How does a child suppository work
Child Suppository Rectal: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing
Uses
This product is used to relieve occasional constipation. Glycerin belongs to a class of drugs known as hyperosmotic laxatives. It works by drawing water into the intestines. This effect usually results in a bowel movement within 15 to 60 minutes.For adults, the normal frequency of bowel movements varies from once daily to 1 to 2 times weekly. For preschool-aged children, the normal frequency of bowel movements varies from once daily to once every other day. Constipation is best treated by drinking plenty of fluids, eating foods high in fiber, and exercising regularly.
How to use Child Suppository Suppository, Rectal
This product is for rectal use only. Read and follow all directions on the product package, or use as directed by your doctor. If you have any questions, ask your doctor or pharmacist. If the suppository is too soft to insert, chill in the refrigerator for 30 minutes or run cold water over it before removing the foil wrapper.
Wash your hands before and after using this product. If the medication is wrapped in foil, remove the foil wrapper. If desired, the suppository may be moistened with lukewarm water. Do not use petroleum jelly or mineral oil. Doing so may cause the product to be less effective.
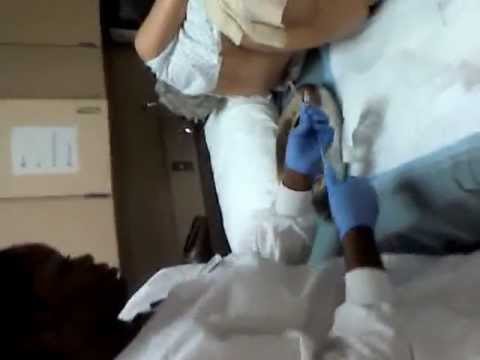
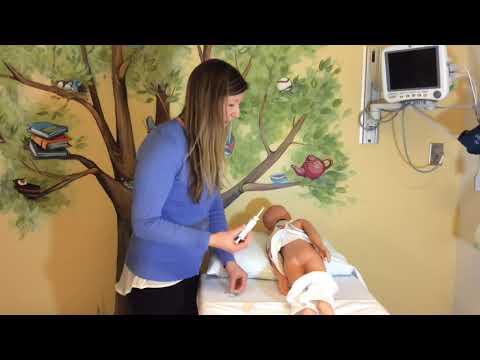
Lie on your left side with the right knee slightly bent. Using your finger, gently insert the suppository well up into the rectum, pointed end first. After insertion, stay in position for 15 to 20 minutes if possible until you feel a strong urge to have a bowel movement. This product does not need to melt completely to produce an effect. If you are helping a child use this product, have the child lie on their side with the lower leg straightened out and the upper leg bent toward the stomach. Using your finger, gently insert the suppository into the rectum, pointed end first. Hold the buttocks together for a few seconds. Then, have your child stay lying down for 15 to 20 minutes if possible to keep the suppository from coming out.
Do not use this product more than once daily unless otherwise directed by your doctor.
If this product is used too frequently, it may cause loss of normal bowel function and an inability to have a bowel movement without using the product (laxative dependence). If you notice symptoms of overuse, such as diarrhea, abdominal pain, decreased weight, or weakness, contact your doctor promptly.
Consult your doctor promptly if you do not have a bowel movement after using this product or if you think you may have a serious medical problem.
Side Effects
Rectal irritation/burning, abdominal discomfort/cramps, or small amounts of mucus in the stool may occur. If any of these effects last or get worse, tell your doctor or pharmacist promptly.
If your doctor has directed you to use this product, remember that your doctor has judged that the benefit to you is greater than the risk of side effects. Many people using this product do not have serious side effects.
Tell your doctor right away if you have any serious side effects, including: abdominal pain that is severe or doesn't go away, bloody stools, rectal bleeding.
Diarrhea that doesn't stop may result in dehydration. Contact your doctor promptly if you notice any symptoms of dehydration, such as unusual decreased urination, unusual dry mouth/thirst, fast heartbeat, or dizziness/lightheadedness.
A very serious allergic reaction to this drug is rare. However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: rash, itching/swelling (especially of the face/tongue/throat), severe dizziness, trouble breathing.
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US - Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada - Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
You may report side effects to Health Canada at 1-866-234-2345.
Precautions
Before using glycerin, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: rectal bleeding, intestinal blockage (obstruction), other bowel problems (such as ulcerative colitis, hemorrhoids), current stomach/abdominal symptoms (such as nausea/vomiting that doesn't stop, pain, cramping).
Consult your doctor before using this product if you have had a sudden change in bowel habits lasting more than 2 weeks or if you need to use a laxative for more than 1 week. These could be symptoms of a serious medical problem.
During pregnancy, this medication should be used only when clearly needed. Discuss the risks and benefits with your doctor.
It is not known whether this drug passes into breast milk. Consult your doctor before breast-feeding.
Interactions
Drug interactions may change how your medications work or increase your risk for serious side effects. This document does not contain all possible drug interactions. Keep a list of all the products you use (including prescription/nonprescription drugs and herbal products) and share it with your doctor and pharmacist. Do not start, stop, or change the dosage of any medicines without your doctor's approval.
Does Child Suppository Suppository, Rectal interact with other drugs you are taking?
Enter your medication into the WebMD interaction checker
Overdose
This medicine may be harmful if swallowed. If someone has overdosed and has serious symptoms such as passing out or trouble breathing, call 911. Otherwise, call a poison control center right away. US residents can call their local poison control center at 1-800-222-1222. Canada residents can call a provincial poison control center.
Canada residents can call a provincial poison control center.
Keep all regular medical and laboratory appointments.
Not applicable.
Refer to storage information on the package label. Protect from high heat. Do not store in the bathroom. If you have any questions about storage, ask your pharmacist. Keep all drug products away from children and pets.
Do not flush medications down the toilet or pour them into a drain unless instructed to do so. Properly discard this product when it is expired or no longer needed. Consult your pharmacist or local waste disposal company.
Images
Next
Related Links
Drug Survey
Have you ever purchased Child Suppository Suppository, Rectal?
Yes, In the past 3 months
Yes, In the past 6 months
Yes, In the past year
Haven't purchased but considering
Don't plan to purchase
This survey is being conducted by the WebMD marketing sciences department.
Free RX Coupon
Save up to 80% on your prescriptions.

Available coupons
Save up to 80% on your prescription with WebMDRx
Selected from data included with permission and copyrighted by First Databank, Inc. This copyrighted material has been downloaded from a licensed data provider and is not for distribution, except as may be authorized by the applicable terms of use.
CONDITIONS OF USE: The information in this database is intended to supplement, not substitute for, the expertise and judgment of healthcare professionals. The information is not intended to cover all possible uses, directions, precautions, drug interactions or adverse effects, nor should it be construed to indicate that use of a particular drug is safe, appropriate or effective for you or anyone else. A healthcare professional should be consulted before taking any drug, changing any diet or commencing or discontinuing any course of treatment.
Child Constipation Education FAQ
Our FAQ section should help you find the answers you need to resolve their constipation problem now and ways to avoid more problems in the future by changing their diet.
General Questions
-
Q What is constipation?
AA division of the National Institutes of Health defines constipation as having a bowel movement fewer than three times a week. Stools are usually hard, dry, small in size and difficult to eliminate. Children with constipation usually either have very large hard stools or hard pellet-like stools. Bowel movements are infrequent and often painful.
The American Academy of Pediatrics outlines normal frequency of bowel movements for children. See table below.
Age Bowel Movements Per Weeka Bowel Movements Per Dayb Adapted from Fontana M, Blanch C, Cataldo F, et al. Bowel frequency in healthy children.
Acta Paediatr Scand 1987;78;682-4.
a Approximately mean 2 SD.
b Mean.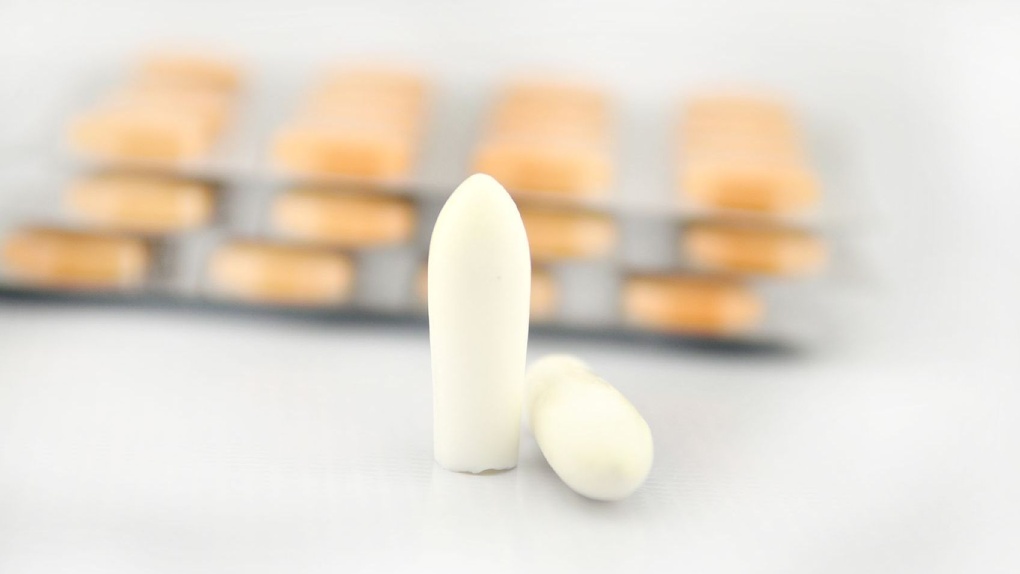
Source: North American Society for Pediatric Gastroenterology, Hepatology and Nutrition0-3 Months Breast-fed 5-40 2.9 Formula-fed 5-28 2.0 6-12 Months 5-28 1.8 1-3 Years 4-21 1.4 More than 3 Years 3-14 1.0 -
Q What causes constipation?
ALots of things can cause constipation. Common causes include not getting enough fiber, fluids or insufficient exercise. In addition, with children, constipation is often caused by “holding it in” too long because they don’t want to stop playing to go to the bathroom. However, constipation can also result from a more serious medical condition. The first time your child experiences constipation, talk to your pediatrician for a complete diagnosis.

-
Q Is constipation normal?
AYes, completely. Constipation is a common problem for kids. Unfortunate, but true.
-
Q Should I panic if my child has constipation?
ANot at all. Constipation is completely normal and a common problem for kids. Unfortunate, but true.
-
Q When should I begin to worry?
AIf constipation lasts for more than seven days after treatment, the child has persistent bouts with constipation or is in acute pain, they should see their pediatrician immediately.
-
Q How do I know it is constipation and not something else?
AWhile it’s perfectly normal for your child to experience occasional constipation, it’s not normal for it to be prolonged or ongoing.
 If constipation lasts for more than seven days after treatment, you need to see a doctor to rule out a more serious medical condition or set up a treatment approach for a chronic constipation condition.
If constipation lasts for more than seven days after treatment, you need to see a doctor to rule out a more serious medical condition or set up a treatment approach for a chronic constipation condition. -
Q How is constipation treated?
AParents can often successfully treat constipation with diet changes and over-the-counter medicine. They should, however, seek the counsel of a healthcare professional for children aged 2 and under and/or the first time their child suffers from constipation.
-
Q How do I prevent constipation?
AThere are several things you can do to help prevent constipation. Make sure your child eats a well-balanced diet with plenty of fiber. They also need to drink lots of fluids and get plenty of exercise. Most importantly, encourage them to use the bathroom when they need to. Let them play a little longer outside or give them a reward when they stop for a bathroom break.
 If potty training, make sure you have scheduled “potty time.”
If potty training, make sure you have scheduled “potty time.” -
Q What is soiling?
ASoiling is what happens when liquid feces leak out around hard, compacted stool. It can happen once or several times a day and is often confused with diarrhea. There is nothing a child can do to withhold it and parents don’t often make the connection between what they think is diarrhea and constipation, making the right diagnosis difficult.
Back to top
Pedia-Lax® Questions
-
Q How does Pedia-Lax® work?
AThe active ingredients in Pedia-Lax® products work in several different ways to help relieve your child’s constipation.
The active ingredient in Pedia-Lax® Chewable Tablets, magnesium hydroxide, increases the amount of water in the intestine, which promotes bowel movement.

The active ingredient in Pedia-Lax® Liquid Stool Softener, docusate sodium, works directly on the stool to help soften it.
The active ingredient in Pedia-Lax® Glycerin Suppositories and Liquid Glycerin Suppositories, glycerin, attracts water into the stool and promotes bowel movement.
The active ingredients in Pedia-Lax® Enema, sodium phosphates, increase the amount of water in the intestine, which promotes bowel movement.
-
Q After I give my child Pedia-Lax®, how long before they’ll go?
AResults will vary with each child and depend on which Pedia-Lax® product you use, anywhere from a few minutes to 72 hours.
-
Q Can I use Pedia-Lax® for my child under 2 years of age?
AIf your child is under age 2, consult your pediatrician.
-
Q Can I use Pedia-Lax® for my child over the age of 11?
APedia-Lax® is indicated for children 2-11.
 Pedia-Lax® would not be unsafe for children over 11, but we recommend using an adult product by Fleet to ensure that the product will be effective for older children.
Pedia-Lax® would not be unsafe for children over 11, but we recommend using an adult product by Fleet to ensure that the product will be effective for older children. -
Q Can pregnant women take Pedia-Lax®?
APedia-Lax® is designed for children ages 2-11. Pregnant women should consult their doctor before taking any medications.
-
Q Can my child take Pedia-Lax® when taking other medications?
AAs with any medication, consult your pediatrician if your child is already taking other medications.
-
Q Which Pedia-Lax® product should I use?
AChoose from five different Pedia-Lax® laxative products. Chewable Tablets, Liquid Stool Softener, Liquid Glycerin Suppositories, Glycerin Suppositories and Pedia-Lax® Enema. The Pedia-Lax® product you choose will be determined by both the severity of the problem and the best delivery system for your child.
 And to help maintain your child's digestive balance* on a regular basis, try our Probiotic Yums.
And to help maintain your child's digestive balance* on a regular basis, try our Probiotic Yums.If your child is uncomfortable because of constipation, we first suggest trying an oral product: chewable tablets or liquid stool softener. If your child’s constipation is more severe and you want the quickest relief possible, we suggest suppositories or enemas.
-
Q What’s the difference between the rectal and oral products?
AOral products are easy to administer and work over a short period of time. Rectal products provide immediate relief. Please see our product information page for details on each product’s reaction time.
-
Q What is the expiration date for these products?
AThe expiration date for Pedia-Lax® laxatives is printed on the outside of the carton.
-
Q How should Pedia-Lax® be stored?
APedia-Lax® should be stored at a controlled temperature between 59°-86° Fahrenheit.

-
Q The dosing directions for Pedia-Lax® are by age range. How do I know what part of the range I should use for my child?
AA dosing range by age provides greater flexibility for finding the right dose. As with any medicine, if you have questions or concerns, ask your pediatrician.
-
Q Is it OK for my child to take Pedia-Lax® laxatives every day?
APedia-Lax® laxatives should not be taken for more than seven days in a row. If your child experiences constipation for more than seven consecutive days, consult your pediatrician.
-
Q Are your products gluten-free?
Back to top
Suppositories and Enema Questions
-
Q What’s the difference between the three rectal products?
APedia-Lax® Enema is a saline laxative.
 Pedia-Lax® Liquid Glycerin Suppositories are a glycerin laxative administered in a liquid form applied with a disposable, no-mess applicator. Pedia-Lax® Glycerin Suppositories are small suppositories. They all relieve occasional constipation.
Pedia-Lax® Liquid Glycerin Suppositories are a glycerin laxative administered in a liquid form applied with a disposable, no-mess applicator. Pedia-Lax® Glycerin Suppositories are small suppositories. They all relieve occasional constipation. -
Q Is your Pedia-Lax® Liquid Glycerin Suppository latex-free?
-
Q Can the Pedia-Lax® Liquid Glycerin applicator be used more than once?
ANo, they are designed to be used only once. Each applicator contains medication for one dose.
-
Q What is the source of your glycerin, animal or vegetable?
AVegetable.
-
Q Are Pedia-Lax® Glycerin Suppositories available in “infant” size?
ANo. Pedia-Lax® Glycerin Suppositories are designed for use in children ages 2-5 years.
 Talk to your pediatrician about use in children below age 2.
Talk to your pediatrician about use in children below age 2. -
Q My child doesn’t do well with suppositories. Every time I get one in, he pushes it out. What can I do?
ATry using Pedia-Lax® Liquid Glycerin Suppositories. They have the same active ingredient in liquid form and can’t be “pushed out” like a regular suppository because they are liquid.
-
Q My child is older than 5. Can I still use Pedia-Lax® Liquid Glycerin or Glycerin Suppositories?
AChildren over the age of 5 can use Fleet Adult Liquid Glycerin or Glycerin Suppositories.
Back to top
Pedia-Lax® Products
Poop happens, but when it doesn't, there's Pedia-Lax®.
Learn more
Where to buy
Pedia-Lax® products are available at many fine retailers.
Find a Store
| 📜 Instructions for use Cefecon ® D 💊 Composition of the drug Cefecon ® D ✅ Application of the drug Tsufon ® D 📅 Storage Conditions Tsecon ® D ⏳ The shelf life of CEFON ® Save Search Description of the drug drug Cefecon ® D (Cefecon D) Based on the official instructions for use of the drug, approved by the manufacturer and prepared for the electronic edition of the Vidal Handbook 2014 year, update date: 2019. Marketing authorization holder:NIZHFARM JSC (Russia)
Contacts for inquiries:STADA (Russia) ATX code: N02BE01 (Paracetamol) Active substance: paracetamol (paracetamol) Rec.INN registered by WHO Dosage forms
Release form, packaging and composition drug Cefecon® Y Rectal suppositories for children white or white with a creamy or yellowish tint, torpedo-shaped.
Auxiliary substances : Viteptopsol. 5 pcs. - cellular contour packings (2) - packs of cardboard. Clinical and pharmacological group: Analgesic-antipyretic Pharmacotherapeutic group: Analgesic non-narcotic agent Pharmacological actionHas analgesic and antipyretic effect. It blocks cyclooxygenase in the central nervous system, affecting the centers of pain and thermoregulation. In the focus of inflammation, cellular peroxidases neutralize the effect of paracetamol on cyclooxygenase, which explains the absence of a significant anti-inflammatory effect. The drug does not adversely affect the water-electrolyte metabolism (does not lead to sodium and water retention) and the gastrointestinal mucosa. PharmacokineticsAbsorption and distribution Rapidly and highly absorbed from the gastrointestinal tract. C max is achieved in 30-60 minutes. Plasma protein binding - 15%. Penetrates through the BBB. Metabolism and excretion Metabolized in the liver; 80% reacts with glucuronic acid and sulfates to form inactive metabolites; 17% undergoes hydroxylation to form active metabolites, which are then conjugated with glutathione to form inactive metabolites. With a lack of glutathione, these metabolites can block the enzyme systems of hepatocytes and cause their necrosis. T 1/2 - 2-3 hours. Within 24 hours, 85-95% of paracetamol is excreted by the kidneys in the form of glucuronides and sulfates, unchanged - 3%. Pharmacokinetics in special clinical situations V d and bioavailability in children (including newborns) are similar to those in adults. In newborns of the first 2 days of life and in children aged 3-10 years, the main metabolite of paracetamol is paracetamol sulfate, in children 12 years and older - conjugated glucuronide. Indications of the drug Cefecon® DThe drug is intended for use in children aged 3 months to 12 years. In children aged 1 to 3 months, a single use of the drug to reduce fever after vaccination is possible (the possibility of using the drug for other indications is decided by the doctor individually). Used as:
Open list of ICD-10 codes
Dosing regimenThe drug is used rectally. Suppositories are introduced into the child's rectum after a cleansing enema or spontaneous bowel movement. The dosing regimen is set according to age and body weight.
When using the drug as an antipyretic, the duration of the course of treatment is 3 days; as an anesthetic - 5 days. If necessary, the doctor can extend the course of treatment. Side effects From the digestive system: possible nausea, vomiting, abdominal pain. Allergic reactions: rash on the skin and mucous membranes, itching, urticaria, Quincke's edema. On the part of the hematopoietic system: rarely - anemia, thrombocytopenia, leukopenia, agranulocytosis. Long-term use in high doses may develop hepatotoxic and nephrotoxic (interstitial nephritis and papillary necrosis) action, hemolytic anemia, aplastic anemia, methemoglobinemia, pancytopenia. Contraindications for use
With caution use the drug for violations of the liver and kidneys, Gilbert's syndrome, Dubin-Johnson, Rotor, diseases of the blood system (anemia, thrombocytopenia, leukopenia), deficiency of the enzyme glucose-6-phosphate dehydrogenase. Use in violation of liver functionUse with caution in violation of liver function. Use in disorders of kidney function Use with caution in case of impaired renal function. Special instructionsThe patient should be warned to consult a doctor if fever persists for more than 3 days and pain persists for more than 5 days. Simultaneous use with other paracetamol-containing drugs should be avoided. this can cause an overdose of paracetamol. When using the drug for more than 5-7 days, peripheral blood parameters and the functional state of the liver should be monitored. Paracetamol distorts the performance of laboratory studies in the quantitative determination of glucose and uric acid in plasma. OverdoseData on overdose of Cefecon D is not available. Drug interactionsInducers of microsomal oxidation in the liver (phenytoin, ethanol, barbiturates, flumecinol, rifampicin, phenylbutazone, tricyclic antidepressants), ethanol, hepatotoxic drugs increase the production of hydroxylated active metabolites, which makes it possible to develop severe intoxication even with a small overdose. Microsomal oxidation inhibitors (cimetidine) reduce the risk of hepatotoxicity. When taken simultaneously with salicylates, the likelihood of developing a nephrotoxic effect increases. When used together with paracetamol, the toxic effects of chloramphenicol are enhanced. When combined with paracetamol, the effect of indirect anticoagulants is enhanced and the effectiveness of uricosuric agents is reduced. Storage conditions of the drug Cefecon® YThe drug should be stored in a dry place, out of the reach of children, at a temperature not exceeding 20°C. Shelf life of the drug Cefecon® DShelf life - 2 years. Terms of saleThe drug is approved for use as an over-the-counter drug.
Contacts for inquiries
Keep |
vaginal and rectal suppositories, 200 mg + 500000 IU 0.084 ‰
Analogs Order in pharmacies Order
drug
All forms of release, dosages, registration certificates, drug manufacturers, drug characteristics
Description Package photos
Photos of packages
06/01/2020
supp. vag. and rect. 200 mg + 500000 IU, No. 10 - 5 pcs. - Pack. contour cell (2) - patch. cardboard.
vag. and rect. 200 mg + 500000 IU, No. 10 - 5 pcs. - Pack. contour cell (2) - patch. cardboard. Active ingredient
Human normal immunoglobulin [IgG + IgM + IgA] + Interferon alfa-2b (Immunoglobulin human normal [IgG + IgA + IgM] + Interferon alfa-2b)
ATX
L03AX Other immunostimulants
Pharmacological group
MIBP-cytokine [Interferons in combinations]
Nosological classification (ICD-10)
ICD-10 code list
- A02 Other salmonella infections
- A03.9 Shigellosis, unspecified
- A09 Diarrhea and gastroenteritis of suspected infectious origin (dysentery, bacterial diarrhea)
- J02.
 9Acute pharyngitis, unspecified
9Acute pharyngitis, unspecified - J06 Acute infections of the upper respiratory tract, multiple and unspecified
- K63.8.0* Dysbacteriosis
- N72 Inflammatory diseases of the cervix
- N74.4 Chlamydia inflammatory diseases of female pelvic organs (A56.1+)
- N76 Other inflammatory diseases of the vagina and vulva
- N76.
 8 Other specified inflammatory diseases of vagina and vulva
8 Other specified inflammatory diseases of vagina and vulva - N86 Erosion and ectropion of the cervix
Composition
| Vaginal and rectal suppositories | 1 supp. |
| Active substances: | |
| Immunoglobulin complex (KIP) | 200 mg |
| Interferon Alfa-2B human, recombinant) | 9000 9000 ME 9005 excipients: special-purpose fat "SolPro" confectionery for chocolate products and sweets - 838 mg; solid petroleum paraffin P-2 - 85 mg; emulsifier "Solid" (T-2) - 85 mg; sodium hydrogen phosphate dodecahydrate - 0.055 mg; sodium dihydrophosphate dihydrate - 0.
Description of dosage form
Suppositories of white, white with a yellowish tinge or light beige color, with a characteristic odor, cylindrical shape with a pointed end, homogeneous on a longitudinal section.
An air core or funnel-shaped recess is allowed on the cut. Color heterogeneity in the form of marbling is allowed.
Pharmacological action
Pharmacological action - antichlamydial , antibacterial , antiviral , anti-inflammatory , immunomodulating .
Pharmacodynamics
Pharmacological action: immunomodulatory, antiviral, antichlamydial.
Pharmacological properties
Kipferon ® suppositories are a complex dosage form containing human recombinant interferon-α2 and CIP.
Interferon-α2 is an activator of innate and acquired immunity, has a pronounced antiviral activity, enhances protection against viral, chlamydial, mycoplasmal and bacterial infections, has an immunomodulating effect in the development of immunological reactions, promotes the growth and preservation of the intestinal and vaginal normal flora.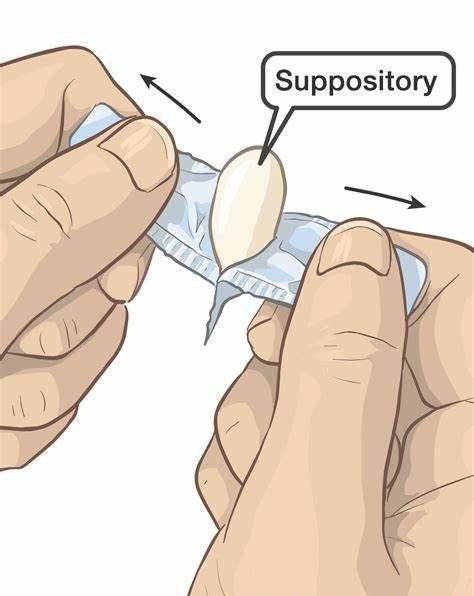 The biological activity of interferon-α2 is realized through interaction with the receptor apparatus of immunocompetent and other cells, leading to an increase in the expression of HLA I and HLA II molecules on the surface of all cell types and the regulation of cell cooperation, an increase in the activity of natural killers, as well as imparting resistance to intact cells against their cytolytic action, proliferation of CD8 T cells. Increases the production of interferon gamma by natural killers.
The biological activity of interferon-α2 is realized through interaction with the receptor apparatus of immunocompetent and other cells, leading to an increase in the expression of HLA I and HLA II molecules on the surface of all cell types and the regulation of cell cooperation, an increase in the activity of natural killers, as well as imparting resistance to intact cells against their cytolytic action, proliferation of CD8 T cells. Increases the production of interferon gamma by natural killers.
CIP contains specific and non-specific Ig classes G, M, A. The combination of antiviral, anti-chlamydial, antibacterial and antitoxic antibodies belonging to different classes of Ig provides agglutination, neutralization and precipitation of etiotropic pathogenic agents. At the site of application, it stabilizes interferon from mucous membrane secretions, normalizes local immunity due to the intake of IgA and IgM with suppositories (substitution effect), increases the activity of locally formed cytokines.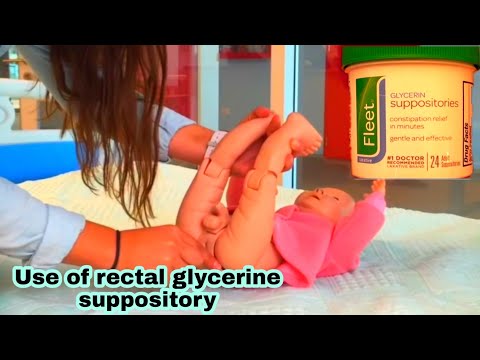
Indications
The drug is used in the complex therapy of diseases accompanied by secondary immunodeficiency states:
acute respiratory diseases, inflammatory diseases of the oropharynx of bacterial and viral etiology in children and adults;
acute viral (rotavirus) and bacterial (salmonellosis, dysentery, coli) intestinal infections, incl. with manifestations of intestinal dysbacteriosis of varying severity in children;
urogenital chlamydia in women (including pregnant women in the II-III trimesters of pregnancy), incl. with manifestations of vaginal dysbacteriosis, vulvovaginitis, uterine cervicitis, cervical erosion.
Contraindications
individual intolerance to individual components;
pregnancy, first trimester;
lactation.
Dosage and administration
Rectally and vaginally.
The drug is used in combination with generally accepted means of pathogenetic therapy of infectious diseases, as well as antibiotics according to indications. The method and dosage regimen, as well as the use in complex treatment, is determined by the doctor, depending on the diagnosis, severity and severity of the disease.
The method and dosage regimen, as well as the use in complex treatment, is determined by the doctor, depending on the diagnosis, severity and severity of the disease.
Acute respiratory diseases, inflammatory diseases of the oropharynx of bacterial and viral etiology: suppositories are administered rectally (mainly after defecation) to children under 1 year old, 1 suppository per day, children under 12 years old - 1 suppository 2 times a day, adults and children over 12 years old - 1 suppository three times a day. The most effective and expedient appointment of the drug Kipferon ® in the acute period of the disease (preferably in the first 3 days). The duration of the course of treatment is determined by the etiology, severity of the disease, the presence of concomitant complications, pathological conditions and is 5-7 days.
Acute viral (rotavirus) and bacterial (salmonellosis, dysentery, coli) intestinal infections, incl. with manifestations of intestinal dysbacteriosis of varying severity in children: suppositories are administered rectally (mainly after defecation) for children under 1 year old, 1 suppository per day, for children under 12 years old - 1 suppository 2 times a day, for adults and children over 12 years old - for 1 suppository three times a day. It is most effective and expedient to prescribe the drug in the acute period of the disease (preferably in the first 3 days). The duration of the course of treatment is determined by the etiology, severity of the disease, the presence of concomitant complications, pathological conditions and is 5-7 days.
It is most effective and expedient to prescribe the drug in the acute period of the disease (preferably in the first 3 days). The duration of the course of treatment is determined by the etiology, severity of the disease, the presence of concomitant complications, pathological conditions and is 5-7 days.
Urogenital chlamydia in women (including pregnant women in the II-III trimesters of pregnancy), incl. with manifestations of vaginal dysbacteriosis, vulvovaginitis, uterine cervicitis, cervical erosion: suppositories are administered deeply intravaginally (before contact with the posterior vaginal fornix and cervix), 1-2 suppositories, depending on the severity of the disease, 2 times a day. The course of treatment averages 10 days; in the presence of erosion of the cervix, the use of the drug is continued until its epithelialization. According to the indications, the course of treatment can be repeated after one month. Treatment should begin in the first days after the end of menstruation. Before administration, it is recommended to remove mucus from the mucous membranes of the vagina and cervix.
Before administration, it is recommended to remove mucus from the mucous membranes of the vagina and cervix.
Side effects
Not registered.
Interactions
No negative effects on other medicinal products were noted.
Overdose
Cases of drug overdose are unknown.
Special instructions
Influence on the ability to drive vehicles. When used in recommended doses, the drug does not affect the behavior or functional performance of the body, and also does not affect the ability to drive vehicles, mechanisms.
Product form
Vaginal and rectal suppositories. 5 suppositories in a blister pack. 1 or 2 blister packs together with instructions for use in a cardboard pack.
Producer
Alpharm LLC, 125212, Russia, Moscow, Admiral Makarov, 10, building 1.
Altfarm LLC, 142073, Russia, Moscow region, Domodedovo district, village. Sudakovo, d / o "Forest".
Packer (secondary packaging) / issuing quality control: Alpharm LLC, 125212, Russia, Moscow, st.
 07.26
07.26  for children 50 mg: 10 pcs.
for children 50 mg: 10 pcs. 

 There is no significant age difference in the rate of elimination of paracetamol and in the total amount of the drug excreted in the urine.
There is no significant age difference in the rate of elimination of paracetamol and in the total amount of the drug excreted in the urine.  8
8  The average single dose is 10-15 mg/kg of the child's body weight. The drug in a single dose is administered 2-3 times / day, after 4-6 hours. The maximum daily dose of the drug should not exceed 60 mg / kg of body weight.
The average single dose is 10-15 mg/kg of the child's body weight. The drug in a single dose is administered 2-3 times / day, after 4-6 hours. The maximum daily dose of the drug should not exceed 60 mg / kg of body weight. 


 6, bld. 2,
6, bld. 2, 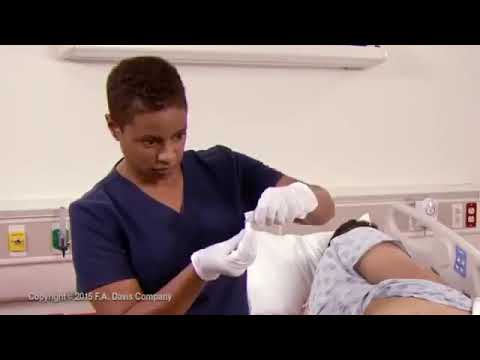 018 mg; sodium chloride - 0.110 mg; purified water - 12 mg
018 mg; sodium chloride - 0.110 mg; purified water - 12 mg