Nuchal translucency nt
Nuchal translucency test Information | Mount Sinai
Nuchal translucency screening; NT; Nuchal fold test; Nuchal fold scan; Prenatal genetic screening; Down syndrome - nuchal translucency
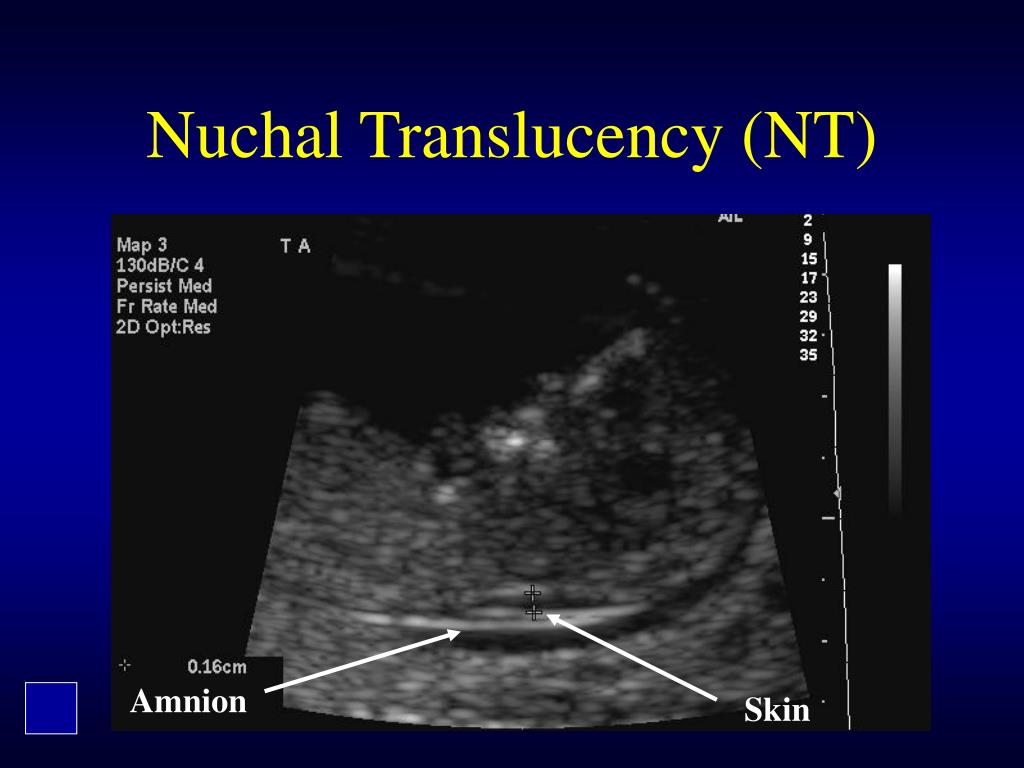
The nuchal translucency test measures the nuchal fold thickness. This is an area of tissue at the back of an unborn baby's neck. Measuring this thickness helps assess the risk for Down syndrome and other genetic problems in the baby.
How the Test is Performed
Your health care provider uses abdominal ultrasound (not vaginal) to measure the nuchal fold. All unborn babies have some fluid at the back of their neck. In a baby with Down syndrome or other genetic disorders, there is more fluid than normal. This makes the space look thicker.
A blood test of the mother is also done. Together, these two tests will tell if the baby could have Down syndrome or another genetic disorder.
How to Prepare for the Test
Having a full bladder will give the best ultrasound picture. You may be asked to drink 2 to 3 glasses of liquid an hour before the test. DO NOT urinate before your ultrasound.
How the Test will Feel
You may have some discomfort from pressure on your bladder during the ultrasound. The gel used during the test may feel slightly cold and wet. You will not feel the ultrasound waves.
The gel used during the test may feel slightly cold and wet. You will not feel the ultrasound waves.
Why the Test is Performed
Your provider may advise this test to screen your baby for Down syndrome. Many pregnant women decide to have this test.
Nuchal translucency is usually done between the 11th and 14th week of pregnancy. It can be done earlier in pregnancy than amniocentesis. Amniocentesis is another test that checks for birth defects.
Normal Results
A normal amount of fluid in the back of the neck during ultrasound means it is very unlikely your baby has Down syndrome or another genetic disorder.
Nuchal translucency measurement increases with gestational age. This is the period between conception and birth. The higher the measurement compared to babies the same gestational age, the higher the risk is for certain genetic disorders.
The measurements below are considered low risk for genetic disorders:
- At 11 weeks -- up to 2 mm
- At 13 weeks, 6 days -- up to 2.8 mm
What Abnormal Results Mean
More fluid than normal in the back of the neck means there is a higher risk for Down syndrome, trisomy 18, trisomy 13, Turner syndrome, or congenital heart disease. But it does not tell for certain that the baby has Down syndrome or another genetic disorder.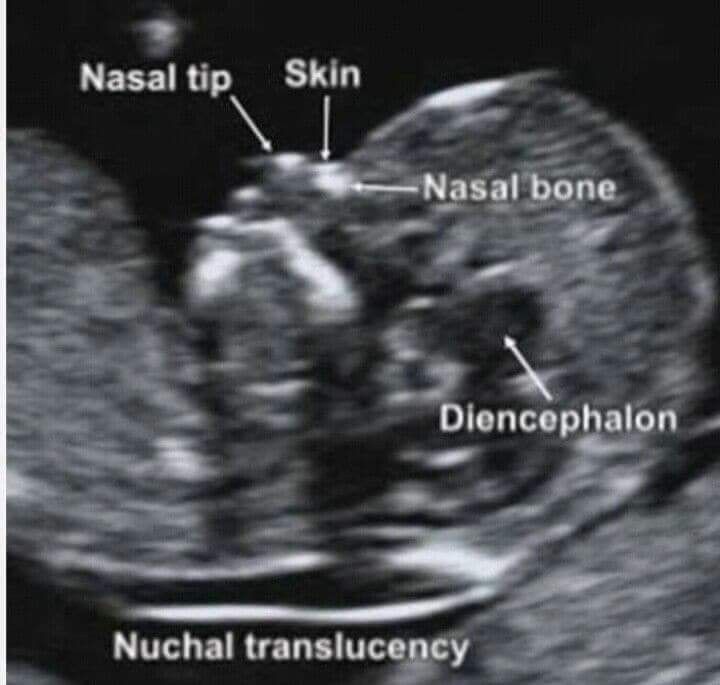
If the result is abnormal, other tests can be done. Most of the time, the other test done is amniocentesis.
Risks
There are no known risks from ultrasound.
Driscoll DA, Simpson JL. Genetic screening and diagnosis. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe's Obstetrics: Normal and Problem Pregnancies. 8th ed. Philadelphia, PA: Elsevier; 2021:chap 10.
Walsh JM, D'Alton ME. Nuchal translucency. In: Copel JA, D'Alton ME, Feltovich H, et al, eds. Obstetric Imaging: Fetal Diagnosis and Care.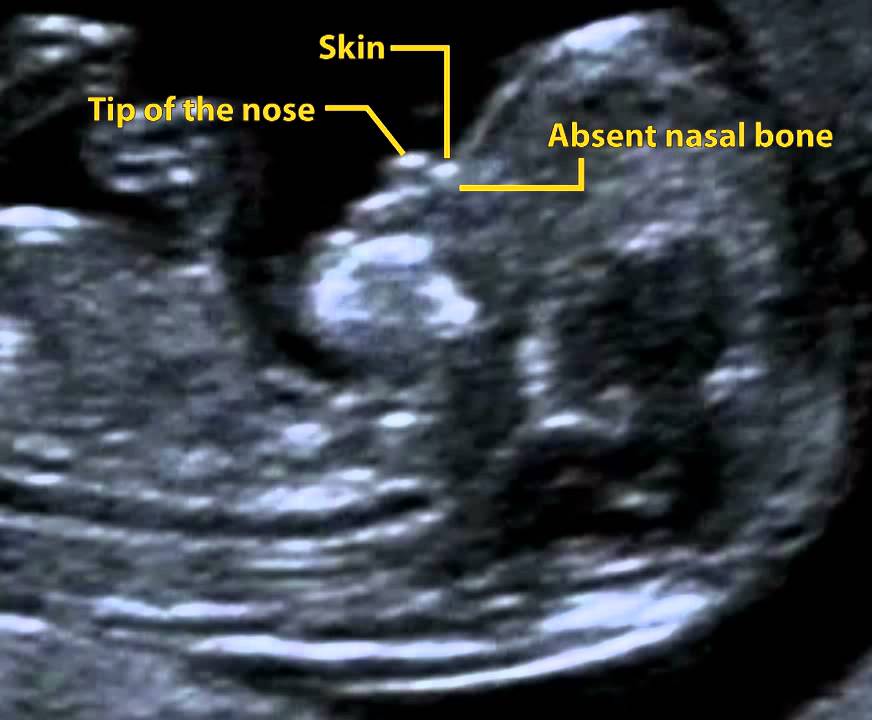 2nd ed. Philadelphia, PA: Elsevier; 2018:chap 45.
2nd ed. Philadelphia, PA: Elsevier; 2018:chap 45.
Wapner RJ, Dugoff L. Prenatal diagnosis of congenital disorders. In: Resnik R, Lockwood CJ, Moore TR, Greene MF, Copel JA, Silver RM, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:chap 32.
Last reviewed on: 4/19/2022
Reviewed by: John D. Jacobson, MD, Department of Obstetrics and Gynecology, Loma Linda University School of Medicine, Loma Linda, CA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.
Normal reference range of fetal nuchal translucency thickness in pregnant women in the first trimester, one center study
J Res Med Sci. 2015 Oct; 20(10): 969–973.
doi: 10.4103/1735-1995.172786
,,,1 and 2
Author information Article notes Copyright and License information Disclaimer
Background:
Considering that establishment of reference value of nuchal translucency (NT)-related to the crown rump length (CRL) during the first trimester will be helpful for determining an appropriate cutoff level for screening of increased NT thickness-related abnormalities, we determined the NT thickness and investigated its relation with different chromosomal and nonchromosomal abnormalities among a large sample size of pregnant Iranian women.
Materials and Methods:
In this analytic cross-sectional study, pregnant women who were in their first trimester were enrolled at their antenatal visit. Using an abdominal ultrasonography, the fetal NT thickness of the studied population was measured. Those with increased NT thickness were determined. The reference value of NT thickness (5th, 25th, 50th, 75th, and 95th percentiles) within each 5-mm range of CRL and during the 11th, 12th, and 13th gestational weeks were determined. The presences of the different chromosomal and nonchromosomal abnormalities were compared in women with different percentiles of NT thickness who underwent amniocentesis and those who did not.
Results:
1,614 pregnant women were evaluated. The mean NT thickness was 1.30 ± 0.54 mm. Increased NT thickness >2 mm and >95th percentile according to their gestational age (GA) was detected in 89 (5.5%) and 58 (3.6%) pregnant women. The reference 95th percentile value range for NT was 1.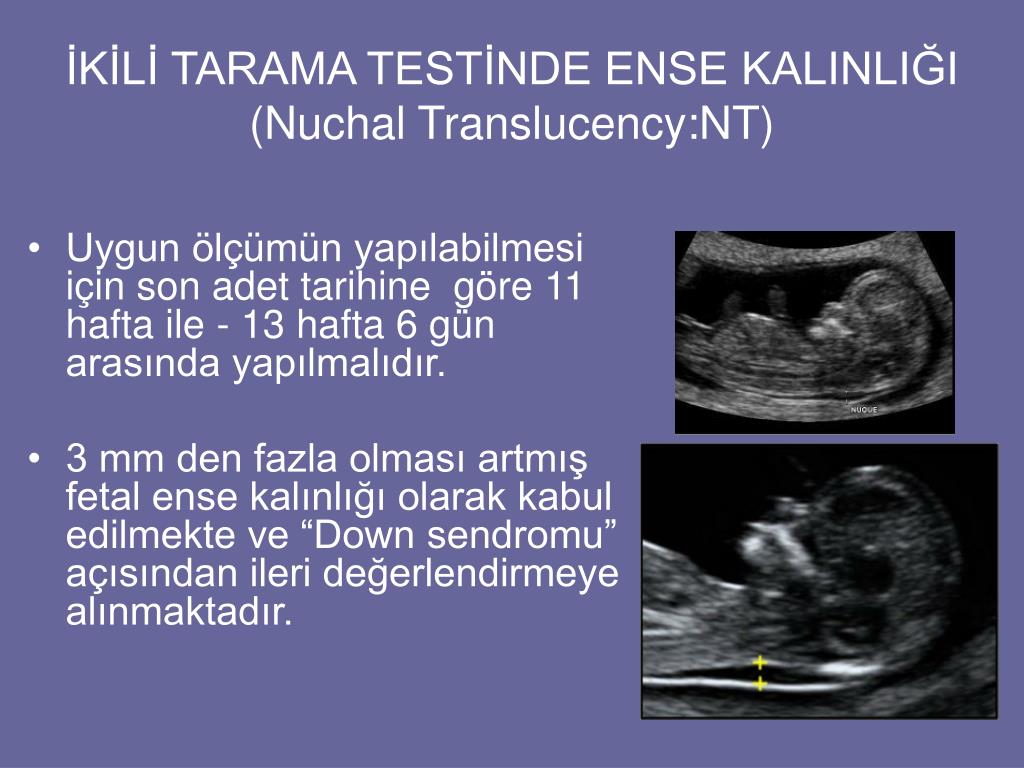 8-2.35 and increased NT thickness according to our obtained values was associated significantly with chromosomal abnormalities.
8-2.35 and increased NT thickness according to our obtained values was associated significantly with chromosomal abnormalities.
Conclusion:
The obtained reference range in our studied population was different from that reported for other ethnic groups and it is suggested that using this values are more favorable for screening of chromosomal abnormalities during the first trimester of pregnancy than the recommended single cutoff value.
Keywords: Crown rump length (CRL), nuchal translucency (NL), reference values
Nuchal translucency (NT) is the normal fluid-filled subcutaneous space between the back of the fetal skin and the overlying skin.[1] NT is visible and can be measured by ultrasonographic imaging between 11 weeks and 14 weeks gestation.[2] Increased NT is associated with different fetal chromosomal and nonchromosomal abnormalities. There is growing evidence that increased NT thickness during the first trimester of pregnancy in a chromosomally normal fetus is associated with numerous fetal structural abnormalities, genetic syndromes, heart defects, and poor perinatal outcomes such as miscarriage and intrauterine death.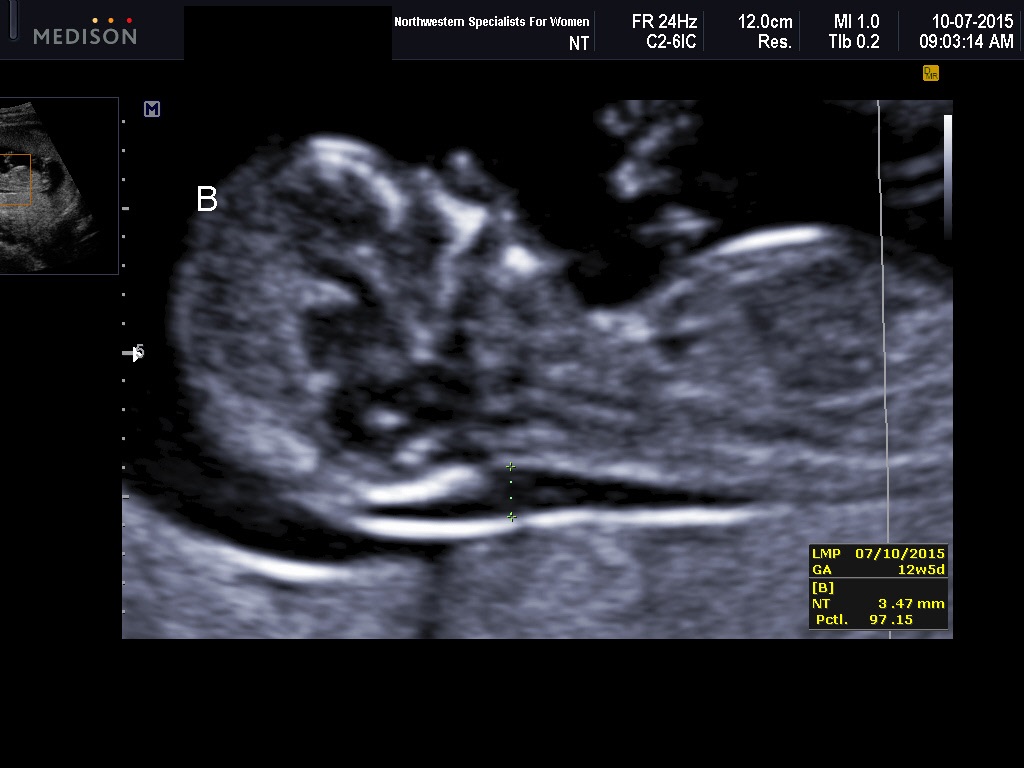 [3,4,5]
[3,4,5]
The first definition for increased NT was a measure >95th percentile for a given crown rump length (CRL) and a NT value of 2.5-3 mm, which was reported as a normal range for the marker. Recently, some studies indicated that NT >99th percentile or NT value that exceeds of 3.5 mm are associated with the most common adverse outcomes.[6,7]
The utility of NT as a sensitive and noninvasive ultrasonographic marker for screening and detection of aneuploidies and major structural anomalies in modern obstetrical practice has been demonstrated recently. Its use as a new screening method for the mentioned purposes has been developed in many developed countries.[8,9,10]
Since the introduction of NT thickness, several studies worldwide have determined the normal range of NT in different populations. The results were different regarding the normative value of NT. One of the explanations for the reported great variety of NT thickness range is ethnic variation.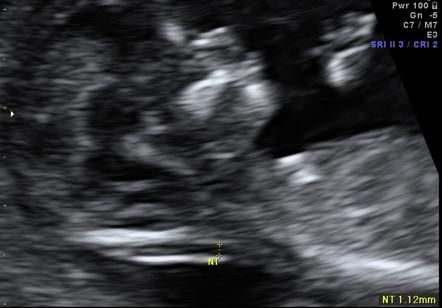 [11,12,13,14,15] However, there are still controversies regarding the role of ethnicity on the value of NT. Some reported a significant role of ethnicity in this regard, whereas others did not support the association.[16,17] However, recently the establishment of reference value for NT in different populations was performed. It is suggested that ethnic and region-specific reference value of NT could have a significant impact on its screening efficacy and using a single cutoff for fetal NT could not be an appropriate tool in this field.[18]
[11,12,13,14,15] However, there are still controversies regarding the role of ethnicity on the value of NT. Some reported a significant role of ethnicity in this regard, whereas others did not support the association.[16,17] However, recently the establishment of reference value for NT in different populations was performed. It is suggested that ethnic and region-specific reference value of NT could have a significant impact on its screening efficacy and using a single cutoff for fetal NT could not be an appropriate tool in this field.[18]
So considering that establishment of reference value of NT related to the CRL during the first trimester will be helpful for determining an appropriate cutoff level for screening of increased NT thickness-related abnormalities and the presence of few reports in this field among the Iranian population, in this study we determined the reference values of NT thickness among Isfahani pregnant women to evaluate the role of ethnicity on the normative value of NT as well as the association of increased NT thickness with chromosomal and nonchromosomal abnormalities during the first trimester.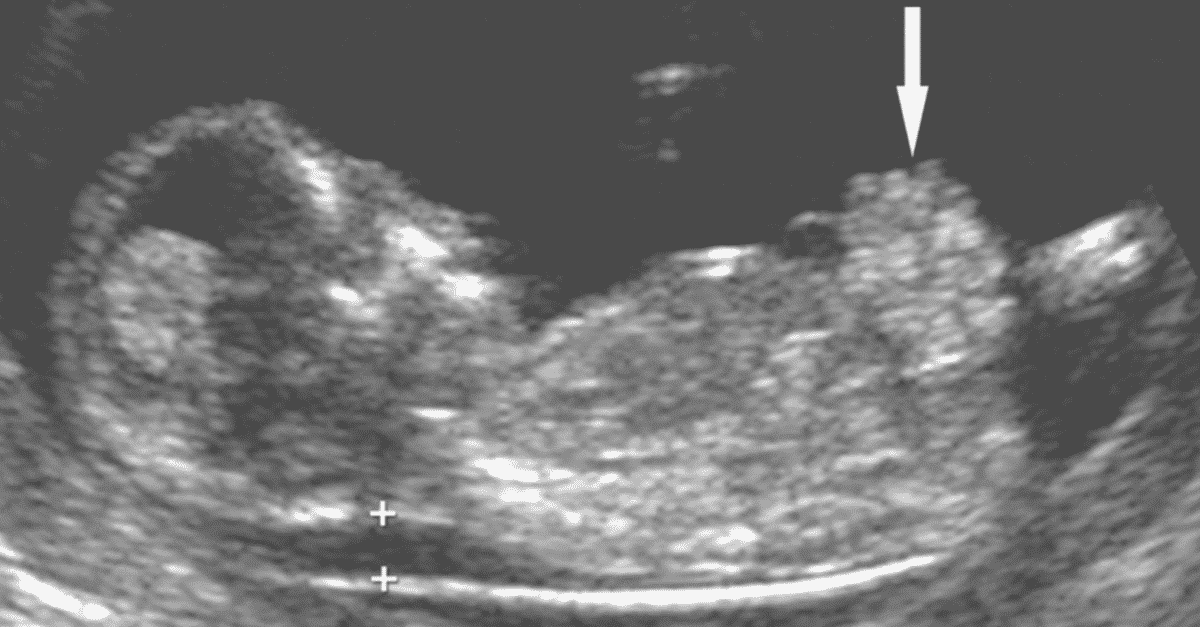
In this analytic cross-sectional study, pregnant women referred to a private radiology center for ultrsonographic assessment during the antenatal visit in their first trimester were enrolled. The study was performed from January 2013 to December 2013 in Isfahan, Isfahan Province, Iran.
The protocol of the study was approved by the Regional Ethics Committee of Isfahan University of Medical Sciences.
Pregnant women with gestational age (GA) of 11-13 weeks and 6 days and/or CRL 45-84 mm were included.
The pregnant women were selected by the consecutive method. Those who did not agree to have the ultrasonography performed, with multiple pregnancies, fetal malformation, and those with inappropriate cooperation were excluded. Written informed consent was obtained from all the selected participants. The selected pregnant women underwent abdominal ultrasonography. The sonography was performed by an expert radiologist. The fetal NT thickness of the studied population was measured.
Those with NT thickness of 2 mm were considered as women with increased NT thickness.[19]
The mean of CRL and GA were compared in women with and without increased NT thickness.
The reference value of NT thickness (5th, 25th, 50th, 75th, and 95th percentiles) within each 5-mm range of CRL and during the 11th, 12th, and 13th gestational weeks were determined.
Women with NT thickness of >95th percentile were determined. The women were followed up and fetal outcomes were evaluated by the neonatologists at birth. The presence of different chromosomal abnormalities as well as nonchromosomal abnormalities including cardiac malformation, genitourinary or renal abnormalities, diaphragmatic hernia, spontaneous miscarriage, and intrauterine fetal death (IUFD) were compared in women with different percentiles of NT thickness who underwent amniocentesis and those who did not.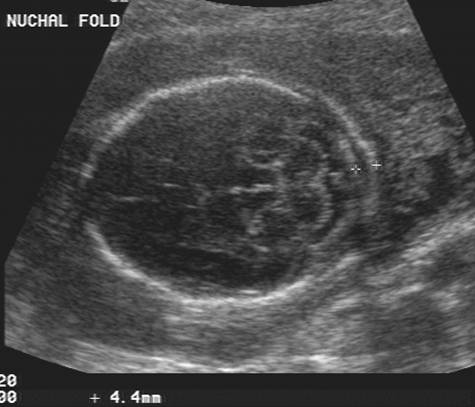
Ultrasonographic measurements
The ultrasonographic measurements were performed in pregnant women in a supine position.
Fetal CRL and NT thickness measurements were performed by transabdominal ultrsonography using a multi fz: 3.5 MHz tranduser (GE Volusun 730). The measurement was performed based on the criteria recommended by the Fetal Medicine Foundation (FMF).[20] According to the criteria, the fetus should be in a neutral position, with the head aligned with the spine in a way that fetus occupied at least 75% of the image. NT was defined as the black area between the inner skin outlines echo and the outer border of the soft tissue overlying the cervical spine.
The maximal thickness of the black area was measured with caliper placed on the inner borders of the NT space, perpendicular to the long axis of the fetus when a sagittal section with a neutral position of the fetus was obtained. The measurements were recorded to the nearest 0.1-mm interval. At least three NK measurements were taken and the largest was recorded.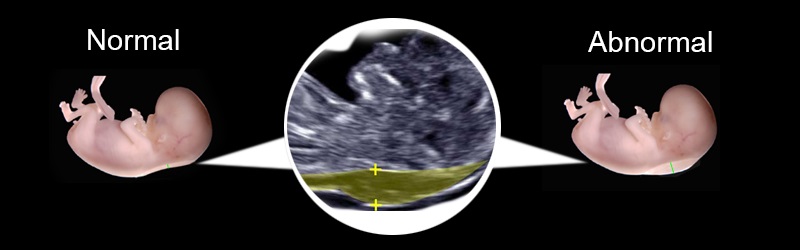
CRL was measured at the same time and recorded.
Statistical analysis
Data were analyzed using Statistical Package for the Social Sciences (SPSS) version 21 (SPSS Inc., Chicago, IL, USA). Using regression equation, the expected 5th, 25th, 50th, 75th, and 5th percentile values of NT thickness according to the CRL categories of CRL (5-mm interval) and GA (11th, 12th, and 13th weeks) were obtained. Quantitive and qualitative values were compared using the t-test and chi-square test, respectively. P value of <0.05 was considered to be statistically significant.
During this study, 1,614 pregnant women were evaluated. Among the studied pregnant women 382 (23.7%), 871 (54.0%), and 361 (22.4) were in the 11th, 12th, and 13th gestational week. The mean of GA, CRL, and NT thickness in the studied population were 12.46 ± 0.62 weeks, 59.35 ± 8.35 mm, and 1.30 ± 0.54 mm, respectively.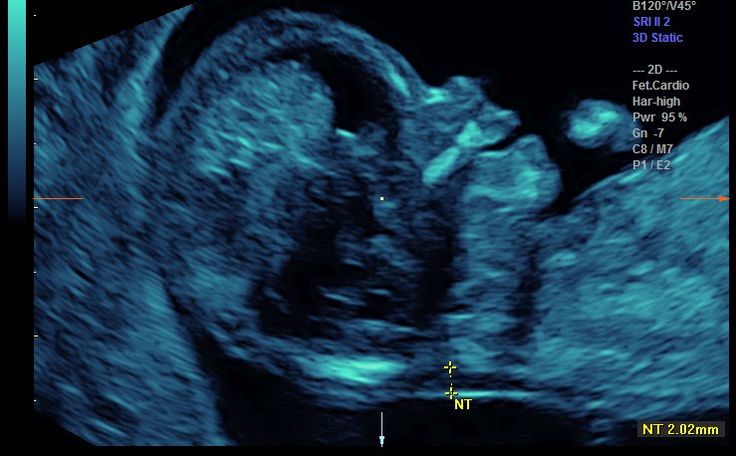 Pearson correlation test indicated that there was a significant positive correlation between NT and CRL (r = 0.238, P < 0.001), NT and GA (r = 0.24, P < 0.001 and GA) and CRL (r = 0.8, P < 0.001).
Pearson correlation test indicated that there was a significant positive correlation between NT and CRL (r = 0.238, P < 0.001), NT and GA (r = 0.24, P < 0.001 and GA) and CRL (r = 0.8, P < 0.001).
Increased NT thickness (NT >2 mm) was detected in 89 (5.5%) pregnant women. The mean of CRL and GA in pregnant women with normal and increased NT thickness are presented in .
Table 1
Mean ± SD of CRL and GA in pregnant women with normal and increased NT thickness
Open in a separate window
The expected 5th, 25th, 50th, 75th, and 95th percentile values of NT thickness to CRL and GA are listed in Tables and . Using the obtained reference value of NT, 58 (3.6%) pregnant women were determined as those with NT thickness >95th percentile according to their GA. During follow-up, 31/58 (53.4%) underwent amniocentesis. Distribution of chromosomal and nonchromosomal abnormalities in pregnant women with NT thickness >95th percentile according to their GA in total and among those with and without amniocentesis are presented in .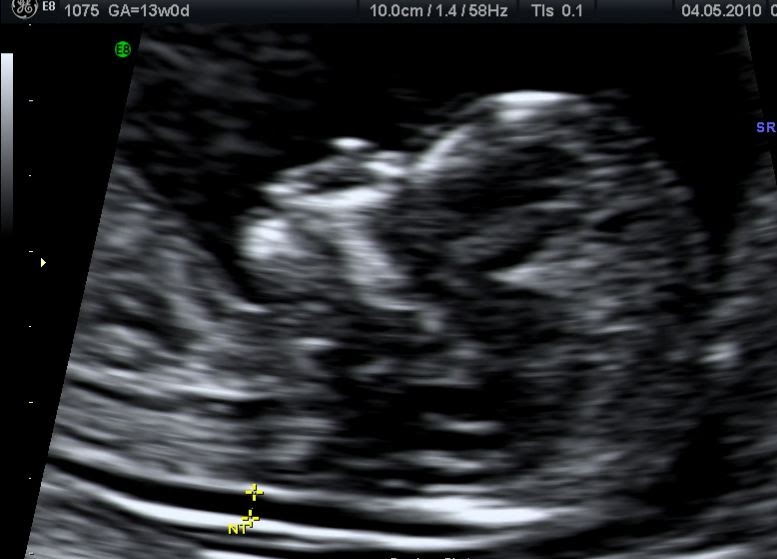 Frequency of chromosomal abnormalities were significantly higher in those pregnant women with increased NT thickness who underwent the amniocentesis procedure (P = 0.001). The frequency of different nonchromosomal abnormalities were not significantly different between the two studied groups (P > 0.05).
Frequency of chromosomal abnormalities were significantly higher in those pregnant women with increased NT thickness who underwent the amniocentesis procedure (P = 0.001). The frequency of different nonchromosomal abnormalities were not significantly different between the two studied groups (P > 0.05).
Table 2
The expected 5th, 25th, 50th, 75th, and 95th percentile values of NT thickness (mm) to CRL
Open in a separate window
Table 3
The expected 5th, 25th, 50th, 75th, and 95th percentile values of NT thickness (mm) to gestational age (GA)
Open in a separate window
Table 4
Distribution of chromosomal and nonchromosomal abnormalities in pregnant women with NT thickness >95th percentile according to their GA in total and among those with and without amniocentesis
Open in a separate window
In this study, we determined the reference values of NT thickness among pregnant Isfahani women to evaluate the role of ethnicity on the normative value of NT as well as the association of increased NT thickness with chromosomal and nonchromosomal abnormalities during the first trimester.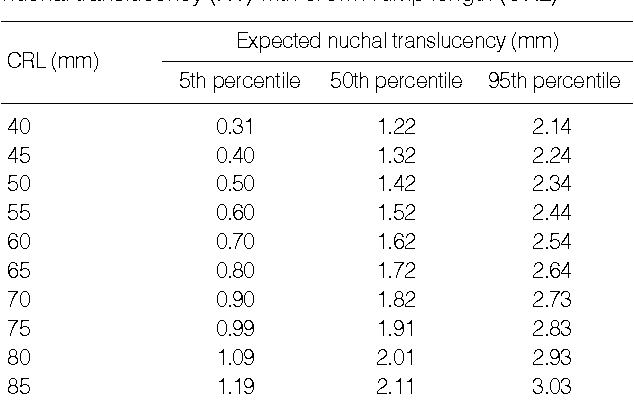 The results indicated that the reference 95th percentile value range for NT was 1.8-2.35 and increased NT thickness according to our obtained values was associated significantly with chromosomal abnormalities.
The results indicated that the reference 95th percentile value range for NT was 1.8-2.35 and increased NT thickness according to our obtained values was associated significantly with chromosomal abnormalities.
Several reports from different parts of the worlds and Iran have demonstrated the utility of NK measurement for screening different chromosomal and nonchromosomal abnormalities.[20,21,22,23]
Most of the studies have used the recommended definition for NT thickness by the FMF (i.e., 2.5-3 mm),[6] whereas recent studies reported that using NT thickness as a continuous variable was more appropriate than using a single cutoff value for the fetal NT and consequently, the outcomes of its increased values and screening programs.[18] So, establishment of reference values of NT have been developed in different regions and ethnic groups worldwide.
Though there were studies in Iran, which investigated the association between increased NT value and Down syndrome[22] and adverse pregnancy outcome including miscarriage, fetal loss, and fetal abnormalities,[23] there was not any study, which reported the normative value of NT thickness for the Iranian population.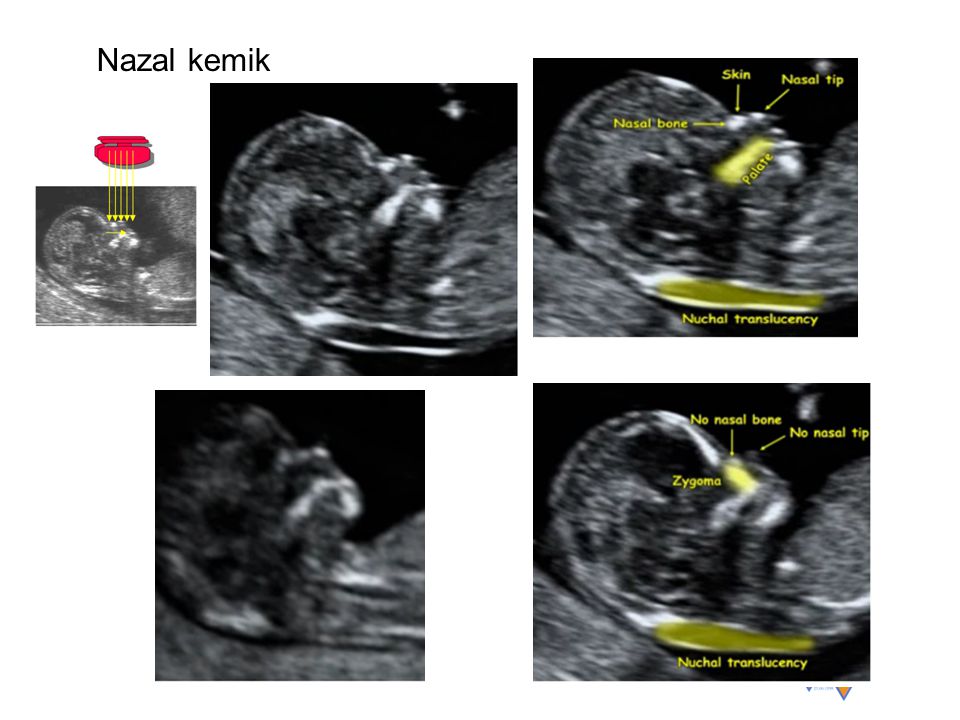 So, this study was designed to determine the ethnic specific reference value of NT thickness for pregnant Iranian women. Our results indicated that the median NT thicknesses for a CRL between 45 mm and 80 mm ranged from 1.00 to 1.65 mm, and the 95th percentiles ranged from 1.8 to 2.35 mm. The median NT thickness for GA were 1.0 mm, 1.2 mm, and 1.4 mm for gestational age of 11 weeks, 12 weeks, and 13 weeks, respectively, and the 95th percentiles of NT thickness were 1.8, 1.9, and 2.2 for gestational age of 11 weeks, 12 weeks, and 13 weeks, respectively.
So, this study was designed to determine the ethnic specific reference value of NT thickness for pregnant Iranian women. Our results indicated that the median NT thicknesses for a CRL between 45 mm and 80 mm ranged from 1.00 to 1.65 mm, and the 95th percentiles ranged from 1.8 to 2.35 mm. The median NT thickness for GA were 1.0 mm, 1.2 mm, and 1.4 mm for gestational age of 11 weeks, 12 weeks, and 13 weeks, respectively, and the 95th percentiles of NT thickness were 1.8, 1.9, and 2.2 for gestational age of 11 weeks, 12 weeks, and 13 weeks, respectively.
The distribution of the NT thickness for CRL has been reported in many studies. The median NT thicknesses has been reported to be 1.2-1.9 mm, 1.22-2.10 mm, and 1.19-1.73 mm for a CRL between 45 mm and 80 mm in Japan, Korea, and Brazil, respectively.[11,12,13] Our reported median value was lower than the other reports.
The 95th NT thickness percentiles have been reported to be 2.1-3.2 mm, 2.14-2.3 mm, 1.57-2. 10 mm, 1.00-2.90 mm, and 1.84-2.35 mm for a CRL between 45 mm and 80 mm in Japan, Korea, Brazil, Thailand, and China, respectively.[11,12,13,14,15] Our results were similar to the reported reference value range of Brazil.[13] Although there was no report from the Eastern Mediterranean region in this field, the values were not similar to the values reported from the Asian countries.
10 mm, 1.00-2.90 mm, and 1.84-2.35 mm for a CRL between 45 mm and 80 mm in Japan, Korea, Brazil, Thailand, and China, respectively.[11,12,13,14,15] Our results were similar to the reported reference value range of Brazil.[13] Although there was no report from the Eastern Mediterranean region in this field, the values were not similar to the values reported from the Asian countries.
Reported variations in the index measurements in the different studies might have been due to factors such as radiologist experience, quality of the ultrasound, method of measurement, and an inappropriate fetal and nuchal cord position. In addition, as mentioned by Kor-anantaku et al. in Thailand some investigators have considered the average of two or three measurements of NT thickness, whereas others considered the largest measurement.[14]
There are controversial reports regarding the impact of ethnicity on NT thickness values and its utility for screening. Thilaganathan et al. have investigated the possible role of ethnicity on NT screening and concluded that the reported differences could not have a significant impact in this regard.[24] Many other studies have also showed that ethnic differences in NT measurements are not clinically significant, especially when it used for screening of Down syndrome.[17,24,25] However, it seems that using ethnic-specific reference values of NT thickness could help us in the first trimester screening programs mainly for chromosomal abnormality, especially when they are integrated with other ultrasonographic and biochemical measurements.
have investigated the possible role of ethnicity on NT screening and concluded that the reported differences could not have a significant impact in this regard.[24] Many other studies have also showed that ethnic differences in NT measurements are not clinically significant, especially when it used for screening of Down syndrome.[17,24,25] However, it seems that using ethnic-specific reference values of NT thickness could help us in the first trimester screening programs mainly for chromosomal abnormality, especially when they are integrated with other ultrasonographic and biochemical measurements.
In this study using the single cutoff value of 2 mm, 5.5% of the studied pregnant women were considered to have high-risk pregnancy and after using our obtained reference value the rate decreased to 3.6%. Thus, it seems that using normative values of NT thickness is more useful for the first trimester screening and it could optimize the screening results by reducing false positive cases.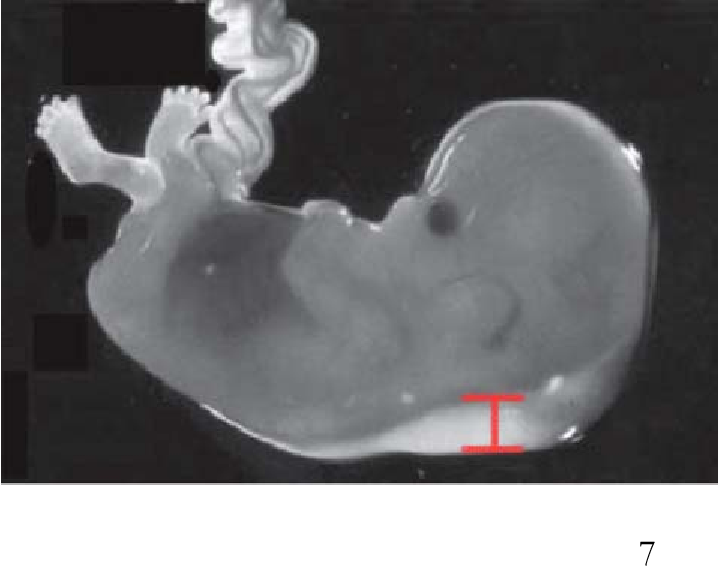
In addition, there was significant association between performing the amniocentesis procedure and detection of chromosomal abnormalities among women with increased NT thickness.
The advantage of the current study was a larger sample size of enrolled pregnant women.
The limitation of the current study was that we did not determine the sex-specific reference value of 95th percentiles of NT and its association with both chromosomal and nonchromosomal abnormalities. We followed up only pregnant women with increased NT thickness and did not determine the frequency of the mentioned abnormalities in pregnant women with normal NT. It was due to the reason that follow-up of that large a sample size was not assessable in the framework of the current study. In addition, we enrolled the patients who were referred to a single referral radiologic center, which could not be a representative sample of the whole population. It is suggested that the large sample size of the studied population could partially alleviate the abovementioned limitation.
Further, the planning of further studies that also determine the 99th percentile values of NT thickness is recommended because recent studies demonstrated that chromosomal and nonchromosomal abnormalities are mainly associated with the 99th percentile value of NT thickness.[7]
The results of our study indicated the reference value of NT thickness in a large sample size of Isfahani pregnant women. The obtained reference range in our studied population was different from that reported for other ethnic groups and it is suggested that using this values are more favorable for screening of chromosomal abnormalities during the first trimester of pregnancy than the recommended single cutoff value. The relation between increased NT thicknesses with chromosomal abnormalities also confirms its utility. The results of the current study could be used as baseline information for other follow-up studies and designing first trimester screening programs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
All authors contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
The study was supported by Isfahan University of Medical Sciences (research project number; 393486).
1. Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: Ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ. 1992;304:867–9. [PMC free article] [PubMed] [Google Scholar]
2. Szabó J, Gellén J. Nuchal fluid accumulation in trisomy-21 detected by vaginosonography in first trimester. Lancet. 1990;336:1133. [PubMed] [Google Scholar]
3. Vogel M, Sharland GK, McElhinney DB, Zidere V, Simpson JM, Miller OI, et al. Prevalence of increased nuchal translucency in fetuses with congenital cardiac disease and a normal karyotype. Cardiol Young. 2009;19:441–5. [PubMed] [Google Scholar]
4. Bilardo CM, Müller MA, Pajkrt E, Clur SA, van Zalen MM, Bijlsma EK. Increased nuchal translucency thickness and normal karyotype: Time for parental reassurance. Ultrasound Obstet Gynecol. 2007;30:11–8. [PubMed] [Google Scholar]
Bilardo CM, Müller MA, Pajkrt E, Clur SA, van Zalen MM, Bijlsma EK. Increased nuchal translucency thickness and normal karyotype: Time for parental reassurance. Ultrasound Obstet Gynecol. 2007;30:11–8. [PubMed] [Google Scholar]
5. Saldanha FA, Brizot Mde L, Moraes EA, Lopes LM, Zugaib M. Increased fetal nuchal translucency thickness and normal karyotype: Prenatal and postnatal follow-up. Rev Assoc Med Bras. 2009;55:575–80. [PubMed] [Google Scholar]
6. Salman Guraya S. The associations of nuchal translucency and fetal abnormalities; significance and implications. J Clin Diagn Res. 2013;7:936–41. [PMC free article] [PubMed] [Google Scholar]
7. Souka AP, Von Kaisenberg CS, Hyett JA, Sonek JD, Nicolaides KH. Increased nuchal translucency with normal karyotype. Am J Obstet Gynecol. 2005;192:1005–21. [PubMed] [Google Scholar]
8. Stefanovic V, Äyräs O, Eronen M, Paavonen1 J, Tikkanen M. Clinical utility of nuchal translucency screening. Res Rep Neonatol. 2014;4:169–76. [Google Scholar]
[Google Scholar]
9. Snijders RJ, Noble P, Sebire N, Souka A, Nicolaides KH. UK Multicentre Project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10-14 weeks of gestation. Fetal Medicine Foundation First Trimester Screening Group. Lancet. 1998;352:343–6. [PubMed] [Google Scholar]
10. Miron P, Côté YP, Lambert J. Nuchal translucency thresholds in prenatal screening for down syndrome and trisomy 18. J Obstet Gynaecol Can. 2009;31:227–35. [PubMed] [Google Scholar]
11. Hasegawa J, Nakamura M, Hamada S, Matsuoka R, Ichizuka K, Sekizawa A, et al. Distribution of nuchal translucency thickness in Japanese fetuses. J Obstet Gynaecol Res. 2013;39:766–9. [PubMed] [Google Scholar]
12. Chung JH, Yang JH, Song MJ, Cho JY, Lee YH, Park SY, et al. The distribution of fetal nuchal translucency thickness in normal Korean fetuses. J Korean Med Sci. 2004;19:32–6. [PMC free article] [PubMed] [Google Scholar]
13. Araujo Júnior E, Pires CR, Martins WP, Nardozza LM, Filho SM. Reference values of nuchal translucency thickness in a Brazilian population sample: Experience from a single center. J Perinat Med. 2014;42:255–9. [PubMed] [Google Scholar]
Reference values of nuchal translucency thickness in a Brazilian population sample: Experience from a single center. J Perinat Med. 2014;42:255–9. [PubMed] [Google Scholar]
14. Kor-Anantakul O, Suntharasaj T, Suwanrath C, Chanprapaph P, Sirichotiyakul S, Ratanasiri T, et al. Distribution of normal nuchal translucency thickness: A multicenter study in Thailand. Gynecol Obstet Invest. 2011;71:124–8. [PubMed] [Google Scholar]
15. Sun Q, Xu J, Hu SQ, Chen M, Ma RM, Lau TK, et al. Distribution and normal reference range of fetal nuchal translucency thickness in Kunming pregnant women in the first trimester. Zhonghua Fu Chan Ke Za Zhi. 2012;47:514–7. [PubMed] [Google Scholar]
16. Huang T, Wang F, Boucher K, O’Donnell A, Rashid S, Summers AM. Racial differences in first trimester nuchal translucency. Prenat Diagn. 2007;27:1174–6. [PubMed] [Google Scholar]
17. Chen M, Lam YH, Tang MH, Lee CP, Sin SY, Tang R, et al. The effect of ethnic origin on nuchal translucency at 10-14 weeks of gestation.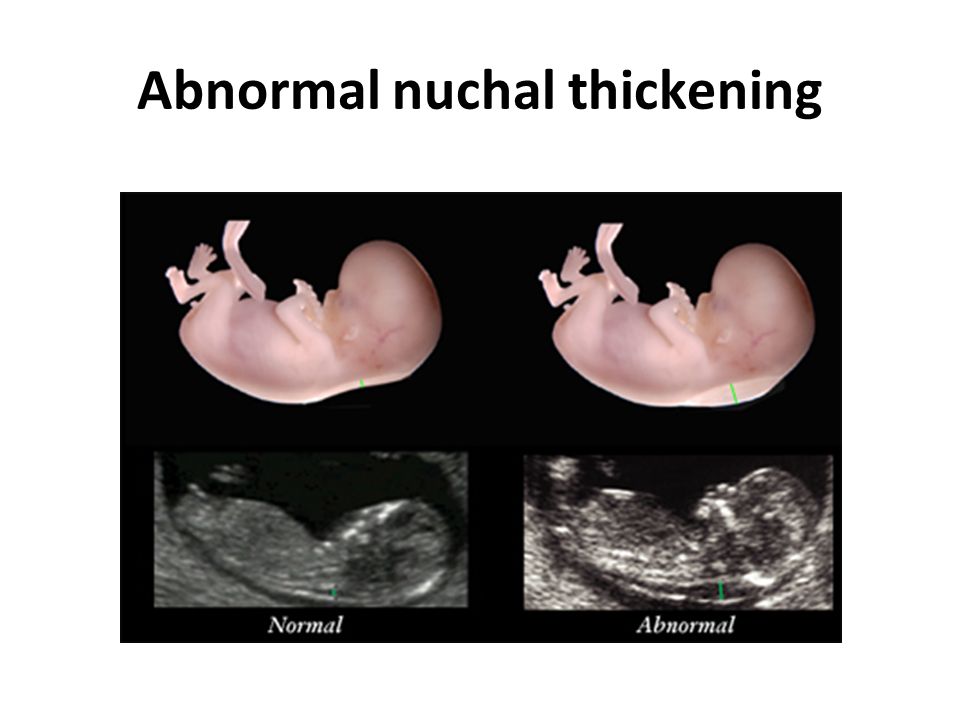 Prenat Diagn. 2002;22:576–8. [PubMed] [Google Scholar]
Prenat Diagn. 2002;22:576–8. [PubMed] [Google Scholar]
18. Taipale P, Hiilesmaa V, Salonen R, Ylöstalo P. Increased nuchal translacency as a marker for fetal chromosomal defects. N Engl J Med. 1997;337:1654–8. [PubMed] [Google Scholar]
19. Kim SM, Jun JK. Simplified protocol of nuchal translucency measurement: Is it still effective? Obstet Gynecol Sci. 2013;56:307–11. [PMC free article] [PubMed] [Google Scholar]
20. Kagan KO, Avgidou K, Molina FS, Gajewska K, Nicolaides KH. Relation between increased fetal nuchal translucency thickness and chromosomal defects. Obstet Gynecol. 2006;107:6–10. [PubMed] [Google Scholar]
21. Barati M, Zargar M, Masihi S, Taherpour S. Evaluation of nuchal translucency measurement in first trimester pregnancy. Int J Fertil Steril. 2011;5:35–8. [PMC free article] [PubMed] [Google Scholar]
22. Elahifar MA, Hasanzadeh M, Dahmardeh H, Elahifar A. The relationship between thickness of nuchal translucency and Down syndrome in the first trimester of pregnancy.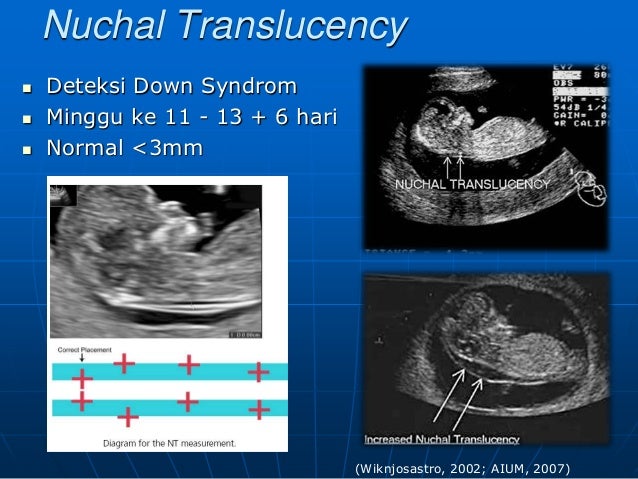 Zahedan J Res Med Sci. 2012;14:26–8. [Google Scholar]
Zahedan J Res Med Sci. 2012;14:26–8. [Google Scholar]
23. Tahmasebpour A, Rafiee NB, Ghaffari S, Jamal A. Increased nuchal translucency and pregnancy outcome. Iran J Public Health. 2012;41:92–7. [PMC free article] [PubMed] [Google Scholar]
24. Thilaganathan B, Khare M, Williams B, Wathen NC. Influence of ethnic origin on nuchal translucency screening for Down's syndrome. Ultrasound Obstet Gynecol. 1998;12:112–4. [PubMed] [Google Scholar]
25. Hsu JJ, Hsieh CC, Chiang CH, Lo LM, Hsieh TT. Preliminary normal reference values of nuchal translucency thickness in Taiwanese fetuses at 11-14 weeks of gestation. Chang Gung Med J. 2003;26:12–9. [PubMed] [Google Scholar]
Fetal Ultrasound | Women's consultation №22
Ultrasound of the fetus is an important diagnostic procedure that is performed in the I, II, III trimesters of pregnancy and is aimed primarily at identifying fetal malformations.
First trimester of pregnancy
Absolutely harmless for both mother and baby, screening includes ultrasound and biochemical examination, carried out from 11 to 13 weeks of pregnancy (first trimester).
It is at these times that it is already possible to diagnose such ultrasonic markers of chromosomal pathology as:
- thickening of the nuchal space of the fetus;
- missing nasal bone.
The thickness of the nuchal space is understood as a certain size between the soft tissues and the skin of the fetus in the cervical spine. The collar space can be both with the normal development of the fetus, and in the presence of chromosomal pathologies (in particular, with Down's disease). But with chromosomal abnormalities, the thickness of the collar space exceeds 2.5 mm. And since the collar space in all fetuses disappears after the 14th week of pregnancy, it is extremely important to undergo an ultrasound precisely in the period from 11 to 13 weeks, when it is well measurable. nine0003
As for the nasal bone , with normal development of the fetus, it is detected by ultrasound from the 10th week of pregnancy. And with chromosomal pathology, the processes of ossification in the fetus occur somewhat later, therefore, ultrasound at this time makes it possible to diagnose the absence of the nasal bone, which is a marker of fetal chromosomal disease.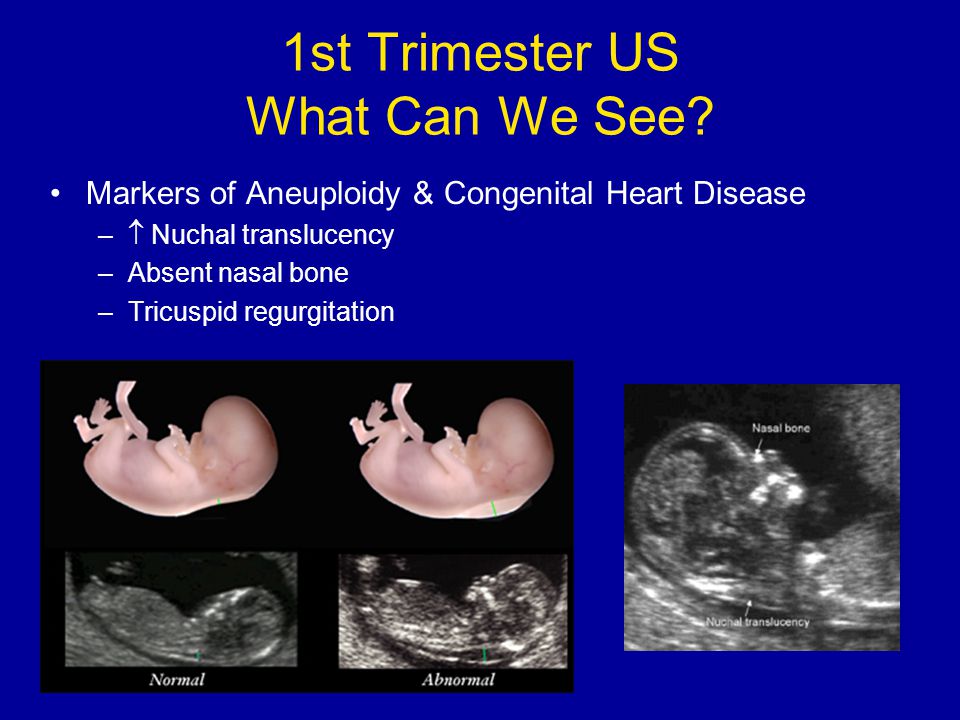
Having become a mandatory examination of pregnant women, ultrasound screening for a period of 11-13 weeks makes it possible to detect in time up to 80% of cases of pathological fetal development (with Down's disease and other chromosomal abnormalities). nine0003
Biochemical screening
A biochemical analysis of the blood of a pregnant woman can examine the concentration of proteins produced in the placenta - PAPP-A and beta-hCG. And since for each specific period of pregnancy there are norms of the studied proteins, any deviation is a sign of chromosomal pathology. The most significant differences in protein concentration during normal development and Down's disease can be diagnosed at 10-12 weeks of gestation. That is why biochemical screening is carried out at these dates, revealing up to 70% of pathologies with Down's disease. nine0003
And with complex screening (ultrasound and biochemical), the effectiveness of detecting chromosomal pathologies increases to 85%, which makes this examination method the most effective prevention of the birth of children with chromosomal pathologies, the most common of which is Down's disease.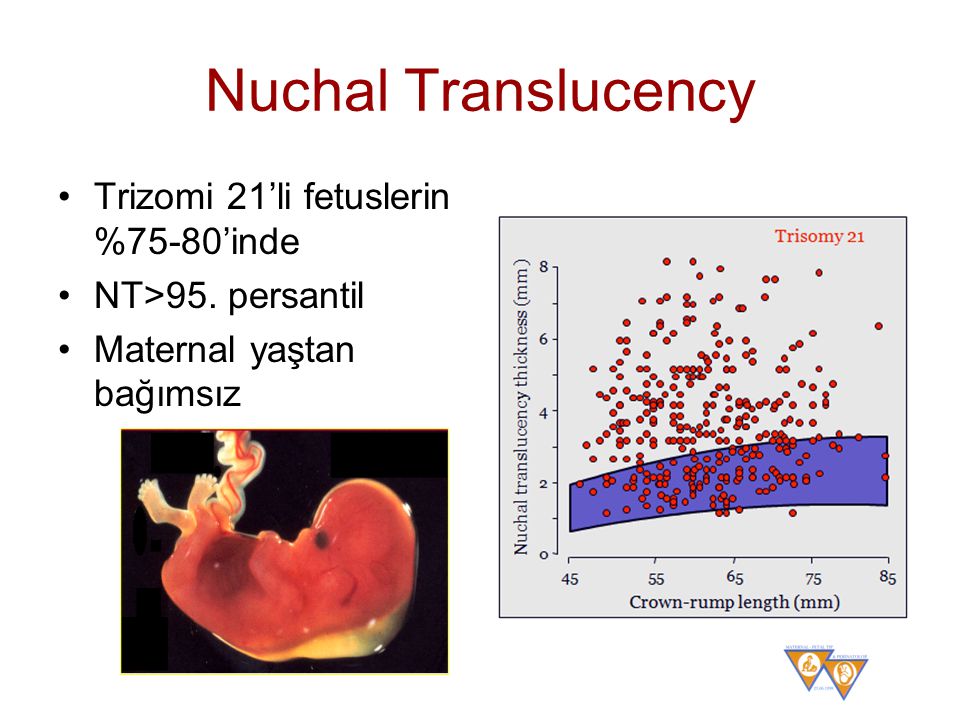
The second screening is done at 18-24 weeks of pregnancy.
On the basis of ultrasound data during pregnancy of the second trimester, a specialist makes a conclusion about the state of the placenta, the woman's pelvic organs and the amount of amniotic fluid. The thickness of the placenta, its location, the distance from the edge of the internal os and the degree of its maturity are assessed. The doctor, using ultrasound in the 2nd trimester of pregnancy, measures the length of the cervix and looks at the state of the internal os, which, normally, should still be closed. During the 2nd ultrasound during pregnancy, the presentation, that is, the position, of the child in the uterus is necessarily examined. This is very important for determining a further strategy for managing pregnancy, choosing tactics for future births and, possibly, prescribing
In the antenatal clinic No. 22, an ultrasound examination is carried out using expert-class equipment Voluson E6 (Austria).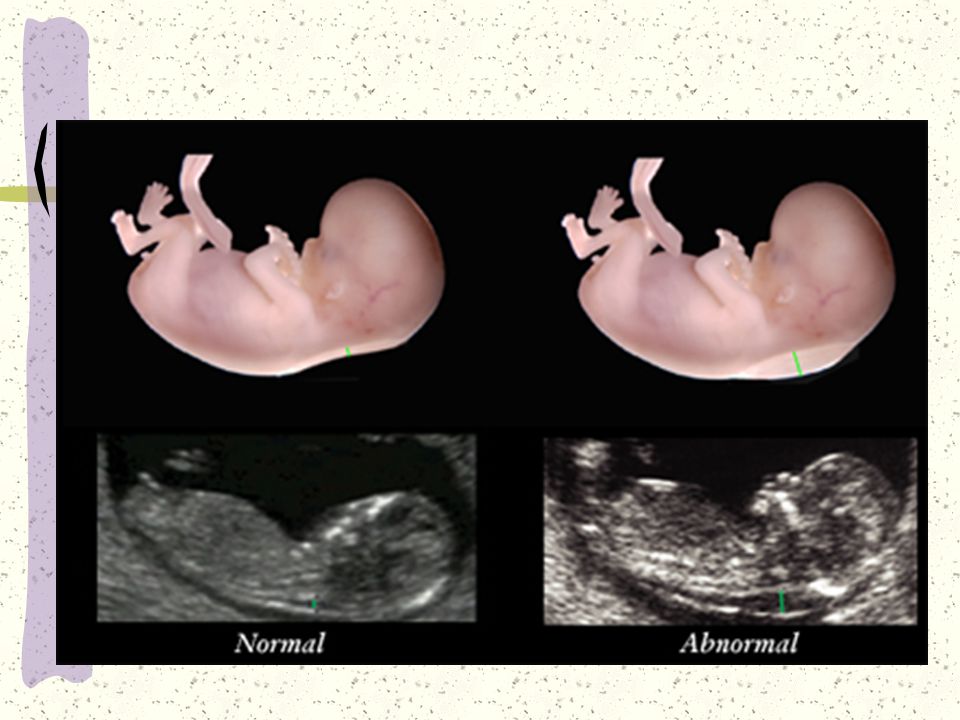
A. The expert level of all studies, diagnostics and conclusions carried out in the St. Petersburg State Budgetary Institution of Healthcare "Women's Consultation No. 22", corresponding to international standards;
B. Using the latest medical equipment equipped with HDlive 3D technology, which allows you to get a more realistic image of the fetus compared to standard 3D ultrasound; nine0003
C. Use of modern computer software, namely the professional program Astraia (certified by the international FMF certificate), which makes ultrasound findings, calculating the duration of pregnancy, fetal weight, individual risks and much more;
D. Thorough and careful examination during ultrasound examination, which lasts an average of 30-45 minutes, and if any pathology of the fetus is suspected, it can take up to 1.5 hours. In the case of multiple pregnancy, the time of diagnosis is at least doubled; nine0003
E. Consultation with a specialist in prenatal medicine, who will “decipher” the ultrasound examination, give the necessary recommendations for further actions and talk about prognosis if any fetal pathology is detected.
Risk group
Necessary understand that the probability of detection pathology and the development of this pathology - it's not the same thing. Elevated detection the risk of any deviation from the normal course of pregnancy or normal fetal development which is not a diagnosis. Pregnant women in the group risk, special additional research to confirm or rule out pathology. When is research done? nine0003
AT I trimester prenatal screening it is recommended to carry out on the 10-14th week pregnancy (optimally - on the 11-13th week). In II trimester examination can be done with 14th to 20th week (optimally - on the 16th-18th week).
Time screening studies also chosen randomly. Wherein a combination of factors is taken into account. Firstly, this is the maximum information indicators, secondly, gestational age, where additional research will be the safest for woman and child. nine0003
Simultaneous using another simple and available method - ultrasonic research - helps to increase accuracy and reliability of prenatal diagnostics. Screening includes ultrasound examination in I trimester of pregnancy (11-13 weeks). During this period, the number fetuses in the uterus and their viability, specifying the gestational age (according to coccyx-parietal size - KTR), gross malformations are excluded and chromosomal markers are determined fetal pathology (collar thickness space, presence or absence nasal bone). The term "thickness collar space "many synonyms found in literature or the conclusion of ultrasound specialists: TVP, cervical transparency, width of the cervical folds, collar zone, NT. In any case, we are talking about the accumulation subcutaneous fluid on the posterior surface fetal neck. Measure the thickness of this layer possible only in I trimester of pregnancy (until the 14th week), since at a later date this the fluid is usually resorbed. Normally, TVP should be no more than 2.7 mm. An increase in this size is typical for Down syndrome. Also, with this pathologies are often not visualized nasal bones in the fetus.
Screening includes ultrasound examination in I trimester of pregnancy (11-13 weeks). During this period, the number fetuses in the uterus and their viability, specifying the gestational age (according to coccyx-parietal size - KTR), gross malformations are excluded and chromosomal markers are determined fetal pathology (collar thickness space, presence or absence nasal bone). The term "thickness collar space "many synonyms found in literature or the conclusion of ultrasound specialists: TVP, cervical transparency, width of the cervical folds, collar zone, NT. In any case, we are talking about the accumulation subcutaneous fluid on the posterior surface fetal neck. Measure the thickness of this layer possible only in I trimester of pregnancy (until the 14th week), since at a later date this the fluid is usually resorbed. Normally, TVP should be no more than 2.7 mm. An increase in this size is typical for Down syndrome. Also, with this pathologies are often not visualized nasal bones in the fetus.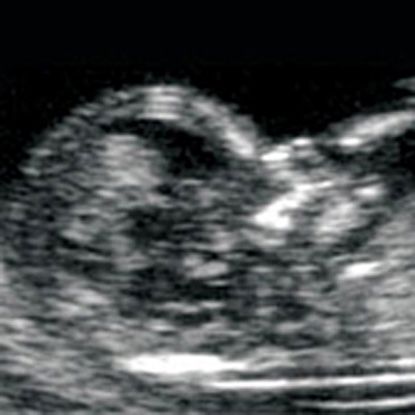 nine0003
nine0003
Necessary know that the concentration of b-hCG, PAPP, estriol and AFP in the blood can change not only with chromosomal pathology and DZNT, but also with other pathology pregnancy: threatened miscarriage or spontaneous abortion, intrauterine developmental delay or death of the fetus, placental insufficiency, late toxicosis (gestosis). Also on the value of biochemical parameters affects the intake of progestogens (Dufaston, Utrozhestan), multiple pregnancy.
Compare the results are the same, but performed in different laboratories incorrect, since the units of measurement and the norms often do not match. It's connected with the fact that for research These laboratories use different test systems. And to calculate the risks pathology at present there are several special programs with their own standards. And the results such an important examination as prenatal screening (wherever it is completed), should be able to assessed by any gynecologist. therefore received data is transferred to MoM (special coefficient).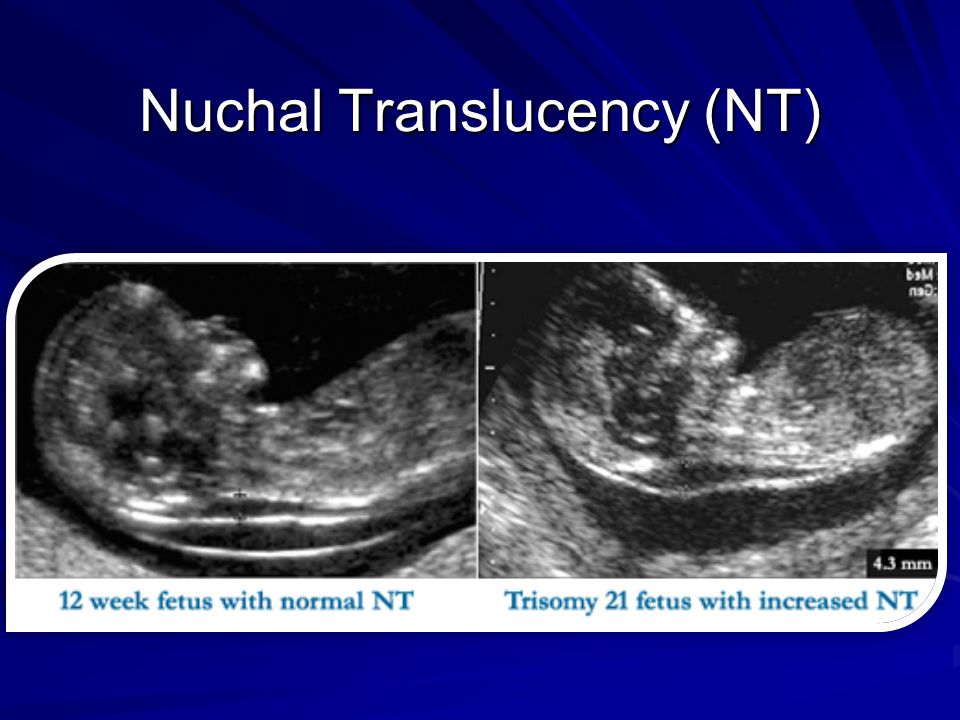 MoM norms for any biochemical markers, and also for collar thickness space (TVP) - from 0.5 to 2.0. nine0003
MoM norms for any biochemical markers, and also for collar thickness space (TVP) - from 0.5 to 2.0. nine0003
For adjusting the MoM value and calculating individual risk are taken into account factors such as the number of fruits, body weight of a pregnant woman, racial affiliation, smoking, sugar diabetes, use of ancillary reproductive technologies (IVF). Taking into account of this data is carried out individually MoM correction, resulting in "adjusted MoM value". Exactly it is then used to calculate individual risks. That's why everything the above factors should be shown on the referral form. As a separate risk factor is taken into account the age of the expectant mother. This is due to the fact that after 35 years the probability of being born child with Down syndrome increases. nine0003
I trimester II trimester Pregnancy (weeks) 10-14 weeks (optimal - 11-13) 14-20 weeks (optimally - 16-18) Biochemical markers (blood test) Free b-hCG PAPP-A AFP Free estriol L-hCG Basic ultrasound markers KTR
TVP
nasal bone
Payment risk of chromosomal abnormalities in the fetus carried out in the laboratory using special programs and is indicated in figures.