Iud miscarriage symptoms
Warning signs, treatments, and prevention
A miscarriage is defined as the spontaneous loss of a fetus before it is viable, which in the United States is the 20th week of pregnancy. The medical term for miscarriage is “spontaneous abortion.”
Miscarriage is one of the most common complications associated with early pregnancy. Sadly, around one-quarter of all pregnancies result in miscarriage.
Most miscarriages occur during the first few months of pregnancy. An estimated 85 percent of miscarriages happen before week 12. A woman may have a miscarriage before she knows she is pregnant.
Although miscarriage is relatively common, it can be an extremely traumatic and devastating experience.
The main sign of miscarriage is vaginal spotting or bleeding, which can vary from slight brownish discharge to very heavy bleeding.
Other symptoms include:
- cramping and pain in the abdomen
- mild to severe back pain
- weight loss
- fluid discharge from the vagina
- tissue or clotted discharge from the vagina
- feeling faint or light-headed
- contractions
If you are pregnant and experience any of these symptoms, contact your doctor, midwife, or antenatal clinic immediately.
Ectopic pregnancy and miscarriage
An ectopic pregnancy is when the fertilized egg settles and grows outside the inner lining of the uterus, instead of inside.
Around 1–2 percent of all pregnancies are ectopic. If left untreated, they can be fatal because of internal bleeding, and the risk of losing the baby is increased.
Symptoms of an ectopic pregnancy are:
Shoulder tip pain – where the shoulder ends and the arm begins; this is more evident when the woman is lying down; also:
- severe abdominal pain
- feeling light-headed
- dizziness
There are a variety of terms that doctors use when discussing miscarriage, these include:
Threatened miscarriage: Some bleeding in early pregnancy with lower backache. Cervix stays closed. In this case, the pregnancy continues.
Inevitable or incomplete miscarriage: Abdominal or back pain, bleeding, and an open cervix. If the cervix is open, the miscarriage is considered inevitable.
Complete miscarriage: The embryo empties out of the uterus. Bleeding and pain subside quickly.
Missed miscarriage: The embryo has died, but there are no other symptoms, such as bleeding or pain.
Recurrent miscarriage: This is defined as three or more miscarriages during the first trimester.
The aim of treatment following or during a miscarriage is to prevent hemorrhaging (bleeding) and infection. Normally, the body expels the fetal tissue on its own, especially earlier in the pregnancy. However, if it does not, a doctor may perform a dilation and curettage (D and C).
During a D and C, a doctor opens the cervix and inserts a thin instrument into the uterus to remove tissue. After the procedure, drugs may be prescribed to control bleeding.
Miscarriage can happen for a range of reasons.
Placental problems: If the placenta develops abnormally, blood supply from the mother to the baby is interrupted.
Chromosome problems: Sometimes, a fetus can receive the wrong number of chromosomes, causing abnormal development of the fetus. Miscarriages that occur during the first trimester are mainly related to chromosomal abnormalities in the baby.
Womb structure abnormalities: Abnormally shaped wombs and the development of fibroids (non-cancerous growths) in the womb can put a developing fetus at risk.
Polycystic ovary syndrome (PCOS): This occurs when the ovaries are too big, causing a hormonal imbalance.
Weakened cervix: The cervix is the neck of the womb. When the muscles of the cervix are weak, they can open up too early during pregnancy, resulting in miscarriage.
Lifestyle factors: Habits such as smoking, drinking alcohol, or using illegal drugs can lead to miscarriage.
Underlying health conditions
Share on PinterestPre-existing kidney conditions can increase the risk of miscarriage.
Underlying health conditions among pregnant woman that are associated with miscarriage include:
- high blood pressure
- coeliac disease
- diabetes
- kidney disease
- lupus
- thyroid gland problems
- HIV
- malaria
- rubella
- chlamydia
- syphilis
- gonorrhea
Being overweight or underweight
Obesity is known to increase the risk of first and subsequent miscarriages.
Women with a low body mass index before they become pregnant are also at a heightened risk of miscarriage. Research published in the International Journal of Obstetrics and Gynaecology reported that underweight women were 72 percent more likely to suffer a miscarriage during their first 3 months of pregnancy, compared with women whose weight was healthy.
Be aware of current medications
It is crucial to check with a doctor which medications are safe to take during pregnancy. Medicines that should be avoided (if possible) while pregnant include:
- retinoids
- non-steroidal anti-inflammatory drugs (NSAIDs)
- methotrexate
- misoprostol
- antidepressants
Limit caffeine
A meta-analysis published in the European Journal of Epidemiology combined data from 60 studies and concluded:
“Greater caffeine intake is associated with an increase in spontaneous abortion, stillbirth, low birth weight, and SGA, but not preterm delivery. ”
”
The World Health Organization (WHO) advises that women who consume more than 300 milligrams (mg) of caffeine per day should reduce their intake.
Miscarriage myths
There are many misconceptions regarding miscarriage. Many people believe that having sex and/or exercising can result in miscarriage, but there is no evidence to suggest this. However, some types of exercise would not be suitable for a woman who is 8 months pregnant. If you are pregnant, ask your doctor which exercises are appropriate.
In many cases, a miscarriage has no apparent cause.
The tests used to diagnose miscarriage are:
Ultrasound scans: Transvaginal ultrasounds involve placing a small probe into the vagina to check for the heartbeat of the fetus. Some women may choose to undergo an external abdominal ultrasound instead to avoid discomfort.
Blood tests: These are useful because they can determine if levels of beta-human chorionic gonadotropin (hCG) and progesterone are normal – both of these hormones are associated with a healthy pregnancy.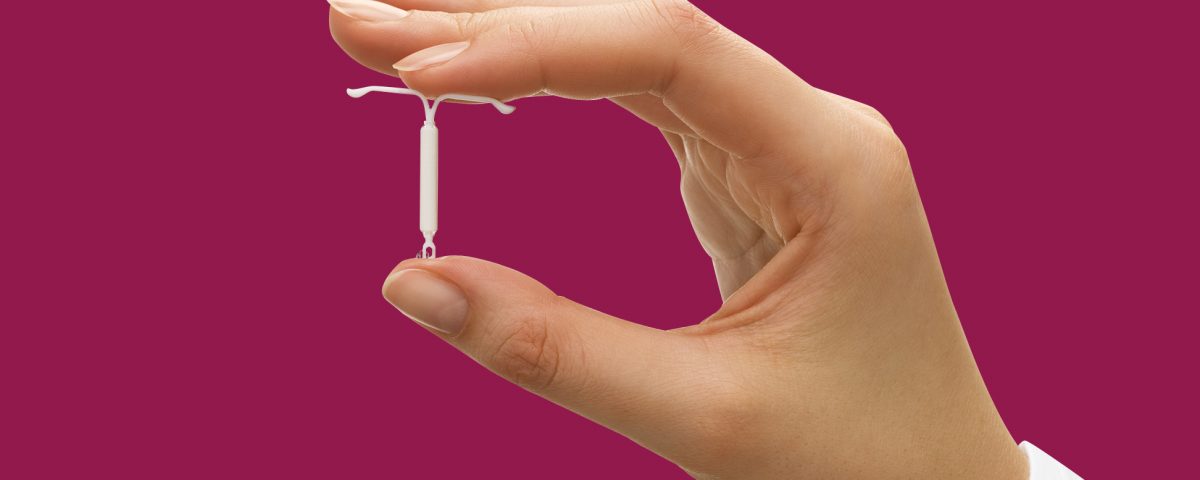
Pelvic exams: These determine whether the cervix has thinned out or opened.
A few simple lifestyle changes can reduce the risk of miscarriage:
- Avoid smoking, drinking alcohol, and using illicit drugs during pregnancy.
- Eat a healthful diet.
- Maintain a healthy weight before and during pregnancy.
- Be careful to avoid certain infections, such as German measles (rubella).
Read the article in Spanish.
Is it possible, and what happens next?
An intrauterine device (IUD) is a T-shaped device that an obstetrician-gynecologist (OB-GYN) places in the uterus to prevent pregnancy. However, some can still become pregnant with an IUD.
The IUD is an effective form of contraception. Fewer than 1 in 100 women will become pregnant within 1 year of use.
If a woman with an IUD thinks that they may be pregnant, they should schedule an appointment with a doctor as soon as possible.
They will be able to help determine the best course of action based on where the embryo has implanted, where the IUD is at the time of the pregnancy, and the woman’s wishes.
This article will look at the symptoms of pregnancy with an IUD, as well as the options available.
Share on PinterestWomen with an IUD should speak to their doctor if they suspect that they are pregnant.An IUD is an effective method of nonpermanent birth control.
According to the Centers for Disease Control and Prevention (CDC), copper IUDs have a failure rate of 0.8%, while the levonorgestrel intrauterine system has a failure rate of 0.1–0.4%
Getting pregnant with an IUD is unlikely, but it can happen. Pregnancy is possible if a woman has penetrative intercourse within 7 days of implantation.
A woman may also become pregnant with an IUD if it falls out of place. Doctors call this IUD expulsion. When an IUD is in the right place, it will sit at the bottom of the uterus, just past the cervix. The IUD strings extend through the cervix into the vagina.
Although IUD expulsion is typically unlikely, it may be more likely in some women.
According to the American College of Obstetricians and Gynecologists (ACOG), the expulsion rate is 2–10% within 1 year of having the IUD inserted. The ACOG also found that women who breastfeed or who get an IUD shortly after giving birth may also be more likely to have their IUD move.
The ACOG also found that women who breastfeed or who get an IUD shortly after giving birth may also be more likely to have their IUD move.
In some cases, when an IUD moves out of place, it will fall out entirely. A woman may notice that they are not able to find it. In other cases, the IUD may shift positions. If this happens, the woman may not know that their IUD has moved, and they might not experience any symptoms.
If it is noticeable, however, a woman may experience:
- IUD strings that are shorter than normal or uneven
- heavy bleeding
- abnormal cramping
- unusual vaginal discharge
If a woman notices any of these symptoms, they should see their OB-GYN as soon as possible.
If a woman becomes pregnant while using an IUD, they may notice some typical pregnancy symptoms — particularly if the embryo has implanted in the uterus.
These symptoms may include:
- nausea
- fatigue
- a missed period
- tender breasts
- cravings
- mood changes
Women who become pregnant while using an IUD may also notice that the strings are out of place, missing, or uneven.
Ectopic pregnancy
Women who become pregnant while using an IUD are more likely to have an ectopic pregnancy. Ectopic pregnancies occur when the embryo implants outside of the uterus — usually in the fallopian tubes.
According to the ACOG, a woman with an ectopic pregnancy may notice:
- lower back pain
- mild abdominal or pelvic pain
- abnormal vaginal bleeding
- mild cramping on one side of the pelvis
As an ectopic pregnancy progresses, a woman may experience some other, more serious symptoms.
These can include:
- sudden and severe pain in the abdomen or pelvis
- weakness
- dizziness or fainting
- shoulder pain
A woman who has these symptoms should seek immediate medical attention.
According to a review of studies that included 221,800 deliveries from women who became pregnant with an IUD in place, there are several possible complications.
These can include:
| Possible complication | Risk if IUD removed before delivery | Risk if IUD retained before delivery | Risk if no IUD before delivery |
| Preterm delivery | 14.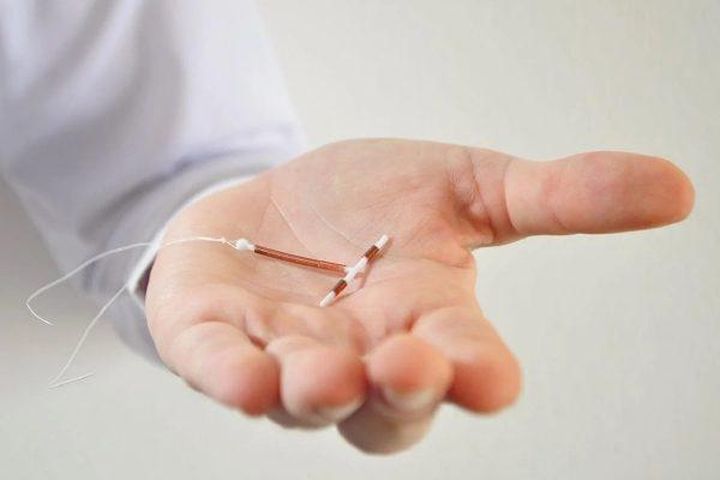 3% 3% | 14.1% | 6.8% |
| Bacterial infection | 5% | 2.7% | 0.6% |
| Low birth weight | 11.3% | 12.1% | 6.6% |
| Pregnancy loss | 2% | 1.3% | 0.5% |
As mentioned above, an ectopic pregnancy is also a potential complication. Ectopic pregnancies can result in a ruptured fallopian tube. If this occurs, there may be internal bleeding, which can lead to death.
If a woman suspects that they are pregnant, they should talk to their healthcare provider, who will first confirm the pregnancy.
They will also confirm whether or not the pregnancy is ectopic. If it is, a health professional may need to perform surgery or prescribe medication to terminate the pregnancy.
If they prescribe medication, it may be an injection of methotrexate, which will stop the embryo cells from growing. The body will then absorb the pregnancy within 4–6 weeks.
According to the ACOG, a typical surgical procedure for an ectopic pregnancy is a laparoscopic procedure. During this procedure, a surgeon makes a small incision in the abdomen. They will then remove the pregnancy.
During this procedure, a surgeon makes a small incision in the abdomen. They will then remove the pregnancy.
If the pregnancy is not ectopic, management will depend on:
- gestational age
- the position of the IUD
- the visibility of the IUD strings
- the woman’s wishes
According to the ACOG, if a woman plans to continue with the pregnancy, a doctor should remove the IUD when the strings are visible or when it is in the cervix during the early stages of pregnancy.
Desire to continue the pregnancy
If the woman wishes to continue with the pregnancy, a doctor will perform a pelvic examination. If the IUD strings are visible, the doctor will remove the IUD gently.
If the IUD strings are not visible, a woman may need to undergo an ultrasound to determine its location. The next course of action depends on the location, as below:
- No IUD found: The woman will need an X-ray to locate the device.
- IUD within the cervix: The doctor will remove the IUD by pulling the strings.

- IUD above the cervix: The doctor will talk about the risks and complications of continuing the pregnancy.
Termination
If the woman desires to terminate the pregnancy, the doctor can remove the IUD at the time of surgical termination or before medication termination.
They will also provide information regarding the potential options available:
| Medication | Surgery |
| Noninvasive | Invasive |
| No sedation | Sedation, if desired |
| Unpredictable completion time | Predictable completion time |
| Available during early pregnancy | Available during early pregnancy |
| High success rate, approximately 95% | High success rate, approximately 99% |
| Heavy bleeding | Light bleeding |
| Requires follow-up sessions to ensure completion of abortion | May not require follow-up in most cases |
| Multiple-step process | Single-step process |
Medication termination
There are a variety of medications a doctor can prescribe, including:
- mifepristone
- misoprostol
- methotrexate
Side effects may include:
- heavy bleeding (requiring roughly two maxi pads per hour for 2 hours)
- nausea
- vomiting
- diarrhea
- headache
- dizziness
- changes in body temperature
Surgical termination
This procedure involves an OB-GYN inserting a speculum into the vagina. If the cervix needs to be dilated during the time of the procedure, they will insert and withdraw a series of dilators to increase the size of the opening.
If the cervix needs to be dilated during the time of the procedure, they will insert and withdraw a series of dilators to increase the size of the opening.
The OB-GYN will insert a plastic tube attached to a suction pump to remove the pregnancy.
Following the procedure, a woman can go home after around an hour. According to the ACOG, however, they should expect cramping for 1–2 days and bleeding that can last for 2 weeks.
It is possible, but not likely, to become pregnant when using an IUD.
The highest chance of pregnancy is during the first few days following the implantation of the IUD. A woman can also get pregnant if the IUD has moved out of place.
If a pregnancy occurs, a doctor will determine where the embryo has implanted to make sure that it is viable. If it is ectopic, they will recommend treatment. If it is viable, they will discuss a woman’s options.
Intrauterine infection of the fetus: a modern view of the problem
The progressive increase in the number of cases of intrauterine infection of the fetus is one of the most urgent problems of modern obstetrics and perinatology. This is facilitated by the polyetiology of this pathology, the lack of a clear relationship between the severity of the clinical manifestations of infection in the mother and the degree of fetal damage, the multifactorial effect of the infectious agent on the fetus.
This is facilitated by the polyetiology of this pathology, the lack of a clear relationship between the severity of the clinical manifestations of infection in the mother and the degree of fetal damage, the multifactorial effect of the infectious agent on the fetus.
Despite increased attention to the problem of intrauterine infection (IUI), many questions remain unresolved. Issues of diagnosis, treatment and prevention of IUI need further research and development. Until now, there are no clear criteria for treatment tactics, data on the effectiveness of complex therapy have not been summarized.
Epidemiology, etiology and pathogenesis of intrauterine infection
In the development of an infectious process in the fetus, the type of pathogen, its virulence, the ways of infection from mother to fetus, the protective reserves of the mother's body and the ability of the fetus to immune response are important [11].
According to modern data [10, 20], the number of cases of IUI varies widely from 6 to 70%. Recently, the structure of the infectious morbidity of pregnant women, parturient women and puerperas, as well as the fetus and newborn has changed [5, 8, 14]. It has been proven that the causative agents of IUI are more than 27 types of bacteria, many viruses, parasites, 6 types of fungi, 4 types of protozoa and rickettsia. So, according to a number of researchers [8, 14, 30], chlamydia (17-50%) and viruses (herpes simplex virus, HSV - 7-47%, cytomegalovirus, CMV - 28-9%) are considered to be the predominant pathogens of antenatal infections.1.6%, enteroviruses - 8-17%). The causative agents of intranatal infections are group B streptococcus (3-12%), staphylococci (1-9%), fungi of the genus Candida (3-7%). Associations of pathogens occupy a leading position (75-95%).
Recently, the structure of the infectious morbidity of pregnant women, parturient women and puerperas, as well as the fetus and newborn has changed [5, 8, 14]. It has been proven that the causative agents of IUI are more than 27 types of bacteria, many viruses, parasites, 6 types of fungi, 4 types of protozoa and rickettsia. So, according to a number of researchers [8, 14, 30], chlamydia (17-50%) and viruses (herpes simplex virus, HSV - 7-47%, cytomegalovirus, CMV - 28-9%) are considered to be the predominant pathogens of antenatal infections.1.6%, enteroviruses - 8-17%). The causative agents of intranatal infections are group B streptococcus (3-12%), staphylococci (1-9%), fungi of the genus Candida (3-7%). Associations of pathogens occupy a leading position (75-95%).
It is known that most bacteria exist in nature in the form of specifically organized biofilms (biofilms) [2, 25, 27]. This form of existence creates a lot of advantages for bacteria. Bacteria in biofilms have an increased survival rate in the presence of aggressive substances, immune defense factors, and antibiotics [27]. In this regard, one of the main problems of practical medicine is the problem of treating diseases of microbial origin.
In this regard, one of the main problems of practical medicine is the problem of treating diseases of microbial origin.
In our study [15, 17], based on the results of bacteriological analysis of the species composition of the vaginal biotope, the strongest influence of Streptococcus faecalis (p=0.00171), E. coli (p=0.01424) and Staphylococcus epidermidis (p=0.02714) for intrauterine infection of the fetus. When assessing the pathogens identified in the cervical canal of pregnant women by polymerase chain reaction (PCR) and enzyme immunoassay (ELISA), the following was established: in the group without the implementation of IUI, mycoplasma, chlamydia and ureaplasma accounted for 8%, CMV - 20%, HSV - 36%, candida - 3%, associations - 60%. When analyzing a group of newborns with the implementation of IUI, the most common pathogens were identified. Thus, mycoplasmas, chlamydia and HSV were found in 50%, CMV infection was detected in 45% of cases, ureaplasmas (20%) and candida (15%) were less common, associations were observed in 95%. The frequency of detection of pathogenic pathogens in newborns with signs of IUI was higher than in patients without infection.
The frequency of detection of pathogenic pathogens in newborns with signs of IUI was higher than in patients without infection.
In the pathogenesis of IUI, there are "maternal", "afterbirth", "fetal" stages of development [23]:
"Maternal" stage reflects the beginning of the infectious process within the lower parts of the urogenital tract.
The "following" stage occurs with hematogenous spread of the inflammatory process, occurs with bacteremia and viremia.
At the "fetal" stage, the infectious process spreads to the organs and tissues of the fetus. This occurs when the uteroplacental and placental-fetal antimicrobial barrier, the boundary of which is the layer of chorionic epithelium, is inconsistent.
The main source of infection in IUI is the mother of the child, from whose body the pathogen enters the fetus (vertical transmission mechanism). In this case, infection occurs both by ascending, transplacental and transovarial routes, as well as by contact and aspiration (directly during childbirth) routes. Moreover, for antenatal infections, the hematogenous route is most typical, and for intranatal infections, the ascending route of infection [4, 10, 20, 21].
Moreover, for antenatal infections, the hematogenous route is most typical, and for intranatal infections, the ascending route of infection [4, 10, 20, 21].
The effect of IUI on the embryo and fetus is the effect of a complex of the following factors [22]:
1. Pathological effect of microorganisms and their toxins (infectious disease, fetal hypoxia, fetal growth retardation).
2. Violation of the process of implantation and placentation (low placentation, placenta previa).
3. Decreased metabolic processes and immune defense of the fetus.
The duration of pregnancy is of particular importance in the pathogenesis of the onset and development of IUI [13, 22]. The fetus does not react to infectious antigens until the 14th week of pregnancy, since it lacks immunocompetent cells, immunoglobulins and does not show immune reactions. With the beginning of the second trimester of pregnancy, the mechanism of action of ascending infection changes due to the merger of decidua vera and decidua capsularis into a single complex decidua parietalis.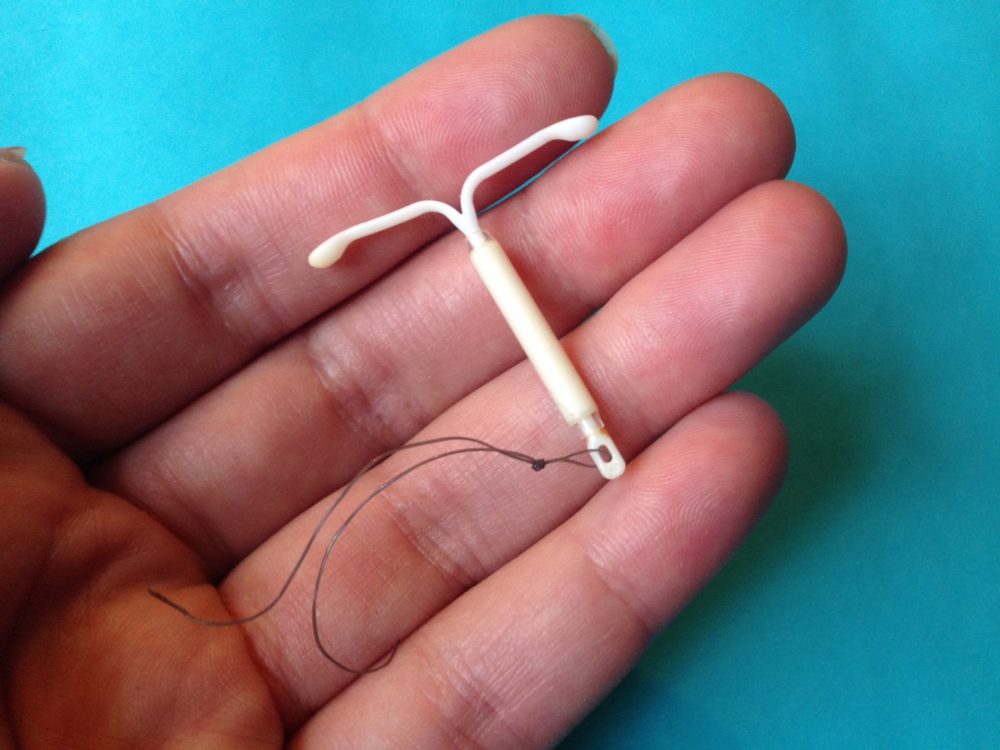 During this time, ascending infection may enter the fetus from the vagina or cervical canal. From this period of pregnancy, the internal pharynx of the cervical canal comes into contact with the water membranes of the fetus and, in the presence of infection, microorganisms penetrate into the amniotic fluid. Amniotic fluid acquires antimicrobial properties only after the 20th week of pregnancy, when, in response to the action of an infectious agent, an inflammatory proliferative reaction develops, which limits further penetration of the infection due to the appearance of lysozyme, complement, interferons, immunoglobulins [1, 22]. In the III trimester of pregnancy, the antibacterial protection of amniotic fluid increases. During this period, the role of the exudative component prevails in the inflammatory reaction of the fetal tissues, when inflammatory leukocyte reactions develop in the fetus (encephalitis, hepatitis, pneumonia, interstitial nephritis) in response to infection [22].
During this time, ascending infection may enter the fetus from the vagina or cervical canal. From this period of pregnancy, the internal pharynx of the cervical canal comes into contact with the water membranes of the fetus and, in the presence of infection, microorganisms penetrate into the amniotic fluid. Amniotic fluid acquires antimicrobial properties only after the 20th week of pregnancy, when, in response to the action of an infectious agent, an inflammatory proliferative reaction develops, which limits further penetration of the infection due to the appearance of lysozyme, complement, interferons, immunoglobulins [1, 22]. In the III trimester of pregnancy, the antibacterial protection of amniotic fluid increases. During this period, the role of the exudative component prevails in the inflammatory reaction of the fetal tissues, when inflammatory leukocyte reactions develop in the fetus (encephalitis, hepatitis, pneumonia, interstitial nephritis) in response to infection [22].
Particularly dangerous in IUI in the II and III trimesters of pregnancy is damage to the fetal brain, which can lead to mental retardation, delayed psychomotor development of children [3, 7]. Intrauterine infection of the CNS structures by pathogens in the fetus is accompanied by various severe disorders in the formation of the brain (hydrocephalus, subependymal cysts, cystic degeneration of the brain substance, anomalies in the development of the cortex, microcephaly). It is also possible to develop ventriculitis (deformity of the choroid plexus, heterogeneity or doubling of the reflection from the ependyma of the ventricles) [11].
Thus, infection of the fetus in the later stages of pregnancy does not, as a rule, lead to the formation of gross malformations, but can disrupt the functional mechanisms of cell and tissue differentiation [10, 11, 13].
Changes in the condition of the fetus and the functioning of the fetoplacental system caused by intrauterine infection of the fetus affect the composition and properties of the amniotic fluid [1, 9, 26, 30]. When an infectious agent enters the amniotic fluid, it reproduces unhindered, followed by the development of chorioamnionitis [1, 9]. The fetus is in an infected environment, which creates favorable conditions for infection of the fetus by contact, i.e. through the skin, mucous membranes, respiratory and gastrointestinal tracts.
When an infectious agent enters the amniotic fluid, it reproduces unhindered, followed by the development of chorioamnionitis [1, 9]. The fetus is in an infected environment, which creates favorable conditions for infection of the fetus by contact, i.e. through the skin, mucous membranes, respiratory and gastrointestinal tracts.
The syndrome of "amniotic fluid infection" develops, the mechanism of which is presented as follows [20]:
1. Ingestion and aspiration of infected water in a newborn shows signs of intrauterine infection (pneumonia, enterocolitis, vesiculosis, omphalitis, conjunctivitis, etc.).
2. At the same time, microorganisms, spreading through the membranes or between them, reach the basal plate of the placenta (deciduitis). Further spread of the inflammatory reaction leads to the development of chorionitis (placentitis), manifested by leukocyte infiltration of the intravillous space and endovasculitis in the chorionic plate. Vasculitis in the decidua, stem and terminal villi leads to vascular obliteration, the appearance of infarcts, calcifications, massive fibrinoid deposits, which can manifest as "premature maturation of the placenta. "
"
3. Polyhydramnios in intrauterine infection is usually secondary and is a manifestation of damage to the kidneys or urinary tract of the fetus. The reason for its development is also a change in the ratio of the processes of production and resorption of amniotic fluid by the cells of the amniotic epithelium against the background of amnionitis.
4. In the genesis of the symptom complex of placental insufficiency in IUI, the main role belongs to vascular disorders.
5. Typical manifestations of intrauterine infection are miscarriage and premature birth [24]. Premature development of labor activity and untimely rupture of the membranes are due to the action of bacterial phospholipases that trigger the prostaglandin cascade and the damaging effect of inflammatory toxins on the membranes.
6. Due to the fact that phospholipases of gram-negative bacteria contribute to the destruction of surfactant in the lungs of the fetus, the newborn develops respiratory disorders.
In modern literature [4, 9, 29] there are many works devoted to the study of the relationship between immunological parameters and the severity of the infectious-inflammatory process during pregnancy. Increasingly, in foreign and domestic literature, data appear on the relationship between bacterial invasion and cytokine synthesis by amnion, chorion, decidua, and fetal tissues [5, 29]. The multiplication of microorganisms in the amniotic fluid leads to an increase in the level of lipopolysaccharides, which activate the synthesis of cytokines by fetal trophoblast cells. It seems promising in IUI to study changes in the cytokine system, which provides the processes of intercellular cooperation, growth and differentiation of lymphoid cells, hematopoiesis, and neuroimmunoendocrine interactions. Cytokines in the pre-implantation period and during the development of pregnancy are actively produced by many maternal and embryonic cells, in particular, decidual cells of the uterus and trophoblast cells. It was found that cell cultures have different ability to synthesize cytokines under the influence of lipopolysaccharides. Thus, tumor necrosis factor (TNF) is produced by amnion cells, interleukins (IL)-6 and IL-8 are produced by amnion and chorion, and IL-1 is produced only by chorion [9, 11, 28]. According to N.V. Ordzhonikidze [14], from a variety of pro-inflammatory (IL-1, IL-2, IL-6, IL-8, IL-15, TNF, etc.) and anti-inflammatory (IL-4, IL-10, IL-13, transforming growth factor, etc.) of cytokines, IL-1, IL-6, IL-10, and TNF are considered to be the main markers of the inflammatory process in human tissues and organs [9, 16, 28].
It was found that cell cultures have different ability to synthesize cytokines under the influence of lipopolysaccharides. Thus, tumor necrosis factor (TNF) is produced by amnion cells, interleukins (IL)-6 and IL-8 are produced by amnion and chorion, and IL-1 is produced only by chorion [9, 11, 28]. According to N.V. Ordzhonikidze [14], from a variety of pro-inflammatory (IL-1, IL-2, IL-6, IL-8, IL-15, TNF, etc.) and anti-inflammatory (IL-4, IL-10, IL-13, transforming growth factor, etc.) of cytokines, IL-1, IL-6, IL-10, and TNF are considered to be the main markers of the inflammatory process in human tissues and organs [9, 16, 28].
Clinical characteristics of intrauterine infection
In the pre-implantation period, under the influence of an infectious agent, the embryo dies (alternative inflammation) or continues to develop.
Infectious damage to the embryo at 3-12 weeks is usually associated with a viral infection that freely penetrates the chorion.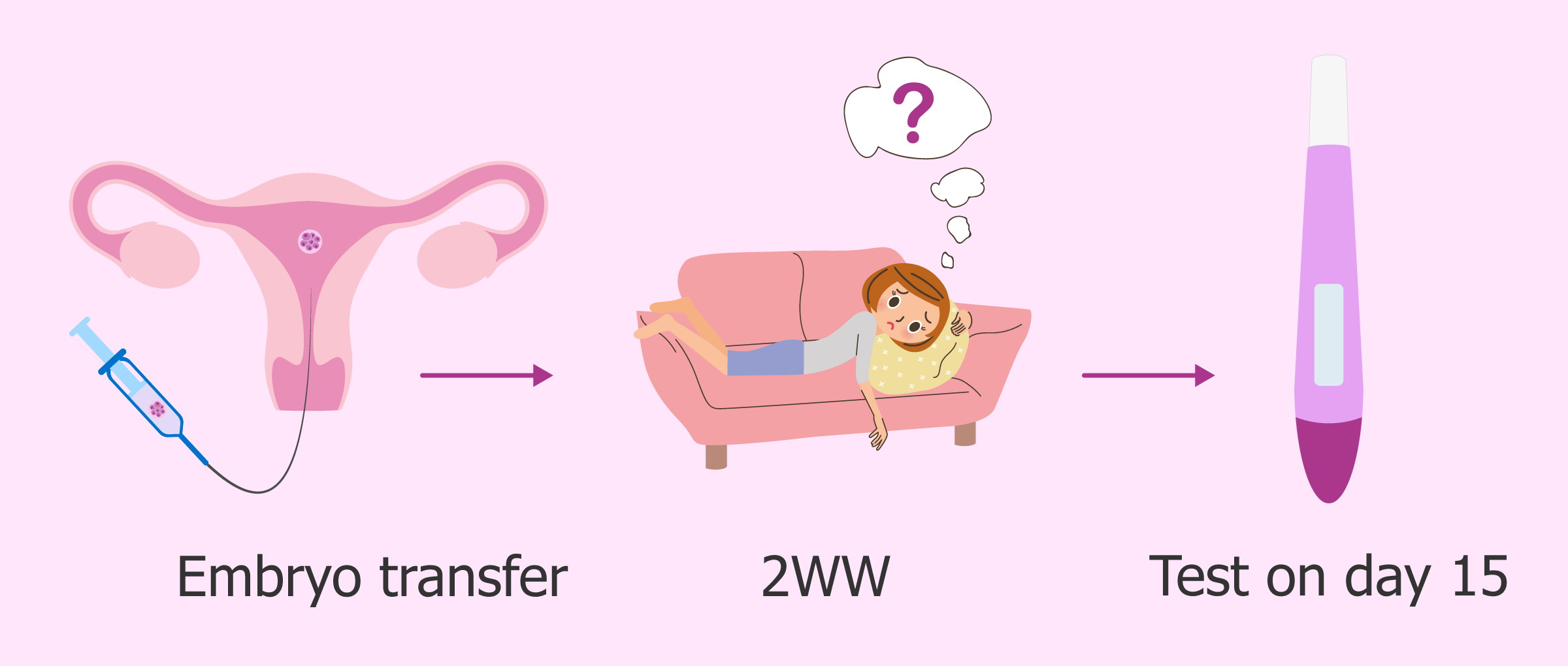 The fetus does not yet have anti-infective protection systems, and during the period of organogenesis, IUI placentation leads to the formation of malformations (teratogenic) or death of the embryo (embryotoxic effect) [5, 23].
The fetus does not yet have anti-infective protection systems, and during the period of organogenesis, IUI placentation leads to the formation of malformations (teratogenic) or death of the embryo (embryotoxic effect) [5, 23].
Infectious fetopathy occurs from the 16th-27th week of gestation, when the infection in the fetus generalizes, the formation of pseudomalformations (myocardial fibroelastosis, polycystic lung disease, hydrocephalus, hydronephrosis) occurs. When infected after 28 weeks, the fetus acquires the ability to a specific local reaction to the introduction of the pathogen, as a result, IUI (encephalitis, pneumonia, hepatitis, interstitial nephritis), miscarriage, intrauterine growth retardation, and fetal death are possible [2, 3, 10].
Currently, the following types of intrauterine lesions in IUI are distinguished [20, 21]:
- blastopathy - with a gestation period of 0-14 days; possible death of the embryo, spontaneous miscarriage or the formation of a systemic pathology similar to genetic diseases;
- embryopathy - with a period of 15-75 days; characteristic malformations at the organ or cellular levels (true malformations), spontaneous miscarriage;
- early fetopathy - with a period of 76-180 days; characteristic is the development of a generalized inflammatory reaction with a predominance of alterative and exudative components and an outcome in fibrosclerotic deformities of organs (false defects), abortion;
- late fetopathy - with a period of 181 days before delivery; it is possible to develop a manifest inflammatory reaction with damage to various organs and systems (hepatitis, encephalitis, thrombocytopenia, pneumonia).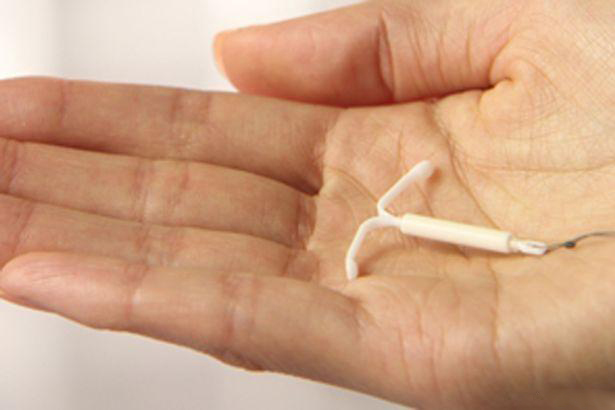
IUI often does not have clear clinical manifestations [4, 10, 15, 17]. Rarely, the first signs in a newborn are present immediately after birth, more often they appear during the first 3 days of life. When infected in the postnatal period, the symptoms of the infectious process are detected at a later date. Clinical manifestations of congenital bacterial or mycotic skin lesions in a newborn may have the character of vesiculo-pustulosis [4]. Conjunctivitis, rhinitis and otitis media that appeared on the 1st-3rd day of life can also be manifestations of IUI. Congenital aspiration pneumonia can also appear on the 2-3rd day of life. From the moment of birth, children have signs of respiratory failure: shortness of breath, cyanosis, often dullness of percussion sound and small bubbling wet rales. The course of intrauterine pneumonia is severe, because as a result of aspiration, large areas of the lung (lower and middle lobes) are turned off from breathing due to bronchial obstruction with infected amniotic fluid containing an admixture of meconium, fetal skin scales. Enterocolitis in newborns occurs as a result of the penetration of the pathogen along with the amniotic fluid into the gastrointestinal tract. Dyspeptic symptoms usually develop on the 2-3rd day of life. Characterized by sluggish sucking, regurgitation, bloating, hepatosplenomegaly, expansion of the venous network of the anterior abdominal wall, frequent loose stools. In the microbiological study of intestinal contents, the predominance of Klebsiella, Proteus and Pseudomonas aeruginosa. Damage to the central nervous system during IUI in newborns can be both primary (meningitis, encephalitis) and secondary, due to intoxication. With damage to the choroid plexuses of the lateral ventricles of the brain, congenital hydrocephalus develops. It is necessary to pay attention to such symptoms as lethargy, poor sucking, regurgitation, delayed recovery or secondary weight loss, delayed healing of the umbilical wound, development of omphalitis. Typical symptoms of infectious intoxication in a newborn are respiratory and tissue metabolism disorders.
Enterocolitis in newborns occurs as a result of the penetration of the pathogen along with the amniotic fluid into the gastrointestinal tract. Dyspeptic symptoms usually develop on the 2-3rd day of life. Characterized by sluggish sucking, regurgitation, bloating, hepatosplenomegaly, expansion of the venous network of the anterior abdominal wall, frequent loose stools. In the microbiological study of intestinal contents, the predominance of Klebsiella, Proteus and Pseudomonas aeruginosa. Damage to the central nervous system during IUI in newborns can be both primary (meningitis, encephalitis) and secondary, due to intoxication. With damage to the choroid plexuses of the lateral ventricles of the brain, congenital hydrocephalus develops. It is necessary to pay attention to such symptoms as lethargy, poor sucking, regurgitation, delayed recovery or secondary weight loss, delayed healing of the umbilical wound, development of omphalitis. Typical symptoms of infectious intoxication in a newborn are respiratory and tissue metabolism disorders. There is a pale cyanotic coloration of the skin with a pronounced vascular pattern. Intoxication is accompanied by a violation of the excretory function of the liver and kidneys, an increase in the spleen and peripheral lymph nodes.
There is a pale cyanotic coloration of the skin with a pronounced vascular pattern. Intoxication is accompanied by a violation of the excretory function of the liver and kidneys, an increase in the spleen and peripheral lymph nodes.
Modern methods of diagnosing intrauterine infections
The prevalence of IUI among the causes of adverse outcomes, the high level of infection in pregnant women and puerperas necessitate the search for reliable methods for its diagnosis. The non-specificity of the clinical manifestations of IUI creates diagnostic difficulties, which dictates the need for the combined use of clinical and laboratory research methods. In the last decade, the main methods for diagnosing IUI have been bacteriological and immunological [10, 11, 21].
There are 3 stages in the diagnosis of intrauterine infection: 1) diagnosis during pregnancy; 2) early diagnosis at the time of birth; 3) diagnosis in the development of clinical signs of infection in the early neonatal period [12].
Of the non-invasive methods of prenatal diagnosis of IUI, the most informative are ultrasound and Doppler sonography [6, 7, 15, 26]. Direct methods of laboratory diagnostics (cordocentesis, dark-field microscopy, PCR, ELISA, culture) can detect the pathogen in biological fluids or tissue biopsies of an infected child. Indirect methods for diagnosing IUI include clinical symptoms of the mother, ultrasound, and help to make only a presumptive diagnosis of IUI [10, 17, 21]. Screening tests for IUI in newborns include studies of smears of amniotic fluid, placenta, cultures of cord blood and stomach contents of the newborn, and sometimes blood culture [6, 7, 10]. The “gold standard” for post-diagnosis of IUI is the histological examination of the placenta, umbilical cord, and fetal membranes [4, 14, 23].
Any changes in homeostasis in the mother's body are reflected in the cellular and chemical parameters of the amniotic fluid, which very finely characterize the course of the pathological process, and therefore the amniotic fluid can serve as an important diagnostic material [1, 6, 16]. According to I.V. Bakhareva [1], the most significant in the diagnosis of IUI is the determination of the antimicrobial activity of the amniotic fluid, based on the migration of leukocytes in it when bacteria accumulate in the amniotic membrane, exceeding 103 CFU/ml. The appearance of a large number of leukocytes in the amniotic fluid, an increase in cytosis due to epithelial cells without detecting microflora may indicate IUI.
According to I.V. Bakhareva [1], the most significant in the diagnosis of IUI is the determination of the antimicrobial activity of the amniotic fluid, based on the migration of leukocytes in it when bacteria accumulate in the amniotic membrane, exceeding 103 CFU/ml. The appearance of a large number of leukocytes in the amniotic fluid, an increase in cytosis due to epithelial cells without detecting microflora may indicate IUI.
Currently, great importance is attached to ultrasound methods of research, with the help of which it is possible to determine indirect signs of IUI of the fetus (polyhydramnios, ventriculomegaly, microcephaly, hepatomegaly, an increase in the thickness of the placenta, a fine suspension in the amniotic fluid) and structural changes in various organs [4, 11, 18, 26].
We have developed the necessary list of diagnostic measures for the early detection of IUI [19].
The complex examination of pregnant women included:
1. General clinical and biochemical blood and urine tests with the definition of standard indicators.
General clinical and biochemical blood and urine tests with the definition of standard indicators.
2. Determination of TORCH-complex pathogens by PCR in vaginal swabs, amniotic fluid.
3. Determination of blood antibodies to chlamydia, mycoplasmas and ureaplasmas, CMV and HSV by ELISA.
4. Carrying out an amine test, pH-metry of vaginal contents.
5. Bacterioscopic examination of the contents of the vagina, cervical canal and urethra.
6. Bacteriological examination of the maternal surface of the placenta, amniotic fluid, intestinal contents.
7. Determination of the level of pro-inflammatory (IL-1β, TNF) and anti-inflammatory (IL-10) cytokines in amniotic fluid, maternal venous blood serum and fetal cord blood.
8. Ultrasound scanning of the fetus, amniotic fluid and placenta.
9. Histomorphological examination of the placenta.
Newborn examination complex included:
1. Apgar score, measurement of body weight and length at birth, dynamics of body weight gain until discharge from the maternity hospital.
2. Determination of blood antibodies to chlamydia, mycoplasmas and ureaplasmas, CMV and HSV by ELISA.
3. Bacteriological examination of scrapings from the conjunctiva, posterior pharyngeal wall and vulva.
4. Identification of clinical signs of IUI together with a neonatologist.
Comprehensive study of the species composition of microorganisms of the birth canal, amniotic fluid, placenta, newborn, determination of antigens and antibodies to the alleged pathogen in cord blood and amniotic fluid, histological examination of the placenta to determine the path of infection of the child, the nature of the pathogen and clarify the scope of additional diagnostic tests for IUI, as well as to determine therapeutic and preventive measures in the early neonatal period.
Diagnosis of threatened miscarriage | Directory of medical laboratory Optimum (Sochi, Adler)
- Home
- How to recognize the disease
- Pregnancy and childbirth
- Miscarriage (spontaneous abortion)
More about the doctor
Miscarriage (spontaneous abortion) is a pathological involuntary rejection of an intrauterine fetus that occurs in the first or second trimester of pregnancy. Most cases occur before 12 weeks of gestation, which is considered an early miscarriage. Late spontaneous abortion can occur up to 22 weeks.
Most cases occur before 12 weeks of gestation, which is considered an early miscarriage. Late spontaneous abortion can occur up to 22 weeks.
Causes of miscarriage
Of the most common causes of miscarriage , the following are distinguished:
- abnormal development of the fetus at the genetic level;
- female hormonal imbalance;
- different rhesus blood groups with the fetus;
- sexually infectious diseases;
- the presence in the body of a woman of viral, chronic diseases;
- taking certain drugs during the first trimester;
- frequent stressful situations;
- physical overexertion;
- falls, injury;
- pathological features of the organism.
Miscarriage can also be caused by previous abortions, hot baths, bad habits.
Symptoms
The following symptoms are common signs of an incipient miscarriage :
- pain in the lower abdomen, shooting into the lower back;
- brown or red vaginal discharge;
- profuse bleeding with clots;
- local or general uterine tension.

After the fetal egg leaves the birth canal, an impulse contraction of the uterus occurs, after which the bleeding stops.
Diagnosis of a threatened miscarriage
During a gynecological examination, an obstetrician-gynecologist compares the parameters of the uterus with the expected weeks of pregnancy. The level of general tension of the uterus, density, diameter of the cervix are also checked. The secreted biological secret is analyzed (by color, consistency, clots, tissue remnants). This study allows you to determine whether the fetal egg has completely come out.
If bleeding of unclear etiology occurs, an ultrasound of the uterus and appendages, as well as the location of the fetus, is prescribed. If the fetal egg is in place, it is checked whether its detachment has occurred. Through ultrasound, the tone of the uterus is determined. If she is in a state of tension, the threat of miscarriage is diagnosed.
Based on the results of the examinations and ultrasound, a final diagnosis is made, and a decision is made on further monitoring of the patient. If there is a risk of spontaneous abortion, the woman is hospitalized in a medical facility.
If there is a risk of spontaneous abortion, the woman is hospitalized in a medical facility.
Treatment for threatened miscarriage
If there is an increased risk of miscarriage it is recommended to stay in bed at all times, getting up only when absolutely necessary.
Medical therapy consists in taking the following drugs:
- hormone-containing drugs;
- uterine antispasmodics;
- vitamin complex;
- sedatives.
In some cases, to maintain pregnancy, the cervix is narrowed with special medical threads. If there are genital infections, anti-inflammatory, antibiotic drugs are prescribed.
Preventive measures
Best prophylaxis is considered to be an examination of both partners before the conception of a child. Pregnant women should adhere to the following recommendations:
- give up bad habits, strong tea, coffee;
- minimize physical activity;
- refrain from stress;
- follow your inner feelings.