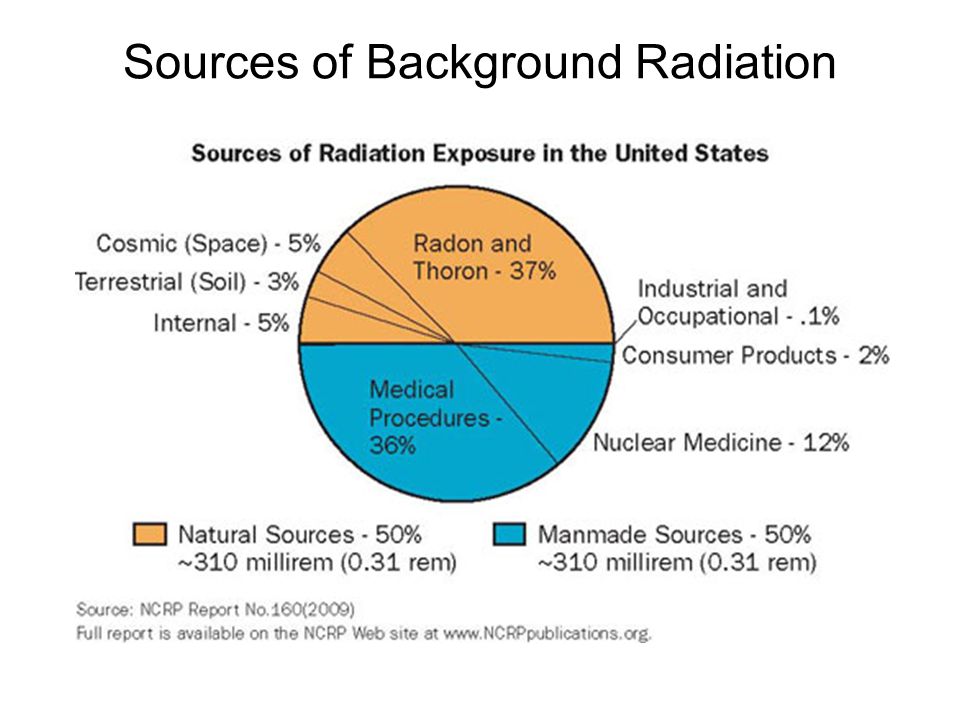 These include data on increased morbidity and mortality of such children in Nagasaki, as well as reports of developmental anomalies observed in x-ray procedures performed at different stages of pregnancy of mothers. Thus, in a summary of 168 such cases (Raf R., 1962), it is stated that irradiation of a human embryo in the period of the first two months leads to 100% damage, in the period from 3 to 5 months - to 64, in the period from 6 to 10 months - to 23% damage to embryos. These data should, of course, be kept in mind, but their value for reliable conclusions regarding the exposure of the human embryo and fetus is limited by the lack of accurate dosimetric parameters.
These include data on increased morbidity and mortality of such children in Nagasaki, as well as reports of developmental anomalies observed in x-ray procedures performed at different stages of pregnancy of mothers. Thus, in a summary of 168 such cases (Raf R., 1962), it is stated that irradiation of a human embryo in the period of the first two months leads to 100% damage, in the period from 3 to 5 months - to 64, in the period from 6 to 10 months - to 23% damage to embryos. These data should, of course, be kept in mind, but their value for reliable conclusions regarding the exposure of the human embryo and fetus is limited by the lack of accurate dosimetric parameters.
An exceptional feature of the embryo is determined by the presence of a large number of undifferentiated primary cells aimed at compensating for cellular losses, due to which the irradiated organism continues to develop as a whole, although the inevitable consequence of irradiation is the occurrence of a state of insufficiency (inferiority), the tolerance of which by the body depends on the degree of replacement of affected cells by cells , which remained undifferentiated.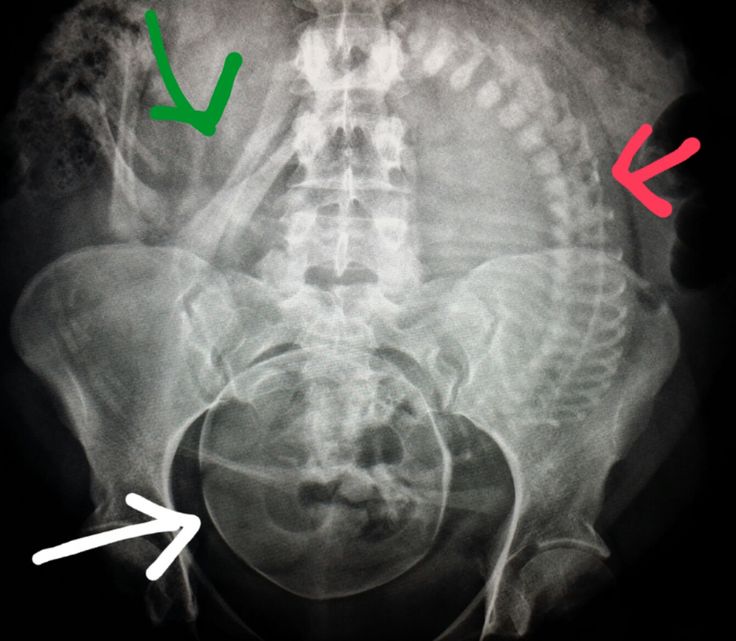
The outcome of the damage to the embryo, thus, is expressed by a certain balance between the initial effect of irradiation and the ability of the "organism as a whole" to recover from the defeat of its individual parts, regenerating through newly oriented viable primary cells.
Regardless of the details of the mechanism of the radioembryological effect, the consequences of irradiation of the embryo or fetus are such that they inevitably lead to the conclusion that it is necessary at all costs to prevent radiation exposure in this period. The threshold dose of radiation that causes an abnormality of the human fetus has not yet been established, but radiation at a dose of 0.05 Gy increases the rate of resorption of a fertilized egg in a mouse at a very early stage (before the cleavage stage).
There are reasons to believe that even diagnostic exposure of pregnant women (at doses of 0.001-0.2 Gy) can cause significant deformities, especially if this occurs during early ontogenesis.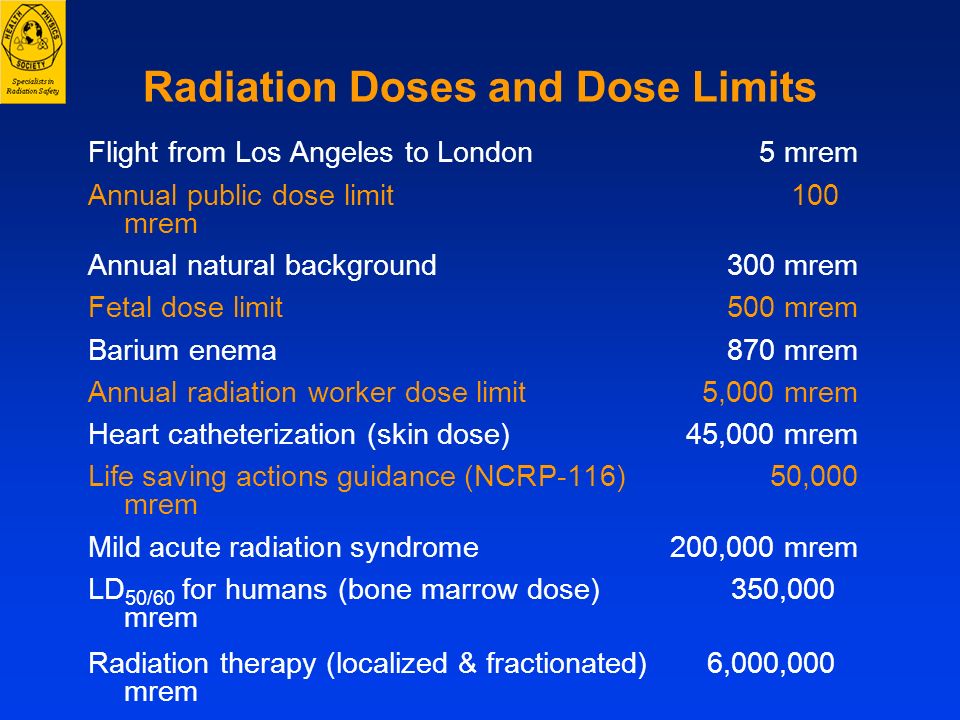
According to D. Wesley (1960), at least 96% of all deaths caused by congenital deformities can be due to background radiation, and the 60% increase in deformities observed in the USA from the 1930s to the 1960s was in his opinion, the result of ill-conceived medical use of x-rays.
In addition, a very important problem affecting the evaluation of the results of irradiation during embryogenesis is the study of the frequency and time of occurrence of long-term effects of irradiation. For example, if the development of a visible picture of cancer in a person occurs 15-30 years after irradiation, will the latent period be just as long if the embryo or fetus is exposed to irradiation? In addition to the examples already given above, which demonstrate the severity of this problem, the following can be added. Conducted by R. Mole (1974) a retrospective analysis of published materials on tumors in children revealed an increased incidence of leukemia and malignant tumors in children born to mothers who underwent radiographic examinations, in 10% of cases these were single fetuses, and in
55% were twins. The multiplicity of observed leukemias and solid tumors gives the author grounds for a confident conclusion about a causal relationship between these diseases and fetal exposure.
The multiplicity of observed leukemias and solid tumors gives the author grounds for a confident conclusion about a causal relationship between these diseases and fetal exposure.
According to A. Stewart (1973), in the first 10 years of life in 15 million single children and 350 thousand twins exposed to radiation in utero, the relative risk of developing leukemia or solid tumors increases by 1.5 times for single children and respectively at 2.2 and 1.6 times for twins.
Summarizing all available data, in the last review of 1997, Doll and Weckford came to the following conclusion. Exposure to low doses of in utero , especially in the last trimester, increases the risk of childhood cancer (registered in the first 10-15 years of life). Already at a dose of about 0.01 Gy (1 rad), the risk of childhood cancer increases by 40% above the spontaneous level. The increase in absolute risk is about 6% per 1 Gy, which is not much different from the risk of adult Japanese survivors of the bombardment.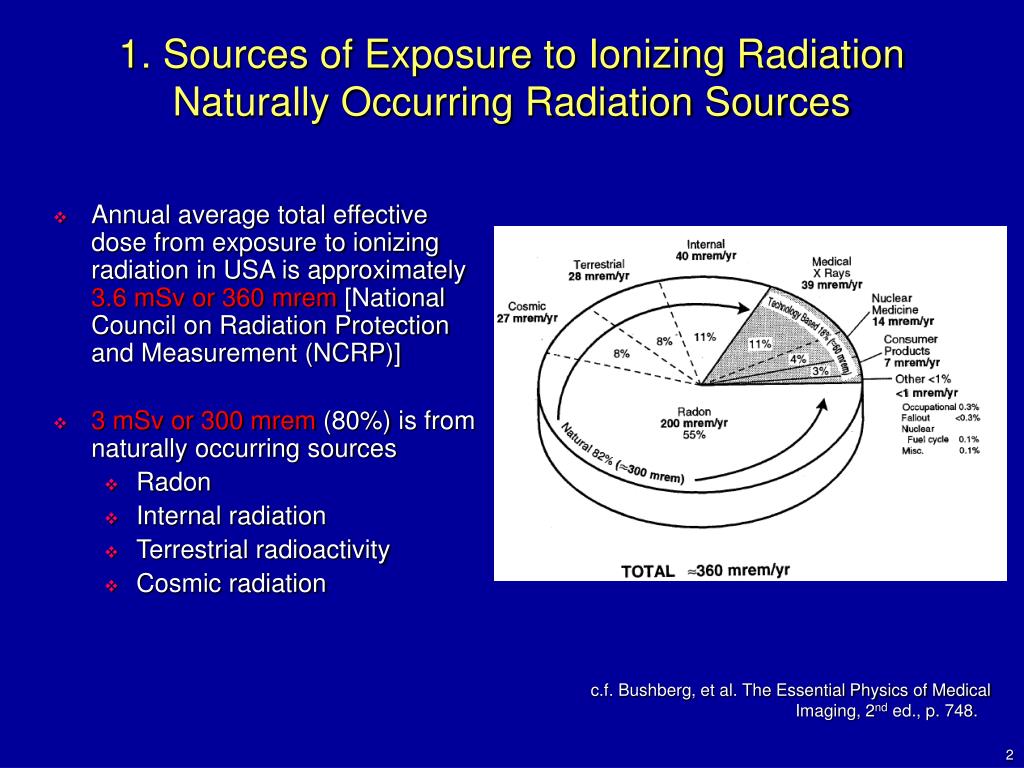
When irradiating the rudiments of the genital organs in embryos at an early stage of development, one can expect other consequences than when irradiating the gonads of an adult organism, if only because of the difference in the radiosensitivity of their germ cells. In addition, changes in a small number of such rudimentary germ cells of the embryo will have a much greater effect, since they are the precursors of a huge number of epithelial cells of the adult gonads, therefore, any effect of radiation on the primary germ cell of the embryo is transmitted to all cells of its subsequent generations.
All of the above provides a basis for confirming the validity of the early (1962) statement of one of the founders of radiation embryology R. Raf: “... ionizing radiation is and will remain in the future the most important tool in the flourishing of civilization, as has already been shown in medicine, fields of science and technology. The average human life expectancy has increased largely due to the development of radiology.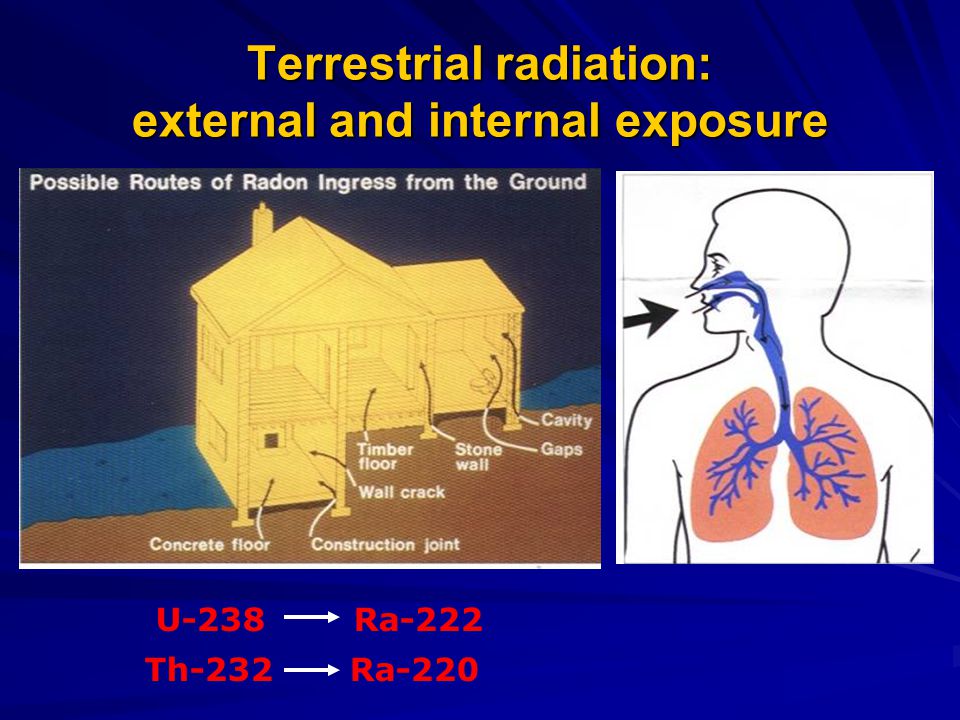 Nevertheless, the task of every radioembryologist is to emphasize that the embryo is extremely radiosensitive and that irradiation of it can cause far-reaching consequences. A demonstration of the point of view expressed by R. Raf 40 years ago is the unaltered conclusion of UNSCEAR-84 that the radiosensitivity of the fetus to induce long-term effects of radiation is 10-300 times greater than that of an adult organism.
Nevertheless, the task of every radioembryologist is to emphasize that the embryo is extremely radiosensitive and that irradiation of it can cause far-reaching consequences. A demonstration of the point of view expressed by R. Raf 40 years ago is the unaltered conclusion of UNSCEAR-84 that the radiosensitivity of the fetus to induce long-term effects of radiation is 10-300 times greater than that of an adult organism.
Thus, we can conclude that:
- the organism of the embryo and fetus has an extremely high radiosensitivity. Irradiation during this period, even in small doses (> 0.1 Gy), causes teratogenic effects in the form of various malformations, mental retardation and deformities;
- the probability of occurrence of specific teratogenic effects depends on the stage of embryonic development at which the exposure occurred, and the number and severity of effects depend on the dose;
- The most sensitive period is from 8 to 15 weeks. after conception;
- embryogenic effects of radiation are mainly the result of direct exposure, the indirect effect of radiation through the mother's body accounts for no more than 5%;
- irradiation of an embryo, even in small doses, increases the spontaneous level of childhood (in the first 10-15 years of life) cancer by 1.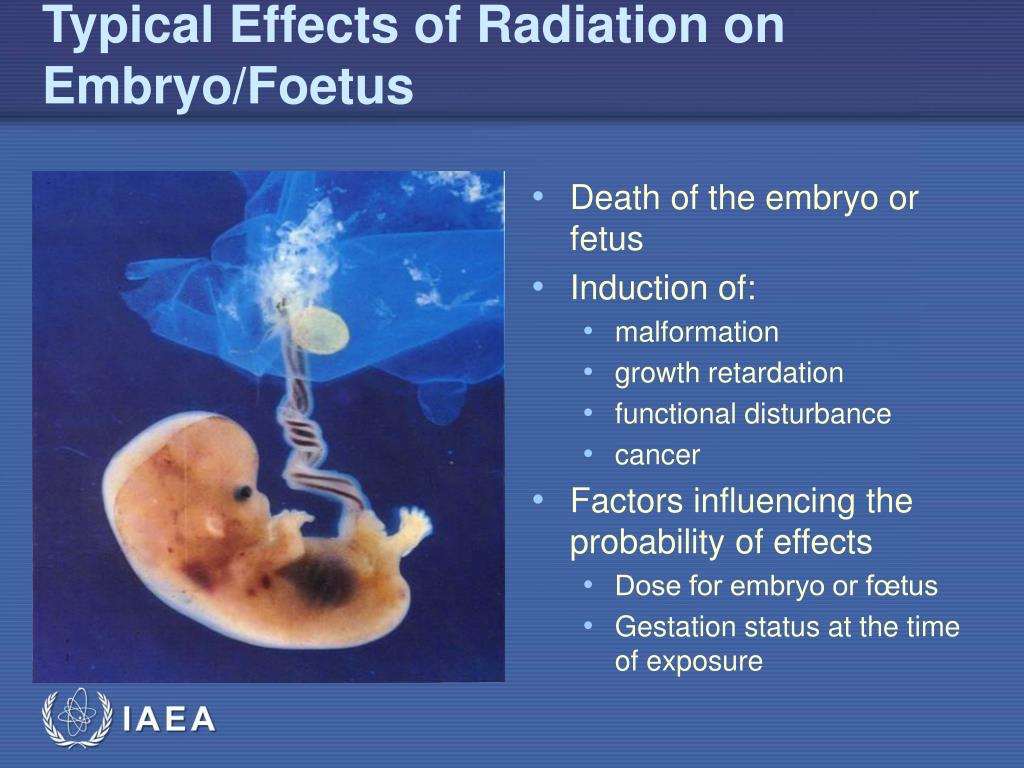

 174, “Preconception and Prenatal Radiation Exposure: Health Effects and Protective Guidance” [NCRP2013].
174, “Preconception and Prenatal Radiation Exposure: Health Effects and Protective Guidance” [NCRP2013].