Poor placental perfusion
Placental Insufficiency - StatPearls - NCBI Bookshelf
Continuing Education Activity
Placental insufficiency is a condition whereby there is a failure of placental vascular remodeling, leading to a failure of placentation resulting in acidosis and fetal hypoxemia. The most common downstream fetal consequences of this condition include intrauterine growth restriction, prematurity, or, unfortunately, fetal demise. In order to reduce the risk of fetal morbidity and mortality, especially in high-risk pregnancies, regular prenatal screening with Doppler ultrasound should be done to increase the chances of detection and diagnosis. This activity reviews the pathophysiology, evaluation, and potential treatments of placental insufficiency, and highlights the role of the interprofessional team in managing patients with this condition.
Objectives:
Review the risk factors associated with placental insufficiency.
Describe the typical imaging findings on Doppler ultrasound associated with placental insufficiency.
Identify the most common adverse events associated with placental insufficiency.
Access free multiple choice questions on this topic.
Introduction
Placental insufficiency is associated with various obstetric disorders such as pre-eclampsia and intrauterine growth restriction, both of which predispose to preterm labor, a leading cause of perinatal morbidity and mortality around the world. Poor placental function is most commonly described by the term ‘placental insufficiency’ within the medical community; however, one study highlighted the problem that there is no standardized definition or consensus for the pathognomonic features pertaining to placental insufficiency.[1]
This poses many challenges when it comes to studying placental insufficiency in the literature, but the general understanding is that placental insufficiency is a process whereby there is a progressive deterioration in placental functioning such that oxygen and nutrient transfer to the fetus via the placenta is decreased, culminating in a decompensated hypoxia and acidosis.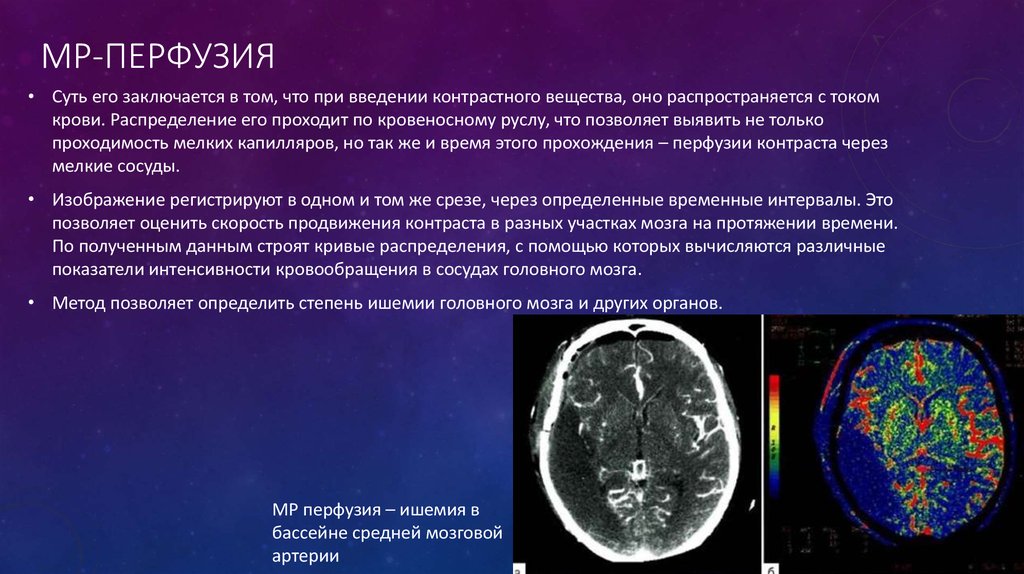 [2][3] This process leads to fetal hypoxemia that then stimulates a downregulation of fetal metabolic demands to preserve what nutrients are already accessible, thus resulting in intrauterine fetal growth restriction. From a histopathologic view, placental insufficiency can be defined when there is chorionic villi fibrosis, uteroplacental thrombosis, placental infarcts, fibrin deposits, or a reduction in number and surface area of the villous capillary tree.[4]
[2][3] This process leads to fetal hypoxemia that then stimulates a downregulation of fetal metabolic demands to preserve what nutrients are already accessible, thus resulting in intrauterine fetal growth restriction. From a histopathologic view, placental insufficiency can be defined when there is chorionic villi fibrosis, uteroplacental thrombosis, placental infarcts, fibrin deposits, or a reduction in number and surface area of the villous capillary tree.[4]
Of note, placental infarcts can be a normal finding, as they are observed in approximately 25% of normal term pregnancies; however, increasing infarction of the placenta has been shown to be associated with placental insufficiency and thus intrauterine growth restriction (IUGR). Both MRI and ultrasound studies looking for placental insufficiency have demonstrated reductions in placental area and volume as well as increased placental thickness, in addition to globular shaped placentas on MRI.[5]
Etiology
To date, the major etiologies that may lead to placental insufficiency are poorly understood and are still being studied. There are known associated maternal risk factors, which include pre-eclampsia or other maternal hypertensive disorders, maternal cigarette use, maternal drug use including cocaine or heroin, maternal alcohol consumption, primiparity, advanced maternal age, and prior history of delivery of IUGR neonate.[2][6]
There are known associated maternal risk factors, which include pre-eclampsia or other maternal hypertensive disorders, maternal cigarette use, maternal drug use including cocaine or heroin, maternal alcohol consumption, primiparity, advanced maternal age, and prior history of delivery of IUGR neonate.[2][6]
Studies analyzing Doppler waveforms in various placental vessels of mothers who smoked cigarettes during pregnancy demonstrated reductions in the blood flow velocity waveforms, thus indicating the nicotine exposure can lead to altered placental vasculature.[6]
Any maternal condition that can lead to a compromise in the fetal circulation puts the fetus at risk for placental insufficiency. Additionally, certain medications such as antineoplastic, anticonvulsants, or anti-coagulants can interfere with fetal growth. Extremes of maternal body mass index, including maternal malnutrition, have also been associated with the development of IUGR neonates.[7] Studies of IUGR complicated pregnancies have demonstrated that there was an incomplete transformation of the placental vasculature early in the pregnancies that can be detected by doppler ultrasound studies. [5][8]
[5][8]
Epidemiology
Prematurity is the leading cause of perinatal death followed by intrauterine fetal growth restriction, which complicates approximately 4% to 6% of known pregnancies. Placental insufficiency is a potential cause of preterm labor, pre-eclampsia, IUGR, and stillbirth, which can affect 10 to 15% of pregnancies. For an IUGR fetus, the risk of spontaneous preterm labor is three-fold greater when compared to a non-growth restricted fetus, and there is also a five to six times higher risk for the development of perinatal death.[2] Unfortunately, approximately 50% of newborns with IUGR are only detected following delivery.
Pathophysiology
Although the underlying etiology for placental insufficiency is unknown, there are proposed mechanisms. Placental insufficiency is associated with a reduction in blood flow across the umbilicus to the fetus, which can be secondary to increased umbilical-placental vascular resistance. This increased resistance can be visualized as abnormal umbilical artery doppler flow velocity waveforms and can be secondary to an abnormality of villi insertion into the placental membrane, a perfusion abnormality between the umbilicus and placenta, or a reduction in uteroplacental blood flow. [2] Studies of umbilical artery Doppler have illustrated that the degree of placental injury is directly related to the degree of fetal injury during pregnancy.[9]
[2] Studies of umbilical artery Doppler have illustrated that the degree of placental injury is directly related to the degree of fetal injury during pregnancy.[9]
The main role of the placenta is to serve as the interface between fetal and maternal circulations. For this to occur, placental adherence and uterine arterial remodeling must be established in order to ensure nutrients can be delivered to the growing fetus. The hallmark for successful placentation is the remodeling of the uterine arteries. Following fertilization, the blastocyst forms, which is composed of an inner cell mass that will eventually become the fetus, and an outer shell called the trophoblast that becomes the fetal portion of the placenta. To aid with placental adherence, the cytotrophoblast, which is the inner layer of the trophoblast, secretes matrix metalloproteinases that breakdown the zona pellucida, and adherence is facilitated by the formation of anchoring villi and expression of adhesion molecules.[10] This invasion of the uteroplacental arteries allows for remodeling into dilated, low-resistance, inelastic vessels that lack maternal vasomotor control that leads to increased uteroplacental perfusion such as that the requirements of the fetus can be met. [5] Any disturbance in the remodeling process can lead to an increase in uteroplacental vascular resistance, leading to hypoperfusion of the placenta and its downstream effects, including coagulation activation, endothelial cell dysfunction, placental thrombosis, and fibrin deposits, which is associated with the development of IUGR.[5][3] Furthermore, if there is a loss of focal adhesion of the endovascular trophoblasts, a reduction in placental surface area can be seen associated with placental insufficiency. This reduction in the placental surface area, along with an increase in the thickness of the placenta, creates a globular appearance to the placenta, which is postulated to act as a compensatory mechanism for placental insufficiency.
[5] Any disturbance in the remodeling process can lead to an increase in uteroplacental vascular resistance, leading to hypoperfusion of the placenta and its downstream effects, including coagulation activation, endothelial cell dysfunction, placental thrombosis, and fibrin deposits, which is associated with the development of IUGR.[5][3] Furthermore, if there is a loss of focal adhesion of the endovascular trophoblasts, a reduction in placental surface area can be seen associated with placental insufficiency. This reduction in the placental surface area, along with an increase in the thickness of the placenta, creates a globular appearance to the placenta, which is postulated to act as a compensatory mechanism for placental insufficiency.
The intrauterine environment is a low-oxygen environment, and as such fetal circulation needs to be flexible in order to adapt to any changes that occur with uteroplacental function.[9] This hypoxic environment stimulates angiogenesis whereby vascular connections are made between the maternal circulation and the intervillous space.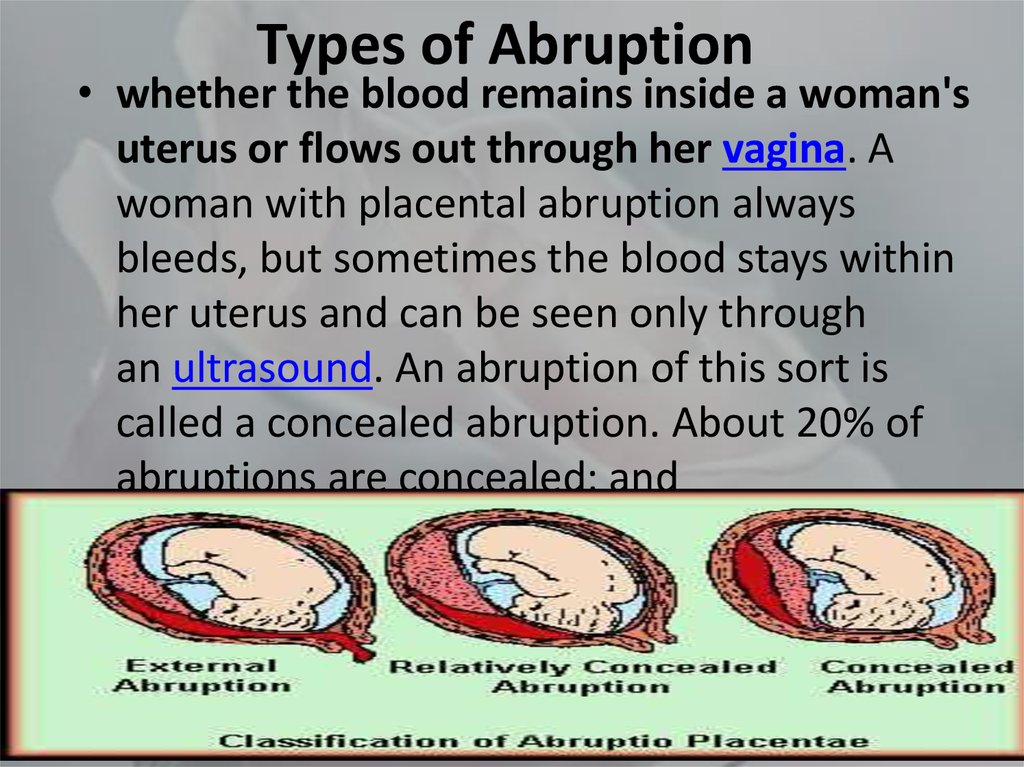 Now that a vascular and nutritional network of support has been established, the villous trophoblast is formed, which consists of maternal microvilli and a fetal basal layer.[10] To maintain placental function requires large amounts of energy, indicated by the fact that in a regular physiologic state, the placenta consumes approximately 70% of the glucose and 40% of the oxygen that is normally supplied to the uterus. Therefore, in order to achieve optimal fetal growth and development, nutrient delivery to the uterus must exceed the placental demand such that there are residual nutrients for fetal utilization.[10] Therefore, any compromise of nutrient delivery to the uterus impacts nutrient delivery to the fetus.
Now that a vascular and nutritional network of support has been established, the villous trophoblast is formed, which consists of maternal microvilli and a fetal basal layer.[10] To maintain placental function requires large amounts of energy, indicated by the fact that in a regular physiologic state, the placenta consumes approximately 70% of the glucose and 40% of the oxygen that is normally supplied to the uterus. Therefore, in order to achieve optimal fetal growth and development, nutrient delivery to the uterus must exceed the placental demand such that there are residual nutrients for fetal utilization.[10] Therefore, any compromise of nutrient delivery to the uterus impacts nutrient delivery to the fetus.
Successful placentation can be negatively affected by lateralization, which is when the placental invasion favors one side, and the placenta is not implanted centrally. If the placenta remains asymmetric until term, doppler ultrasound will demonstrate persistent notching on the non-implanted side, leading to a relative placental insufficiency. Placental lateralization has also been associated with increased risk for the development of maternal pre-eclampsia, and thus downstream placental insufficiency.[9][11]
Placental lateralization has also been associated with increased risk for the development of maternal pre-eclampsia, and thus downstream placental insufficiency.[9][11]
History and Physical
As intrauterine growth restriction is one of the primary outcomes associated with placental insufficiency, most neonates that survive present either prematurely and are known as ‘extremely low birth weight’ (ELBW) neonates or depending on when the insult to the vascular supply occurred, they may present with altered body proportions at birth.[10] If there is a failure of placentation, downstream effects such as IUGR are clinically evident on Doppler studies at approximately 26 weeks gestation.[9]
When there is a concern for IUGR, whether it is secondary to placental insufficiency or another etiology, the fetus will benefit from frequent combined monitoring using both Doppler and biophysical profile screens. This combination of screening techniques allows for the recognition of the declining performance observed when IUGR is related to placental problems.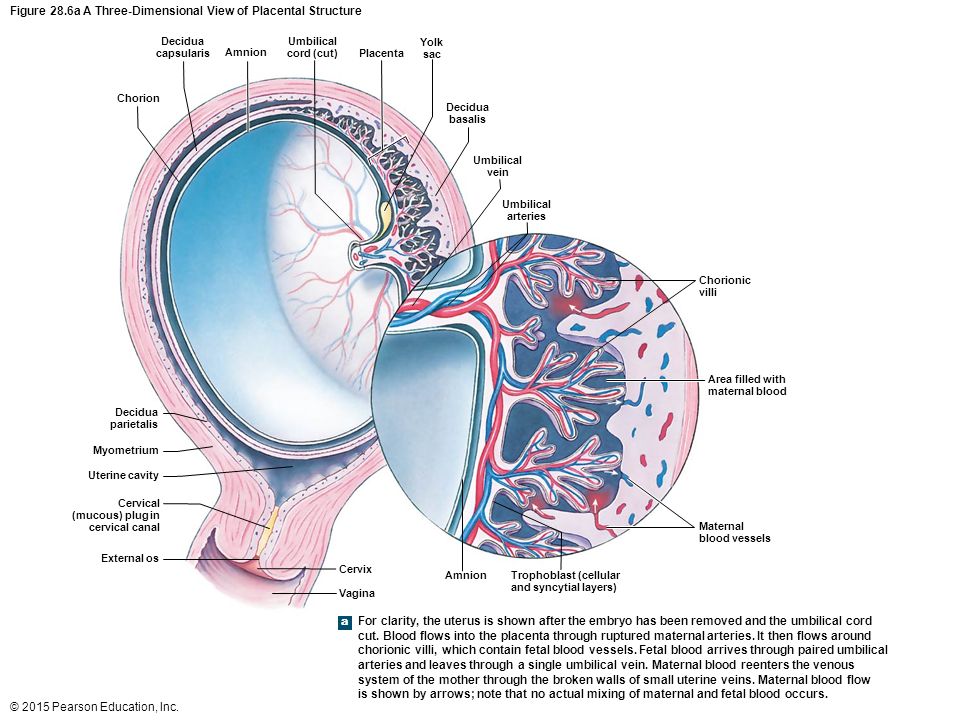 Utilizing doppler studies, failed placentation can be visualized by presenting of notching in the uterine arteries as well as increased resistance in the umbilical artery with progression to either absent- or reversed end-diastolic volumes. One of the first signs seen on a biophysical profile when there is a concern for fetal compromise is a non-reactive fetal heart rate tracing. The following will either be poor or loss of fetal body movements, breathing movements, and tone. These features of declining doppler status and poor biophysical profiles are typical when there is fetal distress, which is common when there is placental insufficiency and may indicate that delivery is required.
Utilizing doppler studies, failed placentation can be visualized by presenting of notching in the uterine arteries as well as increased resistance in the umbilical artery with progression to either absent- or reversed end-diastolic volumes. One of the first signs seen on a biophysical profile when there is a concern for fetal compromise is a non-reactive fetal heart rate tracing. The following will either be poor or loss of fetal body movements, breathing movements, and tone. These features of declining doppler status and poor biophysical profiles are typical when there is fetal distress, which is common when there is placental insufficiency and may indicate that delivery is required.
Evaluation
Currently, the criteria to diagnose placental insufficiency lacks as there are also no standardized methods for diagnosis. Part of the problem is due to the wide variety of terminology used to describe what is known as ‘placental insufficiency.’[1] However, as technology has improved, Doppler ultrasound has proven to be a useful tool to aid in the evaluation of fetal and placental circulations in both healthy and diseased states.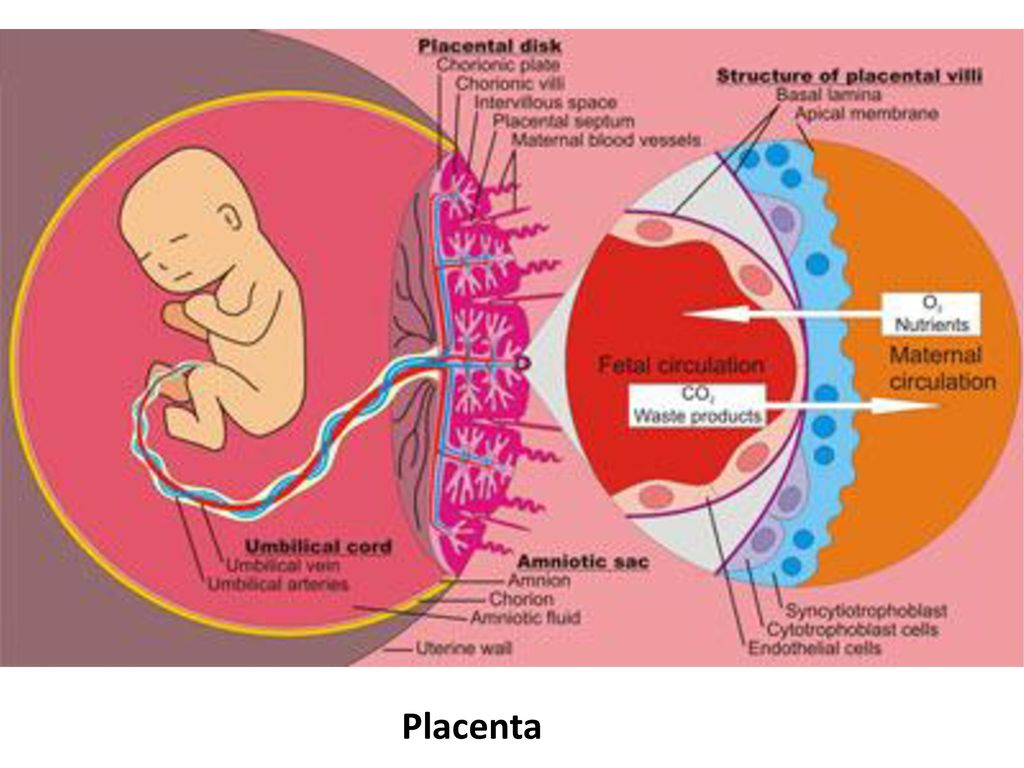 Four doppler techniques have been found to be fundamental in providing useful information regarding fetal-maternal circulation, including umbilical artery studies, uterine artery studies, middle cerebral artery studies, and ductus venosus studies.[9] As a fetus continues to mature during gestation towards term, there are many circulatory changes that occur that can be evaluated by Doppler ultrasound.
Four doppler techniques have been found to be fundamental in providing useful information regarding fetal-maternal circulation, including umbilical artery studies, uterine artery studies, middle cerebral artery studies, and ductus venosus studies.[9] As a fetus continues to mature during gestation towards term, there are many circulatory changes that occur that can be evaluated by Doppler ultrasound.
Prior to the onset of pregnancy, uterine arteries show low diastolic flow, high resistance, and elastic recoil noted in the form of early diastolic notches. Successful placentation involves removal of the intimal muscle from the vasculature such that the blood vessels will have a vigorous diastolic flow, minimal resistance, and no elastic properties. When placentation is successful, Doppler ultrasound demonstrates that the remodeling occurs rapidly, such that by 12 weeks gestation, there is a loss of notching, and resistance is low by 20 weeks gestation or earlier. When there is a failure of placentation, notching persists, and resistance remains high, which has shown to be correlated with maternal-hypertensive related fetal complications, including IUGR, pre-eclampsia, and fetal demise. [9] Using the uterine artery for Doppler screening to evaluate for notching and resistance to help identify these high-risk situations has been shown to be approximately 85% sensitive for detecting severe IUGR and pre-eclampsia.
[9] Using the uterine artery for Doppler screening to evaluate for notching and resistance to help identify these high-risk situations has been shown to be approximately 85% sensitive for detecting severe IUGR and pre-eclampsia.
As resistance in the placenta increases, doppler studies of the umbilical artery can demonstrate either normal, reduced, absent, or reversed end-diastolic velocities.[12][8] It is normal for placental resistance to be high in the early stages of pregnancy, and therefore it can be expected that there will be absent end-diastolic velocity on Doppler studies up to 12 to 14 weeks of gestation. When the placental successfully invades, resistance drops and doppler studies of the umbilical artery should demonstrate continuous flow by 14 to 18 weeks of gestation.[9] Persistent umbilical artery resistance over the duration of pregnancy is an indicator of increased risk of placental insufficiency.
While umbilical artery Doppler studies provide important information pertaining to possible placental compromise, a valuable adjunct is to also use the middle cerebral artery (MCA) Doppler.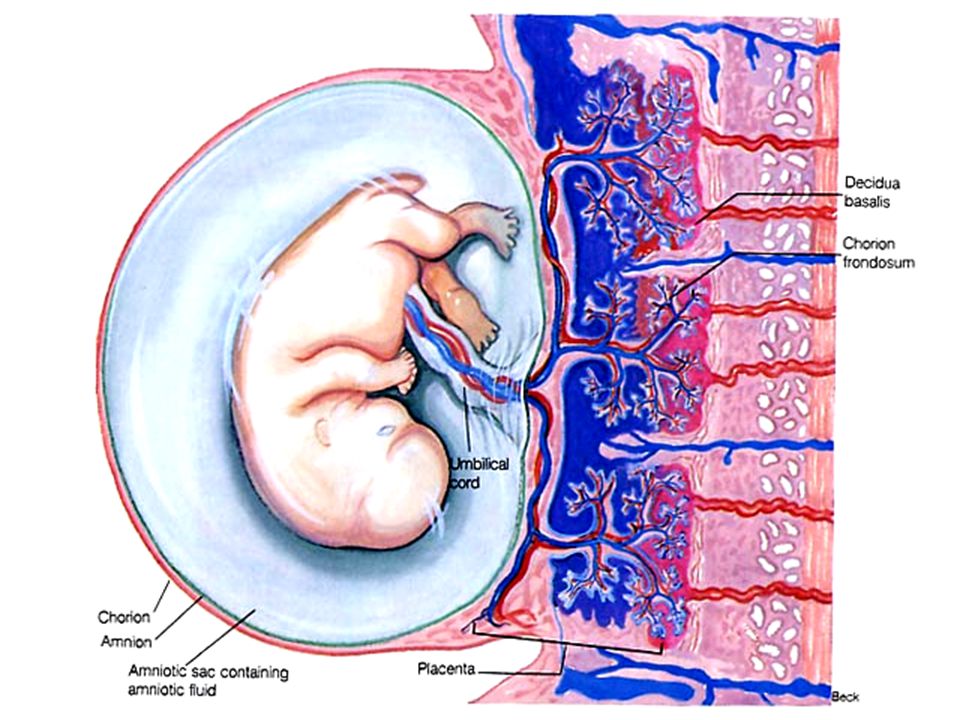 The MCA provides information regarding systemic circulatory responses in the developing fetus, as it represents downstream resistance in the cerebral microcirculation. The normal MCA Doppler displays high resistance throughout pregnancy; however, the placental disease can be identified when there is increased diastolic flow along with declining pulsatility index. Therefore, MCA Doppler studies can provide important additional information when there is a concern for severe IUGR, indicating that there may be fetal compromise and intervention may be required.
The MCA provides information regarding systemic circulatory responses in the developing fetus, as it represents downstream resistance in the cerebral microcirculation. The normal MCA Doppler displays high resistance throughout pregnancy; however, the placental disease can be identified when there is increased diastolic flow along with declining pulsatility index. Therefore, MCA Doppler studies can provide important additional information when there is a concern for severe IUGR, indicating that there may be fetal compromise and intervention may be required.
Another form of Doppler that provides insight into placental and fetal health is the venous Doppler, which relays information pertaining to cardiac data when the fetal circulation is experiencing stress. The venous waveform that has been shown to provide the best clinical data is the ductus venosus. There are many benefits of using the ductus venosus over other venous waveforms, which include its responsiveness to oxygenation changes, it is one of the primary regulators of venous return in abnormal and normal fetal circulations, it is independent of cardiac function, it serves as a direct conduit to view right atrial retrograde pulse waves, and lastly, because it has elevated and focal velocity color Doppler signal from as early as 12 weeks gestation up to 40 weeks gestation, it is very easily imaged. [9] If an abnormal ductus venosus or retrograde atrial-wave is visualized on Doppler at around 12 to 14 weeks gestation, there is an increased risk for fetal cardiac abnormalities, and it also serves as a possible precursor indicating severe placental-based IUGR.
[9] If an abnormal ductus venosus or retrograde atrial-wave is visualized on Doppler at around 12 to 14 weeks gestation, there is an increased risk for fetal cardiac abnormalities, and it also serves as a possible precursor indicating severe placental-based IUGR.
MRI imaging has been found to provide additional information in order to detect and diagnose placental insufficiency. Flow voids occur when there is a loss of MRI signal observed in a blood vessel where blood flows vigorously. When using T2-weighted Rapid Acquisition with Relaxation Enhancement (RARE) imaging, placental insufficiency can be detected when decreased flow voids are observed between the placenta and the uterus, as this can be considered to reflect a reduction in uteroplacental perfusion.[5] An additional advantage of MRI imaging is that it provides high soft-tissue contrast. Therefore placental vascular abnormalities, including hemorrhages and infarctions, can be detected on placental-MRI, indicating high risk for placental insufficiency and downstream IUGR.
Treatment / Management
Currently, there is no known treatment for placental insufficiency, other than delivering the fetus if it is at a viable timepoint. Low-dose aspirin and the use of antioxidant therapies, including vitamins C and E, have been shown to promote improved placentation in cases where there is uncertainty for successful placentation.[9] Studies have demonstrated that there is an approximate 38% reduction in perinatal mortality when early Doppler ultrasound is used for cases of suspected IUGR during pregnancy.[12][13]
High-risk women, such as those with chronic hypertension, coagulopathies, or a history of pre-eclampsia, can benefit from having Doppler ultrasound screening at 12 to 14 weeks gestation because if bilateral notching is evident, then low-dose aspirin therapy should be initiated.
In vitro studies have demonstrated that heparin can stimulate neo-angiogenesis and also improve placental perfusion. Heparin’s anticoagulant properties are exhibited by its ability to mobilize tissue inhibition factor into circulation and by the fact that it also enhances antithrombin activity. Additional benefits of heparin when considering placental insufficiency and its downstream consequences are that heparin promotes trophoblastic proliferation, reduces inflammation by downregulating the complement cascade, reduces apoptosis, and it also acts indirectly as a growth factor.[3]
Additional benefits of heparin when considering placental insufficiency and its downstream consequences are that heparin promotes trophoblastic proliferation, reduces inflammation by downregulating the complement cascade, reduces apoptosis, and it also acts indirectly as a growth factor.[3]
Heparin has also been shown to upregulate certain proteins involved in placental angiogenesis and development. These include angiopoietin-2, which is responsible for chorionic villi vascular remodeling, leptin, which is responsible for the regulation of nutrient transfer, as well as vascular endothelial growth factor receptor-3, tissue inhibitor matrix metalloprotease-1, tumor necrosis factor-alpha, and angiostatin. Based on its properties and preliminary data, some studies have demonstrated that there may be a role for heparin as a preventive treatment for the placental disease.
Differential Diagnosis
There are many factors that can contribute to placental insufficiency, and it is known that IUGR is a major downstream complication; however, it can be difficult at times to differentiate a small-for-gestational-age (SGA) neonate versus an IUGR neonate. One of the major benefits of doppler studies, especially of the umbilical artery, is that abnormal umbilical artery Dopplers can be used to differentiate between pathologic IUGR and a small-for-gestational-age neonate, thus guiding which pregnancy would require high-level surveillance versus routine monitoring.[9][14]
One of the major benefits of doppler studies, especially of the umbilical artery, is that abnormal umbilical artery Dopplers can be used to differentiate between pathologic IUGR and a small-for-gestational-age neonate, thus guiding which pregnancy would require high-level surveillance versus routine monitoring.[9][14]
When the mean estimated fetal weight is below the 10th percentile for a specific gestational age, that fetus is SGA. Conversely, a fetus that is IUGR will not be able to achieve normal growth usually secondary to placental insufficiency, a genetic disorder, or infection.[14] Therefore, an SGA neonate will be physically small but overall healthy versus an IUGR neonate who may also be physically small but will likely have its health compromised.
In considering differential diagnoses that contribute to placental insufficiency there are known associated diseases, including but not limited to preeclampsia, maternal hypertensive disorders as these both interfere with placental resistance and uteroplacental blood flow. Other illnesses to consider include oligohydramnios and maternal malnutrition or calorie restriction.
Other illnesses to consider include oligohydramnios and maternal malnutrition or calorie restriction.
Prognosis
If a neonate suffered from IUGR as a consequence of placental insufficiency and survives the perinatal period, they are at higher risk compared to a non-growth restricted neonate for developing cognitive deficits in childhood, including cerebral palsy and seizure disorders. Patients who suffer from placental insufficiency often have abnormal umbilical artery doppler flow velocity waveforms (DFVWs), and when one study compared DFVWs of infants who experienced placental insufficiency in-utero versus those infants who did not, they found that the infants who had had abnormal DFVWs also had lower IQs when they were 5-years-old. There is also evidence that suffering from IUGR as an infant predisposes to chronic illness as an adult, including increased risk for developing coronary artery disease, hypertension, and diabetes.[2]
In order to lead to a better prognosis for the neonate, the priority should be to focus on interventions that allow for maximizing gestational age at birth. It is estimated that for each week pregnancy is prolonged for fetuses between 24- and 28-weeks gestation, survival without associated sequelae is increased by approximately 10% to 15%.[3]
It is estimated that for each week pregnancy is prolonged for fetuses between 24- and 28-weeks gestation, survival without associated sequelae is increased by approximately 10% to 15%.[3]
Complications
The downstream effects of placental insufficiency on the developing fetus are complex and multifactorial; however, the major effects tend to be a placental respiratory failure and fetal hypoxemia, both of which contribute to intrauterine growth restriction and its associated effects including prematurity.[10][14]
The most detrimental complication is obviously a complete lack of placentation and thus miscarriage. For the developing fetus, the degree of umbilical artery abnormalities seen on doppler is correlated with acidosis, resuscitation requirements, pressor support, ventilatory support as well as multisystem organ failure, which tends to occur when there is hypoxemia as this triggers a redistribution of blood flow in the developing fetus to essential organs such the as the brain and heart at the expense of other pertinent organs, such as the bowels and kidneys. [14] Furthermore, when Doppler studies demonstrate absent or reversed end-diastolic flow, there is an increased frequency of intraventricular hemorrhage in the neonate.[9]
[14] Furthermore, when Doppler studies demonstrate absent or reversed end-diastolic flow, there is an increased frequency of intraventricular hemorrhage in the neonate.[9]
With increasing placental resistance during pregnancy, the already IUGR fetus is at further risk for hypoglycemia, hypoxic-ischemic encephalopathy, thrombocytopenia, leukopenia, and anemia.[8] Furthermore, there is evidence indicating that infants are at risk for developing cognitive deficits in childhood, and later developing chronic illness as an adult.
Deterrence and Patient Education
It is imperative that regular prenatal screening and ultrasound monitoring occur when a woman is pregnant to provide the best possible outcome for the neonate. Although there are no definitive preventive measures for placental insufficiency, once it has been recognized either via ultrasound, MRI, BPP, or any combination of these methods, interventions such as heparin therapy can be initiated with the hopes to prolong gestation with the hopes of minimizing acidosis and hypoxia and resultant IUGR, prematurity or fetal demise. Further studies need to be done on interventions and treatments, and when during pregnancy, they will provide the most benefit to the developing fetus.
Further studies need to be done on interventions and treatments, and when during pregnancy, they will provide the most benefit to the developing fetus.
Pearls and Other Issues
There is no definite cure for placental insufficiency, but the consequences can be minimized if diagnosed early, and the mother receives adequate prenatal care. Recent metanalysis revealed that heparin might promote fetal growth and prolong pregnancy if heparin is initiated in whom there is a high suspicion of placental insufficiency, but no benefit in reducing adverse outcomes in the neonates.
Enhancing Healthcare Team Outcomes
Based on Doppler studies, the earliest signs of failed placentation are likely not to be detected until at least after 12 weeks gestation, which correlates with a prospective cohort study that demonstrated screening placental function in high-risk pregnancies in the second versus first trimester was a better predictor for adverse perinatal outcomes.[15]
When discussing placental insufficiency, there are going to be at least two teams, if not more, contributing to the care of the patients, as one team will be responsible for monitoring the mother and the fetus prenatally, and another team that will care for the neonate post-natally. Managing both mother and an IUGR-baby requires an interdisciplinary team composed of Ob/Gyn’s, neonatal intensivists, both Ob and NICU nurses, respiratory therapists, pharmacists, pulmonologists, and sometimes other specialties including surgery, infectious disease, or genetics.
Managing both mother and an IUGR-baby requires an interdisciplinary team composed of Ob/Gyn’s, neonatal intensivists, both Ob and NICU nurses, respiratory therapists, pharmacists, pulmonologists, and sometimes other specialties including surgery, infectious disease, or genetics.
For the IUGR neonate, they often develop chronic lung disease secondary to their prematurity and may require multiple medications as well as home oxygen management, or they may develop multiple infections including necrotizing enterocolitis or late-onset sepsis, all of which requires coordination between the above-mentioned teams to provide improved outcomes for the patient. At times, depending on the outcome of the pregnancy, it will be important to involve an ethics committee or a palliative care team. Often it is easier for the families if family meetings can be organized where at least one member from each specialty team is present, as this improves communication between all teams and affords families the chance to ask their questions from all the specialists at once.
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
References
- 1.
Hunt K, Kennedy SH, Vatish M. Definitions and reporting of placental insufficiency in biomedical journals: a review of the literature. Eur J Obstet Gynecol Reprod Biol. 2016 Oct;205:146-9. [PubMed: 27591716]
- 2.
Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003 Sep 22;110 Suppl 1:S99-107. [PubMed: 12965097]
- 3.
Mazarico E, Molinet-Coll C, Martinez-Portilla RJ, Figueras F. Heparin therapy in placental insufficiency: Systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020 Feb;99(2):167-174. [PubMed: 31519033]
- 4.
Agarwal R, Tiwari A, Wadhwa N, Radhakrishnan G. Placental histopathological findings in preterm/term and early/late onset small for gestation age: Are they significant? Indian J Pathol Microbiol.
 2017 Apr-Jun;60(2):232-235. [PubMed: 28631641]
2017 Apr-Jun;60(2):232-235. [PubMed: 28631641]- 5.
Ohgiya Y, Nobusawa H, Seino N, Miyagami O, Yagi N, Hiroto S, Munechika J, Hirose M, Takeyama N, Ohike N, Matsuoka R, Sekizawa A, Gokan T. MR Imaging of Fetuses to Evaluate Placental Insufficiency. Magn Reson Med Sci. 2016;15(2):212-9. [PMC free article: PMC5600058] [PubMed: 26607809]
- 6.
Pintican D, Poienar AA, Strilciuc S, Mihu D. Effects of maternal smoking on human placental vascularization: A systematic review. Taiwan J Obstet Gynecol. 2019 Jul;58(4):454-459. [PubMed: 31307732]
- 7.
Audette MC, Kingdom JC. Screening for fetal growth restriction and placental insufficiency. Semin Fetal Neonatal Med. 2018 Apr;23(2):119-125. [PubMed: 29221766]
- 8.
Baschat AA, Harman CR, Gembruch U. Haematological consequences of placental insufficiency. Arch Dis Child Fetal Neonatal Ed. 2004 Jan;89(1):F94. [PMC free article: PMC1721655] [PubMed: 14711871]
- 9.
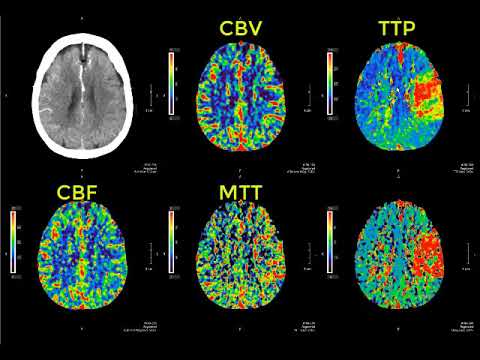
Harman CR, Baschat AA. Comprehensive assessment of fetal wellbeing: which Doppler tests should be performed? Curr Opin Obstet Gynecol. 2003 Apr;15(2):147-57. [PubMed: 12634607]
- 10.
Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004 Oct;111(10):1031-41. [PubMed: 15383103]
- 11.
Yousuf S, Ahmad A, Qadir S, Gul S, Tali SH, Shaheen F, Akhtar S, Dar R. Utility of Placental Laterality and Uterine Artery Doppler Abnormalities for Prediction of Preeclampsia. J Obstet Gynaecol India. 2016 Oct;66(Suppl 1):212-6. [PMC free article: PMC5016443] [PubMed: 27651606]
- 12.
Seyam YS, Al-Mahmeid MS, Al-Tamimi HK. Umbilical artery Doppler flow velocimetry in intrauterine growth restriction and its relation to perinatal outcome. Int J Gynaecol Obstet. 2002 May;77(2):131-7. [PubMed: 12031563]
- 13.
Alfirevic Z, Neilson JP. Doppler ultrasonography in high-risk pregnancies: systematic review with meta-analysis.
 Am J Obstet Gynecol. 1995 May;172(5):1379-87. [PubMed: 7755042]
Am J Obstet Gynecol. 1995 May;172(5):1379-87. [PubMed: 7755042]- 14.
Kalache KD, Dückelmann AM. Doppler in obstetrics: beyond the umbilical artery. Clin Obstet Gynecol. 2012 Mar;55(1):288-95. [PubMed: 22343245]
- 15.
Costa SL, Proctor L, Dodd JM, Toal M, Okun N, Johnson JA, Windrim R, Kingdom JC. Screening for placental insufficiency in high-risk pregnancies: is earlier better? Placenta. 2008 Dec;29(12):1034-40. [PubMed: 18930542]
Placental Insufficiency - StatPearls - NCBI Bookshelf
Continuing Education Activity
Placental insufficiency is a condition whereby there is a failure of placental vascular remodeling, leading to a failure of placentation resulting in acidosis and fetal hypoxemia. The most common downstream fetal consequences of this condition include intrauterine growth restriction, prematurity, or, unfortunately, fetal demise. In order to reduce the risk of fetal morbidity and mortality, especially in high-risk pregnancies, regular prenatal screening with Doppler ultrasound should be done to increase the chances of detection and diagnosis.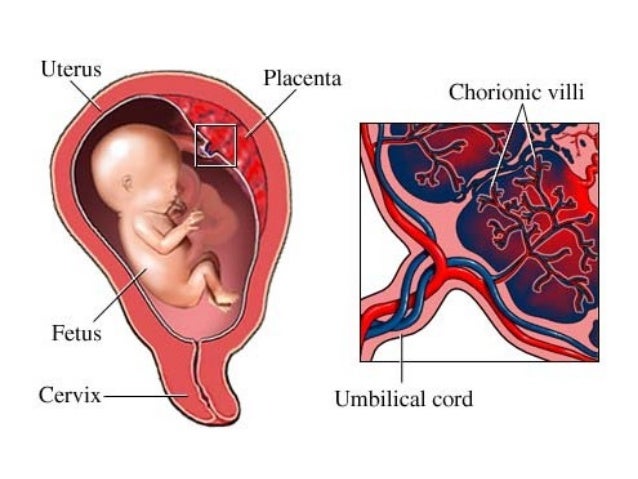 This activity reviews the pathophysiology, evaluation, and potential treatments of placental insufficiency, and highlights the role of the interprofessional team in managing patients with this condition.
This activity reviews the pathophysiology, evaluation, and potential treatments of placental insufficiency, and highlights the role of the interprofessional team in managing patients with this condition.
Objectives:
Review the risk factors associated with placental insufficiency.
Describe the typical imaging findings on Doppler ultrasound associated with placental insufficiency.
Identify the most common adverse events associated with placental insufficiency.
Access free multiple choice questions on this topic.
Introduction
Placental insufficiency is associated with various obstetric disorders such as pre-eclampsia and intrauterine growth restriction, both of which predispose to preterm labor, a leading cause of perinatal morbidity and mortality around the world. Poor placental function is most commonly described by the term ‘placental insufficiency’ within the medical community; however, one study highlighted the problem that there is no standardized definition or consensus for the pathognomonic features pertaining to placental insufficiency. [1]
[1]
This poses many challenges when it comes to studying placental insufficiency in the literature, but the general understanding is that placental insufficiency is a process whereby there is a progressive deterioration in placental functioning such that oxygen and nutrient transfer to the fetus via the placenta is decreased, culminating in a decompensated hypoxia and acidosis.[2][3] This process leads to fetal hypoxemia that then stimulates a downregulation of fetal metabolic demands to preserve what nutrients are already accessible, thus resulting in intrauterine fetal growth restriction. From a histopathologic view, placental insufficiency can be defined when there is chorionic villi fibrosis, uteroplacental thrombosis, placental infarcts, fibrin deposits, or a reduction in number and surface area of the villous capillary tree.[4]
Of note, placental infarcts can be a normal finding, as they are observed in approximately 25% of normal term pregnancies; however, increasing infarction of the placenta has been shown to be associated with placental insufficiency and thus intrauterine growth restriction (IUGR). Both MRI and ultrasound studies looking for placental insufficiency have demonstrated reductions in placental area and volume as well as increased placental thickness, in addition to globular shaped placentas on MRI.[5]
Both MRI and ultrasound studies looking for placental insufficiency have demonstrated reductions in placental area and volume as well as increased placental thickness, in addition to globular shaped placentas on MRI.[5]
Etiology
To date, the major etiologies that may lead to placental insufficiency are poorly understood and are still being studied. There are known associated maternal risk factors, which include pre-eclampsia or other maternal hypertensive disorders, maternal cigarette use, maternal drug use including cocaine or heroin, maternal alcohol consumption, primiparity, advanced maternal age, and prior history of delivery of IUGR neonate.[2][6]
Studies analyzing Doppler waveforms in various placental vessels of mothers who smoked cigarettes during pregnancy demonstrated reductions in the blood flow velocity waveforms, thus indicating the nicotine exposure can lead to altered placental vasculature.[6]
Any maternal condition that can lead to a compromise in the fetal circulation puts the fetus at risk for placental insufficiency. Additionally, certain medications such as antineoplastic, anticonvulsants, or anti-coagulants can interfere with fetal growth. Extremes of maternal body mass index, including maternal malnutrition, have also been associated with the development of IUGR neonates.[7] Studies of IUGR complicated pregnancies have demonstrated that there was an incomplete transformation of the placental vasculature early in the pregnancies that can be detected by doppler ultrasound studies.[5][8]
Additionally, certain medications such as antineoplastic, anticonvulsants, or anti-coagulants can interfere with fetal growth. Extremes of maternal body mass index, including maternal malnutrition, have also been associated with the development of IUGR neonates.[7] Studies of IUGR complicated pregnancies have demonstrated that there was an incomplete transformation of the placental vasculature early in the pregnancies that can be detected by doppler ultrasound studies.[5][8]
Epidemiology
Prematurity is the leading cause of perinatal death followed by intrauterine fetal growth restriction, which complicates approximately 4% to 6% of known pregnancies. Placental insufficiency is a potential cause of preterm labor, pre-eclampsia, IUGR, and stillbirth, which can affect 10 to 15% of pregnancies. For an IUGR fetus, the risk of spontaneous preterm labor is three-fold greater when compared to a non-growth restricted fetus, and there is also a five to six times higher risk for the development of perinatal death.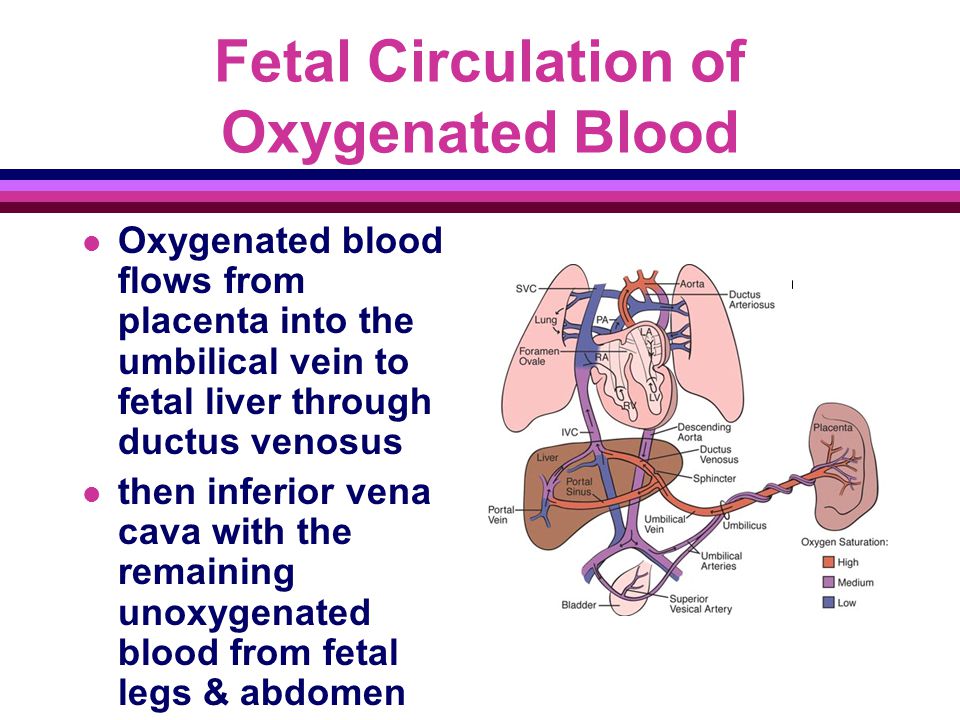 [2] Unfortunately, approximately 50% of newborns with IUGR are only detected following delivery.
[2] Unfortunately, approximately 50% of newborns with IUGR are only detected following delivery.
Pathophysiology
Although the underlying etiology for placental insufficiency is unknown, there are proposed mechanisms. Placental insufficiency is associated with a reduction in blood flow across the umbilicus to the fetus, which can be secondary to increased umbilical-placental vascular resistance. This increased resistance can be visualized as abnormal umbilical artery doppler flow velocity waveforms and can be secondary to an abnormality of villi insertion into the placental membrane, a perfusion abnormality between the umbilicus and placenta, or a reduction in uteroplacental blood flow.[2] Studies of umbilical artery Doppler have illustrated that the degree of placental injury is directly related to the degree of fetal injury during pregnancy.[9]
The main role of the placenta is to serve as the interface between fetal and maternal circulations. For this to occur, placental adherence and uterine arterial remodeling must be established in order to ensure nutrients can be delivered to the growing fetus.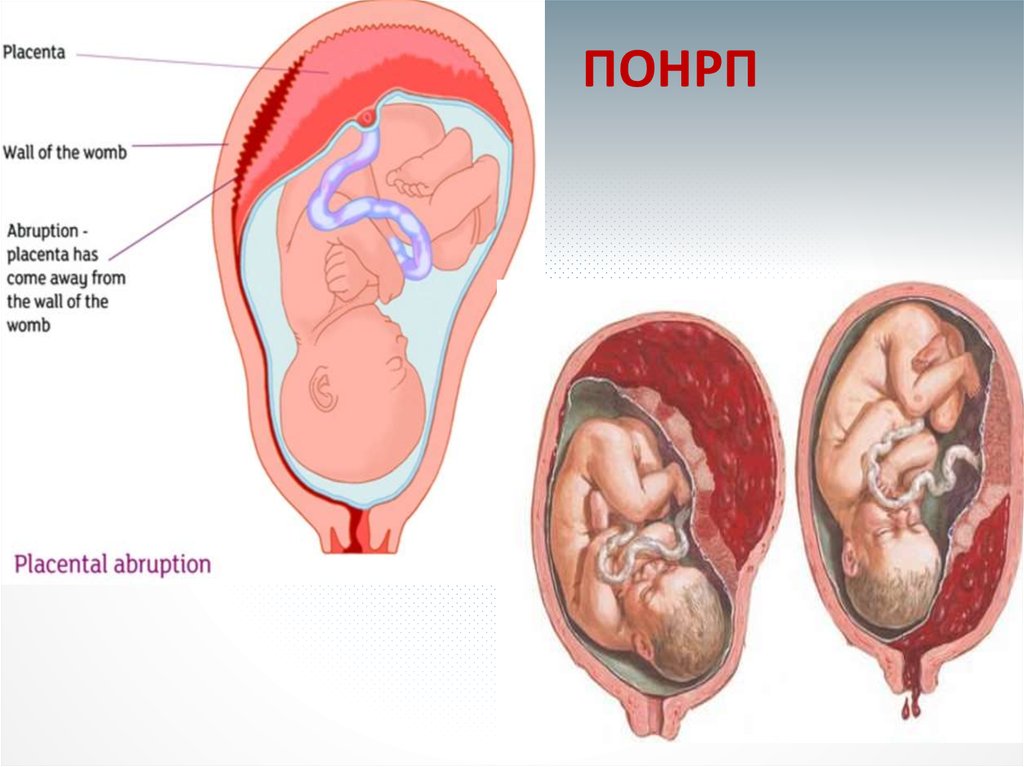 The hallmark for successful placentation is the remodeling of the uterine arteries. Following fertilization, the blastocyst forms, which is composed of an inner cell mass that will eventually become the fetus, and an outer shell called the trophoblast that becomes the fetal portion of the placenta. To aid with placental adherence, the cytotrophoblast, which is the inner layer of the trophoblast, secretes matrix metalloproteinases that breakdown the zona pellucida, and adherence is facilitated by the formation of anchoring villi and expression of adhesion molecules.[10] This invasion of the uteroplacental arteries allows for remodeling into dilated, low-resistance, inelastic vessels that lack maternal vasomotor control that leads to increased uteroplacental perfusion such as that the requirements of the fetus can be met.[5] Any disturbance in the remodeling process can lead to an increase in uteroplacental vascular resistance, leading to hypoperfusion of the placenta and its downstream effects, including coagulation activation, endothelial cell dysfunction, placental thrombosis, and fibrin deposits, which is associated with the development of IUGR.
The hallmark for successful placentation is the remodeling of the uterine arteries. Following fertilization, the blastocyst forms, which is composed of an inner cell mass that will eventually become the fetus, and an outer shell called the trophoblast that becomes the fetal portion of the placenta. To aid with placental adherence, the cytotrophoblast, which is the inner layer of the trophoblast, secretes matrix metalloproteinases that breakdown the zona pellucida, and adherence is facilitated by the formation of anchoring villi and expression of adhesion molecules.[10] This invasion of the uteroplacental arteries allows for remodeling into dilated, low-resistance, inelastic vessels that lack maternal vasomotor control that leads to increased uteroplacental perfusion such as that the requirements of the fetus can be met.[5] Any disturbance in the remodeling process can lead to an increase in uteroplacental vascular resistance, leading to hypoperfusion of the placenta and its downstream effects, including coagulation activation, endothelial cell dysfunction, placental thrombosis, and fibrin deposits, which is associated with the development of IUGR.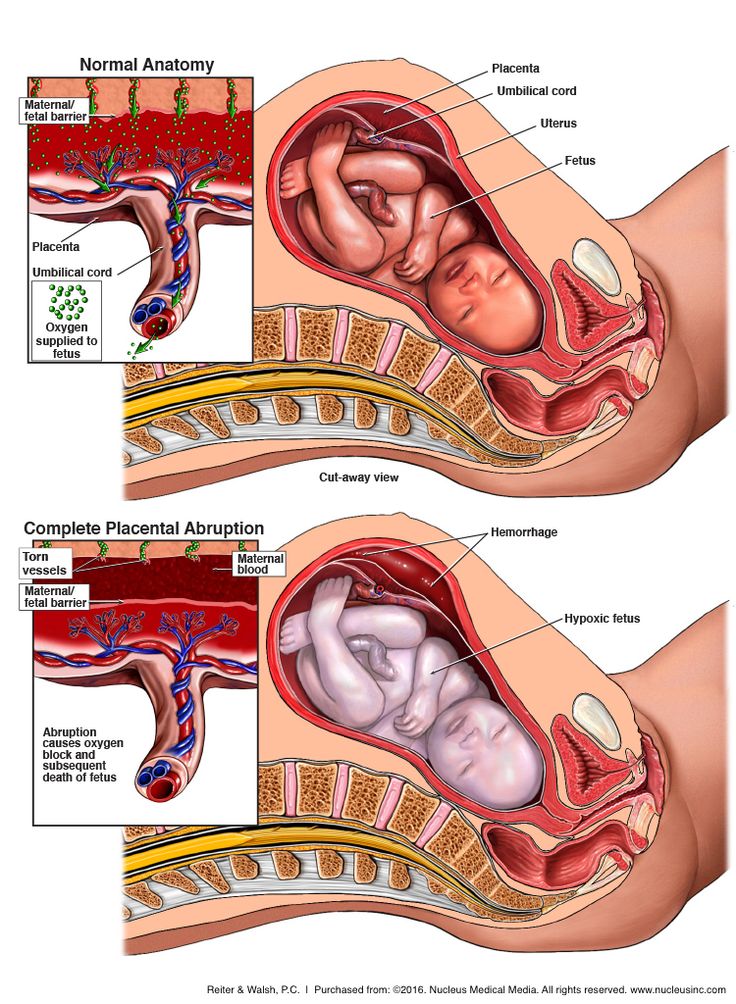 [5][3] Furthermore, if there is a loss of focal adhesion of the endovascular trophoblasts, a reduction in placental surface area can be seen associated with placental insufficiency. This reduction in the placental surface area, along with an increase in the thickness of the placenta, creates a globular appearance to the placenta, which is postulated to act as a compensatory mechanism for placental insufficiency.
[5][3] Furthermore, if there is a loss of focal adhesion of the endovascular trophoblasts, a reduction in placental surface area can be seen associated with placental insufficiency. This reduction in the placental surface area, along with an increase in the thickness of the placenta, creates a globular appearance to the placenta, which is postulated to act as a compensatory mechanism for placental insufficiency.
The intrauterine environment is a low-oxygen environment, and as such fetal circulation needs to be flexible in order to adapt to any changes that occur with uteroplacental function.[9] This hypoxic environment stimulates angiogenesis whereby vascular connections are made between the maternal circulation and the intervillous space. Now that a vascular and nutritional network of support has been established, the villous trophoblast is formed, which consists of maternal microvilli and a fetal basal layer.[10] To maintain placental function requires large amounts of energy, indicated by the fact that in a regular physiologic state, the placenta consumes approximately 70% of the glucose and 40% of the oxygen that is normally supplied to the uterus.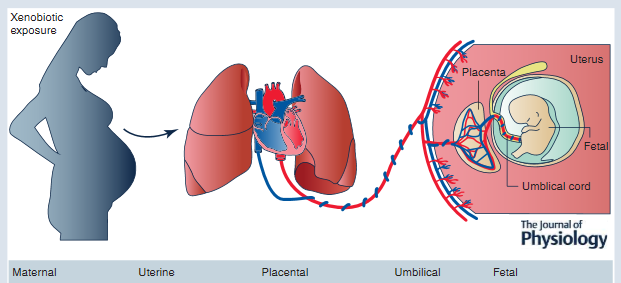 Therefore, in order to achieve optimal fetal growth and development, nutrient delivery to the uterus must exceed the placental demand such that there are residual nutrients for fetal utilization.[10] Therefore, any compromise of nutrient delivery to the uterus impacts nutrient delivery to the fetus.
Therefore, in order to achieve optimal fetal growth and development, nutrient delivery to the uterus must exceed the placental demand such that there are residual nutrients for fetal utilization.[10] Therefore, any compromise of nutrient delivery to the uterus impacts nutrient delivery to the fetus.
Successful placentation can be negatively affected by lateralization, which is when the placental invasion favors one side, and the placenta is not implanted centrally. If the placenta remains asymmetric until term, doppler ultrasound will demonstrate persistent notching on the non-implanted side, leading to a relative placental insufficiency. Placental lateralization has also been associated with increased risk for the development of maternal pre-eclampsia, and thus downstream placental insufficiency.[9][11]
History and Physical
As intrauterine growth restriction is one of the primary outcomes associated with placental insufficiency, most neonates that survive present either prematurely and are known as ‘extremely low birth weight’ (ELBW) neonates or depending on when the insult to the vascular supply occurred, they may present with altered body proportions at birth.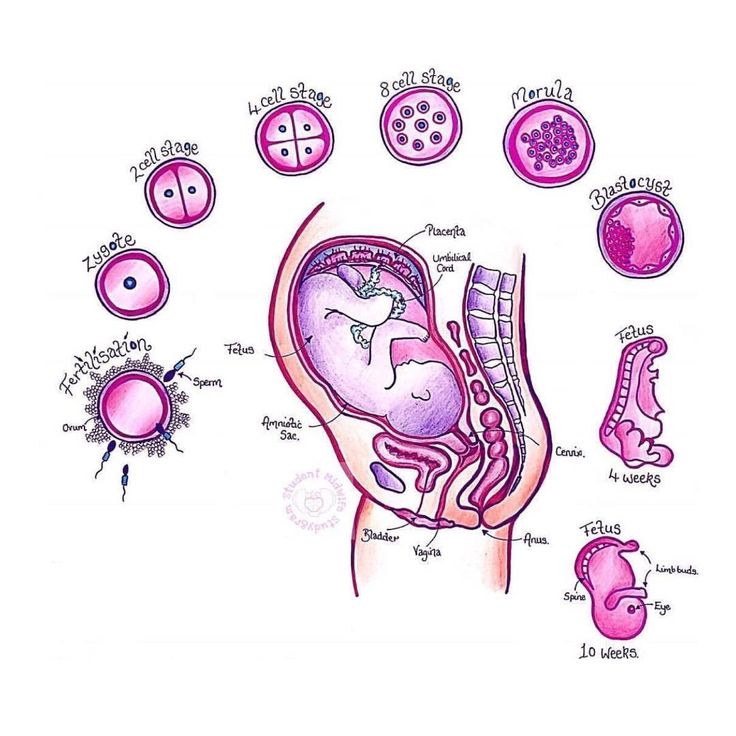 [10] If there is a failure of placentation, downstream effects such as IUGR are clinically evident on Doppler studies at approximately 26 weeks gestation.[9]
[10] If there is a failure of placentation, downstream effects such as IUGR are clinically evident on Doppler studies at approximately 26 weeks gestation.[9]
When there is a concern for IUGR, whether it is secondary to placental insufficiency or another etiology, the fetus will benefit from frequent combined monitoring using both Doppler and biophysical profile screens. This combination of screening techniques allows for the recognition of the declining performance observed when IUGR is related to placental problems. Utilizing doppler studies, failed placentation can be visualized by presenting of notching in the uterine arteries as well as increased resistance in the umbilical artery with progression to either absent- or reversed end-diastolic volumes. One of the first signs seen on a biophysical profile when there is a concern for fetal compromise is a non-reactive fetal heart rate tracing. The following will either be poor or loss of fetal body movements, breathing movements, and tone. These features of declining doppler status and poor biophysical profiles are typical when there is fetal distress, which is common when there is placental insufficiency and may indicate that delivery is required.
These features of declining doppler status and poor biophysical profiles are typical when there is fetal distress, which is common when there is placental insufficiency and may indicate that delivery is required.
Evaluation
Currently, the criteria to diagnose placental insufficiency lacks as there are also no standardized methods for diagnosis. Part of the problem is due to the wide variety of terminology used to describe what is known as ‘placental insufficiency.’[1] However, as technology has improved, Doppler ultrasound has proven to be a useful tool to aid in the evaluation of fetal and placental circulations in both healthy and diseased states. Four doppler techniques have been found to be fundamental in providing useful information regarding fetal-maternal circulation, including umbilical artery studies, uterine artery studies, middle cerebral artery studies, and ductus venosus studies.[9] As a fetus continues to mature during gestation towards term, there are many circulatory changes that occur that can be evaluated by Doppler ultrasound.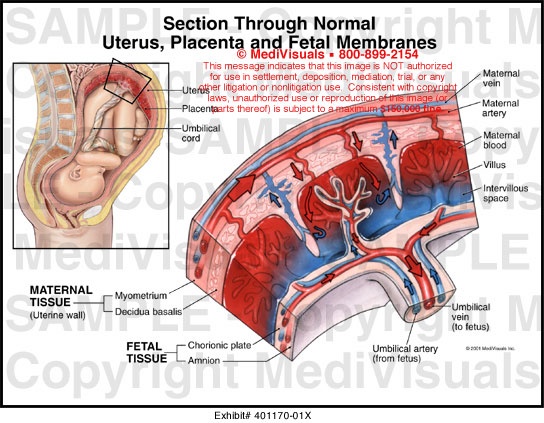
Prior to the onset of pregnancy, uterine arteries show low diastolic flow, high resistance, and elastic recoil noted in the form of early diastolic notches. Successful placentation involves removal of the intimal muscle from the vasculature such that the blood vessels will have a vigorous diastolic flow, minimal resistance, and no elastic properties. When placentation is successful, Doppler ultrasound demonstrates that the remodeling occurs rapidly, such that by 12 weeks gestation, there is a loss of notching, and resistance is low by 20 weeks gestation or earlier. When there is a failure of placentation, notching persists, and resistance remains high, which has shown to be correlated with maternal-hypertensive related fetal complications, including IUGR, pre-eclampsia, and fetal demise.[9] Using the uterine artery for Doppler screening to evaluate for notching and resistance to help identify these high-risk situations has been shown to be approximately 85% sensitive for detecting severe IUGR and pre-eclampsia.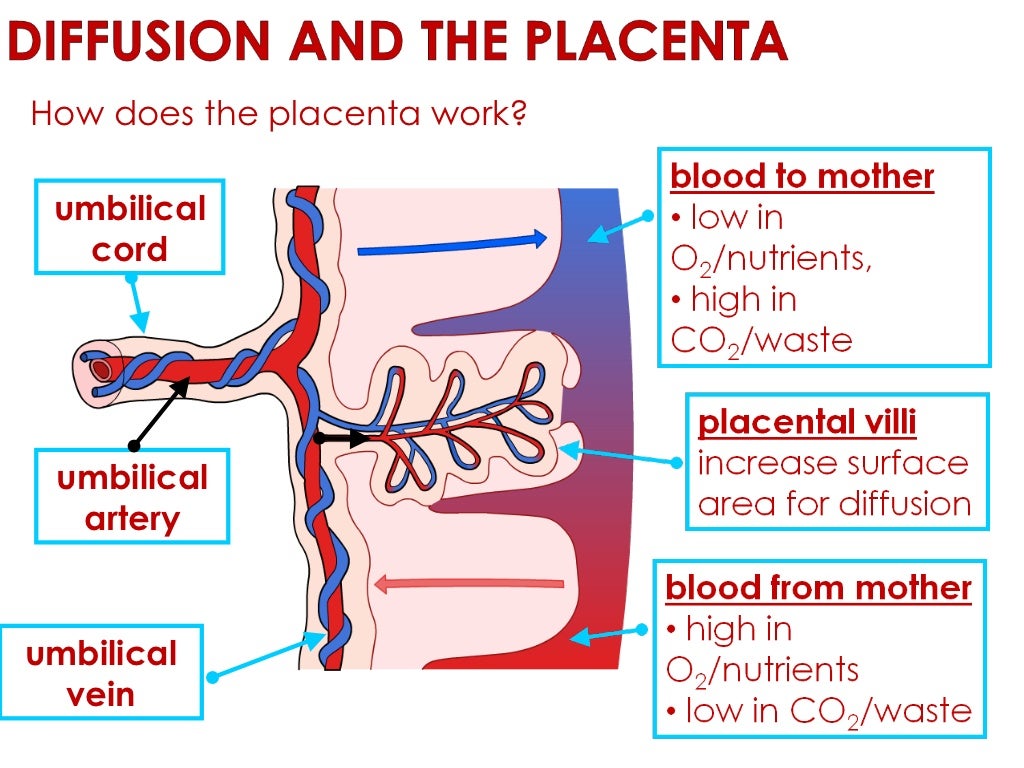
As resistance in the placenta increases, doppler studies of the umbilical artery can demonstrate either normal, reduced, absent, or reversed end-diastolic velocities.[12][8] It is normal for placental resistance to be high in the early stages of pregnancy, and therefore it can be expected that there will be absent end-diastolic velocity on Doppler studies up to 12 to 14 weeks of gestation. When the placental successfully invades, resistance drops and doppler studies of the umbilical artery should demonstrate continuous flow by 14 to 18 weeks of gestation.[9] Persistent umbilical artery resistance over the duration of pregnancy is an indicator of increased risk of placental insufficiency.
While umbilical artery Doppler studies provide important information pertaining to possible placental compromise, a valuable adjunct is to also use the middle cerebral artery (MCA) Doppler. The MCA provides information regarding systemic circulatory responses in the developing fetus, as it represents downstream resistance in the cerebral microcirculation.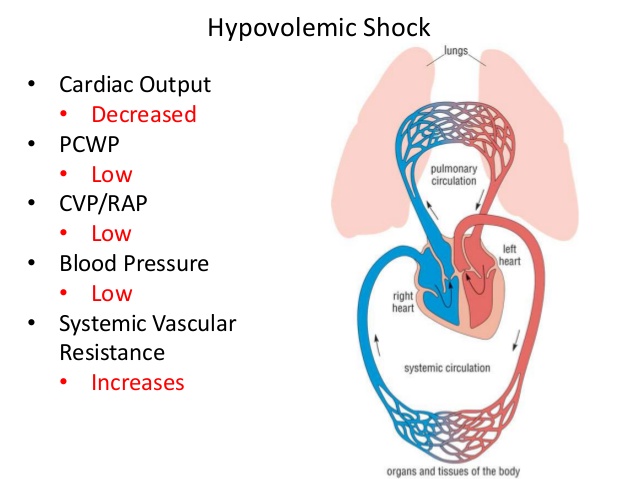 The normal MCA Doppler displays high resistance throughout pregnancy; however, the placental disease can be identified when there is increased diastolic flow along with declining pulsatility index. Therefore, MCA Doppler studies can provide important additional information when there is a concern for severe IUGR, indicating that there may be fetal compromise and intervention may be required.
The normal MCA Doppler displays high resistance throughout pregnancy; however, the placental disease can be identified when there is increased diastolic flow along with declining pulsatility index. Therefore, MCA Doppler studies can provide important additional information when there is a concern for severe IUGR, indicating that there may be fetal compromise and intervention may be required.
Another form of Doppler that provides insight into placental and fetal health is the venous Doppler, which relays information pertaining to cardiac data when the fetal circulation is experiencing stress. The venous waveform that has been shown to provide the best clinical data is the ductus venosus. There are many benefits of using the ductus venosus over other venous waveforms, which include its responsiveness to oxygenation changes, it is one of the primary regulators of venous return in abnormal and normal fetal circulations, it is independent of cardiac function, it serves as a direct conduit to view right atrial retrograde pulse waves, and lastly, because it has elevated and focal velocity color Doppler signal from as early as 12 weeks gestation up to 40 weeks gestation, it is very easily imaged. [9] If an abnormal ductus venosus or retrograde atrial-wave is visualized on Doppler at around 12 to 14 weeks gestation, there is an increased risk for fetal cardiac abnormalities, and it also serves as a possible precursor indicating severe placental-based IUGR.
[9] If an abnormal ductus venosus or retrograde atrial-wave is visualized on Doppler at around 12 to 14 weeks gestation, there is an increased risk for fetal cardiac abnormalities, and it also serves as a possible precursor indicating severe placental-based IUGR.
MRI imaging has been found to provide additional information in order to detect and diagnose placental insufficiency. Flow voids occur when there is a loss of MRI signal observed in a blood vessel where blood flows vigorously. When using T2-weighted Rapid Acquisition with Relaxation Enhancement (RARE) imaging, placental insufficiency can be detected when decreased flow voids are observed between the placenta and the uterus, as this can be considered to reflect a reduction in uteroplacental perfusion.[5] An additional advantage of MRI imaging is that it provides high soft-tissue contrast. Therefore placental vascular abnormalities, including hemorrhages and infarctions, can be detected on placental-MRI, indicating high risk for placental insufficiency and downstream IUGR.
Treatment / Management
Currently, there is no known treatment for placental insufficiency, other than delivering the fetus if it is at a viable timepoint. Low-dose aspirin and the use of antioxidant therapies, including vitamins C and E, have been shown to promote improved placentation in cases where there is uncertainty for successful placentation.[9] Studies have demonstrated that there is an approximate 38% reduction in perinatal mortality when early Doppler ultrasound is used for cases of suspected IUGR during pregnancy.[12][13]
High-risk women, such as those with chronic hypertension, coagulopathies, or a history of pre-eclampsia, can benefit from having Doppler ultrasound screening at 12 to 14 weeks gestation because if bilateral notching is evident, then low-dose aspirin therapy should be initiated.
In vitro studies have demonstrated that heparin can stimulate neo-angiogenesis and also improve placental perfusion. Heparin’s anticoagulant properties are exhibited by its ability to mobilize tissue inhibition factor into circulation and by the fact that it also enhances antithrombin activity. Additional benefits of heparin when considering placental insufficiency and its downstream consequences are that heparin promotes trophoblastic proliferation, reduces inflammation by downregulating the complement cascade, reduces apoptosis, and it also acts indirectly as a growth factor.[3]
Additional benefits of heparin when considering placental insufficiency and its downstream consequences are that heparin promotes trophoblastic proliferation, reduces inflammation by downregulating the complement cascade, reduces apoptosis, and it also acts indirectly as a growth factor.[3]
Heparin has also been shown to upregulate certain proteins involved in placental angiogenesis and development. These include angiopoietin-2, which is responsible for chorionic villi vascular remodeling, leptin, which is responsible for the regulation of nutrient transfer, as well as vascular endothelial growth factor receptor-3, tissue inhibitor matrix metalloprotease-1, tumor necrosis factor-alpha, and angiostatin. Based on its properties and preliminary data, some studies have demonstrated that there may be a role for heparin as a preventive treatment for the placental disease.
Differential Diagnosis
There are many factors that can contribute to placental insufficiency, and it is known that IUGR is a major downstream complication; however, it can be difficult at times to differentiate a small-for-gestational-age (SGA) neonate versus an IUGR neonate. One of the major benefits of doppler studies, especially of the umbilical artery, is that abnormal umbilical artery Dopplers can be used to differentiate between pathologic IUGR and a small-for-gestational-age neonate, thus guiding which pregnancy would require high-level surveillance versus routine monitoring.[9][14]
One of the major benefits of doppler studies, especially of the umbilical artery, is that abnormal umbilical artery Dopplers can be used to differentiate between pathologic IUGR and a small-for-gestational-age neonate, thus guiding which pregnancy would require high-level surveillance versus routine monitoring.[9][14]
When the mean estimated fetal weight is below the 10th percentile for a specific gestational age, that fetus is SGA. Conversely, a fetus that is IUGR will not be able to achieve normal growth usually secondary to placental insufficiency, a genetic disorder, or infection.[14] Therefore, an SGA neonate will be physically small but overall healthy versus an IUGR neonate who may also be physically small but will likely have its health compromised.
In considering differential diagnoses that contribute to placental insufficiency there are known associated diseases, including but not limited to preeclampsia, maternal hypertensive disorders as these both interfere with placental resistance and uteroplacental blood flow. Other illnesses to consider include oligohydramnios and maternal malnutrition or calorie restriction.
Other illnesses to consider include oligohydramnios and maternal malnutrition or calorie restriction.
Prognosis
If a neonate suffered from IUGR as a consequence of placental insufficiency and survives the perinatal period, they are at higher risk compared to a non-growth restricted neonate for developing cognitive deficits in childhood, including cerebral palsy and seizure disorders. Patients who suffer from placental insufficiency often have abnormal umbilical artery doppler flow velocity waveforms (DFVWs), and when one study compared DFVWs of infants who experienced placental insufficiency in-utero versus those infants who did not, they found that the infants who had had abnormal DFVWs also had lower IQs when they were 5-years-old. There is also evidence that suffering from IUGR as an infant predisposes to chronic illness as an adult, including increased risk for developing coronary artery disease, hypertension, and diabetes.[2]
In order to lead to a better prognosis for the neonate, the priority should be to focus on interventions that allow for maximizing gestational age at birth. It is estimated that for each week pregnancy is prolonged for fetuses between 24- and 28-weeks gestation, survival without associated sequelae is increased by approximately 10% to 15%.[3]
It is estimated that for each week pregnancy is prolonged for fetuses between 24- and 28-weeks gestation, survival without associated sequelae is increased by approximately 10% to 15%.[3]
Complications
The downstream effects of placental insufficiency on the developing fetus are complex and multifactorial; however, the major effects tend to be a placental respiratory failure and fetal hypoxemia, both of which contribute to intrauterine growth restriction and its associated effects including prematurity.[10][14]
The most detrimental complication is obviously a complete lack of placentation and thus miscarriage. For the developing fetus, the degree of umbilical artery abnormalities seen on doppler is correlated with acidosis, resuscitation requirements, pressor support, ventilatory support as well as multisystem organ failure, which tends to occur when there is hypoxemia as this triggers a redistribution of blood flow in the developing fetus to essential organs such the as the brain and heart at the expense of other pertinent organs, such as the bowels and kidneys. [14] Furthermore, when Doppler studies demonstrate absent or reversed end-diastolic flow, there is an increased frequency of intraventricular hemorrhage in the neonate.[9]
[14] Furthermore, when Doppler studies demonstrate absent or reversed end-diastolic flow, there is an increased frequency of intraventricular hemorrhage in the neonate.[9]
With increasing placental resistance during pregnancy, the already IUGR fetus is at further risk for hypoglycemia, hypoxic-ischemic encephalopathy, thrombocytopenia, leukopenia, and anemia.[8] Furthermore, there is evidence indicating that infants are at risk for developing cognitive deficits in childhood, and later developing chronic illness as an adult.
Deterrence and Patient Education
It is imperative that regular prenatal screening and ultrasound monitoring occur when a woman is pregnant to provide the best possible outcome for the neonate. Although there are no definitive preventive measures for placental insufficiency, once it has been recognized either via ultrasound, MRI, BPP, or any combination of these methods, interventions such as heparin therapy can be initiated with the hopes to prolong gestation with the hopes of minimizing acidosis and hypoxia and resultant IUGR, prematurity or fetal demise. Further studies need to be done on interventions and treatments, and when during pregnancy, they will provide the most benefit to the developing fetus.
Further studies need to be done on interventions and treatments, and when during pregnancy, they will provide the most benefit to the developing fetus.
Pearls and Other Issues
There is no definite cure for placental insufficiency, but the consequences can be minimized if diagnosed early, and the mother receives adequate prenatal care. Recent metanalysis revealed that heparin might promote fetal growth and prolong pregnancy if heparin is initiated in whom there is a high suspicion of placental insufficiency, but no benefit in reducing adverse outcomes in the neonates.
Enhancing Healthcare Team Outcomes
Based on Doppler studies, the earliest signs of failed placentation are likely not to be detected until at least after 12 weeks gestation, which correlates with a prospective cohort study that demonstrated screening placental function in high-risk pregnancies in the second versus first trimester was a better predictor for adverse perinatal outcomes.[15]
When discussing placental insufficiency, there are going to be at least two teams, if not more, contributing to the care of the patients, as one team will be responsible for monitoring the mother and the fetus prenatally, and another team that will care for the neonate post-natally. Managing both mother and an IUGR-baby requires an interdisciplinary team composed of Ob/Gyn’s, neonatal intensivists, both Ob and NICU nurses, respiratory therapists, pharmacists, pulmonologists, and sometimes other specialties including surgery, infectious disease, or genetics.
Managing both mother and an IUGR-baby requires an interdisciplinary team composed of Ob/Gyn’s, neonatal intensivists, both Ob and NICU nurses, respiratory therapists, pharmacists, pulmonologists, and sometimes other specialties including surgery, infectious disease, or genetics.
For the IUGR neonate, they often develop chronic lung disease secondary to their prematurity and may require multiple medications as well as home oxygen management, or they may develop multiple infections including necrotizing enterocolitis or late-onset sepsis, all of which requires coordination between the above-mentioned teams to provide improved outcomes for the patient. At times, depending on the outcome of the pregnancy, it will be important to involve an ethics committee or a palliative care team. Often it is easier for the families if family meetings can be organized where at least one member from each specialty team is present, as this improves communication between all teams and affords families the chance to ask their questions from all the specialists at once.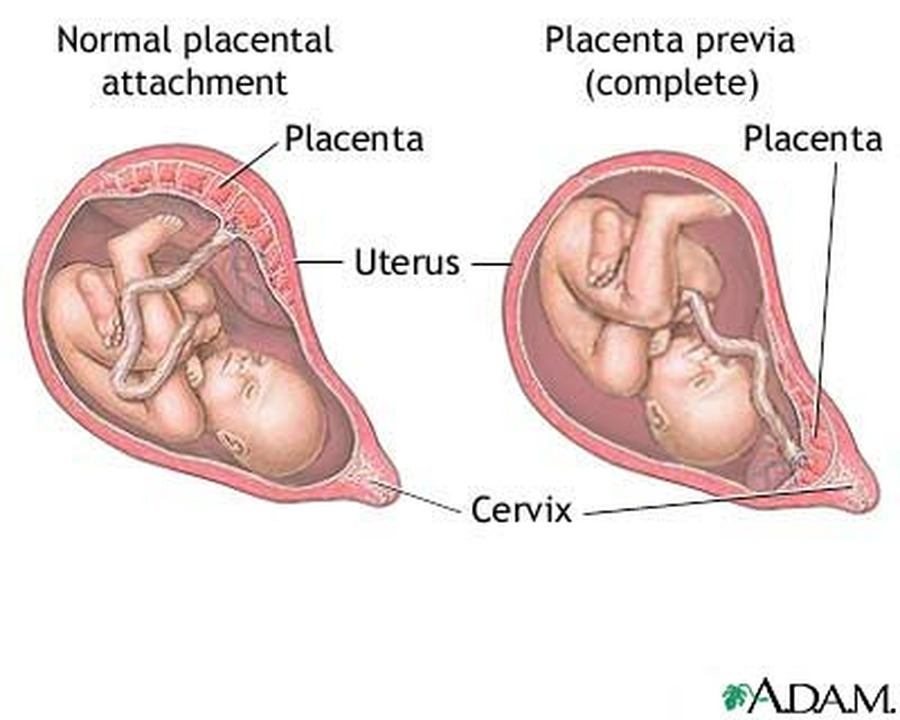
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
References
- 1.
Hunt K, Kennedy SH, Vatish M. Definitions and reporting of placental insufficiency in biomedical journals: a review of the literature. Eur J Obstet Gynecol Reprod Biol. 2016 Oct;205:146-9. [PubMed: 27591716]
- 2.
Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003 Sep 22;110 Suppl 1:S99-107. [PubMed: 12965097]
- 3.
Mazarico E, Molinet-Coll C, Martinez-Portilla RJ, Figueras F. Heparin therapy in placental insufficiency: Systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020 Feb;99(2):167-174. [PubMed: 31519033]
- 4.
Agarwal R, Tiwari A, Wadhwa N, Radhakrishnan G. Placental histopathological findings in preterm/term and early/late onset small for gestation age: Are they significant? Indian J Pathol Microbiol.
 2017 Apr-Jun;60(2):232-235. [PubMed: 28631641]
2017 Apr-Jun;60(2):232-235. [PubMed: 28631641]- 5.
Ohgiya Y, Nobusawa H, Seino N, Miyagami O, Yagi N, Hiroto S, Munechika J, Hirose M, Takeyama N, Ohike N, Matsuoka R, Sekizawa A, Gokan T. MR Imaging of Fetuses to Evaluate Placental Insufficiency. Magn Reson Med Sci. 2016;15(2):212-9. [PMC free article: PMC5600058] [PubMed: 26607809]
- 6.
Pintican D, Poienar AA, Strilciuc S, Mihu D. Effects of maternal smoking on human placental vascularization: A systematic review. Taiwan J Obstet Gynecol. 2019 Jul;58(4):454-459. [PubMed: 31307732]
- 7.
Audette MC, Kingdom JC. Screening for fetal growth restriction and placental insufficiency. Semin Fetal Neonatal Med. 2018 Apr;23(2):119-125. [PubMed: 29221766]
- 8.
Baschat AA, Harman CR, Gembruch U. Haematological consequences of placental insufficiency. Arch Dis Child Fetal Neonatal Ed. 2004 Jan;89(1):F94. [PMC free article: PMC1721655] [PubMed: 14711871]
- 9.
Harman CR, Baschat AA. Comprehensive assessment of fetal wellbeing: which Doppler tests should be performed? Curr Opin Obstet Gynecol. 2003 Apr;15(2):147-57. [PubMed: 12634607]
- 10.
Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004 Oct;111(10):1031-41. [PubMed: 15383103]
- 11.
Yousuf S, Ahmad A, Qadir S, Gul S, Tali SH, Shaheen F, Akhtar S, Dar R. Utility of Placental Laterality and Uterine Artery Doppler Abnormalities for Prediction of Preeclampsia. J Obstet Gynaecol India. 2016 Oct;66(Suppl 1):212-6. [PMC free article: PMC5016443] [PubMed: 27651606]
- 12.
Seyam YS, Al-Mahmeid MS, Al-Tamimi HK. Umbilical artery Doppler flow velocimetry in intrauterine growth restriction and its relation to perinatal outcome. Int J Gynaecol Obstet. 2002 May;77(2):131-7. [PubMed: 12031563]
- 13.
Alfirevic Z, Neilson JP. Doppler ultrasonography in high-risk pregnancies: systematic review with meta-analysis.
 Am J Obstet Gynecol. 1995 May;172(5):1379-87. [PubMed: 7755042]
Am J Obstet Gynecol. 1995 May;172(5):1379-87. [PubMed: 7755042]- 14.
Kalache KD, Dückelmann AM. Doppler in obstetrics: beyond the umbilical artery. Clin Obstet Gynecol. 2012 Mar;55(1):288-95. [PubMed: 22343245]
- 15.
Costa SL, Proctor L, Dodd JM, Toal M, Okun N, Johnson JA, Windrim R, Kingdom JC. Screening for placental insufficiency in high-risk pregnancies: is earlier better? Placenta. 2008 Dec;29(12):1034-40. [PubMed: 18930542]
Placental insufficiency - what is it and how to treat it
- Types and causes of placental insufficiency
- Diagnosis of placental insufficiency
- Treatment of placental insufficiency
Most women know that the placenta connects mother and baby during pregnancy and provides nutrients and oxygen to the baby.
Are there situations when the placenta ceases to perform its function correctly and fully? Is it possible to somehow prevent this? nine0012
What is the function of the placenta
So, the placenta is an important organ that is formed only during pregnancy. The placenta is formed from the chorion - the embryonic membranes of the fetus. At the very beginning of pregnancy, chorionic villi - outgrowths of the membrane - evenly cover the entire surface of the fetal egg, starting from the second month of pregnancy, on one side of the fetal egg, the villi begin to lengthen, increase in size and form the placenta.
The placenta is formed from the chorion - the embryonic membranes of the fetus. At the very beginning of pregnancy, chorionic villi - outgrowths of the membrane - evenly cover the entire surface of the fetal egg, starting from the second month of pregnancy, on one side of the fetal egg, the villi begin to lengthen, increase in size and form the placenta.
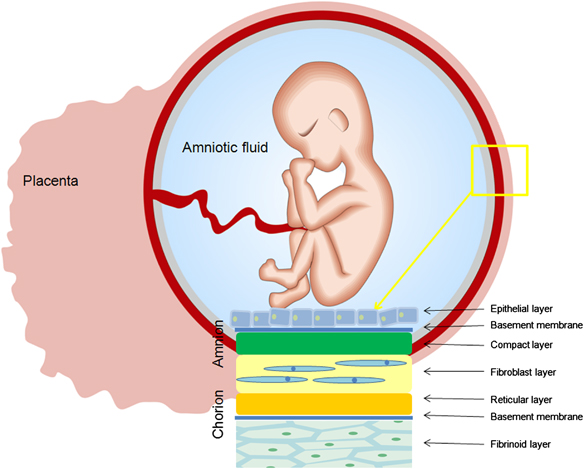
Inside the villi flows the blood of the baby, and outside they are bathed in the blood of the mother. Between the blood flow of mother and baby there is only one layer of cells, which plays the role of a barrier between the body of mother and child. Thanks to this membrane, the blood of the mother and fetus does not mix. nine0013
However, in recent years it has become known that fetal blood cells still penetrate the placental barrier into the mother's bloodstream, and thanks to this, it has become possible to conduct genetic analyzes and determine chromosomal abnormalities, Rh factor and fetal sex from the blood of a pregnant woman (non-invasive prenatal test) .
In the placenta, there is a constant exchange of substances between mother and child. Oxygen and nutrients are supplied from the mother's blood to the fetus, carbon dioxide and metabolic products from the fetus back to the mother, to be removed from the body. nine0013
The placental barrier performs an immunological function, as it allows some protective antibodies - blood cells that fight infectious agents, in addition, it is impervious to certain harmful substances, viruses and bacteria. Unfortunately, the placental barrier is easily overcome by drugs, alcohol, nicotine, components of many drugs and some viruses.
An important function of the placenta is the production of hormones and biologically active substances. First of all, these are hormones that are important for successful pregnancy, for example, chorionic gonadotropin, placental lactogen, estrogens, etc.
Unfortunately, things don't always go well. Due to a variety of reasons, deviations in the development and functioning of the placenta may occur at different stages of pregnancy. These changes never go unnoticed for mom and baby, and often have dire consequences.
These changes never go unnoticed for mom and baby, and often have dire consequences.
If the placenta ceases to perform its functions to the fullest, the so-called placental insufficiency develops. In fact, it consists in the deterioration of blood circulation in the mother-placenta-fetus system. nine0013
Types and causes of placental insufficiency
Doctors distinguish between acute and chronic placental insufficiency:
Acute placental insufficiency
This is a condition that requires urgent medical intervention. It is characterized by a rapid deterioration in placental blood flow. Acute placental insufficiency occurs mainly as a result of placental abruption or the death of certain areas of placental tissue, for example, during the formation of blood clots in the vessels. Abdominal trauma, antiphospholipid syndrome can serve as the cause of detachment. nine0013
Phospholipids are complex fats that are part of the membranes of all body cells. In some cases, the body's immune system produces a large number of antibodies to some of its own phospholipids and proteins that bind these lipids.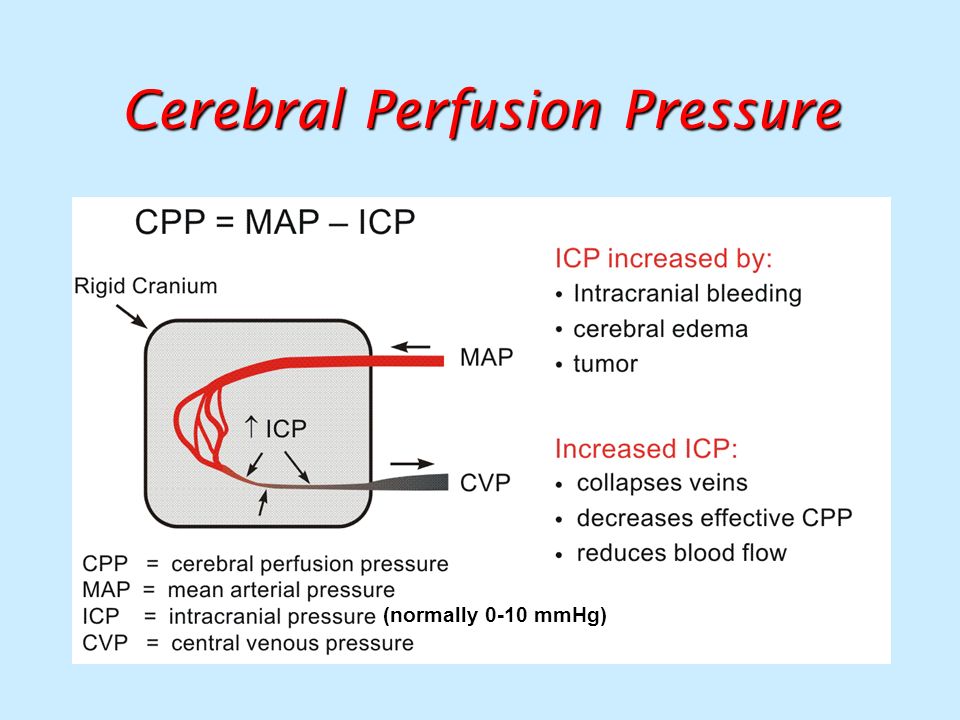 They are called antiphospholipid antibodies and, when interacting with the cells of the body, cause cell damage and activation of the blood coagulation system, which leads to thrombosis.
They are called antiphospholipid antibodies and, when interacting with the cells of the body, cause cell damage and activation of the blood coagulation system, which leads to thrombosis.
Antiphospholipid syndrome is the most common cause of thrombotic complications in pregnancy, including placental abruption and acute placental insufficiency. nine0047 A severe course of gestosis, a formidable complication of the second half of pregnancy, manifested by edema, increased pressure and the appearance of protein in the urine, can also cause placental abruption.
Acute placental insufficiency develops when more than 2/3 of the placental surface is detached.
In case of acute placental insufficiency, it is necessary to perform a caesarean section as soon as possible to save the life of the baby and mother.
Chronic placental insufficiency
Chronic placental insufficiency is much more common in pregnant women. In this case, there is a violation of the formation and maturation of the placenta, the uteroplacental and fetal-placental blood flow decreases, gas exchange and metabolism in the placenta are limited, and the synthesis of placental hormones decreases. All these changes determine the insufficient supply of oxygen and nutrients to the baby, cause a delay in the growth and development of the fetus.
All these changes determine the insufficient supply of oxygen and nutrients to the baby, cause a delay in the growth and development of the fetus.
The most common causes of placental insufficiency are previous abortions, especially surgical abortion during the first pregnancy, smoking, while the number and strength of cigarettes smoked do not matter, since tobacco smoke, not nicotine, has a negative effect on the formation of defective placental vessels. nine0013
The risk group for the development of placental insufficiency also includes women with chronic diseases such as arterial hypertension, iron deficiency anemia, pyelonephritis, diabetes, thyroid disease.
In recent years, there has been a significant increase in placental insufficiency caused by bacteria, viruses, fungi. The reason for this can be both an acute infection suffered by the expectant mother during pregnancy, and the activation of a chronic infectious process in the body of a pregnant woman. nine0013
Of no small importance in the formation of chronic placental insufficiency is the pathology of the uterus: endometriosis, malformations of the uterus (saddle-shaped, bicornuate). Doctors also consider uterine fibroids to be a risk factor. Of course, a number of drugs have an adverse effect on the formation of the placenta and the development of the fetus. Currently, a list of drugs that are not approved for use during pregnancy has been defined.
Doctors also consider uterine fibroids to be a risk factor. Of course, a number of drugs have an adverse effect on the formation of the placenta and the development of the fetus. Currently, a list of drugs that are not approved for use during pregnancy has been defined.
Also of great importance in the development of placental insufficiency is thrombophilia - an increased tendency of the body to form blood clots - blood clots in the vessels. nine0013
In some cases, placental insufficiency may be due to the presence of chromosomal abnormalities in the fetus, in particular in Down syndrome (the presence of an additional chromosome 21 in the fetus) or Edwards syndrome (an additional chromosome 18 in the fetus), placental dysfunction is diagnosed already in the early stages of pregnancy.
It should be noted that among the complications of pregnancy, most often leading to the development of chronic placental insufficiency, a significant factor is preeclampsia (or late preeclampsia) - a complication of the second half of pregnancy, manifested by edema, increased pressure and the appearance of protein in the urine. nine0047 Regardless of the factors contributing to the development of placental insufficiency, it is based on circulatory disorders in the uterine-placental complex, leading to disruption of all functions of the placenta. Consequently, the symptoms of chronic placental insufficiency will be due to a lack of oxygen and nutrients to the fetus.
nine0047 Regardless of the factors contributing to the development of placental insufficiency, it is based on circulatory disorders in the uterine-placental complex, leading to disruption of all functions of the placenta. Consequently, the symptoms of chronic placental insufficiency will be due to a lack of oxygen and nutrients to the fetus.
This is, first of all, intrauterine fetal growth retardation - a lag in the size of the fetus and a slowdown in its growth. Often there is a change in the motor activity of the fetus. At first there may be some increase in movements, and then a decrease. Violation of the protective function of the placenta leads to intrauterine infection of the fetus under the action of pathogenic (pathogenic) microorganisms penetrating the placenta. The fetus, the development of which occurs in conditions of placental insufficiency, is much more at risk of trauma during childbirth, they have a violation of adaptation to extrauterine life, increased morbidity in the first year of life. nine0013
nine0013
According to the time of occurrence, doctors divide placental insufficiency into early and late.
Early (or primary) placental insufficiency
Develops before 16 weeks of gestation. It occurs already at the stage of placenta formation and is associated with diseases of a pregnant woman that are present before pregnancy, for example, with uterine pathology, chronic arterial hypertension, and endocrinological diseases. In this case, the formation of defective vessels in the placenta occurs. nine0013
Late (or secondary) placental insufficiency
Occurs after 16 weeks of pregnancy and is most often associated with diseases that have already occurred during pregnancy. Most often, these are iron deficiency anemia (that is, a decrease in the concentration of hemoglobin and iron in the blood), gestational diabetes mellitus (that is, a violation of the absorption of glucose by the body that occurred during pregnancy), past viral and bacterial infections.
It is important to subdivide placental insufficiency into compensated and decompensated forms. nine0013
Compensated placental insufficiency
It develops, for example, with the threat of abortion and mild forms of late preeclampsia, if these complications are successfully amenable to medical correction.
Decompensated placental insufficiency
Causes the development of fetal growth retardation, chronic intrauterine hypoxia, up to fetal death.
Learn more about services:
- Ultrasound for pregnant women
- Ultrasound of the 1st trimester of pregnancy
- Ultrasound of vessels during pregnancy
- Consultation with a gynecologist
Diagnosis of placental insufficiency
It is almost impossible to treat an already developed placental insufficiency, so doctors actively seek to identify pregnant women who are at risk of developing placental dysfunction. If placental insufficiency is detected in the 3rd trimester of pregnancy, there is no effective treatment, unfortunately. Therefore, all methods of identifying in the early stages of pregnancy those women whose formation of the placenta has been disturbed are being very actively used. nine0013
Therefore, all methods of identifying in the early stages of pregnancy those women whose formation of the placenta has been disturbed are being very actively used. nine0013
First of all, when registering for pregnancy, the most significant risk factors are identified - smoking, abortions, aggravated heredity (low birth weight, tendency to thrombosis), the presence of chronic diseases of the heart, blood vessels, diabetes mellitus.
Preventive measures against the development of placental insufficiency are especially relevant and necessary until 16-17 weeks of pregnancy, when the formation of placental structures occurs.
Significant assistance in assessing the risk of developing placental insufficiency is provided by prenatal screening, which is carried out at 11-14 weeks of pregnancy. It is carried out to detect Down syndrome, Edwards syndrome and other chromosomal diseases in the fetus. Currently, the most relevant is to conduct a comprehensive early screening of a pregnant woman to predict the risk of developing placental insufficiency, preeclampsia and intrauterine growth retardation. Since this type of diagnostics is one of the most modern and advanced, unfortunately, it is not yet included in the list of services provided in the antenatal clinic within the framework of compulsory medical insurance, but is available to everyone in prenatal diagnostic centers. nine0013
Since this type of diagnostics is one of the most modern and advanced, unfortunately, it is not yet included in the list of services provided in the antenatal clinic within the framework of compulsory medical insurance, but is available to everyone in prenatal diagnostic centers. nine0013
Determination of proteins produced by the placenta
First of all, the PAPP-A protein is determined, it is also a marker of chromosomal abnormalities of the fetus. A decrease in the concentration of PAPP-A in the blood at 11-14 weeks of gestation occurs in pregnant women who have a high risk of placental insufficiency and fetal growth retardation.
The second placental hormone that helps in assessing the risk of placental insufficiency is PIGF (placental growth factor). Its concentration in the blood decreases long before the first manifestations of placental insufficiency. Its definition is not as widely used as PAPP-A, but many laboratories have already included this protein in 1st trimester prenatal screening.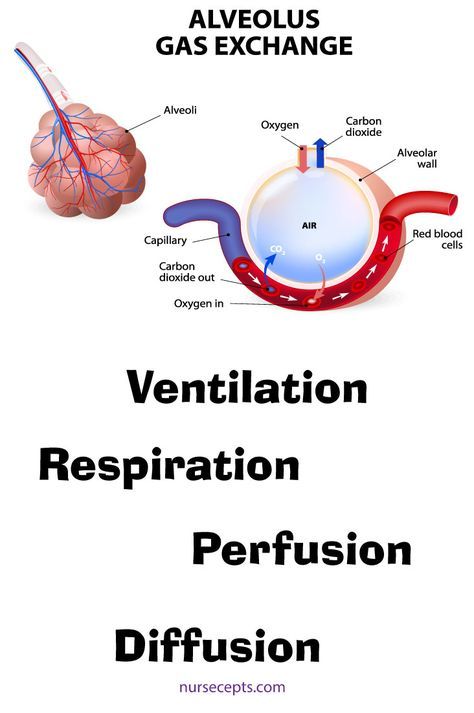 It is extremely important when screening the 1st trimester to measure blood flow in the vessels of the uterus. It has been unambiguously proven that the narrowing of the vessels of the uterus, determined during the study, indicates the inferiority of the formation of the placenta, which will worsen with increasing gestational age and lead to a decrease in the nutrition of the baby and the supply of oxygen, that is, to the development of placental insufficiency and fetal growth retardation. With normal sizes of uterine vessels at 11-14 weeks of gestation, the risk of severe placental insufficiency is negligible. nine0013
It is extremely important when screening the 1st trimester to measure blood flow in the vessels of the uterus. It has been unambiguously proven that the narrowing of the vessels of the uterus, determined during the study, indicates the inferiority of the formation of the placenta, which will worsen with increasing gestational age and lead to a decrease in the nutrition of the baby and the supply of oxygen, that is, to the development of placental insufficiency and fetal growth retardation. With normal sizes of uterine vessels at 11-14 weeks of gestation, the risk of severe placental insufficiency is negligible. nine0013
The next mandatory screening ultrasound is at 20-21 weeks of gestation. In this case, measurements of the fetus must be carried out in order to assess whether there is a lag in growth. After all, with oxygen starvation, the growth rate of the fetus slows down and its size begins to lag behind the norm for each period of pregnancy. In addition, the doctor must evaluate the condition and maturity of the placenta. During ultrasound, dopplerometry of the uterine vessels is also performed to detect early changes that precede the clinical manifestations of placental insufficiency. nine0013
During ultrasound, dopplerometry of the uterine vessels is also performed to detect early changes that precede the clinical manifestations of placental insufficiency. nine0013
In patients belonging to the high-risk group, in addition to ultrasound and dopplerometry, daily monitoring of blood pressure fluctuations is also carried out, the amount of protein in the urine sample collected per day is determined, and the indicators of the blood coagulation system are evaluated.
The third ultrasound is performed for all expectant mothers at 30-34 weeks of pregnancy. The doctor measures the circumference of the head and abdomen of the crumbs, the length of the bones of his arms and legs, and calculates the estimated weight of the fetus. These measurements allow the doctor to make sure that the baby is developing normally. The structure of the placenta is also important, the presence of signs of aging in it, as a result of which it usually ceases to fully supply the baby with blood, which means that it ceases to have enough oxygen and nutrients and the development of the child is disturbed. During ultrasound, the amount and type of amniotic fluid is assessed, which can also change with intrauterine fetal suffering. nine0013
During ultrasound, the amount and type of amniotic fluid is assessed, which can also change with intrauterine fetal suffering. nine0013
Doppler
Doppler of the placental and umbilical cord vessels (method of studying blood flow velocities in these vessels) also allows you to assess the well-being of the baby. The doctor examines the blood flow in the arteries of the uterus, umbilical cord, heart and brain of the child. This study allows you to determine whether the placenta is working well, whether there are signs of a lack of oxygen in the baby, or the development of preeclampsia in the mother. With a decrease in the speed of blood flow in any vessel, one can speak of fetal malnutrition of varying severity. nine0013
A well-timed examination makes it possible to identify the initial stages of blood supply deficiency. In such cases, treatment can prevent formidable complications, such as hypoxia and intrauterine growth retardation of the baby. Dopperometry is carried out at 20-21 weeks and at 30-32 weeks of pregnancy, if there are changes, control is carried out at least every two weeks.
Cardiotocography
This is an important method for assessing the condition of the fetus. CTG is performed at a gestational age of 33 weeks or more, since only at this stage of the intrauterine development of the baby is a complete regulation of the activity of the cardiovascular system of the fetus established by the centers of the spinal cord and brain. Recording of fetal heartbeats is carried out for 20-40 minutes, and if necessary, the study can be extended up to 1.5 hours. nine0013
The device registers and records the baby's heart rate. The obstetrician-gynecologist evaluates the heartbeat recording curve, episodes of slowing down and a sharp increase in the fetal heart rate, and based on these data, makes a conclusion about how comfortable the baby feels in the mother's stomach. For example, with a decrease in the concentration of oxygen in the blood of the fetus, its supply to the cells of the nervous system also decreases, which in turn affects the heart rate.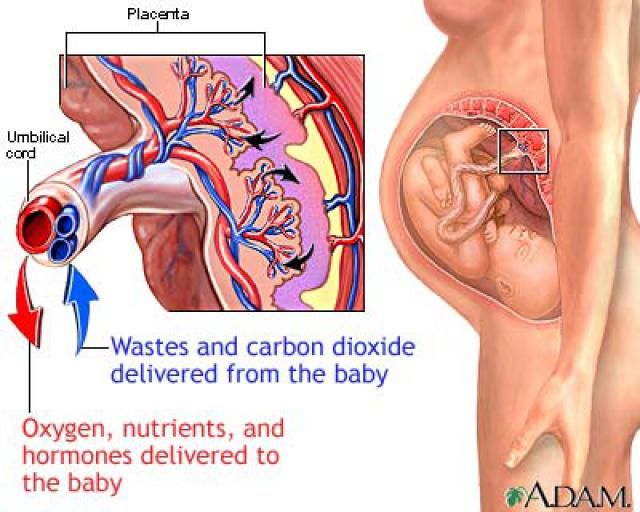 In the normal course of pregnancy, CTG is performed after 33 weeks 1 time in 10-14 days, sometimes more often. Some clinics currently offer the service of continuous CTG monitoring, which becomes relevant in the presence of signs of placental insufficiency. A pregnant woman is given a monitor that records changes in the baby's heart activity and these data are transmitted via the Internet to the attending physician. nine0013
In the normal course of pregnancy, CTG is performed after 33 weeks 1 time in 10-14 days, sometimes more often. Some clinics currently offer the service of continuous CTG monitoring, which becomes relevant in the presence of signs of placental insufficiency. A pregnant woman is given a monitor that records changes in the baby's heart activity and these data are transmitted via the Internet to the attending physician. nine0013
Treatment of placental insufficiency
There are currently no specific treatments for placental insufficiency because there are no drugs that selectively improve uteroplacental blood flow. That is why all measures to combat placental insufficiency are aimed at prevention. If the patient is at high risk for the development of placental insufficiency, from early pregnancy she is prescribed drugs whose effectiveness is well proven and which prevent the early development of severe placental dysfunction. nine0013
If during the additional methods of assessing the condition of the fetus, initial disturbances in the supply of oxygen to the baby are detected, drug treatment is carried out aimed at increasing the flow of blood and oxygen through the placenta and mandatory control examinations against the background of ongoing therapy.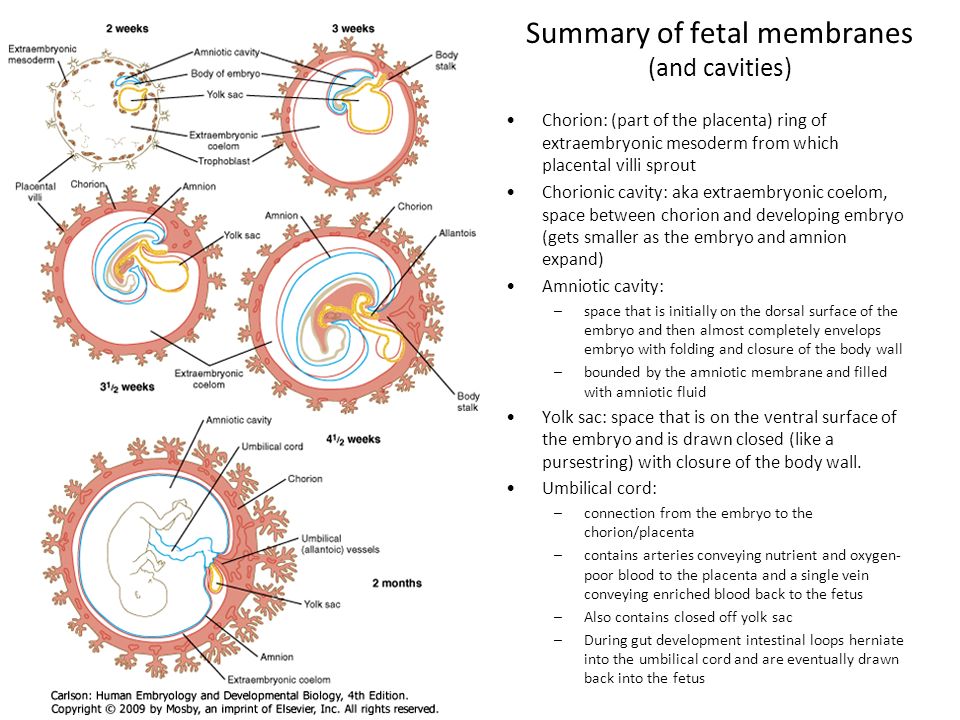 If the changes are serious and the baby experiences a pronounced deficiency of oxygen and nutrients, his condition suffers, then in such cases an emergency delivery is performed.
If the changes are serious and the baby experiences a pronounced deficiency of oxygen and nutrients, his condition suffers, then in such cases an emergency delivery is performed.
Violation of uteroplacental blood flow - causes, symptoms, diagnosis and treatment
Violation of uteroplacental blood flow - a symptom complex that develops during pregnancy due to dysfunction of the placenta or morphological changes occurring in its structure. On the mother's side, the clinic may be absent. Against the background of obstetric pathology, fetal hypoxia occurs, manifested by an increase or slowdown in heart rate, and a decrease in activity. Diagnosis of disorders of the uteroplacental blood flow is carried out by means of ultrasound, CTG, dopplerometry. Treatment is carried out in a hospital in a conservative way using drugs that improve hemodynamics in the vessels of the placenta. nine0013
General information
Violation of uteroplacental blood flow is an obstetric pathology resulting from a disorder of hemodynamic functions in the "woman-placenta-child" system.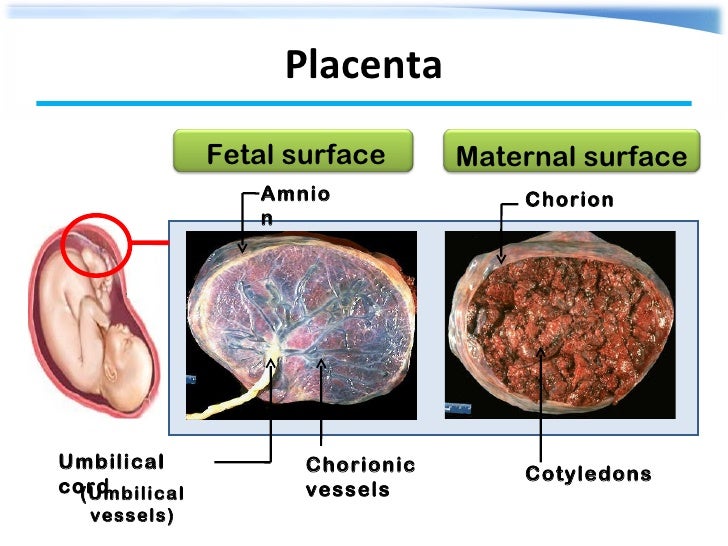 Such an anomaly is diagnosed in about 4% of pregnant women. In 25% of cases, the disease develops against the background of existing extragenital diseases of the patient. Violation of the uteroplacental blood flow poses a threat to the health and life of the fetus, since it can lead to insufficient intake of nutrients, which is complicated by intrauterine growth retardation, hypoxia, and even the possible death of the child. nine0013
Such an anomaly is diagnosed in about 4% of pregnant women. In 25% of cases, the disease develops against the background of existing extragenital diseases of the patient. Violation of the uteroplacental blood flow poses a threat to the health and life of the fetus, since it can lead to insufficient intake of nutrients, which is complicated by intrauterine growth retardation, hypoxia, and even the possible death of the child. nine0013
The risk of impaired uteroplacental blood flow depends on the severity and duration of this obstetric pathology. The less nutrients the child receives, the higher the likelihood of abnormalities. According to statistics, about 85% of newborns prone to such a pathology are born with signs of hypoxia or congenital anomalies of varying severity. Violation of the uteroplacental blood flow can occur at different stages of pregnancy, most often it is diagnosed in the 2nd-3rd trimester of gestation. A hemodynamic disorder that has developed before 16 weeks often ends in spontaneous miscarriages. nine0013
nine0013
Violation of uteroplacental blood flow
Causes of violation of uteroplacental blood flow
Violation of uteroplacental blood flow develops as a result of improper formation of the villous layer of the fetal membranes even during the period of placental laying or as a result of the influence of adverse factors on the mother's body that cause hemodynamic disorders in normal placenta. The pathogenesis of the disease consists in inadequate uteroplacental perfusion, leading to insufficient oxygen supply to the fetus. As a result, a violation of the uteroplacental blood flow triggers the mechanism of hypoxic changes that contribute to the delay in fetal development. nine0013
Endogenous and exogenous causes can provoke a violation of the uteroplacental blood flow. The first group includes factors that influence the future mother's body from within. The risk of developing pathology is observed if a woman has diabetes mellitus, diseases of the kidneys, heart and blood vessels, against the background of thyroid dysfunction. The formation of a violation of the uteroplacental blood flow contributes to a burdened obstetric history - late preeclampsia, threats of interruption, multiple abortions and miscarriages, benign tumors of the uterus. A high risk of hemodynamic disorders is observed during pregnancy with an Rhesus conflict, as well as if the patient suffered from infertility. nine0013
The formation of a violation of the uteroplacental blood flow contributes to a burdened obstetric history - late preeclampsia, threats of interruption, multiple abortions and miscarriages, benign tumors of the uterus. A high risk of hemodynamic disorders is observed during pregnancy with an Rhesus conflict, as well as if the patient suffered from infertility. nine0013
Violation of the uteroplacental blood flow often develops against the background of genetic disorders in the fetus and in the presence of congenital malformations of the mother's reproductive system (with a bicornuate or saddle uterus, partitions in the organ cavity). The likelihood of obstetric pathology also exists with sexual infections, as well as if the patient has had viral diseases, for example, influenza, SARS. Exogenous factors contributing to the violation of uteroplacental blood flow include work in hazardous industries, drug and alcohol use, and smoking. Poor nutrition also has an adverse effect. The risk group for the development of impaired uteroplacental blood flow includes women under the age of 18 and over 35 years. The risk of abnormal hemodynamics is present with constant stress, intense physical exertion. nine0013
The risk group for the development of impaired uteroplacental blood flow includes women under the age of 18 and over 35 years. The risk of abnormal hemodynamics is present with constant stress, intense physical exertion. nine0013
Classification of uteroplacental blood flow disorders
Depending on the location of pathological changes in obstetrics, there are several degrees of severity of uteroplacental blood flow disorders:
- 1a - is characterized by a hemodynamic disorder between the uterus and the placenta, while a sufficient amount of nutrients enters the child.
- 1b - circulatory disorders occur in the "fetus-placenta" circle. nine0004
- 2nd degree - violation of uteroplacental blood flow is observed in the circle "fetus-placenta-mother", however, hypoxia is slightly pronounced.
- 3 degree - accompanied by a critical disorder of hemodynamic parameters, can lead to the death of a child or spontaneous abortion.
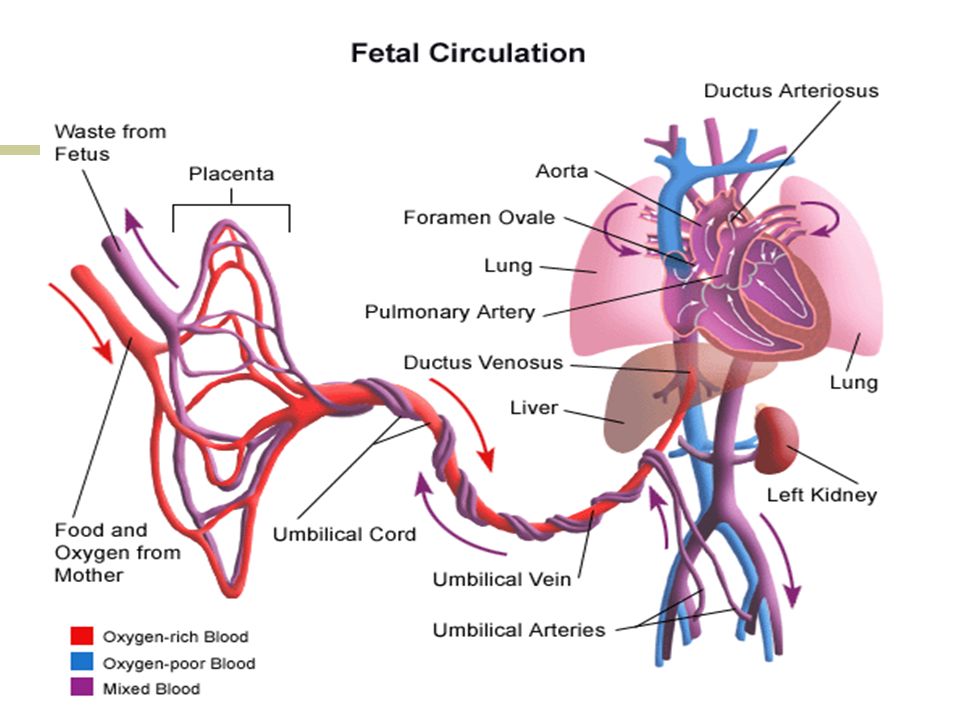
Taking into account the gestational age, at which there is a violation of the uteroplacental blood flow, the following types of pathology can be distinguished:
- Primary - occurs in the first trimester, usually develops against the background of abnormal implantation, disorders in the formation or attachment of the placenta.
- Secondary - diagnosed after 16 weeks of embryogenesis, usually provoked by negative external factors or the health of the mother.
Symptoms of impaired uteroplacental blood flow
Clinical manifestations of impaired uteroplacental blood flow depend on the severity of the obstetric anomaly. On the part of the mother, pathological signs are not always observed. The patient may develop preeclampsia, often there is a threat of miscarriage or premature birth, which is accompanied by pain in the abdomen and in the groin area. There may be bloody mucus from the genital tract. Against the background of a violation of the uteroplacental blood flow, the activity of the conditionally pathogenic flora is activated, colpitis often occurs. This complication of impaired uteroplacental blood flow can cause intrauterine infection of the fetus. nine0013
Against the background of a violation of the uteroplacental blood flow, the activity of the conditionally pathogenic flora is activated, colpitis often occurs. This complication of impaired uteroplacental blood flow can cause intrauterine infection of the fetus. nine0013
Violation of uteroplacental blood flow is more pronounced on the part of the child. In some cases, the patient herself may suspect signs of fetal hypoxia. The pathological condition is manifested by a decrease in the motor activity of the child. During the examination, the obstetrician-gynecologist reveals an increase or decrease in the heart rate in the baby, which is also a reliable sign of a violation of the uteroplacental blood flow. The lack of nutrients can cause premature detachment of the placenta. At the same time, the condition of the woman and the fetus is rapidly deteriorating, and a threat to life may arise. nine0013
Diagnosis and treatment of uteroplacental blood flow disorders
uteroplacental blood flow disorders can be detected during ultrasound. The presence of obstetric pathology is evidenced by the pathology of the placenta and intrauterine growth retardation of the fetus, which is manifested by a discrepancy between the size of the anatomical parts and the gestational age. It is possible to assess the degree of violation of the uteroplacental blood flow using dopplerography. CTG is used to assess the functionality of the child's cardiovascular system. A characteristic sign is tachycardia or bradycardia, which arose against the background of hypoxia. nine0013
The presence of obstetric pathology is evidenced by the pathology of the placenta and intrauterine growth retardation of the fetus, which is manifested by a discrepancy between the size of the anatomical parts and the gestational age. It is possible to assess the degree of violation of the uteroplacental blood flow using dopplerography. CTG is used to assess the functionality of the child's cardiovascular system. A characteristic sign is tachycardia or bradycardia, which arose against the background of hypoxia. nine0013
Treatment of disorders of uteroplacental blood flow is carried out in a hospital. The patient is shown bed rest, the exclusion of stress and intense physical activity. Conservative therapy consists in the use of drugs to relieve uteroplacental blood flow disorders and improve fetal oxygenation. Antiplatelet agents and agents that improve the nutrition of brain tissues are also used. In case of violation of the uteroplacental blood flow, the use of vitamins, calcium channel blockers is indicated. The latter are used to eliminate uterine hypertonicity. nine0013
The latter are used to eliminate uterine hypertonicity. nine0013
In case of violation of the uteroplacental blood flow, all the efforts of specialists are aimed at prolonging the management of pregnancy up to 37-38 weeks. Subject to sufficient effectiveness of drug therapy after 4 weeks, the patient is transferred to outpatient treatment. If it is not possible to cope with the signs of impaired uteroplacental blood flow and the condition of the fetus continues to deteriorate, premature delivery is carried out by emergency caesarean section. If the pregnancy has been carried to 38 weeks, childbirth can occur naturally. In the second period, the use of vacuum extraction of the fetus or the imposition of obstetric forceps is shown. In the event of a violation of the uteroplacental blood flow against the background of other diseases, the mother undergoes a planned caesarean section in a period of 38 weeks. nine0013
Prognosis and prevention of uteroplacental blood flow disorders
Timely treatment of uteroplacental blood flow disorders allows a woman to prolong pregnancy up to 37 weeks of gestation and give birth to an absolutely healthy baby.