Assisted delivery methods
Childbirth Options, Differences & Benefits
Overview
What are the types of delivery methods?
It’s hard to know exactly what will happen when you give birth. Most people have a plan in mind for how they hope their labor and delivery goes. When it comes to delivering your baby, it’s good to know there are many methods pregnancy care providers use. Types of delivery include:
- Vaginal delivery.
- Assisted vaginal delivery (vacuum or forceps).
- C-section (Cesarean birth).
- VBAC (vaginal birth after cesarean).
What type of delivery is best?
A vaginal delivery is the safest and most common type of childbirth. Vaginal deliveries account for about 68% of all births in the United States. Most medical organizations and obstetricians recommend a vaginal delivery unless there is a medical reason for a C-section.
Vaginal delivery
What is a vaginal delivery?
In a vaginal birth, your baby is born through your vagina or birth canal. It’s the most preferred and most common way to deliver a baby because it carries the lowest risk (in most cases). A vaginal delivery occurs most often between weeks 37 and 42 of pregnancy. A vaginal delivery has three stages: labor, birth and delivering the placenta.
Some benefits of a vaginal delivery include:
- Faster recovery.
- Safest for the pregnant person and the baby.
- Lower rates of infection.
- Babies are at lower risk for respiratory problems and have a stronger immune system.
- Lactation and breastfeeding are usually easier.
A vaginal delivery can be spontaneous or induced:
- Spontaneous vaginal delivery: A vaginal delivery that happens on its own and without labor-inducing drugs. Going into labor naturally at 40 weeks of pregnancy is ideal.
- Induced vaginal delivery: Drugs or other techniques start labor and soften or open your cervix for delivery. Pregnancy care providers often recommend inducing labor when a pregnant person has a medical condition or is past due.
 Labor is usually induced with Pitocin®, a synthetic form of the drug oxytocin.
Labor is usually induced with Pitocin®, a synthetic form of the drug oxytocin.
What happens if you don’t push during a vaginal delivery?
In most cases, once your cervix is fully dilated and your healthcare team is in place, your provider will ask you to push during a contraction. Pregnancy care providers have differing opinions on when to push, how long to push, delayed pushing or waiting until you feel the urge to push.
It’s hard to say what will happen if you don’t or can’t push during a vaginal delivery, because your birthing experience is so unique. However, studies show that resisting the urge to push or delaying pushing (laboring down) can cause complications like infection, bleeding or damage to your pelvis.
It’s best to discuss pushing with your pregnancy care provider ahead of time so you know what to expect during labor.
Assisted vaginal delivery
What is an assisted vaginal delivery?
An assisted vaginal delivery is when your obstetrician uses forceps or a vacuum device to get your baby out of your vagina. Assisted deliveries often happen when:
Assisted deliveries often happen when:
- You’ve been in labor a long time.
- Your labor isn’t progressing.
- You become too fatigued to continue pushing.
- You or your baby are showing signs of distress.
Assisted deliveries only occur when certain conditions are met.
What are examples of assisted deliveries?
The procedure your obstetrician recommends will depend on the conditions that arise while you’re in labor. Assisted delivery procedures can include the following:
- Forceps delivery: Forceps are a tong-like surgical tool obstetricians use to grasp your baby’s head in order to guide them out of the birth canal.
- Vacuum extraction delivery: In a vacuum extraction, your obstetrician places a small suction cup on your baby’s head. The cup is attached to a pump that pulls on your baby while you push.
Vacuum extraction and forceps delivery are similar in their advantages and disadvantages, and often the choice between them comes down to the experience of your obstetrician.
C-section
What is a C-section?
During a C-section birth, your obstetrician delivers your baby through surgical incisions made in your abdomen and uterus. A C-section delivery might be planned in advance if a medical reason calls for it, or it might be unplanned and take place during your labor if certain problems arise. There are about 1.2 million C-section deliveries in the United States each year.
Your provider may recommend a planned cesarean delivery if you:
- Had a previous C-section delivery.
- Are expecting multiples.
- Have placenta previa.
- Have a breech baby.
- Have a baby with fetal macrosomia or a large baby.
- Have a uterine fibroid or other obstruction.
Sometimes, your labor and delivery changes, and a cesarean birth becomes necessary for the health and safety of you or your baby. An unplanned C-section might be needed if any of the following conditions arise during your labor:
- Fetal distress (your baby isn’t tolerating labor).

- Labor isn’t progressing.
- Umbilical cord prolapse.
- Placental abruption.
- Hemorrhage or excessive bleeding.
Risks of C-section deliveries
Like any surgery, a cesarean birth involves some risks. In general, there is more risk associated with a C-section than with a vaginal delivery.
These might include:
- Infection.
- Loss of blood or need for a blood transfusion.
- A blood clot that may break off and enter the bloodstream (embolism).
- Injury to the bowel or bladder.
- Longer recovery and longer hospital stay.
- Abdominal adhesions.
Benefits of C-section deliveries
Some people prefer a C-section birth because it gives them more control on choosing a due date. This is called an elective C-section. Some providers may allow elective C-sections for nonmedical reasons, however, this is usually discouraged. In most cases, a C-section birth occurs because it’s medically necessary. The American Congress of Obstetrics and Gynecologists (ACOG) recommends that scheduled cesareans not be performed before 39 weeks gestation, unless medically indicated.
The American Congress of Obstetrics and Gynecologists (ACOG) recommends that scheduled cesareans not be performed before 39 weeks gestation, unless medically indicated.
Some benefits of a C-section as compared to a vaginal delivery are:
- Lower risk of your baby having trauma from passing through your vagina.
- Less risk of your baby being oxygen-deprived during delivery.
- Possible lower risk of incontinence or sexual dysfunction.
VBAC (vaginal birth after cesarean)
What is a VBAC?
If you’ve already had a cesarean birth, you may be able to have your next baby vaginally. This is a VBAC, or vaginal birth after cesarean. Because a surgical cut results in a scar on your uterus, the concern is that the pressure of labor in a vaginal delivery could cause your uterus to open (rupture) along the previous C-section scar. For this reason, certain criteria must be met in order for your obstetrician to attempt a vaginal birth after C-section.
Can I have a baby vaginally after a C-section?
People who have had a cesarean delivery might be able to deliver vaginally in a future pregnancy. If you meet the following criteria, your chances of a successful vaginal birth after cesarean (VBAC) are high:
- Your obstetrician made a low transverse incision during your cesarean. This is the typical way to perform a C-section, unless they need to deliver your baby in a hurry.
- You don’t have other uterine scars or abnormalities.
- You had a prior vaginal delivery.
- You haven’t had a previous uterine rupture.
What else should I know about delivery?
There are several other terms you should be familiar with in case your pregnancy care provider discusses them during labor and delivery.
Episiotomy
An episiotomy is a surgical incision that widens the opening of your vagina. This allows your baby’s head to pass through more easily. Most people will not need an episiotomy.
There are two types of episiotomy incisions: the midline, made directly back toward your anus, and the mediolateral, which slants away from your anus.
Amniotomy (breaking your bag of waters)
An amniotomy is the artificial rupture of the amniotic membranes, or sac, which contains the fluid surrounding your baby. Your pregnancy care provider may artificially rupture your membranes (AROM) to:
- Induce or progress labor.
- Place an internal monitor to assess your contractions.
- Place an internal monitor on your baby’s scalp to assess their well-being.
- Check for meconium (a greenish-brown substance, which is your baby’s first poop).
Your provider will use an amniohook, which looks like a crochet hook, to rupture the sac. Once the procedure is complete, delivery should take place within 24 hours to prevent infection.
Fetal monitoring
Fetal monitoring is the process of watching your baby’s heart rate during labor. This can be external or internal. Knowing how your baby is handling labor helps your pregnancy care provider decide if labor can continue or if delivery is necessary.
This can be external or internal. Knowing how your baby is handling labor helps your pregnancy care provider decide if labor can continue or if delivery is necessary.
- In external fetal monitoring, an ultrasound device is placed on your abdomen to record information about your baby’s heart rate, and the frequency and duration of your contractions.
- Internal monitoring involves the use of a small electrode to record your baby’s heart rate. Your membranes must be ruptured before the electrodes can be attached to your baby’s scalp. A pressure sensor can also be placed near your baby to measure the strength of contractions.
Frequently Asked Questions
Which type of delivery is most painful?
This may come down to personal opinion. There are many factors involved — for example, using pain medication, the type of pain medication or your pain tolerance. You should discuss pain relief with your pregnancy care provider before labor so you know the risks and benefits of each type.
There are two general options: no medication (drug-free or natural delivery) or using pain medications.
A drug-free delivery means you intend to give birth vaginally without any pain medication. You can’t have a C-section without medication.
Your options for pain relief during childbirth could consist of:
- Analgesics: Analgesics relieve pain without causing complete loss of feeling or muscle movement. The most common example of an analgesic used during childbirth is an epidural. You can receive an epidural for a vaginal or a cesarean delivery.
- Anesthetics: Anesthetics (or anesthesia) keep you from feeling pain by blocking signals from your brain. These drugs are given as a shot or through an intravenous (IV) line. During a C-section, you may receive general anesthesia, which puts you to sleep. Another option during childbirth could be local anesthesia, which involves a shot of medication in a specific area of your body, like the nerves in your vagina and vulva.

Another factor in deciding what type of delivery is most painful is the recovery. Generally, a vaginal delivery is easier to recover from than a C-section delivery.
A note from Cleveland Clinic
Your labor and delivery experience will be unique to you. During pregnancy, it’s a good idea to familiarize yourself with the different types of delivery and other terms associated with childbirth. Your pregnancy care provider will recommend the safest delivery method based on your medical history and pregnancy.
Assisted delivery (forceps or ventouse)
Assisted delivery (forceps or ventouse) | Pregnancy Birth and Baby beginning of content3-minute read
Listen
An assisted delivery, sometimes called an ‘instrumental delivery’, is when your doctor will help in the birthing process by using instruments such as a ventouse (vacuum extractor) or forceps to help you deliver your baby.
Both options are safe for you and your baby and are only used when necessary.
Some reasons why your doctor or obstetrician may need to assist during delivery is because:
- there are concerns about the baby's heart rate
- your baby is in an awkward position
- you're too exhausted
If the baby's head is in an awkward position, it will need turning (rotating) to allow the birth. A paediatrician may be present to check your baby's condition after the birth. A local anaesthetic is usually given to numb the vagina and perineum (the skin between the vagina and anus) if you haven't already had an epidural.
If your obstetrician has any concerns, you may be moved to an operating theatre so that a caesarean section can be carried out if needed, for example if the baby can't be easily delivered by forceps or a ventouse. This is more likely if your baby's head needs turning.
Sometimes, a cut, known as an ‘episiotomy’ may be needed as the baby is being born to make the vaginal opening bigger. Any tear or cut will be repaired with stitches. Depending on the circumstances, your baby can be delivered and placed onto your tummy, and your birthing partner may still be able to cut the cord if they want to.
Any tear or cut will be repaired with stitches. Depending on the circumstances, your baby can be delivered and placed onto your tummy, and your birthing partner may still be able to cut the cord if they want to.
Ventouse (vacuum extraction)
A ventouse (vacuum extractor) is an instrument that is attached to the baby's head by suction. A plastic or metal cup is attached by a tube to a suction device. The cup fits firmly onto your baby's head. During a contraction and with the help of your pushing, the obstetrician or midwife gently pulls to help deliver your baby.
The suction cup leaves a small swelling on your baby's head, called a 'chignon'. This disappears quickly. The cup may also leave a bruise on your baby's head, called a 'cephalhaematoma'. A ventouse is not used if you're giving birth at less than 34 weeks pregnant, because your baby's head is too soft.
A ventouse is less likely to cause vaginal tearing than forceps.
Forceps
Forceps are smooth metal instruments that look like large spoons or tongs.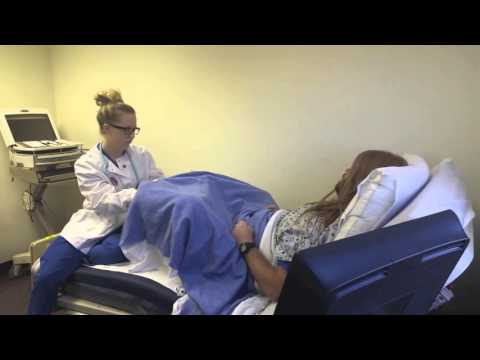 They're curved to fit around the baby's head. The forceps are carefully positioned around your baby's head and joined together at the handles. With a contraction and your pushing, an obstetrician gently pulls to help deliver your baby.
They're curved to fit around the baby's head. The forceps are carefully positioned around your baby's head and joined together at the handles. With a contraction and your pushing, an obstetrician gently pulls to help deliver your baby.
There are many different types of forceps. Some forceps are specifically designed to turn the baby to the right position to be born, for example, if your baby is lying facing upwards (known as 'occipito-posterior' position) or to one side (known as 'occipito-lateral' position).
Forceps can leave small marks on your baby's face but these will disappear quite quickly.
Afterwards
You will sometimes need a catheter (a small tube that drains your bladder) for up to 24 hours. You're more likely to need this if you have had an epidural because you may not have fully regained sensation in your bladder (and therefore don't know when it's full).
You should discuss these procedures with your midwife or doctor during your pregnancy so you have an understanding of what is happening if they are needed during your labour.
Sources:
Royal Australasian College of Obstetricians and Gynaecologists (Assisted birth), Royal Women's Hospital (Assisted birth)Learn more here about the development and quality assurance of healthdirect content.
Last reviewed: September 2019
Back To Top
Related pages
- Fetal heart rate monitoring
- Giving birth - stages of labour
- Episiotomy
- Interventions during labour
Need more information?
Assisted Birth
Read more on RANZCOG - Royal Australian and New Zealand College of Obstetricians and Gynaecologists website
Episiotomy
An episiotomy is a procedure performed during labour to assist with the delivery of your baby.
Read more on Pregnancy, Birth & Baby website
Malpresentation
Malpresentation is when your baby is in an unusual position as the birth approaches. Sometimes it’s possible to move the baby, but a caesarean maybe safer.
Read more on Pregnancy, Birth & Baby website
Interventions during labour
An ‘intervention’ is an action taken by a midwife or doctor that literally intervenes in the birthing process. Read about the different types of intervention.
Read more on Pregnancy, Birth & Baby website
Giving birth to twins
Twins are more likely to be born early, often before 38 weeks, so it's important to understand your birth options.
Read more on Pregnancy, Birth & Baby website
Slow progress in labour
Slow progress in labour (also known as failure to progress in labour or prolonged labour) occurs when labour doesn’t go as quickly as expected. Your doctor may need to intervene to help you have your baby.
Read more on Pregnancy, Birth & Baby website
Disclaimer
Pregnancy, Birth and Baby is not responsible for the content and advertising on the external website you are now entering.
OKNeed further advice or guidance from our maternal child health nurses?
1800 882 436
Video call
- Contact us
- About us
- A-Z topics
- Symptom Checker
- Service Finder
- Linking to us
- Information partners
- Terms of use
- Privacy
Pregnancy, Birth and Baby is funded by the Australian Government and operated by Healthdirect Australia.
Pregnancy, Birth and Baby is provided on behalf of the Department of Health
Pregnancy, Birth and Baby’s information and advice are developed and managed within a rigorous clinical governance framework. This website is certified by the Health On The Net (HON) foundation, the standard for trustworthy health information.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
This information is for your general information and use only and is not intended to be used as medical advice and should not be used to diagnose, treat, cure or prevent any medical condition, nor should it be used for therapeutic purposes.
The information is not a substitute for independent professional advice and should not be used as an alternative to professional health care. If you have a particular medical problem, please consult a healthcare professional.
Except as permitted under the Copyright Act 1968, this publication or any part of it may not be reproduced, altered, adapted, stored and/or distributed in any form or by any means without the prior written permission of Healthdirect Australia.
Support this browser is being discontinued for Pregnancy, Birth and Baby
Support for this browser is being discontinued for this site
- Internet Explorer 11 and lower
We currently support Microsoft Edge, Chrome, Firefox and Safari. For more information, please visit the links below:
- Chrome by Google
- Firefox by Mozilla
- Microsoft Edge
- Safari by Apple
You are welcome to continue browsing this site with this browser. Some features, tools or interaction may not work correctly.
Create your own shipping method for WooCommerce
What will you createWooCommerce is great. For a free e-commerce solution, it has an incredibly useful set of features.
But sometimes you need something extra. One time I often find that WooCommerce doesn't give me everything I need is when it comes to setting up custom shipping methods.
Out of the box, WooCommerce allows you to set shipping rates for your country or state, as well as the rest of the world. Which is pretty limited. What if you want to set different shipping rates for different US states or different parts of Europe?
If you're feeling ambitious, you can write your own shipping method. But if you don't want to go for it, CodeCanyon's WooCommerce Advanced Shipping Plugin gives you as much flexibility as you need.
In this tutorial, I'll show you how to set up custom shipping methods using a plugin. Since I live in the UK, I'm going to set rates for the UK, the rest of Europe, the US and the rest of the world.
As you'll see as we work through this process, you can use several conditions to define shipping methods. These may include weight, shipping address, number of units, and a host of other options.
In my store, I will use location and shipping weight to determine my shipping methods, but you can easily add additional terms to further improve your shipping methods.
So let's get started.
To follow this tutorial you will need:
- a WordPress site with WooCommerce installed and configured
- WooCommerce Extended Shipping Plugin
- some products have been added to your store with assigned weights
WooCommerce gives you very little flexibility when it comes to shipping zones. You can define a delivery zone for your territory and for the rest of the world, but this is:
If you are shipping to multiple countries or to other US states, this will not be enough for you.
Using the plugin, you can add additional shipping zones as well as shipping classes and link them together with shipping methods.
Before continuing, let's look at the difference between delivery zones, classes, and methods. I don't know about you, but I might find the terminology confusing.
- Delivery zone is a location or territory. It can be a state, a country, a continent, or a combination of them.
 Therefore, if you are in the US, you may need shipping zones for individual states or groups of states, as well as for other countries. Your shipping areas will depend on how much you have to pay for shipping to different parts of the world.
Therefore, if you are in the US, you may need shipping zones for individual states or groups of states, as well as for other countries. Your shipping areas will depend on how much you have to pay for shipping to different parts of the world. - Delivery class is a group of goods with certain characteristics or a complete order with these characteristics. General criteria for shipping classes will include product weight, dimensions and fragility. You can create a class to group individual products or to define a complete order.
- Delivery method combines zones and classes. This is the rate that is applied to an order that is delivered to a given zone and has goods of a certain class.
Shipping methods are not provided by WooCommerce out of the box. But the Advanced Shipping plugin gives you the ability to add as many shipping methods as you need and make them as complex as you need.
Let's start creating one.
I'm going to create a set of shipping methods based on location and weight. Each location requires a shipping method for orders under 1kg and orders over 1kg. Zones I will be using:
- United Kingdom
- Rest of Europe
- US
- Rest of the world
Before we start, we need to delete all shipping zones already defined in the shipping settings. If we don't, customers will have a choice between our new shipping methods and our existing ones. On screen Delivery zones , delete all existing delivery zones.
Optionally, you can first define shipping classes based on weight and then use them as one of the criteria when setting up a shipping method. I won't do this here, but you may find it saves you time.
Now it's time to create your own shipping method. Let's start by creating a relatively simple shipping method for orders under 1kg in the UK.
Go to WooCommerce > Settings > Shipping > Shipping Methods . You will be taken to the Delivery Methods screen provided by the plugin.
You will be taken to the Delivery Methods screen provided by the plugin.
The first step is to define the criteria. You can use "and" criteria or "or" criteria. You can also use criteria to rule out certain conditions, which I'll demonstrate shortly.
My criteria are based on location and weight, so I'll add both criteria together. First, I want to set the country. I choose country , then is , then UK .
Next, I determine the weight. I select Weight , then Less than or equal to , then I dial 1 kg .
Note: Make sure you use the same weight units that you use in your store as a whole. You can access this at WooCommerce > Settings > Products .
Here is my defined shipping class:
Now scroll down and determine how much your shipping method will cost.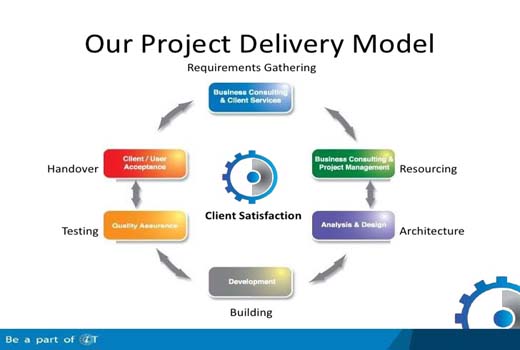
This will depend on how much it costs to ship the products. Options you have:
- Delivery name: name you remember
- Shipping cost : order value
- processing fee : fixed order processing fee. In my store, I'm going to set the same handling fee for all of my shipping methods, with a different shipping cost for each.
- Cost per unit: Shipping cost per unit. You can use this in place of or in addition to the order shipping cost if you need to.
- Cost per weight: shipping cost for each kg of weight. You can use this as an alternative to creating multiple shipping methods for multiple weight bands. Or you can do both, with different prices for different weight groups.
- Tax status: Is shipping taxable or not? This will depend on your tax regime.
It is unlikely that you will use all of these, but you will probably use one or two.
In my case, I set a flat handling fee and shipping cost for the order:
Then I can set a second class shipping for the UK that is greater than or equal to 1.01 kg.
The beauty of this plugin is that it allows you to combine "and" and "or" conditions, and allows you to use negative conditions to create exactly the shipping method you need.
For my next pair of shipping methods, I'll focus on the rest of Europe. Therefore, I want the continent to be Europe and the country not to be Great Britain.
So I will need the following:
- Continent (or Country: it still works) equals Europe.
- The country is not equal to the United Kingdom.
- Weight equal to or less than 1 kg.
This gives me three criteria, as you can see in the screenshot:
If I wanted to get even more detailed information, I could use the "or" groups to select individual countries, for example, if I had one shipping method for France and Germany, and another for the rest of Europe.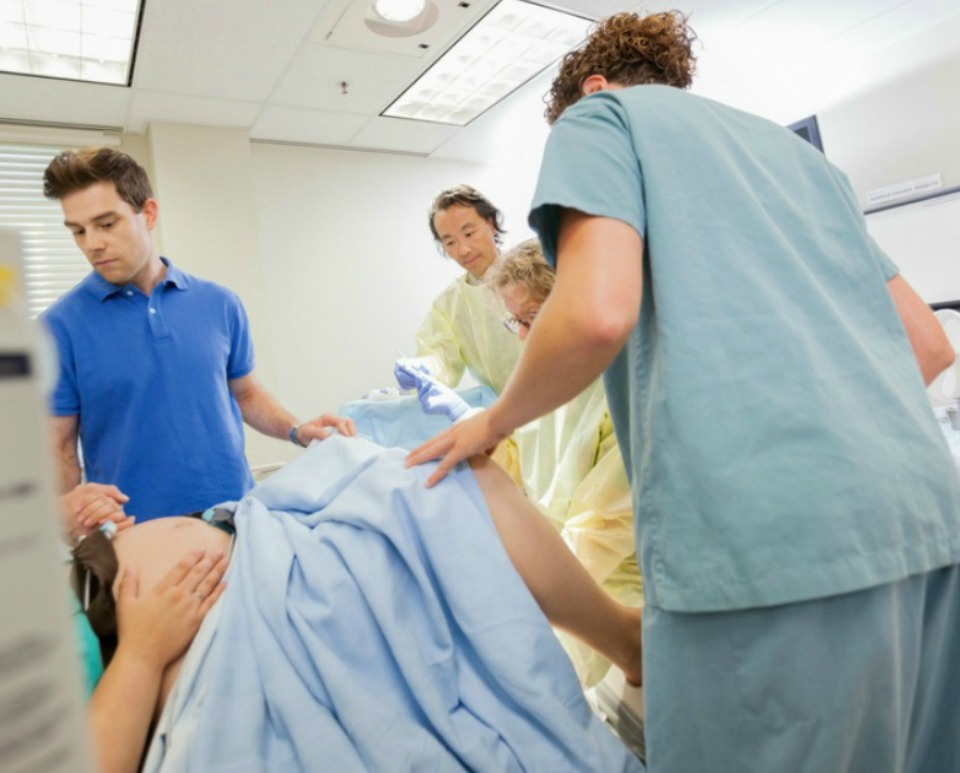
You can get as much as you want; The plugin gives you a lot of flexibility.
I now have two other sets of shipping methods: one for the US and one for the rest of the world.
- For the USA, I will use two terms, one for country and one for weight.
- For the rest of the US, I will use three terms: continent not equal to Europe, country not equal to the US, and weight.
I have now set up all the shipping methods that I see on the overview screen Advanced Shipping :
Now let's see what happens when I place an order.
I have several pacifiers in my shop and I have assigned a weight to them. I assigned a weight of 0.6 kg to the belt and 0.5 kg to the sweatshirt (I gained weight: in a real store you need to know the actual weight).
I will first place an order to ship to the UK for the belt only. This will place my order on the first shipping method I set up for the UK, as it will be under 1kg.
Here is my shopping cart with shipping charges to the UK:
Now if I add a sweatshirt the shipping will increase:
Now if I keep these two items in my shopping cart but change the shipping address, the shipping charges will increase again. This time I'm shipping to a US address and the shipping costs go up to £16.
This time I'm shipping to a US address and the shipping costs go up to £16.
The plugin took my shipping address and the total weight of the items in my cart and calculated the shipping cost. Clever!
If you plan to sell to customers worldwide, it's important to make sure you're not losing money by not charging the correct shipping cost.
By setting up as many advanced shipping methods as you need, you can bill your customers the exact amount, no matter where they live or what they buy. By installing the WooCommerce Advanced Shipping plugin and following the steps below, you will be able to set up shipping exactly the way you want.
And while you're at it, check out some of our other posts on WooCommerce plugins.
-
WooCommerce
Top 20 WooCommerce Plugins for Shipping and Pricing
Nona Blackman
-
WooCommerce
Best WooCommerce Coupon Plugins
Daniel Strongin
-
WooCommerce
The 12 Best WooCommerce Plugins for Your Online Store
Jane Baker
Intranasal administration as a method of drug delivery to the brain (review) | Porfiryeva
1. Crowe T. P., Greenlee M. H. W., Kanthasamy A. G., Hsu W. H. Mechanism of intranasal drug delivery directly to the brain. life sciences. 2018;195:44–52. DOI: 10.1016/j.lfs.2017.12.025.
Crowe T. P., Greenlee M. H. W., Kanthasamy A. G., Hsu W. H. Mechanism of intranasal drug delivery directly to the brain. life sciences. 2018;195:44–52. DOI: 10.1016/j.lfs.2017.12.025.
2. Brown R. C., Lockwood A. H., Sonawane B. R. Neurodegenerative Diseases: An Overview of Environmental Risk Factors. Environmental Health Perspectives. 2005;113(9):1250–1256. DOI: 10.1289/ehp.7567.
3. Patel A., Surti N., Mahajan A. Intranasal drug delivery: Novel delivery route for effective management of neurological disorders. Journal of Drug Delivery Science and Technology. 2019;52:130–137. DOI: 10.1016/j.jddst.2019.04.017.
4. Nguyen T. T., Nguyen T. T. D., Nguyen T. K. O., Vo T. K., Vo V. G. Advances in developing therapeutic strategies for Alzheimer’s disease. Biomedicine & Pharmacotherapy. 2021;139:111623. DOI: 10.1016/j.biopha.2021.111623.
5. Tolosa E., Garrido A., Scholz S.W., Poewe W. Challenges in the diagnosis of Parkinson’s disease. The Lancet Neurology. 2021;20(5):385–397. DOI: 10.1016/S1474-4422(21)00030-2.
DOI: 10.1016/S1474-4422(21)00030-2.
6. Wright G. E. B., Black H. F., Collins J. A., Gall-Duncan T., Caron N. S., Pearson C. E., Hayden M. R. Interrupting sequence variants and age of onset in Huntington's disease: clinical implications and emerging therapies. The Lancet Neurology. 2020;19(11):930–939. DOI: 10.1016/S1474-4422(20)30343-4.
7. Schuchman E. H., Desnick R. J. Types A and B Niemann-Pick disease. Molecular Genetics and Metabolism. 2017;120(1–2):27–33. DOI: 10.1016/j.ymgme.2016.12.008.
8. Deramecourt V., Slade J. Y., Oakley A. E., Perry R. H., Ince P. G., Maurage C.-A., Kalaria R. N. Staging and natural history of cerebrovascular pathology in dementia. Neurology. 2012;78(14):1043–1050. DOI: 10.1212/WNL.0b013e31824e8e7f.
9. O'Brien J. T., Erkinjuntti T., Reisberg B., Roman G., Sawada T., Pantoni L., Bowler J. V., Ballard C., DeCarli C., Gorelick P. B., Rockwood K., Burns A. , Gauthier S., DeKosky S. T. Vascular cognitive impairment. The Lancet Neurology. 2003;2(2):89-98. DOI: 10.1016/S1474-4422(03)00305-3.
2003;2(2):89-98. DOI: 10.1016/S1474-4422(03)00305-3.
10. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology. 2010;9(7):689–701. DOI: 10.1016/S1474-4422(10)70104-6.
11. Charidimou A., Pantoni L., Love S. The concept of sporadic cerebral small vessel disease: A road map on key definitions and current concepts. International Journal of Stroke. 2016;11(1):6–18. DOI: 10.1177/1747493015607485.
12. Wardlaw J. M., Smith C., Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. The Lancet Neurology. 2013;12(5):483–497. DOI: 10.1016/S1474-4422(13)70060-7.
13. Seyfried T. N., Kiebish M. A., Marsh J., Shelton L. M., Huysentruyt L. C., Mukherjee P. Metabolic management of brain cancer. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2011;1807(6):577–594. DOI: 10.1016/j.bbabio.2010.08.009.
14. Han L., Jiang C. Evolution of blood–brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharmaceutica Sinica B. 2021;11(8):2306–2325. DOI: 10.1016/j.apsb.2020.11.023.
Acta Pharmaceutica Sinica B. 2021;11(8):2306–2325. DOI: 10.1016/j.apsb.2020.11.023.
15. Abbott N. J., Patabendige A. A. K., Dolman D. E. M., Yusof S. R., Begley D. J. Structure and function of the blood–brain barrier. Neurobiology of Disease. 2010;37(1):13–25. DOI: 10.1016/j.nbd.2009.07.030.
16. Lee C.S., Leong K.W. Advances in microphysiological blood-brain barrier (BBB) models towards drug delivery. Current Opinion in Biotechnology. 2020;66:78–87. DOI: 10.1016/j.copbio.2020.06.009.
17. Sharma G., Sharma A. R., Lee S.-S., Bhattacharya M., Nam J-.S., Chakraborty C. Advances in nanocarriers enabled brain targeted drug delivery across blood brain barrier. International Journal of Pharmaceutics. 2019;559:360–372. 18. Costa C. P., Moreira J. N., Sousa Lobo J. M., Silva A. C. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: A overview of in vivo studies. Acta Pharmaceutica Sinica B. 2021;11(4):925–940. DOI: 10.1016/j. apsb.2021.02.012.
apsb.2021.02.012.
19. Lochhead J. J., Thorne R. G. Intranasal delivery of biologics to the central nervous system. Advanced Drug Delivery Reviews. 2012;64(7):614–628. DOI: 10.1016/j.addr.2011.11.002.
20. Misra A., Kher G. Drug Delivery Systems from Nose to Brain. Current Pharmaceutical Biotechnology. 2012;13(12):2355–2379. DOI: 10.2174/138920112803341752.
21. Costa C., Moreira J. N., Amaral M. H., Sousa Lobo J. M., Silva A. C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. Journal of Controlled Release. 2019;295:187–200. DOI: 10.1016/j.jconrel.2018.12.049.
22. Arora P., Sharma S., Garg S. Permeability issues in nasal drug delivery. Drug Discovery Today. 2002;7(18):967–975. DOI: 10.1016/S1359-6446(02)02452-2.
23. Grassin-Delyle S., Buenestado A., Naline E., Faisy C., Blouquit-Laye S., Couderc L.-J., Le Guen M., Fischler M., Devillier P. Intranasal drug delivery : An efficient and non-invasive route for systemic administration. Pharmacology & Therapeutics. 2012;134(3):366–379. DOI: 10.1016/j.pharmthera.2012.03.003.
Pharmacology & Therapeutics. 2012;134(3):366–379. DOI: 10.1016/j.pharmthera.2012.03.003.
24. Watelet J. B., Van Cauwenberge P. Applied anatomy and physiology of the nose and paranasal sinuses. Allergy. 1999;54(s57):14–25. DOI: 10.1111/j.1398-9995.1999.tb04402.x.
25. Erdő F., Bors L.A., Farkas D., Bajza A., Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Research Bulletin. 2018;143:155–170. DOI: 10.1016/j.brainresbull.2018.10.009.
26. Mittal D., Ali A., Md S., Baboota S., Sahni J. K., Ali J. Insights into direct nose to brain delivery: current status and future perspective. drug delivery. 2014;21(2):75–86. DOI: 10.3109/10717544.2013.838713.
27. Gänger S., Schindowski K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. pharmaceuticals. 2018;10(3):116. DOI: 10.3390/pharmaceutics10030116.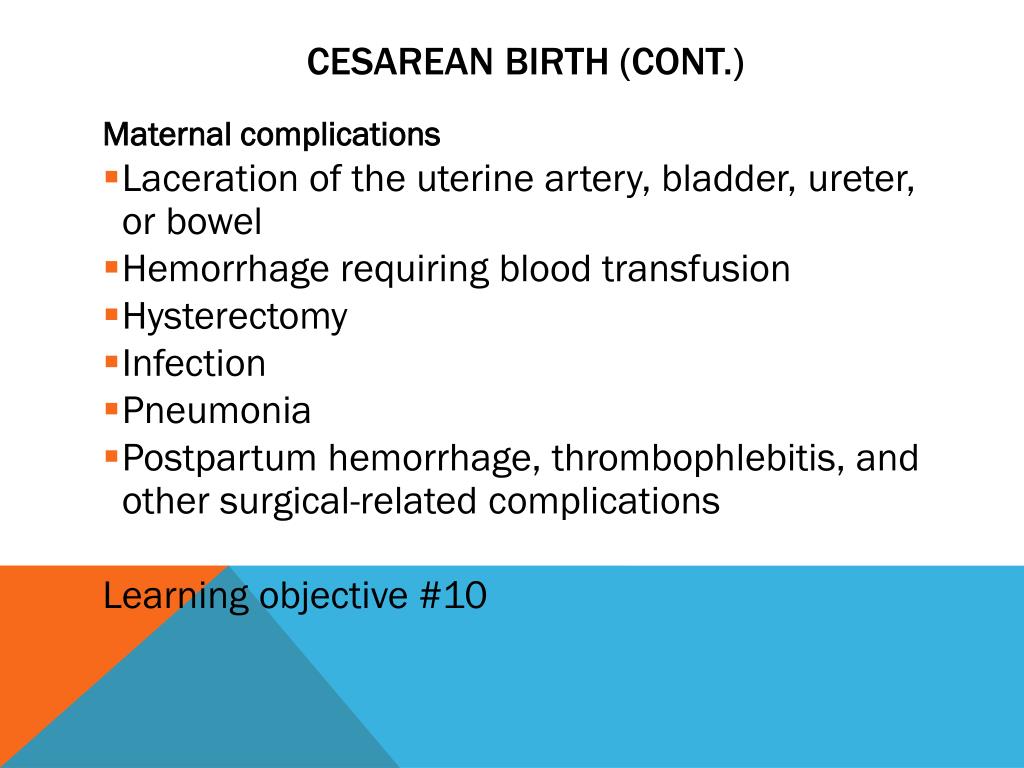
28. Bourganis V., Kammona O., Alexopoulos A., Kiparissides C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. European Journal of Pharmaceutics and Biopharmaceutics. 2018;128:337–362. DOI: 10.1016/j.ejpb.2018.05.009.
29. Ugwoke M. I., Verbeke N., Kinget R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. Journal of Pharmacy and Pharmacology. 2001;53(1):3–21. DOI: 10.1211/0022357011775145.
30. Dhuria S. V., Hanson L. R., Frey W. H . Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. Journal of Pharmaceutical Sciences. 2010;99(4):1654–1673. DOI: 10.1002/jps.21924.
31. Inoue D., Tanaka A., Kimura S., Kiriyama A., Katsumi H., Yamamoto A., Ogawara K-I., Kimura T., Higaki K., Yutani R., Sakane T., Furubayashi T The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. European Journal of Pharmaceutical Sciences.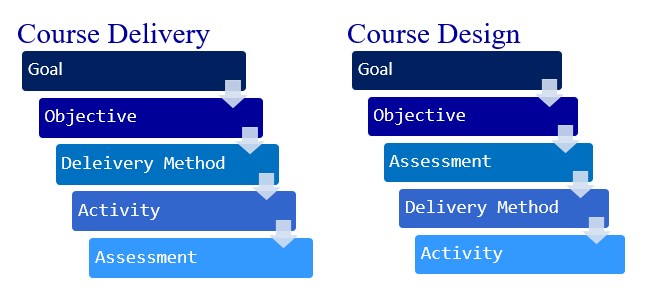 2018;117:21–26. DOI: 10.1016/j.ejps.2018.01.032.
2018;117:21–26. DOI: 10.1016/j.ejps.2018.01.032.
32. Mall M. A. Role of Cilia, Mucus, and Airway Surface Liquid in Mucociliary Dysfunction: Lessons from Mouse Models. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2008;21(1):13–24. DOI: 10.1089/jamp.2007.0659.
33. Lansley A. B. Mucociliary clearance and drug delivery via the respiratory tract. Advanced Drug Delivery Reviews. 1993;11:299–327. DOI: 10.1016/0169-409X(93)
-U.
34. Braiman A., Priel Z. Efficient mucociliary transport relies on efficient regulation of ciliary beating. Respiratory Physiology & Neurobiology. 2008;163(1–3):202–207. DOI: 10.1016/j.resp.2008.05.010.
35. Alsarra I. A., Hamed A. Y., Alanazi F. K., El Maghraby G. M. Vesicular Systems for Intranasal Drug Delivery. In: Drug Delivery to the Central Nervous System. Totowa: Humana Press; 2009. P. 175–203. DOI: 10.1007/978-1-60761-529-3_8.
36. Zarshenas M. M., Zargaran A., Müller J., Mohagheghzadeh A. Nasal Drug Delivery in Traditional Persian Medicine. Jundishapur Journal of Natural Pharmaceutical Products. 2013;8(3):144–148. DOI: 10.17795/jjnpp-9990.
Jundishapur Journal of Natural Pharmaceutical Products. 2013;8(3):144–148. DOI: 10.17795/jjnpp-9990.
37. Chan A. S., Cheung M., Sze S. L., Leung W. W., Shi D. An Herbal Nasal Drop Enhanced Frontal and Anterior Cingulate Cortex Activity. Evidence Based Complementary and Alternative Medicine. 2011;2011:543648–543656. DOI: 10.1093/ecam/nep198.
38. Al-Ghananeem A. M., Traboulsi A. A., Dittert L. W., Hussain A. A. Targeted brain delivery of 17β-estradiol via nasally administered water soluble prodrugs. AAPS PharmSciTech. 2002;3(1):40–47. DOI: 10.1208/pt030105.
39. Kao H. D., Traboulsi A., Itoh S., Dittert L., Hussain A. Enhancement of the systemic and CNS specific delivery of L-dopa by the nasal administration of its water soluble prodrugs. pharmaceutical research. 2000;17(8):978–984. DOI: 10.1023/A:1007583422634.
40. Serralheiro A., Alves G., Fortuna A., Falcão A. Intranasal administration of carbamazepine to mice: A direct delivery pathway for brain targeting. European Journal of Pharmaceutical Sciences. 2014;60:32–39. DOI: 10.1016/j.ejps.2014.04.019.
European Journal of Pharmaceutical Sciences. 2014;60:32–39. DOI: 10.1016/j.ejps.2014.04.019.
41. Sin B., Wiafe J., Ciaramella C., Valdez L., Motov S. M. The use of intranasal analgesia for acute pain control in the emergency department: A literature review. The American Journal of Emergency Medicine. 2018;36(2):310–318. DOI: 10.1016/j.ajem.2017.11.043.
42. Agrawal M., Saraf S., Saraf S., Antimisiaris S. G., Chougule M. B., Shoyele S. A., Alexander A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs . Journal of Controlled Release. 2018;281:139–177. DOI: 10.1016/j.jconrel.2018.05.011.
43. Costantino H. R., Illum L., Brandt G., Johnson P. H., Quay S. C. Intranasal delivery: Physicochemical and therapeutic aspects. International Journal of Pharmaceutics. 2007;337(1–2):1–24. DOI: 10.1016/j.ijpharm.2007.03.025.
44. Keech B., Crowe S., Hocking D. R. Intranasal oxytocin, social cognition and neurodevelopmental disorders: A meta-analysis. Psychoneuroendocrinology. 2018;87:9–19. DOI: 10.1016/j.psyneuen.2017.09.022.
Psychoneuroendocrinology. 2018;87:9–19. DOI: 10.1016/j.psyneuen.2017.09.022.
45. Oppong-Damoah A., Zaman R. U., D’Souza M. J., Murnane K. S. Nanoparticle encapsulation increases the brain penetrance and duration of action of intranasal oxytocin. Hormones and Behavior. 2019;108:20–29. DOI: 10.1016/j.yhbeh.2018.12.011.
46. Ozsoy Y., Gungor S., Cevher E. Nasal Delivery of High Molecular Weight Drugs. Molecules. 2009;14(9):3754–3779. DOI: 10.3390/molecules14093754.
47. Rhea E. M., Salameh T. S., Banks W. A. Routes for the delivery of insulin to the central nervous system: A comparative review. Experimental Neurology. 2019;313:10–15. DOI: 10.1016/j.expneurol.2018.11.007.
48. Craft S. Intranasal Insulin Therapy for Alzheimer Disease and Amnestic Mild Cognitive Impairment. Archives of Neurology. 2012;69(1):29–38. DOI: 10.1001/archneurol.2011.233.
49. Simon K. U., Neto E. W., dos Santos Tramontin N., Canteiro P. B., da Costa Pereira B., Zaccaron R. P., Silveira P. C. L., Muller A. P. Intranasal insulin treatment modulates the neurotropic, inflammatory, and oxidant mechanisms in the cortex and hippocampus in a low-grade inflammation model. Peptides. 2020;123:170175. DOI: 10.1016/j.peptides.2019.170175.
P., Silveira P. C. L., Muller A. P. Intranasal insulin treatment modulates the neurotropic, inflammatory, and oxidant mechanisms in the cortex and hippocampus in a low-grade inflammation model. Peptides. 2020;123:170175. DOI: 10.1016/j.peptides.2019.170175.
50. Salameh T. S., Bullock K. M., Hujoel I. A., Niehoff M. L., Wolden-Hanson T., Kim J., Morley J. E., Farr S. A., Banks W. A. Central Nervous System Delivery of Intranasal Insulin: Mechanisms of Uptake and Effects on Cognition. Journal of Alzheimer's Disease. 2015;47(3):715–728. DOI: 10.3233/JAD-150307.
51. Yu H., Kim K. Direct nose-to-brain transfer of a growth hormone releasing neuropeptide, hexarelin after intranasal administration to rabbits. International Journal of Pharmaceutics. 2009;378(1–2):73–79. DOI: 10.1016/j.ijpharm.2009.05.057.
52. Ren Z., Zhao Y., Liu J., Ji X., Meng L., Wang T., Sun W., Zhang K., Sang X., Yu Z., Li Y., Feng N ., Wang H., Yang D., Yang Z., Ma Y., Gao Y., Xia X. Intramuscular and intranasal immunization with an H7N9 influenza viruslike particle vaccine protects mice against lethal influenza virus challenge.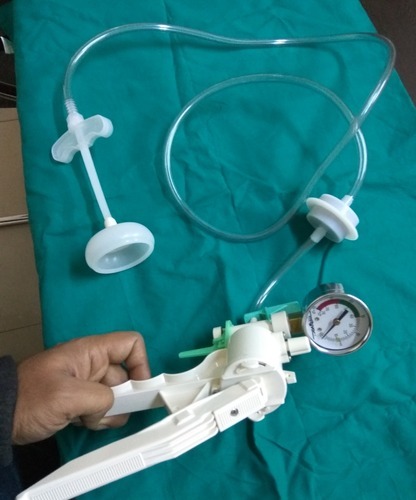 International Immunopharmacology. 2018;58:109–116. DOI: 10.1016/j.intimp.2017.12.020.
International Immunopharmacology. 2018;58:109–116. DOI: 10.1016/j.intimp.2017.12.020.
53. Bahadur S., Pathak K. Physicochemical and physiological considerations for efficient nose-to-brain targeting. Expert Opinion on Drug Delivery. 2012;9(1):19–31. DOI: 10.1517/17425247.2012.636801.
54. Pires A., Fortuna A., Alves G., Falcão A. Intranasal Drug Delivery: How, Why and What for? Journal of Pharmacy & Pharmaceutical Sciences. 2009;12(3):288–311. DOI: 10.18433/J3NC79.
55. Tian B., Liu Y., Liu J. Chitosan-based nanoscale and non-nanoscale delivery systems for anticancer drugs: A review. European Polymer Journal. 2021;154:110533. DOI: 10.1016/j.eurpolymj.2021.110533.
56. Pacheco C., Sousa F., Sarmento B. Chitosan-based nanomedicine for brain delivery: Where are we heading? Reactive and Functional Polymers. 2020;146:104430. DOI: 10.1016/j.reactfunctpolym.2019.104430.
57. García-González C. A., Uy J. J., Alnaief M., Smirnova I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydrate Polymers. 2012;88(4):1378–1386. DOI: 10.1016/j.carbpol.2012.02.023.
Carbohydrate Polymers. 2012;88(4):1378–1386. DOI: 10.1016/j.carbpol.2012.02.023.
58. Kundu D., Banerjee T. Development of microcrystalline cellulose based hydrogels for the in vitro delivery of Cephalexin. Helion. 2020;6(1):e03027. DOI: 10.1016/j.heliyon.2019.e03027.
59. Vasvani S., Kulkarni P., Rawtani D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. International Journal of Biological Macromolecules. 2020;151:1012–1029. DOI: 10.1016/j.ijbiomac.2019.11.066.
60. Varshosaz J. Dextran conjugates in drug delivery. Expert Opinion on Drug Delivery. 2012;9(5):509–523. DOI: 10.1517/17425247.2012.673580.
61. Lei C., Liu X.-R., Chen Q.-B., Li Y., Zhou J.-L., Zhou L.-Y., Zou T. Hyaluronic acid and albumin based nanoparticles for drug delivery. Journal of Controlled Release. 2021;331:416-433. DOI: 10.1016/j.jconrel.2021.01.033.
62. Jahanban-Esfahlan R., Derakhshankhah H., Haghshenas B., Mas-soumi B., Abbasian M., Jaymand M. A bioinspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. International Journal of Biological Macromolecules. 2020;156:438–445. DOI: 10.1016/j.ijbiomac.2020.04.074.
Jahanban-Esfahlan R., Derakhshankhah H., Haghshenas B., Mas-soumi B., Abbasian M., Jaymand M. A bioinspired magnetic natural hydrogel containing gelatin and alginate as a drug delivery system for cancer chemotherapy. International Journal of Biological Macromolecules. 2020;156:438–445. DOI: 10.1016/j.ijbiomac.2020.04.074.
63. Liu S., Qin S., He M., Zhou D., Qin Q., Wang H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Composites Part B: Engineering. 2020;199:108238. DOI: 10.1016/j.compositesb.2020.108238.
64. Kipper M. J., Shen E., Determan A., Narasimhan B. Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. biomaterials. 2002;23(22):4405–4412. DOI: 10.1016/S0142-9612(02)00181-3.
65. Porfiryeva N. N., Moustafine R. I., Khutoryanskiy V. V. PEGylated Systems in Pharmaceutics. Polymer Science, Series C. 2020;61:62–74. DOI: 10.1134/S181123822001004X.
66. Wei X., Gong C., Gou M., Fu S., Guo Q., Shi S., Luo F., Guo G., Qiu L., Qian Z. Biodegradable poly(ε-caprolactone) –poly(ethylene glycol) copolymers as a drug delivery system. International Journal of Pharmaceutics. 2009;381(1):1–18. DOI: 10.1016/j.ijpharm.2009.07.033.
Wei X., Gong C., Gou M., Fu S., Guo Q., Shi S., Luo F., Guo G., Qiu L., Qian Z. Biodegradable poly(ε-caprolactone) –poly(ethylene glycol) copolymers as a drug delivery system. International Journal of Pharmaceutics. 2009;381(1):1–18. DOI: 10.1016/j.ijpharm.2009.07.033.
67. Molavi F., Barzegar-Jalali M., Hamishehkar H. Polyester based polymeric nano and microparticles for pharmaceutical purposes: A review on formulation approaches. Journal of Controlled Release. 2020;320:265–282. DOI: 10.1016/j.jconrel.2020.01.028.
68. Gunatillake P., Adhikari R. Biodegradable synthetic polymers for tissue engineering. European Cells and Materials. 2003;5:1–16 DOI: 10.22203/eCM.v005a01.
69. Bruschi M. L., de Souza Ferreira S. B., Bassi da Silva J. Mucoadhesive and mucus-penetrating polymers for drug delivery. In: Nanotechnology for Oral Drug Delivery. Cambridge: Academic Press; 2020. P. 77–141.
70. Smart J. The basics and underlying mechanisms of mucoadhesion. Advanced Drug Delivery Reviews. 2005;57(11):1556–1568. DOI: 10.1016/j.addr.2005.07.001.
2005;57(11):1556–1568. DOI: 10.1016/j.addr.2005.07.001.
71. Khutoryanskiy V. V. Advances in Mucoadhesion and Mucoadhesive Polymers. macromolecular bioscience. 2011;11(6):748–764. DOI: 10.1002/mabi.201000388.
72. Peppas N. A., Buri P. A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. Journal of Controlled Release. 1985;2:257–275. DOI: 10.1016/0168-3659(85)
-1.
73. Zhao D., Yu S., Sun B., Gao Sh., Guo S., Zhao K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. polymers. 2018;10(4):462. DOI: 10.3390/polym10040462.
74. Sahin A., Yoyen-Ermis D., Caban-Toktas S., Horzum U., Aktas Y., Couvreur P., Esendagli G., Capan Y. Evaluation of brain-targeted chitosan nanoparticles through blood–brain barrier cerebral microvessel endothelial cells. Journal of Microencapsulation. 2017;34(7):659–666. DOI: 10.1080/02652048.2017.1375039.
75. Raj R., Wairkar S., Sridhar V., Gaud R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. International Journal of Biological Macromolecules. 2018;109:27–35. DOI: 10.1016/j.ijbiomac.2017.12.056.
International Journal of Biological Macromolecules. 2018;109:27–35. DOI: 10.1016/j.ijbiomac.2017.12.056.
76. Rukmangathen R., Yallamalli I. M., Yalavarthi P. R. Formulation and biopharmaceutical evaluation of risperidoneloaded chitosan nanoparticles for intranasal delivery. Drug Development and Industrial Pharmacy. 2019;45:1342–1350. DOI: 10.1080/03639045.2019.1619759.
77. Keely S., Rullay A., Wilson C., Carmichael A., Carrington S., Corfield A., Haddleton D. M., Brayden D. R. In Vitro and ex Vivo Intestinal Tissue Models to Measure Mucoadhesion of Poly (Methacrylate) and N-Trimethylated Chitosan Polymers. pharmaceutical research. 2005;22:38–39. DOI: 10.1007/s11095-004-9007-1.
78. Patel M. M., Smart J. D., Nevell T. G., Ewen R. J., Eaton P. J., Tsibouklis J. Mucin/Poly(acrylic acid) Interactions: A Spectroscopic Investigation of Mucoadhesion. biomacromolecules. 2003;4:1184–1190. DOI: 10.1021/bm034028p.
79. Porfiryeva N. N., Semina I. I., Salakhov I. A. , Moustafine R. I., Khutoryanskiy V. V. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2021;37:102432. DOI: 10.1016/j.nano.2021.102432.
, Moustafine R. I., Khutoryanskiy V. V. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomedicine: Nanotechnology, Biology and Medicine. 2021;37:102432. DOI: 10.1016/j.nano.2021.102432.
80. Brannigan R. P., Khutoryanskiy V. V. Progress and Current Trends in the Synthesis of Novel Polymers with Enhanced Mucoadhesive Properties. macromolecular bioscience. 2019;19(10):1
4. DOI: 10.1002/mabi.2014.81. Bernkop-Schnürch A. Thiomers: A new generation of mucoadhesive polymers. Advanced Drug Delivery Reviews. 2005;57:1569–1582. DOI: 10.1016/j.addr.2005.07.002.
82. Leitner V. M., Guggi D., Bernkop-Schnürch A. Thiomers in noninvasive polypeptide delivery: In vitro and in vivo characterization of a polycarbophil-cysteine/glutathione gel formulation for human growth hormone. Journal of Pharmaceutical Sciences. 2004;93(7):1682–1691. DOI: 10.1002/jps.20069.
83. Porfiryeva N. N., Nasibullin S. F., Abdullina S. G., Tukhbatullina I. K., Moustafine R. I., Khutoryanskiy V. V. Acrylated Eudragit® E PO as a novel polymeric excipient with enhanced mucoadhesive properties for application in nasal drug delivery. International Journal of Pharmaceutics. 2019;562:241–248. DOI: 10.1016/j.ijpharm.2019.03.027.
G., Tukhbatullina I. K., Moustafine R. I., Khutoryanskiy V. V. Acrylated Eudragit® E PO as a novel polymeric excipient with enhanced mucoadhesive properties for application in nasal drug delivery. International Journal of Pharmaceutics. 2019;562:241–248. DOI: 10.1016/j.ijpharm.2019.03.027.
84. Tonglairoum P., Brannigan R. P., Opanasopit P., Khutoryanskiy V. V. Maleimide-bearing nanogels as novel mucoadhesive materials for drug delivery. Journal of Materials Chemistry B. 2016;4(40):6581–6587. DOI: 10.1039/C6TB02124G.
85. Kaldybekov D. B., Tonglairoum P., Opanasopit P., Khutoryanskiy V. V. Mucoadhesive maleimide-functionalised liposomes for drug delivery to urinary bladder. European Journal of Pharmaceutical Sciences. 2018;111:83–90. DOI: 10.1016/j.ejps.2017.09.039.
86. Kaldybekov D. B., Filippov S. K., Radulescu A., Khutoryanskiy V. V. Maleimide-functionalised PLGA-PEG nanoparticles as mucoadhesive carriers for intravesical drug delivery. European Journal of Pharmaceutics and Biopharmaceutics.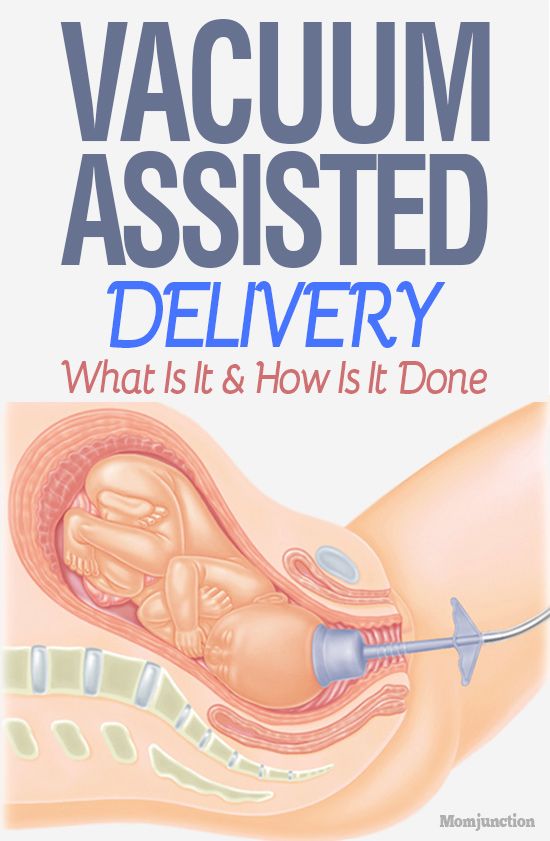 2019;143:24–34. DOI: 10.1016/j.ejpb.2019.08.007.
2019;143:24–34. DOI: 10.1016/j.ejpb.2019.08.007.
87. Mainardes R. M., Khalil N. M., Gremião M. P. D. Intranasal delivery of zidovudine by PLA and PLA–PEG blend nanoparticles. International Journal of Pharmaceutics. 2010;395:266–271. DOI: 10.1016/j.ijpharm.2010.05.020.
88. Guerra-Crespo M., Sistos A., Gleason D., Fallon J. H . Intranasal Administration of PEGylated Transforming Growth Factor-α Improves Behavioral Deficits in a Chronic Stroke Model. Journal of Stroke and Cerebrovascular Diseases. 2010;19:3–9. DOI: 10.1016/j.jstrokecerebrovasdis.2009.09.005.
89. Khutoryanskiy V. V. Beyond PEGylation: Alternative surface-modification of nanoparticles with mucusinert biomaterials. Advanced Drug Delivery Reviews. 2018;124:140–149. DOI: 10.1016/j.addr.2017.07.015.
90. Matsuyama T., Morita T., Horikiri Y., Yamahara H., Yoshino H. Enhancement of nasal absorption of large molecular weight compounds by combination of mucolytic agent and nonionic surfactant. Journal of Controlled Release. 2006;110(2):347–352. DOI: 10.1016/j.jconrel.2005.09.047.
2006;110(2):347–352. DOI: 10.1016/j.jconrel.2005.09.047.
91. Agrawal M., Saraf Sh., Saraf S., Dubey S. K., Puri A., Gupta U., Kesharwani P., Ravichandiran V., Kumar P., Naidu V. G. M., Murty U. S., Ajazuddin, Alexander A. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. Journal of Controlled Release. 2020;327:235–265. DOI: 10.1016/j.jconrel.2020.07.044.
92. Karavasili C., Fatouros D. G. Smart materials: in situ gelforming systems for nasal delivery. Drug Discovery Today. 2016;21(1):157–166. DOI: 10.1016/j.drudis.2015.10.016.
93. Attwood D., Collett J., Tait C. The micellar properties of the poly(oxyethylene) – poly(oxypropylene) copolymer Pluronic F127 in water and electrolyte solution. International Journal of Pharmaceutics. 1985;26:25–33. DOI: 10.1016/0378-5173(85)
-8. 94. Naik A., Nair H. Formulation and Evaluation of Thermosensitive Biogels for Nose to Brain Delivery of Doxepin. BioMed Research International. 2014;2014:847547. DOI: 10.1155/2014/847547.
DOI: 10.1155/2014/847547.
95. Singh R. M., Kumar A., Pathak K. Mucoadhesive in situ nasal gelling drug delivery systems for modulated drug delivery. Expert Opinion on Drug Delivery. 2013;10:115–130. DOI: 10.1517/17425247.2013.746659.
96. Gabal Y. M., Kamel A. O., Sammour O. A., Elshafeey A. H. Effect of surface charge on the brain delivery of nanostructured lipid carriers in situ gels via the nasal route. International Journal of Pharmaceuticals. 2014;473(1–2):442–457. DOI: 10.1016/j.ijpharm.2014.07.025.
97. Cao S., Zhang Q., Jiang X. Preparation of ion-activated in situ gel systems of scopolamine hydrobromide and evaluation of its antimotion sickness efficacy. Acta Pharmacologica Sinica. 2007;28(4):584–590. DOI: 10.1111/j.1745-7254.2007.00540.x.
98. Mathure D., Madan J. R., Gujar K. N., Tupsamundre A., Ranpise H. A., Dua K. Formulation and Evaluation of Niosomal in situ Nasal Gel of a Serotonin Receptor Agonist, Buspirone Hydrochloride for the Brain Delivery via Intranasal Route.