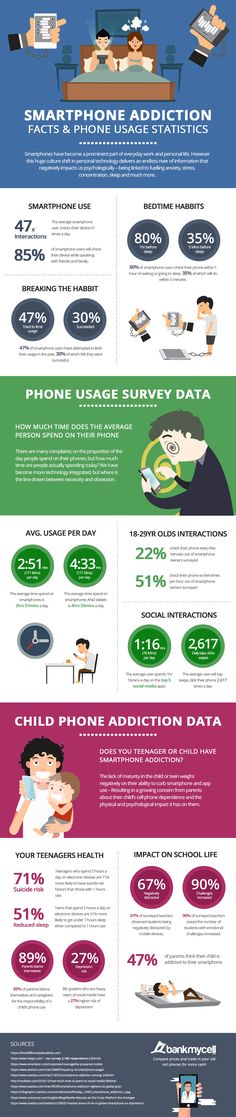
How much zofran can a child take
Ondansetron Effective for Gastroenteritis with Vomiting
MARK EBELL, M.D., M.S.
Am Fam Physician. 2006;74(5):830-831
Clinical Question: Is ondansetron (Zofran) safe and effective for dehydrated children with gastroenteritis?
Setting: Emergency department
Study Design: Randomized controlled trial (double-blinded)
Allocation: Concealed
Synopsis: Although oral rehydration is the treatment of choice for children with gastroenteritis, it can be a challenge when the child cannot keep anything down. This leads to overuse of intravenous hydration, particularly given the time pressures in the emergency department. The authors considered for inclusion in the study any mildly to moderately dehydrated child who had had at least one episode of diarrhea and one episode of vomiting in the previous four hours. Those with a body weight of less than 17 lb 10 oz (8 kg), who were severely dehydrated using standardized symptoms (e.g., clammy or cool skin, very dry mucosa, no tears, moderate tachycardia, no urine for at least six hours, limp, lethargic), and those with significant comorbidities were excluded.
Of the 243 children asked to enroll, 215 underwent randomization (allocation concealed) to ondansetron or placebo. The dose of ondansetron was 2 mg for children who weighed between 17 lb 10 oz and 33 lb (15 kg), 4 mg for those who weighed 33 lb to 66 lb (30 kg), and 8 mg for those who weighed more than 66 lb. The dose was repeated if children vomited within 15 minutes of taking the medicine. The mean age of the children was 28 months, 57 percent were boys, and they had a mean of nine episodes of vomiting and six episodes of diarrhea in the previous 24 hours. Groups were similar at baseline, and analysis was by intention to treat.
The mean age of the children was 28 months, 57 percent were boys, and they had a mean of nine episodes of vomiting and six episodes of diarrhea in the previous 24 hours. Groups were similar at baseline, and analysis was by intention to treat.
Children receiving ondansetron were less likely to vomit while being given liquids (14 versus 35 percent; P = .001; number needed to treat [NNT] = 5), had fewer vomiting episodes (0.18 versus 0.65; P < .001), and were less likely to require intravenous rehydration (14 versus 31 percent; P = .003; NNT = 5). There was no difference in the number of children requiring hospitalization or the percentage returning to the emergency department. The drug was well tolerated, although there was a mean of 0.9 additional episodes of diarrhea for children who received ondansetron.
Bottom Line: Ondansetron, when given to children who are mildly to moderately dehydrated because of diarrhea and vomiting, improves their ability to comply with oral rehydration and reduces the need for intravenous hydration. (Level of Evidence: 1b)
(Level of Evidence: 1b)
POEMs (patient-oriented evidence that matters) are provided by Essential Evidence Plus, a point-of-care clinical decision support system published by Wiley-Blackwell. For more information, see http://www.essentialevidenceplus.com. Copyright Wiley-Blackwell. Used with permission.
For definitions of levels of evidence used in POEMs, see http://www.essentialevidenceplus.com/product/ebm_loe.cfm?show=oxford.
To subscribe to a free podcast of these and other POEMs that appear in AFP, search in iTunes for “POEM of the Week” or go to http://goo.gl/3niWXb.
This series is coordinated by Sumi Sexton, MD, editor-in-chief.
A collection of POEMs published in AFP is available at https://www.aafp.org/afp/poems.
Using ondansetron for kids with nausea
You may have had Zofran (ondansetron) prescribed to you for nausea after a particularly bad stomach flu, gastroenteritis, or food poisoning, but it is most often known as a chemotherapy drug. However, if your child is having severe nausea or vomiting, you may want to do anything in your power to make their symptoms stop and give them a chance to rehydrate and recover. Here, we go over if Zofran may be an option for children.
However, if your child is having severe nausea or vomiting, you may want to do anything in your power to make their symptoms stop and give them a chance to rehydrate and recover. Here, we go over if Zofran may be an option for children.
What is Zofran?
Zofran (ondansetron) is commonly prescribed in hospital settings like emergency rooms for severe vomiting or to prevent nausea and vomiting in people who are undergoing chemotherapy. The dosage will vary based on the person, including for children. Zofran has been approved for children as young as 6 months—but you should never give it to a child without their own prescription.
Zofran for kids
Zofran is prescribed for children with vomiting who are unable to keep food or fluids down so they can regain their strength as they get better.
“Zofran is useful in kids with stomach bugs where the main symptom is vomiting as it is an antiemetic (anti-vomiting) drug,” says Pierrette Mimi Poinsett, MD, a pediatrician and consultant for Mom Loves Best. “Zofran makes it possible for a child to keep fluids down and prevent dehydration. This is known as supportive care. Zofran does not treat the stomach bug directly.”
“Zofran makes it possible for a child to keep fluids down and prevent dehydration. This is known as supportive care. Zofran does not treat the stomach bug directly.”
But in stomach bugs where children are experiencing diarrhea, Zofran should be avoided. “Zofran can cause diarrhea and may make diarrhea worse,” Dr. Poinsett says. Since many stomach bugs often have vomiting and diarrhea, this means that Zofran may not always be the best drug for alleviating common stomach flu symptoms.
In fact, Zofran should really be a used as a last resort in the case of stomach bugs “because it slows the gut motility,” explains Aniruddh Setya, MD, a pediatric gastroenterologist at
Kidz Medical Services. Dr. Setya also adds that Zofran can slow down the time to expel the toxin or virus, which would cause more harm than benefit.
Even though it is hard to watch your child suffer, getting rid of the virus is an important part of the healing process. Before Zofran, first try alternative supportive care such as sipping water, broth, electrolyte drinks, Pedialyte, Ensure, and other fluids, or eating bland foods like gelatin, applesauce, popsicles, or crackers. “Wait 30 minutes to an hour after vomiting before giving fluids or soft foods,” Dr. Poinsett says.
“Wait 30 minutes to an hour after vomiting before giving fluids or soft foods,” Dr. Poinsett says.
In addition to stomach bugs, Zofran might be prescribed for a child after having surgery if they are feeling nauseated from anesthesia. It also might be prescribed for a child going through cancer treatment if they experience nausea as a side effect of chemotherapy.
Always follow healthcare provider recommendations on the use of Zofran as there are some associated risks and contraindications outlined below.
Zofran side effects
Zofran is usually safe to use, but it does have some common side effects, including:
- Dizziness
- Constipation
- Diarrhea
- Tiredness
- Weakness
- Headache
If your child is not tolerating the side effects well, you suspect they are allergic to Zofran, or their vomiting or nausea is not getting better, contact your healthcare provider for additional advice or treatment.
Zofran risks, warnings, contraindications
As with any medication, there are several risks associated with taking Zofran. Some medications and health conditions are contraindicated with Zofran so give your child’s full medical history to all healthcare providers.
“Zofran can potentially cause changes in the heart rhythm—known as QT prolongation—and slowing of the heart rate, which can be detrimental to a child, especially those with an underlying, undiagnosed cardiac illness or electrolyte disturbances,” Dr. Setya says. Speak to your healthcare provider about any cardiac health concerns you have before starting a new medication with your child.
Some people are allergic to Zofran so look out for potential signs of allergic reaction:
- Rash
- Dizziness
- Trouble breathing
- Itching
- swelling, especially in the mouth, tongue, or throat
Seek medical attention immediately if you notice these symptoms in your child.
Alternative anti-nausea treatments
Besides comfort care, there are other medications for nausea and vomiting, called antiemetics, that may help people who are unable to keep food or water down.
Other medications, some of which are over the counter and some may be prescribed, include:
- Dimenhydrinate
- Meclizine
- Metoclopramide
- Prochlorperazine
However, many of these medications are not recommended for children under the age of 12 and you should never give any medication to a child or take medication for the first time without consulting your healthcare provider.
There are some natural antiemetics, which can be used as tolerated by your child. These include:
- Eating ginger or cumin
- Smelling lemon
- Eating or smelling peppermint
- Acupuncture or acupressure
- Deep breathing
READ NEXT: What can you take for nausea relief? 20 nausea medications and remedies
Zofran, 2 mg/ml, solution for intravenous and intramuscular administration, 2 ml, 5 pcs.
Zofran - antiemetic.
Pharmacodynamics
Ondansetron is a selective 5-HT3 receptor antagonist. Drugs for cytostatic chemotherapy and radiotherapy can cause an increase in the level of serotonin, which, by activating vagal afferent fibers containing 5-HT 3 receptors, causes a gag reflex. Ondansetron inhibits the appearance of a gag reflex by blocking 5-HT 3 receptors at the level of neurons of both the central nervous system and the peripheral nervous system.
Pharmacokinetics
Ondansetron is completely absorbed from the gastrointestinal tract after oral administration and undergoes first pass metabolism through the liver. Cmax in plasma is reached approximately 1.5 hours after ingestion. Bioavailability is somewhat increased with concomitant food intake, but does not change with antacids.
The distribution of ondansetron is the same for oral, intramuscular and intravenous administration; T 1 / 2 is approximately 3 hours, in elderly patients it can reach 5 hours, and with severe renal failure - 15-20 hours. The volume of distribution when an equilibrium concentration is reached is about 140 liters. Plasma protein binding is 70-76%. After rectal administration, ondansetron is determined in plasma after 15-60 minutes. The concentration of the active substance increases linearly, Cmax is reached after about 6 hours and is 20–30 ng/ml. The decrease in plasma concentration occurs at a slower rate than after oral administration (due to continued absorption). T 1/2 - 6 hours. Absolute bioavailability with rectal administration - 60%.
The volume of distribution when an equilibrium concentration is reached is about 140 liters. Plasma protein binding is 70-76%. After rectal administration, ondansetron is determined in plasma after 15-60 minutes. The concentration of the active substance increases linearly, Cmax is reached after about 6 hours and is 20–30 ng/ml. The decrease in plasma concentration occurs at a slower rate than after oral administration (due to continued absorption). T 1/2 - 6 hours. Absolute bioavailability with rectal administration - 60%.
It is eliminated from the systemic circulation mainly as a result of metabolism in the liver, which occurs with the participation of several enzyme systems. The absence of the CYP2D6 enzyme (spartein-debrisoquine type polymorphism) does not affect the pharmacokinetics of ondansetron. Less than 5% of the administered dose is excreted unchanged in the urine.
At doses above 8 mg, blood levels increase disproportionately, because when prescribing high doses orally, metabolism during the first passage through the liver may decrease.
The pharmacokinetic parameters of ondansetron do not change with repeated administration.
In patients with moderate renal insufficiency (Cl creatinine - 15-60 ml / min), both systemic clearance and volume of distribution of ondansetron are reduced, resulting in a small and clinically insignificant increase in its T 1/2 (up to 5.4 h) . The pharmacokinetics of ondansetron remains virtually unchanged in patients with severe renal impairment on chronic hemodialysis. In patients with severe hepatic impairment, the systemic clearance of ondansetron is sharply reduced, resulting in an increase in its T 1/2 (up to 15-32 hours), and oral bioavailability reaches 100% due to a decrease in first-pass metabolism.
Special patient groups
Sex
The pharmacokinetics of ondansetron depends on the sex of the patients. Women have lower systemic clearance and volume of distribution (values adjusted for body weight) than men.
Children and adolescents (aged 1 month to 17 years)
In a clinical study, children aged 1 to 24 months (51 patients) received ondansetron at a dose of 0. 1 mg/kg or 0.2 mg/kg up to surgical intervention. In patients aged 1 to 4 months, Cl was approximately 30% lower than in patients aged 5 to 24 months, but comparable to this indicator in patients aged 3 to 12 years (with correction for indicators depending on weight bodies). T 1/2 in the group of patients aged 1-4 months averaged 6.7 hours; in the age groups 5–24 months and 3–12 years — 2.9h. In patients aged 1 to 4 months, dose adjustment is not required, since a single intravenous injection of ondansetron is used to treat postoperative nausea and vomiting in this category of patients. Differences in pharmacokinetic parameters are partly explained by a higher volume of distribution in patients aged 1 to 4 months.
1 mg/kg or 0.2 mg/kg up to surgical intervention. In patients aged 1 to 4 months, Cl was approximately 30% lower than in patients aged 5 to 24 months, but comparable to this indicator in patients aged 3 to 12 years (with correction for indicators depending on weight bodies). T 1/2 in the group of patients aged 1-4 months averaged 6.7 hours; in the age groups 5–24 months and 3–12 years — 2.9h. In patients aged 1 to 4 months, dose adjustment is not required, since a single intravenous injection of ondansetron is used to treat postoperative nausea and vomiting in this category of patients. Differences in pharmacokinetic parameters are partly explained by a higher volume of distribution in patients aged 1 to 4 months.
In a study in children aged 3–12 years (21 patients) undergoing elective surgery under general anesthesia, the absolute values of Cl and volume of distribution of ondansetron after a single intravenous dose of 2 mg (from 3 to 7 years) or 4 mg (8 to 12 years) were reduced compared to adult values. Both parameters increased linearly with body weight, in patients aged 12 years, these values approached those in adults. When correcting the values of clearance and volume of distribution, depending on body weight, these parameters were similar in different age groups. The weight-adjusted dose (0.1 mg/kg, up to a maximum of 4 mg) compensates for these changes and for the systemic exposure of ondansetron in children.
Both parameters increased linearly with body weight, in patients aged 12 years, these values approached those in adults. When correcting the values of clearance and volume of distribution, depending on body weight, these parameters were similar in different age groups. The weight-adjusted dose (0.1 mg/kg, up to a maximum of 4 mg) compensates for these changes and for the systemic exposure of ondansetron in children.
A population pharmacokinetic analysis was performed in 74 patients aged 6 to 48 months who received ondansetron 0.15 mg/kg IV every 4 hours for 3 doses for the relief of chemotherapy-induced nausea and vomiting and in 41 patients aged 1 to 24 months after surgery who received ondansetron at a single dose of 0.1 or 0.2 mg/kg. Based on the pharmacokinetic parameters in this group, for patients aged 1 to 48 months, the administration of ondansetron IV at a dose of 0.15 mg/kg every 4 hours in the amount of 3 doses should lead to a systemic exposure comparable to that observed with the use of the drug at the same doses in children aged 5 to 24 months in surgical interventions, as well as in previous studies in children with cancer (aged 4 to 18 years) and in surgical intervention (aged 3 to 12 years ).