Lysine during pregnancy
Lysine Requirements of Healthy Pregnant Women are Higher During Late Stages of Gestation Compared to Early Gestation
Save citation to file
Format: Summary (text)PubMedPMIDAbstract (text)CSV
Add to Collections
- Create a new collection
- Add to an existing collection
Name your collection:
Name must be less than 100 characters
Choose a collection:
Unable to load your collection due to an error
Please try again
Add to My Bibliography
- My Bibliography
Unable to load your delegates due to an error
Please try again
Your saved search
Name of saved search:
Search terms:
Test search terms
Email: (change)
Which day? The first SundayThe first MondayThe first TuesdayThe first WednesdayThe first ThursdayThe first FridayThe first SaturdayThe first dayThe first weekday
Which day? SundayMondayTuesdayWednesdayThursdayFridaySaturday
Report format: SummarySummary (text)AbstractAbstract (text)PubMed
Send at most: 1 item5 items10 items20 items50 items100 items200 items
Send even when there aren't any new results
Optional text in email:
Create a file for external citation management software
. 2018 Jan 1;148(1):94-99.
doi: 10.1093/jn/nxx034.
Magdalene Payne 1 2 , Trina Stephens 1 2 , Kenneth Lim 1 3 , Ronald O Ball 4 , Paul B Pencharz 5 , Rajavel Elango 1 2 6
Affiliations
Affiliations
- 1 BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada.
- 2 Pediatrics, Obstetrics and Gynecology, and School of Population and Public Health, University of British Columbia, British Columbia, Canada.

- 3 Obstetrics and Gynecology, and School of Population and Public Health, University of British Columbia, British Columbia, Canada.
- 4 Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada.
- 5 The Research Institute, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada.
- 6 School of Population and Public Health, University of British Columbia, British Columbia, Canada.
- PMID: 29378056
- DOI: 10.
 1093/jn/nxx034
1093/jn/nxx034
Magdalene Payne et al. J Nutr. .
. 2018 Jan 1;148(1):94-99.
doi: 10.1093/jn/nxx034.
Authors
Magdalene Payne 1 2 , Trina Stephens 1 2 , Kenneth Lim 1 3 , Ronald O Ball 4 , Paul B Pencharz 5 , Rajavel Elango 1 2 6
Affiliations
- 1 BC Children's Hospital Research Institute, Vancouver, British Columbia, Canada.

- 2 Pediatrics, Obstetrics and Gynecology, and School of Population and Public Health, University of British Columbia, British Columbia, Canada.
- 3 Obstetrics and Gynecology, and School of Population and Public Health, University of British Columbia, British Columbia, Canada.
- 4 Department of Agricultural, Food and Nutritional Science, University of Alberta, Edmonton, Alberta, Canada.
- 5 The Research Institute, Hospital for Sick Children, 555 University Avenue, Toronto, Ontario, Canada.
- 6 School of Population and Public Health, University of British Columbia, British Columbia, Canada.

- PMID: 29378056
- DOI: 10.1093/jn/nxx034
Abstract
Background: Lysine is the first limiting amino acid in cereal proteins and is found mainly in animal-derived products. Current Dietary Reference Intake (DRI) recommendations extrapolate lysine requirements during pregnancy from nonpregnant adult data, and may underestimate true requirements.
Objective: Our objective is to define a quantitative lysine requirement in healthy pregnant women and to determine whether requirements vary between 2 phases of gestation.
Methods: Fourteen pregnant women in early (12-19 wk) and 19 women in late (33-39 wk) gestation were studied using the indicator amino acid oxidation technique. Individual lysine intakes (6-84 mg · kg-1 · d-1, deficient to excess) were tested on each study day as a crystalline amino acid mixture based on egg protein composition. Isonitrogenous diets maintained protein intake at 1.5 g · kg-1 · d-1 and calorie intake at 1.7 times resting energy expenditure during each study day. Phenylalanine and tyrosine intakes were held constant across all lysine intakes. Breath and urine samples were collected at baseline and isotopic steady state. Lysine requirements were determined by measuring the oxidation of L-[1-13C]-phenylalanine to 13CO2 (F13CO2). Biphase linear regression crossover analysis was used to determine a breakpoint (which represents the estimated average requirement, EAR) in F13CO2.
Individual lysine intakes (6-84 mg · kg-1 · d-1, deficient to excess) were tested on each study day as a crystalline amino acid mixture based on egg protein composition. Isonitrogenous diets maintained protein intake at 1.5 g · kg-1 · d-1 and calorie intake at 1.7 times resting energy expenditure during each study day. Phenylalanine and tyrosine intakes were held constant across all lysine intakes. Breath and urine samples were collected at baseline and isotopic steady state. Lysine requirements were determined by measuring the oxidation of L-[1-13C]-phenylalanine to 13CO2 (F13CO2). Biphase linear regression crossover analysis was used to determine a breakpoint (which represents the estimated average requirement, EAR) in F13CO2.
Results: The EAR for lysine during early gestation was determined to be 36.6 mg · kg-1 · d-1 (R2 = 0.484, upper 95% CI = 46.2 mg · kg-1 · d-1), similar to an earlier adult requirement of 36 mg · kg-1 · d-1. The EAR for lysine during late gestation was determined to be 50.3 mg · kg-1 · d-1 (R2 = 0.664, upper 95% CI = 60.4 mg · kg-1 · d-1), 23% higher than the current pregnancy DRI EAR recommendation of 41 mg · kg-1 · d-1.
The EAR for lysine during late gestation was determined to be 50.3 mg · kg-1 · d-1 (R2 = 0.664, upper 95% CI = 60.4 mg · kg-1 · d-1), 23% higher than the current pregnancy DRI EAR recommendation of 41 mg · kg-1 · d-1.
Conclusions: Our results suggest that lysine requirements are higher during late gestation compared to early gestation, and that current dietary lysine recommendations during late stages of pregnancy may be underestimated. The results have implications for populations consuming cereal-based diets as their primary source of protein. This trial was registered at clinicaltrials.gov as NCT01776931.
Keywords: dietary reference intakes; indicator amino acid oxidation; lysine; pregnancy; requirements.
© 2018 American Society for Nutrition. All rights reserved.
Similar articles
-
Protein requirements of healthy pregnant women during early and late gestation are higher than current recommendations.
Stephens TV, Payne M, Ball RO, Pencharz PB, Elango R. Stephens TV, et al. J Nutr. 2015 Jan;145(1):73-8. doi: 10.3945/jn.114.198622. Epub 2014 Sep 24. J Nutr. 2015. PMID: 25527661 Clinical Trial.
-
Glycine, a Dispensable Amino Acid, Is Conditionally Indispensable in Late Stages of Human Pregnancy.
Rasmussen BF, Ennis MA, Dyer RA, Lim K, Elango R. Rasmussen BF, et al. J Nutr. 2021 Feb 1;151(2):361-369. doi: 10.1093/jn/nxaa263. J Nutr. 2021. PMID: 32939556 Free PMC article.
-
Dietary phenylalanine requirements during early and late gestation in healthy pregnant women.
Ennis MA, Rasmussen BF, Lim K, Ball RO, Pencharz PB, Courtney-Martin G, Elango R. Ennis MA, et al. Am J Clin Nutr.
 2020 Feb 1;111(2):351-359. doi: 10.1093/ajcn/nqz288. Am J Clin Nutr. 2020. PMID: 31758682 Free PMC article.
2020 Feb 1;111(2):351-359. doi: 10.1093/ajcn/nqz288. Am J Clin Nutr. 2020. PMID: 31758682 Free PMC article. -
Protein/energy ratios of current diets in developed and developing countries compared with a safe protein/energy ratio: implications for recommended protein and amino acid intakes.
Millward DJ, Jackson AA. Millward DJ, et al. Public Health Nutr. 2004 May;7(3):387-405. doi: 10.1079/PHN2003545. Public Health Nutr. 2004. PMID: 15153271 Review.
-
Protein and Amino Acid Requirements during Pregnancy.
Elango R, Ball RO. Elango R, et al. Adv Nutr. 2016 Jul 15;7(4):839S-44S. doi: 10.3945/an.115.011817. Print 2016 Jul. Adv Nutr. 2016. PMID: 27422521 Free PMC article. Review.
See all similar articles
Cited by
-
In Silico Genomic and Metabolic Atlas of Limosilactobacillus reuteri DSM 20016: An Insight into Human Health.

Smythe P, Efthimiou G. Smythe P, et al. Microorganisms. 2022 Jul 2;10(7):1341. doi: 10.3390/microorganisms10071341. Microorganisms. 2022. PMID: 35889060 Free PMC article.
-
Maternal Serum Albumin Redox State Is Associated with Infant Birth Weight in Japanese Pregnant Women.
Wada Y, Ehara T, Tabata F, Komatsu Y, Izumi H, Kawakami S, Noshiro K, Umazume T, Takeda Y. Wada Y, et al. Nutrients. 2021 May 22;13(6):1764. doi: 10.3390/nu13061764. Nutrients. 2021. PMID: 34067270 Free PMC article.
-
Dietary Aromatic Amino Acid Requirements During Early and Late Gestation in Healthy Pregnant Women.
Ennis MA, Ong AJ, Lim K, Ball RO, Pencharz PB, Courtney-Martin G, Elango R. Ennis MA, et al. J Nutr.
 2020 Dec 10;150(12):3224-3230. doi: 10.1093/jn/nxaa317. J Nutr. 2020. PMID: 33188409 Free PMC article.
2020 Dec 10;150(12):3224-3230. doi: 10.1093/jn/nxaa317. J Nutr. 2020. PMID: 33188409 Free PMC article. -
Amino Acids and Developmental Origins of Hypertension.
Hsu CN, Tain YL. Hsu CN, et al. Nutrients. 2020 Jun 12;12(6):1763. doi: 10.3390/nu12061763. Nutrients. 2020. PMID: 32545526 Free PMC article. Review.
-
Preconceptional diet quality is associated with birth outcomes among low socioeconomic status minority women in a high-income country.
Abu-Saad K, Kaufman-Shriqui V, Freedman LS, Belmaker I, Fraser D. Abu-Saad K, et al. Eur J Nutr. 2021 Feb;60(1):65-77. doi: 10.1007/s00394-020-02221-4. Epub 2020 Mar 17. Eur J Nutr. 2021. PMID: 32185478
See all "Cited by" articles
Publication types
MeSH terms
Substances
Grant support
- MT 10321/Canadian Institutes of Health Research/International
Full text links
Silverchair Information Systems
Cite
Format: AMA APA MLA NLM
Send To
Cold sores in pregnancy | Pregnancy Birth and Baby
Cold sores in pregnancy | Pregnancy Birth and Baby beginning of content6-minute read
Listen
What is HSV?
If you or your partner has the virus that causes cold sores or genital herpes, you might be worried about what could happen when you're pregnant or have a new baby. Here's what you need to know about herpes simplex virus (HSV), and how to protect your baby from infection.
Here's what you need to know about herpes simplex virus (HSV), and how to protect your baby from infection.
The herpes simplex virus is a very common virus carried by most people. Sometimes it causes cold sores or genital herpes.
Cold sores are blisters that form on the lips, around the mouth and nose. Genital herpes is blisters or sores around the genitals or anus. The blisters may form a crust after about 3 days. The sores go away by themselves within 2 weeks.
There are two main types of HSV:
- HSV-1 causes mostly cold sores on the face and lips, and sometimes on the genitals
- HSV-2 causes mostly genital herpes
Both viruses are transferred though contact of the skin, saliva or genitals, and the viruses stay in the body for life. The viruses do not always cause symptoms, so you can carry the virus without knowing it.
HSV-1 is very common, with about 8 in 10 Australians carrying it in their bloodstream. But only 1 in 3 people with the virus has ever had a cold sore.:strip_icc():format(jpeg)/kly-media-production/medias/2785562/original/028627600_1556001360-shutterstock_1019963743.jpg) HSV-2 is less common.
HSV-2 is less common.
It is common for women who have had cold sores in the past to experience an outbreak while pregnant.
What happens if I get cold sores or genital herpes during pregnancy?
Having the HSV virus does not affect your chance of becoming pregnant.
It is quite common for women to have a cold sore during their pregnancy, even if they haven’t had one for a long time. Cold sores should not affect your unborn baby. But they are infectious, so it’s a good idea to treat them.
Genital herpes should not affect the baby if you have your first outbreak or it comes back in the first 34 weeks. But it can be transferred to your baby during the birth, especially if it’s your first outbreak.
If you've ever had a cold sore or genital herpes and you become pregnant, or you develop these conditions during pregnancy, it's important to tell your doctor or midwife about it. Together you can make a plan for managing herpes during pregnancy and birth.
How is HSV treated during pregnancy or breastfeeding?
You can treat cold sores and genital herpes with:
- aciclovir cream, available from a pharmacist without prescription
- aciclovir or valaciclovir tablets, for which you need a prescription
These 'antiviral' drugs are known to be safe for pregnant and breastfeeding women and are effective most of the time.
Famciclovir tablets are not recommended to take during pregnancy. Speak to your doctor if the cold sore is severe.
How is genital herpes treated during pregnancy?
You should take acyclovir or valaciclovir tablets, as above.
If it's your first outbreak of genital herpes, your baby may be more at risk because you haven't had time to develop immune protection against the virus, which also helps protect the baby.
Some women experiencing an outbreak of genital herpes might be advised to have a caesarean. This would prevent the herpes virus passing from mother to baby during a vaginal birth. But most women in Australia with genital herpes do give birth safely to healthy babies vaginally.
It's often recommended that women who have ever had genital herpes take antiviral tablets prior to the birth, even if the herpes isn't active at the time.
Talk to your doctor or midwife about the best course of action for your situation.
How do I protect my baby from herpes?
Herpes can cause serious problems in a baby, such as infections to the eyes and throat, brain damage and even death.
A newborn baby can catch HSV-1 and HSV-2 from being kissed or touched by someone with cold sores, or during childbirth.
Fortunately, most babies born to women who carry the virus are not affected. But if you or your partner has a cold sore or genital herpes, talk to your doctor about keeping it under control during the pregnancy and after the birth. It is also important to treat these conditions aggressively while you are breastfeeding.
It's important to maintain strict hygiene habits when caring for a new baby if you, or anyone in close contact with the baby, have cold sores.
If you have cold sores, you should:
- cover cold sores when you're around the baby
- avoid kissing your baby until the sores are completely healed
- avoid touching the cold sores then touching your baby
- wash your hands thoroughly before touching your baby
What if I have a cold sore while breastfeeding?
If you have cold sores, it is safe to breastfeed your baby as long as the cold sores are not on the breast or nipple.
If they are, it may be wise to breastfeed from the unaffected nipple only until the lesions have cleared up. You would express and dispose of breastmilk from the affected nipple. Breastmilk itself doesn't contain the herpes virus but it can be contaminated through the skin lesions.
Talk to your doctor or midwife as soon as you notice any cold sores on your breast or nipple.
What happens if my baby gets herpes?
It is very important that herpes in a newborn is recognised and treated by a doctor in hospital immediately. Signs of herpes in a newborn include:
- blisters on the skin
- fever
- irritability
- tiredness
- lack of appetite
If you think your baby might have the herpes virus, don't wait to see if they get better — seek medical help. Tell medical staff if you or your partner carry the herpes virus.
Resource and Support
If you are worried about your baby, see a doctor or midwife, or take them to the hospital.
FIND A HEALTH SERVICE — The Service Finder can help you find doctors, pharmacies, hospitals and other health services.
If you're not sure what to do or want more information, you call Pregnancy Birth Baby on 1800 882 436 to speak with a maternal child nurse.
Sources:
Family Planning NSW (Genital herpes), The Royal Woman’s Hospital (Genital herpes), Mothersafe (Cold sores in pregnancy and breastfeeding), Queensland Government Health (Genital Herpes and Pregnancy), SA Health (Cold sores (herpes simplex type 1) - including symptoms, treatment and prevention), Safer Care Victoria (Herpes simplex virus in neonates)Learn more here about the development and quality assurance of healthdirect content.
Last reviewed: May 2021
Back To Top
Need more information?
Cold sores - Better Health Channel
Cold sores are blisters around the mouth and nose, caused by the herpes simplex virus.
Read more on Better Health Channel website
Cold sores overview - MyDr.com.au
A cold sore is a skin infection that is caused by the herpes simplex virus (HSV). Cold sores usually occur on or around the lips or nose and are very common. They have nothing to do with colds.
Read more on myDr website
Cold sores: self-care - MyDr.com.au
Cold sores are caused by the herpes simplex type 1 virus. Most people carry this virus in their bodies. Find out what products are available for cold sores.
Read more on myDr website
Cold sores | SA Health
Herpes simplex virus type 1 (HSV1) causes cold sores on the face or lips - it is spread by skin or mucous membrane contact with infected saliva
Read more on SA Health website
Cold sore infections - MyDr.
 com.au
com.au Find the answers to common questions about cold sores, irritating blisters which are caused by the herpes simplex type 1 virus and can be triggered by stress, fatigue or exposure to sunlight.
Read more on myDr website
Cold sores: children & teens | Raising Children Network
Cold sores are quite common in older children and teenagers. Cold sores usually clear up by themselves, but see your GP if you’re concerned.
Read more on raisingchildren.net.au website
Genital Herpes | Family Planning NSW
Genital herpes is a common sexually transmitted infection (STI) caused by the herpes simplex virus (HSV). There are two types of herpes simplex virus.
Read more on Family Planning NSW website
Genital Herpes and Pregnancy
If you are pregnant and you get genital herpes, it is important to tell your midwife or obstetrician.
Read more on Queensland Health website
Genital herpes
Genital herpes is a sexually transmitted infection (STI) which shows as blisters or sores on the genitals. This is caused by the herpes simplex virus (HSV).
Read more on WA Health website
Genital herpes: what is it? - MyDr.com.au
Genital herpes is a viral infection characterised by outbreaks of blisters and sores around your genital area.
Read more on myDr website
Disclaimer
Pregnancy, Birth and Baby is not responsible for the content and advertising on the external website you are now entering.
OKNeed further advice or guidance from our maternal child health nurses?
1800 882 436
Video call
- Contact us
- About us
- A-Z topics
- Symptom Checker
- Service Finder
- Linking to us
- Information partners
- Terms of use
- Privacy
Pregnancy, Birth and Baby is funded by the Australian Government and operated by Healthdirect Australia.
Pregnancy, Birth and Baby is provided on behalf of the Department of Health
Pregnancy, Birth and Baby’s information and advice are developed and managed within a rigorous clinical governance framework. This website is certified by the Health On The Net (HON) foundation, the standard for trustworthy health information.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
This information is for your general information and use only and is not intended to be used as medical advice and should not be used to diagnose, treat, cure or prevent any medical condition, nor should it be used for therapeutic purposes.
The information is not a substitute for independent professional advice and should not be used as an alternative to professional health care. If you have a particular medical problem, please consult a healthcare professional.
Except as permitted under the Copyright Act 1968, this publication or any part of it may not be reproduced, altered, adapted, stored and/or distributed in any form or by any means without the prior written permission of Healthdirect Australia.
Support this browser is being discontinued for Pregnancy, Birth and Baby
Support for this browser is being discontinued for this site
- Internet Explorer 11 and lower
We currently support Microsoft Edge, Chrome, Firefox and Safari. For more information, please visit the links below:
- Chrome by Google
- Firefox by Mozilla
- Microsoft Edge
- Safari by Apple
You are welcome to continue browsing this site with this browser. Some features, tools or interaction may not work correctly.
| 📜 Instructions for use L-lysine aescinat ® 💊 Composition of the drug L-lysin Escinate ® ✅ The use of L-lysine Escinate ® 📅 Storage conditions of L-lysinate ® ⏳ LIZINA LIZINAS ® Keep for yourself Search for analogues Description of the medicinal product L-lysine aescinat ® (L-lysine-aescinat) Based on the official instructions for use of the drug and prepared for the electronic edition of the 2010 Vidal guide, updated on 2020. Marketing authorization holder:ART-PHARM, OOO (Russia)
Manufactured and packaged:GALYCHPHARM JSC (Ukraine) ATX code: C05CX (drugs that reduce capillary permeability, others) Dosage form
Release form, packaging and composition the drug L-lysine Escinate®
5 ml - ampoules (5) - blister packs (2) - cardboard packs. Clinical and pharmacological group: The drug is used for violations of venous circulation. Angioprotector Pharmacotherapeutic group: Angioprotective agent Pharmacological action L-lysine aescinate reduces the activity of lysosomal hydrolases, which prevents the breakdown of mucopolysaccharides in the walls of capillaries and in the connective tissue that surrounds them, and thus normalizes increased vascular tissue permeability and has anti-exudative (anti-edematous) and analgesic action. PharmacokineticsNot studied. Indications of the drug L-lysine aescinate®
Open list of ICD-10 codes
Dosing regimenThe drug is administered strictly in / in slowly, as a rule, drip (intra-arterial administration is not allowed) in a daily dose of 5-10 ml. To prepare an infusion solution of L-lysine, aescinat is diluted in 50-100 ml of 0.9% sodium chloride solution. If necessary, the drug can be administered by IV bolus very slowly. For intravenous injection of L-lysine, aescinat is diluted in 10-15 ml of 0.9% sodium chloride solution. Without preliminary dilution, the drug is not used. In conditions that threaten the life of the patient (craniocerebral trauma, postoperative edema of the brain and spinal cord with symptoms of edema-swelling, swelling of large sizes due to extensive soft tissue injuries), the daily dose is increased to 20 ml, divided into 2 doses. Side effectsIn case of individual hypersensitivity to escin in some patients, the following are possible: Allergic reactions: skin rash (papular, petechial, erythematous), itching, flushing of the skin of the face, lips, fever, urticaria; in isolated cases - Quincke's edema, anaphylactic shock. From the side of the central nervous system: headache, dizziness, tremor; in isolated cases - ataxia, short-term loss of consciousness. From the side of the liver and biliary system: increased activity of transaminases and bilirubin. From the digestive system: nausea; in isolated cases - vomiting, diarrhea. From the side of the cardiovascular system: decrease in blood pressure, increase in blood pressure, tachycardia, chest pain. From the respiratory system: isolated cases - a feeling of lack of air, shortness of breath, bronchial obstruction, dry cough. Local reactions: burning along the vein during injection, phlebitis, pain in the arm. Other: general weakness, chills, fever. Contraindications for use
Use in pregnancy and lactationContraindicated in pregnancy and lactation. Use in hepatic dysfunctionContraindicated in severe hepatic impairment. Use with caution in liver disease. Use in impaired renal functionContraindicated in severe renal impairment. Use in childrenContraindicated in children under 18 years of age. Special instructions In some patients with hepatocholecystitis, when prescribing the drug, a short-term increase in the activity of transaminases and bilirubin (direct fraction) is possible, which do not pose a threat to patients and do not require discontinuation of the drug. The concentrate for the preparation of a solution for intravenous administration contains 23.8% ethanol by volume, that is, up to 2.4 ml of ethanol in a single dose and up to a maximum of 6.1 ml / day, which corresponds to 122 ml of beer or 50.8 ml of wine per day. The drug should be prescribed with caution in alcoholism, as well as in patients with liver disease and epilepsy. Influence on the ability to drive vehicles and mechanisms The use of the drug may affect the ability to drive vehicles and mechanisms, but due to the severity of the injuries in which the use is indicated, this factor usually does not matter. OverdoseSymptoms: fever, tachycardia, nausea, heartburn, epigastric pain. Treatment: symptomatic therapy. Drug interactionsThe drug should not be used simultaneously with aminoglycosides due to the possibility of increasing their nephrotoxicity. In the case of recent, prior to the appointment of L-lysine aescinate, long-term anticoagulant therapy or, if necessary, the simultaneous appointment of L-lysine aescinate and anticoagulants, it is necessary to adjust the dose of the latter under the control of the prothrombin index. The binding of escin to plasma proteins is hampered by the simultaneous use of cephalosporin antibiotics, which can increase the concentration of free escin in the blood with the risk of side effects of the latter. The preparation contains ethyl alcohol 96%. It is necessary to take into account the possible interaction of alcohol with other drugs. Storage conditions for L-lysine aescinate®Store in a place protected from light at a temperature of 15°C to 25°C. Keep out of the reach of children. Shelf life of L-lysine aescinat®Shelf life - 2 years. Do not use the drug after the expiration date indicated on the package. Terms of saleThe drug is dispensed by prescription. Keep L-Lysine - ingredient description, instructions for use, indications and contraindications ProWellnessTable of Contents
Disclaimer Please note that all information posted on the site Prowellness is provided for informational purposes only and is not a personal program, a direct recommendation for action, or medical advice. Description of L-LysineLysine is an essential amino acid. It is not synthesized in the human body, but is essential for maintaining its vital functions. You can get it from meat or dairy products, some plant foods or supplements. Lysine exists in the form of two isomers - L and D. Only the first one, L-lysine, has biological value. It is necessary for the full growth of muscles and the synthesis of carnitine, a substance found in most cells of the human body. In addition, the amino acid is involved in the transport of fats into cells to release energy from them. Food sources of L-lysineThe leading foods for L-lysine content are:
Signs of deficiencyLack of lysine in the body leads to loss of appetite, excessive hair loss, fatigue, mood swings, reproductive disorders, nausea, dizziness, stress, growth retardation, anemia. Possible formation of kidney stones. Consumption ratesThe human body needs 23 mg of lysine per 1 kg of weight per day. The amino acid consumption rate for children is slightly higher - 170 mg / kg. They are actively growing, so they need an increased intake of building material for protein in the body. Biological value of L-lysine Together with other essential amino acids, lysine is involved in almost all processes occurring in the body. Attention! The main functions of the substance are to maintain the full functioning of the internal organs, supply the body with building material for the synthesis of proteins, which make up all the cells and tissues of the body. Benefits for the bodyLysine is important for the maintenance of the immune system as it is involved in the production of antibodies. Due to its antiviral properties, it is used in the production of ointments intended for the treatment of herpes sores and some infectious diseases. Being directly involved in the synthesis of muscle fibers, L-lysine accelerates the healing of damaged muscle tissues and stimulates the formation of new ones. By taking the supplement on training days, you can increase muscle endurance and prevent catabolic processes. Amino acid helps relieve symptoms of anxiety. According to the principle of anti-stress action, it is similar to the joy hormone serotonin: it binds to receptors and blocks the feeling of anxiety. Lysine is essential for normal brain function. With its deficiency, short-term memory deteriorates. In addition, it increases concentration, improves the activity of the cardiovascular system, prevents the formation of cholesterol plaques on the walls of blood vessels and is an excellent source of energy. Attention! L-lysine improves the absorption of calcium and inhibits its excretion from the body, providing bone strength and reducing the risk of fractures. Contraindications and side effectsL-lysine supplements should not be taken during pregnancy and lactation, in the presence of individual intolerance to individual components, liver and kidney diseases. With food, the amino acid can be consumed in any quantity. However, dietary supplements should not be abused. Signs of an overdose of the drug - flatulence, diarrhea. Instructions for use L-lysine is taken before meals, 23 mg per 1 kg of body weight per day. If lysine is used as a sports supplement, then it is taken before or immediately after training. Attention! For better muscle recovery, you can drink dietary supplements before bedtime. This will help prevent catabolic processes at night. Disclaimer Please note that all information posted on the site Prowellness is provided for informational purposes only and is not a personal program, a direct recommendation for action, or medical advice. Do not use these materials for diagnosis, treatment, or any medical procedure. Consult your physician before using any technique or using any product. This site is not a specialized medical portal and does not replace the professional advice of a specialist. |
 08.10
08.10  19
19  The drug increases vascular tone, has a moderate hypoglycemic effect.
The drug increases vascular tone, has a moderate hypoglycemic effect. 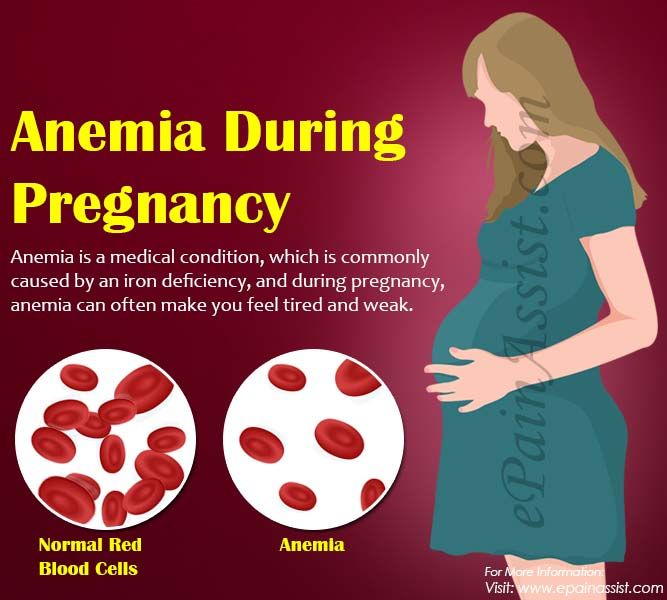 0
0 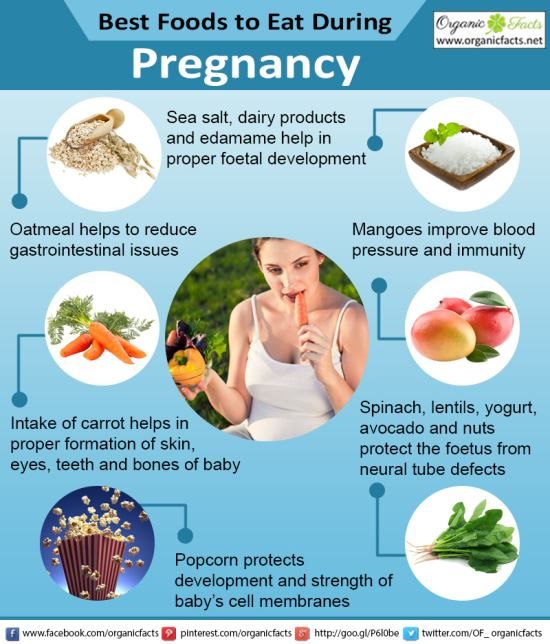 The duration of the course of treatment is from 2 to 8 days, depending on the patient's condition and the effectiveness of therapy.
The duration of the course of treatment is from 2 to 8 days, depending on the patient's condition and the effectiveness of therapy. 


 Do not use these materials for diagnosis, treatment, or any medical procedure. Consult your physician before using any technique or using any product. This site is not a specialized medical portal and does not replace the professional advice of a specialist. The Site Owner is not liable to any party who has suffered indirect or direct damage as a result of misuse of materials posted on this resource.
Do not use these materials for diagnosis, treatment, or any medical procedure. Consult your physician before using any technique or using any product. This site is not a specialized medical portal and does not replace the professional advice of a specialist. The Site Owner is not liable to any party who has suffered indirect or direct damage as a result of misuse of materials posted on this resource. 


 If the supplement is used for medicinal purposes, the dosage may be increased. For example, with acute manifestations of herpes, it is recommended to drink 3-6 capsules daily.
If the supplement is used for medicinal purposes, the dosage may be increased. For example, with acute manifestations of herpes, it is recommended to drink 3-6 capsules daily.