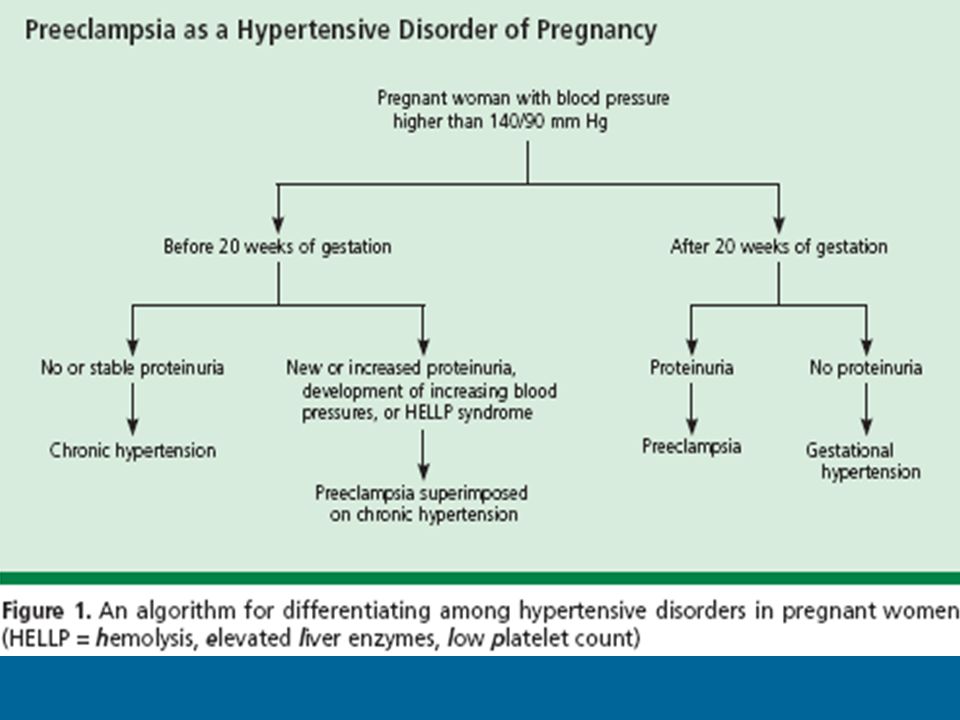
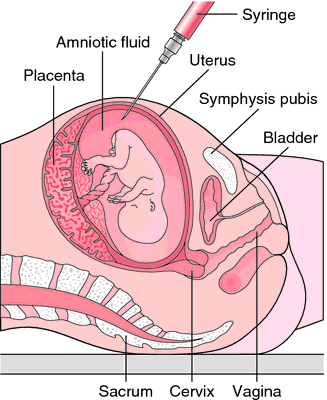
Induction process in pregnancy
Inducing labour - NHS
An induced labour is one that's started artificially. Every year, 1 in 5 labours are induced in the UK.
Sometimes labour can be induced if your baby is overdue or there's any risk to you or your baby's health.
This risk could be if you have a health condition such as high blood pressure, for example, or your baby is not growing.
Induction will usually be planned in advance. You'll be able to discuss the advantages and disadvantages with your doctor and midwife, and find out why they think your labour should be induced.
It's your choice whether to have your labour induced or not.
If your pregnancy lasts longer than 42 weeks and you decide not to have your labour induced, you should be offered increased monitoring to check your baby's wellbeing.
Why you might be induced
- if you're overdue
- if your waters have broken
- if you or your baby have a health problem
If you're overdue
Induction will be offered if you do not go into labour naturally by 42 weeks, as there will be a higher risk of stillbirth or problems for the baby.
If your waters break early
If your waters break more than 24 hours before labour starts, there's an increased risk of infection to you and your baby.
If your waters break after 34 weeks, you'll have the choice of induction or expectant management.
Expectant management is when your healthcare professionals monitor your condition and your baby's wellbeing, and your pregnancy can progress naturally as long as it's safe for both of you.
Your midwife or doctor should discuss your options with you before you make a decision.
They should also let you know about the newborn (neonatal) special care hospital facilities in your area.
If your baby is born earlier than 37 weeks, they may be vulnerable to problems related to being premature.
If your waters break before 34 weeks, you'll only be offered induction if there are other factors that suggest it's the best thing for you and your baby.
If you have a health condition or your baby is not thriving
You may be offered an induction if you have a condition that means it'll be safer to have your baby sooner, such as diabetes, high blood pressure or intrahepatic cholestasis of pregnancy.
If this is the case, your doctor and midwife will explain your options to you so you can decide whether or not to have your labour induced.
Membrane sweep
Before inducing labour, you'll be offered a membrane sweep, also known as a cervical sweep, to bring on labour.
To carry out a membrane sweep, your midwife or doctor sweeps their finger around your cervix during an internal examination.
This action should separate the membranes of the amniotic sac surrounding your baby from your cervix. This separation releases hormones (prostaglandins), which may start your labour.
Having a membrane sweep does not hurt, but expect some discomfort or slight bleeding afterwards.
If labour does not start after a membrane sweep, you'll be offered induction of labour.
Induction is always carried out in a hospital maternity unit. You'll be looked after by midwives and doctors will be available if you need their help.
How labour is induced
If you're being induced, you'll go into the hospital maternity unit.
Contractions can be started by inserting a tablet (pessary) or gel into your vagina.
Induction of labour may take a while, particularly if the cervix (the neck of the uterus) needs to be softened with pessaries or gels.
You will usually stay in the hospital maternity unit while you wait for it to work.
If you've had no contractions after 6 hours, you may be offered another tablet or gel.
If you have a controlled-release pessary inserted into your vagina, it can take 24 hours to work. If you are not having contractions after 24 hours, you may be offered another dose.
Sometimes a hormone drip is needed to speed up the labour. Once labour starts, it should proceed normally, but it can sometimes take 24 to 48 hours to get you into labour.
What induced labour feels like
Induced labour is usually more painful than labour that starts on its own, and you may want to ask for an epidural.
Your pain relief options during labour are not restricted by being induced. You should have access to all the pain relief options usually available in the maternity unit.
If you are induced you'll be more likely to have an assisted delivery, where forceps or ventouse suction are used to help the baby out.
If induction of labour does not work
Induction is not always successful, and labour may not start.
Your obstetrician and midwife will assess your condition and your baby's wellbeing, and you may be offered another induction or a caesarean section.
Your midwife and doctor will discuss all your options with you.
Natural ways to start labour
There are no proven ways of starting your labour yourself at home.
You may have heard that certain things can trigger labour, such as herbal supplements and having sex, but there's no evidence that these work.
Other methods that are not supported by scientific evidence include acupuncture, homeopathy, hot baths, castor oil and enemas.
Having sex will not cause harm, but you should avoid having sex if your waters have broken as there's an increased risk of infection.
For more information on induction, you can read the NICE information for the public on induction of labour.
Video: When would I be induced and what's involved?
In this video, a midwife describes what an induction of labour is and what is involved.
Media last reviewed: 20 March 2020
Media review due: 20 March 2023
Labor induction - Mayo Clinic
Overview
Labor induction — also known as inducing labor — is prompting the uterus to contract during pregnancy before labor begins on its own for a vaginal birth.
A health care provider might recommend inducing labor for various reasons, primarily when there's concern for the mother's or baby's health. An important factor in predicting whether an induction will succeed is how soft and expanded the cervix is (cervical ripening). The gestational age of the baby as confirmed by early, regular ultrasounds also is important.
The gestational age of the baby as confirmed by early, regular ultrasounds also is important.
If a health care provider recommends labor induction, it's typically because the benefits outweigh the risks. If you're pregnant, understanding why and how labor induction is done can help you prepare.
Products & Services
- Book: Mayo Clinic Family Health Book, 5th Edition
- Book: Mayo Clinic Guide to a Healthy Pregnancy
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Why it's done
To determine if labor induction is necessary, a health care provider will likely evaluate several factors. These include the mother's health and the status of the cervix. They also include the baby's health, gestational age, weight, size and position in the uterus. Reasons to induce labor include:
- Nearing 1 to 2 weeks beyond the due date without labor starting (postterm pregnancy).
- When labor doesn't begin after the water breaks (prelabor rupture of membranes).
- An infection in the uterus (chorioamnionitis).
- When the baby's estimated weight is less than the 10th percentile for gestational age (fetal growth restriction).
- When there's not enough amniotic fluid surrounding the baby (oligohydramnios).
- Possibly when diabetes develops during pregnancy (gestational diabetes), or diabetes exists before pregnancy.
- Developing high blood pressure in combination with signs of damage to another organ system (preeclampsia) during pregnancy. Or having high blood pressure before pregnancy, developing it before 20 weeks of pregnancy (chronic high blood pressure) or developing the condition after 20 weeks of pregnancy (gestational hypertension).
- When the placenta peels away from the inner wall of the uterus before delivery — either partially or completely (placental abruption).
- Having certain medical conditions. These include heart, lung or kidney disease and obesity.
Elective labor induction is the starting of labor for convenience when there's no medical need. It can be useful for women who live far from the hospital or birthing center or who have a history of fast deliveries.
It can be useful for women who live far from the hospital or birthing center or who have a history of fast deliveries.
A scheduled induction might help avoid delivery without help. In such cases, a health care provider will confirm that the baby's gestational age is at least 39 weeks or older before induction to reduce the risk of health problems for the baby.
As a result of recent studies, women with low-risk pregnancies are being offered labor induction at 39 to 40 weeks. Research shows that inducing labor at this time reduces several risks, including having a stillbirth, having a large baby and developing high blood pressure as the pregnancy goes on. It's important that women and their providers share in decisions to induce labor at 39 to 40 weeks.
Request an Appointment at Mayo Clinic
From Mayo Clinic to your inbox
Sign up for free, and stay up to date on research advancements, health tips and current health topics, like COVID-19, plus expertise on managing health.
To provide you with the most relevant and helpful information, and understand which information is beneficial, we may combine your email and website usage information with other information we have about you. If you are a Mayo Clinic patient, this could include protected health information. If we combine this information with your protected health information, we will treat all of that information as protected health information and will only use or disclose that information as set forth in our notice of privacy practices. You may opt-out of email communications at any time by clicking on the unsubscribe link in the e-mail.
Risks
Uterine incisions used during C-sections
Uterine incisions used during C-sections
A C-section includes an abdominal incision and a uterine incision. After the abdominal incision, the health care provider will make an incision in the uterus. Low transverse incisions are the most common (top left).
After the abdominal incision, the health care provider will make an incision in the uterus. Low transverse incisions are the most common (top left).
Labor induction carries various risks, including:
- Failed induction. An induction might be considered failed if the methods used don't result in a vaginal delivery after 24 or more hours. In such cases, a C-section might be necessary.
- Low fetal heart rate. The medications used to induce labor — oxytocin or a prostaglandin — might cause the uterus to contract too much, which can lessen the baby's oxygen supply and lower the baby's heart rate.
- Infection. Some methods of labor induction, such as rupturing the membranes, might increase the risk of infection for both mother and baby. The longer the time between membrane rupture and labor, the higher the risk of an infection.
-
Uterine rupture. This is a rare but serious complication in which the uterus tears along the scar line from a prior C-section or major uterine surgery.
 Rarely, uterine rupture can also occur in women who have not had previous uterine surgery.
Rarely, uterine rupture can also occur in women who have not had previous uterine surgery.An emergency C-section is needed to prevent life-threatening complications. The uterus might need to be removed.
- Bleeding after delivery. Labor induction increases the risk that the uterine muscles won't properly contract after giving birth, which can lead to serious bleeding after delivery.
Labor induction isn't for everyone. It might not be an option if:
- You've had a C-section with a classical incision or major uterine surgery
- The placenta is blocking the cervix (placenta previa)
- Your baby is lying buttocks first (breech) or sideways (transverse lie)
- You have an active genital herpes infection
- The umbilical cord slips into the vagina before delivery (umbilical cord prolapse)
If you have had a C-section and have labor induced, your health care provider is likely to avoid certain medications to reduce the risk of uterine rupture.
How you prepare
Labor induction is typically done in a hospital or birthing center. That's because mother and baby can be monitored there, and labor and delivery services are readily available.
What you can expect
During the procedure
There are various ways of inducing labor. Depending on the circumstances, the health care provider might use one of the following ways or a combination of them. The provider might:
-
Ripen the cervix. Sometimes prostaglandins, versions of chemicals the body naturally produces, are placed inside the vagina or taken by mouth to thin or soften (ripen) the cervix. After prostaglandin use, the contractions and the baby's heart rate are monitored.
In other cases, a small tube (catheter) with an inflatable balloon on the end is inserted into the cervix. Filling the balloon with saline and resting it against the inside of the cervix helps ripen the cervix.
- Sweep the membranes of the amniotic sac.
 With this technique, also known as stripping the membranes, the health care provider sweeps a gloved finger over the covering of the amniotic sac near the fetus. This separates the sac from the cervix and the lower uterine wall, which might help start labor.
With this technique, also known as stripping the membranes, the health care provider sweeps a gloved finger over the covering of the amniotic sac near the fetus. This separates the sac from the cervix and the lower uterine wall, which might help start labor. -
Rupture the amniotic sac. With this technique, also known as an amniotomy, the health care provider makes a small opening in the amniotic sac. The hole causes the water to break, which might help labor go forward.
An amniotomy is done only if the cervix is partially dilated and thinned, and the baby's head is deep in the pelvis. The baby's heart rate is monitored before and after the procedure.
- Inject a medication into a vein. In the hospital, a health care provider might inject a version of oxytocin (Pitocin) — a hormone that causes the uterus to contract — into a vein. Oxytocin is more effective at speeding up labor that has already begun than it is as at cervical ripening.
 The provider monitors contractions and the baby's heart rate.
The provider monitors contractions and the baby's heart rate.
How long it takes for labor to start depends on how ripe the cervix is when the induction starts, the induction techniques used and how the body responds to them. It can take minutes to hours.
After the procedure
In most cases, labor induction leads to a vaginal birth. A failed induction, one in which the procedure doesn't lead to a vaginal birth, might require another induction or a C-section.
By Mayo Clinic Staff
Related
Products & Services
Induction of labor or induction of labor
The purpose of this informational material is to familiarize the patient with the induction of labor procedure and to provide information on how and why it is performed.
In most cases, labor begins between the 37th and 42nd weeks of pregnancy. Such births are called spontaneous. If drugs or medical devices are used before the onset of spontaneous labor, then the terms "stimulated" or "induced" labor are used in this case. nine0003
nine0003
Labor should be induced when further pregnancy is for some reason unsafe for the mother or baby and it is not possible to wait for spontaneous labor to begin.
The purpose of stimulation is to start labor by stimulating uterine contractions.
When inducing labor, the patient must be in the hospital so that both mother and baby can be closely monitored.
Labor induction methods
The choice of labor induction method depends on the maturity of the cervix of the patient, which is assessed using the Bishop scale (when viewed through the vagina, the position of the cervix, the degree of its dilatation, consistency, length, and the position of the presenting part of the fetus in the pelvic area are assessed). Also important is the medical history (medical history) of the patient, for example, a past caesarean section or operations on the uterus.
The following methods are used to induce (stimulate) labor:
- Oral misoprostol is a drug that is a synthetic analogue of prostaglandins found in the body.
 It prepares the body for childbirth, under its action the cervix becomes softer and begins to open.
It prepares the body for childbirth, under its action the cervix becomes softer and begins to open. - Balloon Catheter - A small tube is placed in the cervix and the balloon attached to the end is filled with fluid to apply mechanical pressure to the cervix. When using this method, the cervix becomes softer and begins to open. The balloon catheter is kept inside until it spontaneously exits or until the next gynecological examination. nine0022
- Amniotomy or opening of the fetal bladder - in this case, during a gynecological examination, when the cervix has already dilated sufficiently, the fetal bladder is artificially opened. When the amniotic fluid breaks, spontaneous uterine contractions will begin, or intravenous medication may be used to stimulate them.
- Intravenously injected synthetic oxytocin - acts similarly to the hormone of the same name produced in the body. The drug is given by intravenous infusion when the cervix has already dilated (to support uterine contractions).
 The dose of the drug can be increased as needed to achieve regular uterine contractions. nine0022
The dose of the drug can be increased as needed to achieve regular uterine contractions. nine0022
When is it necessary to induce labor?
Labor induction is recommended when the benefits outweigh the risks.
Induction of labor may be indicated in the following cases:
- The patient has a comorbid condition complicating pregnancy (eg, high blood pressure, diabetes mellitus, preeclampsia, or some other condition).
- The duration of pregnancy is already exceeding the norm - the probability of intrauterine death of the fetus increases after the 42nd week of pregnancy. nine0022
- Fetal problems, eg, problems with fetal development, abnormal amount of amniotic fluid, changes in fetal condition, various fetal disorders.
- If the amniotic fluid has broken and uterine contractions have not started within the next 24 hours, there is an increased risk of inflammation in both the mother and the fetus. This indication does not apply in case of preterm labor, when preparation of the baby's lungs with a special medicine is necessary before delivery.
 nine0022
nine0022 - Intrauterine fetal death.
What are the risks associated with labor induction?
Labor induction is not usually associated with significant complications.
Occasionally, after receiving misoprostol, a patient may develop fever, chills, vomiting, diarrhea, and too frequent uterine contractions (tachysystole). In case of too frequent contractions to relax the uterus, the patient is injected intravenously relaxing muscles uterus medicine. It is not safe to use misoprostol if you have had a previous caesarean section as there is a risk of rupture of the uterine scar.
The use of a balloon catheter increases the risk of inflammation inside the uterus.
When using oxytocin, the patient may rarely experience a decrease in blood pressure, tachycardia (rapid heartbeat), hyponatremia (lack of sodium in the blood), which may result in headache, loss of appetite, nausea, vomiting, abdominal pain, depression strength and sleepiness. nine0003
nine0003
Induction of labor, compared with spontaneous labor, increases the risk of prolonged labor, the need for instrumentation
(use of vacuum or forceps), postpartum hemorrhage, uterine rupture, the onset of too frequent uterine contractions and the associated deterioration of the fetus, prolapse umbilical cord, as well as premature detachment of the placenta.
If induction of labor is not successful
The time frame for induction of labor varies from patient to patient, on average labor begins within 24-72 hours. Sometimes more than one method is required. nine0003
The methods used do not always work on different patients in the same way and quickly. If the cervix does not dilate as a result of induction of labor, your doctor will tell you about your next options (which may include inducing labor later, using a different method, or delivering by caesarean section).
ITK833
This informational material was approved by the Women's Clinic on 01/01/2022.
Malignant tumors of the ovaries in pregnant women | Dobrokhotova Yu.E., Payanidi Yu.G., Borovkova E.I., Morozova K.V., Nagaitseva E.A., Arutyunyan A.M. nine0001
The article presents the results of a systematic analysis of data on the tactics of managing pregnant women with malignant neoplasms of the ovaries
Introduction
The risk of developing ovarian cancer (OC) is 1.7% and occurs mainly in the postmenopausal period [1]. The probability of developing OC in reproductive age does not exceed 0.01%. Most ovarian tumors are of germ cell origin (30%), 21% are borderline tumors, 28% are epithelial carcinomas, 3% are Krukenberg tumors, and 8% are other types of neoplasms [2, 3]. Among all tumor processes in pregnant women, OC ranks 5th after cancer of the cervix, breast, thyroid glands, and Hodgkin's lymphoma [1]. nine0003
The prevalence of OC increases with age, amounting to 0.2–1.4 cases up to 20 years old, 1.8–2.2 cases at 20–29 years old, 3. 1–5.1 cases at 20–39 years old, and 3.1–5.1 cases at 20–39 years old. 40-49 years old - 9.0-15.2 cases, at 50-59 years old - 21.8-28.3 cases, at 60-69 years old - 36.2-41.5 cases and after 70 years old - 47, 6–56.7 cases per 100,000 women [1].
1–5.1 cases at 20–39 years old, and 3.1–5.1 cases at 20–39 years old. 40-49 years old - 9.0-15.2 cases, at 50-59 years old - 21.8-28.3 cases, at 60-69 years old - 36.2-41.5 cases and after 70 years old - 47, 6–56.7 cases per 100,000 women [1].
During pregnancy, on average, 0.2–2% of ovarian formations are diagnosed, and approximately 1 to 6% of them are malignant [2, 3]. There are not very many publications on the topic of OC during pregnancy. According to a large study conducted since 1958 to 2007, OC was verified in 41 pregnant women [4]. The average age of the patients was 32.6 years (from 23 to 46 years), the stage of the disease was established in 39 cases: 59% - FIGO I, 5% - FIGO II, 26% - FIGO III, 10% - FIGO IV.
Epithelial ovarian tumors account for 50% of all tumors in pregnant women, germ cell tumors - one third, and the rest - stromal and other types of tumors (sarcoma, metastatic tumors).
About 50% of epithelial ovarian tumors found during pregnancy have a low malignant potential, and the remaining 50% are invasive. Epithelial ovarian tumors with low malignant potential during pregnancy can change their ultrastructure; morphological examination can reveal atypical signs characteristic of invasive cancer (nuclear polymorphism, anisocytosis, multifocal microinvasion). According to some studies, in 8 out of 10 serous neoplasms diagnosed during pregnancy, microscopic signs of a malignant process were detected, which regressed after childbirth [5–7]. nine0003
Epithelial ovarian tumors with low malignant potential during pregnancy can change their ultrastructure; morphological examination can reveal atypical signs characteristic of invasive cancer (nuclear polymorphism, anisocytosis, multifocal microinvasion). According to some studies, in 8 out of 10 serous neoplasms diagnosed during pregnancy, microscopic signs of a malignant process were detected, which regressed after childbirth [5–7]. nine0003
Clinical manifestations
Clinical manifestations of OC during pregnancy are usually absent. Diagnosis is by chance finding on ultrasound, during caesarean section, or by clinical manifestation of the disease in the postpartum period [4]. According to the results of a retrospective study of 8330 caesarean sections, 68 cases of newly diagnosed ovarian tumors with a diameter of more than 5 cm were described, and only 1 of them was confirmed to be malignant [8]. nine0003
Nonspecific symptoms of OC include abdominal and back pain, constipation, bloating, and dysuria [9, 10]. Acute pain may be associated with partial or complete adnexal torsion, which occurs in 5% of pregnant women. With a mass of 6 to 8 cm, the probability of torsion reaches 22%, in 60% it occurs in the range from 10 to 17 weeks. pregnancy. After 20 weeks pregnancy, the probability of torsion does not exceed 6% [3, 11].
Acute pain may be associated with partial or complete adnexal torsion, which occurs in 5% of pregnant women. With a mass of 6 to 8 cm, the probability of torsion reaches 22%, in 60% it occurs in the range from 10 to 17 weeks. pregnancy. After 20 weeks pregnancy, the probability of torsion does not exceed 6% [3, 11].
Diagnosis
Instrumental methods
Instrumental diagnostic methods, such as ultrasound, MRI and CT, allow you to make a preliminary diagnosis. Signs of a malignant process that suggest the presence of a neoplasm on ultrasound are the detection of volumetric formations, single- or multi-chambered, with a heterogeneous echostructure, a parietal component, and in some cases without a clear capsule [12–14]. A review of cases of detection of ovarian formations during pregnancy, in which a malignant process was subsequently verified, showed the predominance of tumors with a diameter of more than 10 cm with a rapid increase in their size by 3. 5 cm per week [11]. nine0003
5 cm per week [11]. nine0003
In most cases, ultrasound provides enough information to make a preliminary diagnosis. However, if differential diagnosis is required, MRI is the method of choice [15, 16].
Indications for CT during pregnancy are limited due to the high dose of ionizing radiation during this type of examination. Although fetal exposure to doses less than 0.05 Gy is not associated with an increased risk of miscarriage, birth defects or worsening perinatal mortality, there is a possibility of a possible increased risk of childhood cancer in the future [17]. nine0003
Table 1 presents the calculated radiation doses for the fetus depending on the diagnostic method used (Table 1) [17, 18].
It has been proven that with external irradiation of 50 Gy, from 0.04 to 0.15 Gy penetrates to the fetus in the first trimester, and up to 2 Gy in the III trimester [19]. Up to 16 weeks pregnancy, the threshold for conditionally safe prenatal exposure to radiation is from 0. 1 to 0.2 Gy (10–20 rad). After 16 weeks it increases to 0.5–0.7 Gy (50–70 rad) [20]. nine0003
1 to 0.2 Gy (10–20 rad). After 16 weeks it increases to 0.5–0.7 Gy (50–70 rad) [20]. nine0003
Tumor markers
A number of serum markers used in screening programs are specific for sex cord tumors and ovarian stromal tumors (alpha-fetoprotein, inhibin A) (Table 2) [12].
An inexplicable increase in these parameters in the blood serum of a pregnant woman may be the first sign of a tumor process [12]. At the same time, the determination of the level of the oncomarker CA 125 (Cancer Antigen 125) is not very informative, since its level usually increases with increasing gestational age (Table 3) [12]. nine0003
Changes in the levels of most markers of the tumor process are difficult to interpret during pregnancy, since many of them (AFP, hCG, CEA, CA 125) are involved in biological functions associated with intrauterine development, differentiation and maturation of fetal organs and systems. The levels of markers are elevated during pregnancy and fluctuate depending on its duration, and can also be abnormally elevated due to a violation of the placentation process or against the background of preeclampsia, Down syndrome, neural tube defect [12]. CA 125 is produced in healthy tissues and can be elevated in early pregnancy and immediately after delivery [12]. CA 125 is informative as a marker of epithelial ovarian cancer in the period from the 15th week. and before childbirth, since its serum values at this time increase slightly. The range from 1000 to 10,000 is likely (but not necessarily) associated with OC. nine0003
CA 125 is produced in healthy tissues and can be elevated in early pregnancy and immediately after delivery [12]. CA 125 is informative as a marker of epithelial ovarian cancer in the period from the 15th week. and before childbirth, since its serum values at this time increase slightly. The range from 1000 to 10,000 is likely (but not necessarily) associated with OC. nine0003
Serum alpha-fetoprotein increases during pregnancy as the term increases and with the development of malformations (neural tube defect). High AFP production is characteristic of germ cell tumors (endodermal cancer, embryonic cancer, mixed tumors). Its level often exceeds 1000 ng/ml, and in endodermal tumors >10,000 ng/ml [5, 21, 22].
The level of lactate dehydrogenase (LDH) increases in women with ovarian dysgerminoma and is a specific marker of this tumor even during pregnancy [21]. This indicator does not change during a normal pregnancy; its relative increase is possible in severe preeclampsia and HELLP syndrome [22]. nine0003
nine0003
Inhibin A is synthesized by syncytiotrophoblast cells, and its concentration in blood serum gradually increases during pregnancy [23, 24]. This limits the value of inhibin as a tumor marker in pregnancy.
The beta subunit of human chorionic gonadotropin (hCG) is a marker for a number of tumors, especially chorionic carcinoma (Table 1). However, this indicator cannot be used as a tumor process marker. nine0003
The production of human epididymal protein 4 (HE4) is significantly increased in OC. The HE4 score is indicated to monitor the effectiveness of therapy, the risk of recurrence or progression of the disease, but not for primary screening. In a study of serum samples from 67 pregnant women without OC, the median HE4 was significantly lower than that of healthy premenopausal nonpregnant women (30.5 vs. 46.6 pmol/l) [25]. The 95th percentiles for HE4 in pregnant women in the first, second, and third trimesters were 49.6, 35.1 and 50.2 pmol/l, respectively.
Management of pregnant women with ovarian cancer
Surgery
The management of pregnant women with cancer always presents the clinician with difficult choices when deciding whether to proceed with therapy. According to the international consensus regarding the treatment of patients with ovarian masses during pregnancy [2, 3, 7], surgical treatment is indicated if:
formation persists in the second trimester of pregnancy and
growth larger than 10 cm in diameter or
the structure of the formation is heterogeneous, with a combination of hyperechoic and anechoic structures, cystic cavities with a suspicion of a malignant process according to ultrasound.
This tactic is justified not only for the early diagnosis of the tumor process, but also to prevent torsion or rupture of the formation [6].
The optimal time for surgical treatment is the beginning of the second trimester.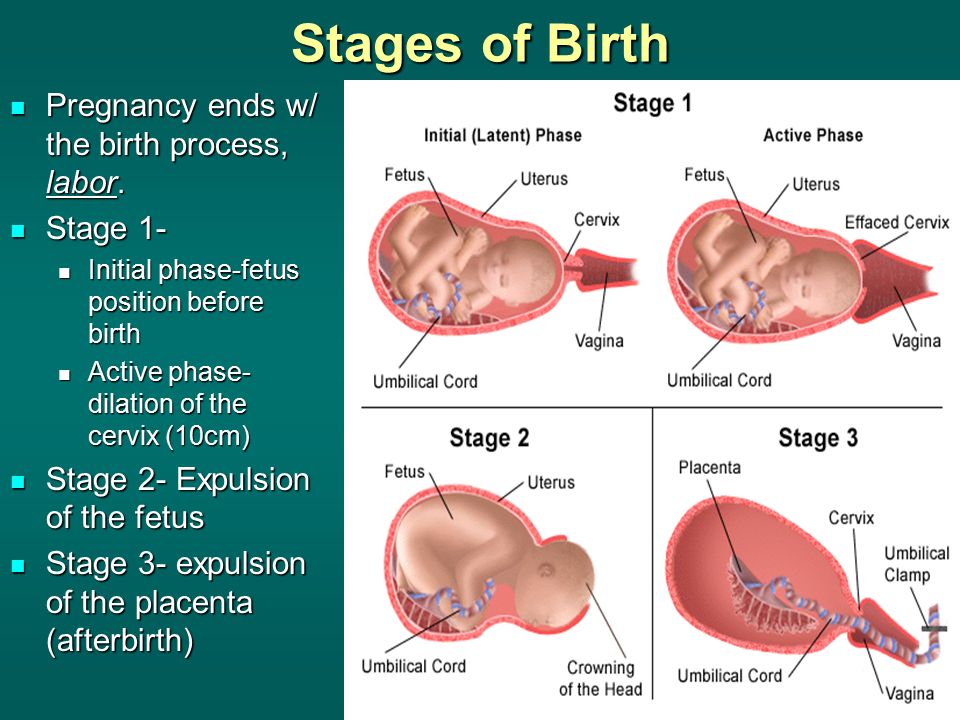 This is due to the following factors: nine0003
This is due to the following factors: nine0003
The processes of organogenesis are almost completed, which expands the possibilities of using drugs and reduces the risk of their teratogenic effects.
The hormonal production of the corpus luteum is completely replaced by the formed placenta; therefore, oophorectomy will not affect the risk of abortion.
The risk of abortion during surgery is minimal in the second trimester.
By the beginning of the second trimester of pregnancy, most functional ovarian cysts undergo reverse development [8]. nine0003
If a malignant process in the ovaries is suspected during pregnancy, the operation should be performed within 14–20 weeks. When performing a surgical intervention for up to 14 weeks. there is a high risk of damage to the corpus luteum, and in the third trimester there is a danger of provoking premature birth. The volume of the operation performed depends on the results of an urgent histological examination.
In the early stages of OC, surgical staging is indicated, including intraperitoneal and retroperitoneal stages. During the revision, the subdiaphragmatic space, the greater and lesser omentum, the small and large intestines and their mesentery, the surface of the parietal and visceral peritoneum, and the retroperitoneal space are carefully examined. In the presence of ascites, its cytological examination is carried out, in its absence, a cytological examination of washings from the peritoneum, including from its diaphragmatic surface, lateral canals and small pelvis. Bilateral salpingo-oophorectomy or unilateral salpingo-oophorectomy with biopsy of the contralateral ovary with urgent histological examination is indicated. Intraoperative damage to the tumor capsule with subsequent dissemination of tumor cells is unacceptable. It is advisable to remove the appendix in case of mucinous cancer, as well as if it is suspected. The minimum level of omentectomy is at the level of the transverse colon in stage I, and in advanced OC, the greater omentum is removed at the level of the greater curvature of the stomach. All affected areas of the peritoneum should be biopsied. In addition, a biopsy of the peritoneum of the walls of the small pelvis, recto-uterine recess, bladder, lateral canals, as well as the right and left domes of the diaphragm is mandatory, even in the absence of metastases according to the examination. At stages IA–IIA of the disease, it is recommended to perform pelvic and para-aortic lymphadenectomy [26]. In this case, the internal, external, common iliac, obturator, preaortic, paraaortic, aortocaval, precaval and paracaval lymph nodes to the level of the renal vessels are subject to removal. Surgery can be performed either laparoscopically (up to 20 weeks gestation) or by laparotomy. nine0003
All affected areas of the peritoneum should be biopsied. In addition, a biopsy of the peritoneum of the walls of the small pelvis, recto-uterine recess, bladder, lateral canals, as well as the right and left domes of the diaphragm is mandatory, even in the absence of metastases according to the examination. At stages IA–IIA of the disease, it is recommended to perform pelvic and para-aortic lymphadenectomy [26]. In this case, the internal, external, common iliac, obturator, preaortic, paraaortic, aortocaval, precaval and paracaval lymph nodes to the level of the renal vessels are subject to removal. Surgery can be performed either laparoscopically (up to 20 weeks gestation) or by laparotomy. nine0003
The ESGO guidelines (2017) do not contain information on the tactics of managing women of reproductive age who suffer from early-stage OC and who want to maintain fertility after delivery [26]. In other cases, after delivery, a complete cytoreductive operation is indicated, which includes extirpation of the uterus with appendages, removal of the greater omentum, pelvic and para-aortic lymphadenectomy (at stages IA–IIA) and all visible manifestations of the tumor process. The decision to conduct chemotherapy after surgery depends on the stage of the process, the morphological variant of the tumor and the degree of its malignancy. Adjuvant chemotherapy is not indicated for stage IA and IB and low-grade tumors. The exception is clear cell adenocarcinomas. With this morphological type of tumor, as well as with any type of tumor of a high degree of malignancy and the absence of full surgical staging procedures for stages IA and IB, 4–6 courses of adjuvant platinum-containing chemotherapy are performed. At stages IC–IV, 6 courses of therapeutic platinum-containing chemotherapy are indicated after surgery. nine0003
The decision to conduct chemotherapy after surgery depends on the stage of the process, the morphological variant of the tumor and the degree of its malignancy. Adjuvant chemotherapy is not indicated for stage IA and IB and low-grade tumors. The exception is clear cell adenocarcinomas. With this morphological type of tumor, as well as with any type of tumor of a high degree of malignancy and the absence of full surgical staging procedures for stages IA and IB, 4–6 courses of adjuvant platinum-containing chemotherapy are performed. At stages IC–IV, 6 courses of therapeutic platinum-containing chemotherapy are indicated after surgery. nine0003
In advanced OC after delivery, it is advisable to perform a complete or optimal cytoreductive operation. According to international studies, non-epithelial malignant neoplasms of the ovaries are diagnosed in the early stages in 90% of cases. In these cases, it is recommended to perform peritoneal surgical staging with preservation of pregnancy; retroperitoneal lymphadenectomy is not indicated. Removal of the affected uterine appendages with preservation of the contralateral ovary is considered to be an adequate volume of surgery in stage I. If the contralateral ovary is not visually altered, it is inappropriate to perform a biopsy. With a widespread tumor process, cytoreductive operations are indicated with the maximum removal of all tumor nodes and preservation of the contralateral ovary. Total lymphadenectomy for malignant germ cell tumors is not indicated even in the presence of metastases in the lymph nodes. nine0003
Removal of the affected uterine appendages with preservation of the contralateral ovary is considered to be an adequate volume of surgery in stage I. If the contralateral ovary is not visually altered, it is inappropriate to perform a biopsy. With a widespread tumor process, cytoreductive operations are indicated with the maximum removal of all tumor nodes and preservation of the contralateral ovary. Total lymphadenectomy for malignant germ cell tumors is not indicated even in the presence of metastases in the lymph nodes. nine0003
The combination of pregnancy and granulosa cell tumors of the ovaries is extremely rare. Nevertheless, patients with early stages of the disease after a thorough visual and palpation revision of the abdominal organs are performed unilateral adnexectomy.
Chemotherapy
After surgical staging, all pregnant women with OC are indicated for chemotherapy. The exception is patients with OC stage IA (G1, G2). In cases of advanced OC, chemotherapy may be the only treatment option to maintain the pregnancy [27]. nine0003
nine0003
At the same time, treatment regimens for pregnant women should not differ from those for non-pregnant women with this pathology. Chemotherapy is contraindicated in the first trimester of pregnancy, since its administration can lead to termination of pregnancy, intrauterine death of the fetus, as well as to malformations of its development [27]. In the second and third trimesters, chemotherapy can cause fetal growth retardation, prematurity, low birth weight, and stillbirth [28]. The side effects of chemotherapy on the mother's body are the same as in non-pregnant women receiving chemotherapy, i.e., it can manifest as myelosuppression, in particular neutropenia. Pregnant women are prescribed the standard chemotherapy regimen for OC: paclitaxel with carboplatin. Studies in animal models have shown that taxanes do not affect organogenesis and cognitive functions. However, the penetration of these drugs through the transplacental barrier in humans is not fully known [29].–31].
Non-epithelial neoplasms of the ovaries (germinogenic tumors, as well as tumors of the sex cord stroma) in pregnant women are diagnosed, as a rule, at stage I, the main method of their treatment is the removal of the affected uterine appendages while maintaining the contralateral ovary. If the contralateral ovary is not visually altered, it is inappropriate to perform a biopsy. For sex cord stromal tumors, postoperative chemotherapy is indicated for patients starting from the IC stage. In patients with malignant germ cell tumors after surgery, chemotherapy is not indicated for immature stage IA GI teratoma or stage IA dysgerminoma. In advanced stages, adjuvant chemotherapy is indicated, the same as in non-pregnant women. For them, the standard regimen is a combination of platinum with etoposide (BEP or EP) [32]. Based on literature data, the main treatment regimen includes a combination of cisplatin (75 mg/m 2 ) with weekly administration of paclitaxel (80 mg/m 2 ) from the second trimester [32–34].
Prognosis
Cancer prognosis
Oncological prognosis in pregnant women with OC was presented by Norwegian researchers H. Stensheim et al. (2009) [35]. There was no significant association between pregnancy and mortality from OC, but this risk increased in lactating women (HR 2.23; 95% CI, 1.05–4.73; p = 0.036). The study, which included 105 pregnant women with OC, showed that the worst prognosis was for poorly differentiated tumors and advanced stages of the disease, the 2-year survival of such patients was 30.0% and 25.0% (stage IV), respectively. nine0003
There are 2 reviews in the literature (n=46 and n=102) on non-epithelial malignant ovarian neoplasms and pregnancy [36]. The vast majority of patients (>76%) were diagnosed with stage I disease and had a favorable oncological prognosis. In patients with sex cord stromal tumors, pregnancy was maintained in 69.4% of cases [36]. Of these, 13% needed chemotherapy, but only 4% received it during pregnancy. Overall 5-year survival was 89.3%. With advanced tumor processes (stages II–IV), survival was significantly lower (5-year survival rate for stages II–IV was 70% versus 100% for stage I, p=0.008). Relapses and rapid disease progression in sex cord stromal tumors occurred in 8.7% and 2.2% of cases, respectively. The second report concerns pregnant women with ovarian germ cell tumors (n=102), most of which were unilateral (84.3%) and were diagnosed at early stages (76.4%) [37–39]. More than half of the patients (52.0%) received systemic chemotherapy according to the scheme of cisplatin with bleomycin. In 7 (6.9%) patients, relapse occurred during pregnancy. Alpha-fetoprotein was elevated only in 4 (57.1%) patients with relapses of the disease, which once again emphasizes the limited possibilities of its use in monitoring pregnant women suffering from this pathology. Most often, in pregnant women, recurrent tumors were localized in the abdominal cavity. Overall 5-year survival was 80.1%. Young patient age (≤20 vs >20), large tumor size (<20 cm vs ≥20 cm), and advanced disease stage (II–IV vs stage I) are the most important prognostic factors associated with poor prognosis.
Overall 5-year survival was 89.3%. With advanced tumor processes (stages II–IV), survival was significantly lower (5-year survival rate for stages II–IV was 70% versus 100% for stage I, p=0.008). Relapses and rapid disease progression in sex cord stromal tumors occurred in 8.7% and 2.2% of cases, respectively. The second report concerns pregnant women with ovarian germ cell tumors (n=102), most of which were unilateral (84.3%) and were diagnosed at early stages (76.4%) [37–39]. More than half of the patients (52.0%) received systemic chemotherapy according to the scheme of cisplatin with bleomycin. In 7 (6.9%) patients, relapse occurred during pregnancy. Alpha-fetoprotein was elevated only in 4 (57.1%) patients with relapses of the disease, which once again emphasizes the limited possibilities of its use in monitoring pregnant women suffering from this pathology. Most often, in pregnant women, recurrent tumors were localized in the abdominal cavity. Overall 5-year survival was 80.1%. Young patient age (≤20 vs >20), large tumor size (<20 cm vs ≥20 cm), and advanced disease stage (II–IV vs stage I) are the most important prognostic factors associated with poor prognosis. At the same time, the stage of the disease remains an independent prognostic factor (HR 21.6, 95% CI 2.06–226, p=0.01).
At the same time, the stage of the disease remains an independent prognostic factor (HR 21.6, 95% CI 2.06–226, p=0.01).
Obstetric prognosis
Pregnancy complicated by cancer is always associated with a high risk of complications, regardless of the methods of treatment. Prematurity, fetal growth retardation, abortion (including stillbirth) are the most common obstetric complications. A population-based study conducted from 1973 to 2012 showed that cancer diagnosed during pregnancy resulted in high neonatal mortality associated mainly with fetal growth retardation and iatrogenic preterm birth [37]. In OC and pregnancy, obstetric complications can be caused not only by the underlying disease, but also by complications due to surgical treatment and chemotherapy. A review published in 2015 described 105 cases of invasive OC during pregnancy. The majority of pregnancies (81.3%) resulted in the birth of live children. The most common cause of fetal and newborn death was preterm birth. More than half of the patients (71.6%) delivered by caesarean section. In women who received chemotherapy during pregnancy, no fetal malformations were detected, in addition, there was no statistically significant difference in fetal growth retardation during chemotherapy. Obstetric outcome in OC mainly depends on the stage of the disease and the histological type of the tumor. nine0003
More than half of the patients (71.6%) delivered by caesarean section. In women who received chemotherapy during pregnancy, no fetal malformations were detected, in addition, there was no statistically significant difference in fetal growth retardation during chemotherapy. Obstetric outcome in OC mainly depends on the stage of the disease and the histological type of the tumor. nine0003
Clinical cases
1. Patient S., 34 years old. Was registered in the antenatal clinic from 6 weeks. No complaints were made. During ultrasound screening in the first trimester, bilateral ovarian masses were detected: the right ovary, 88 × 47 × 57 mm in size, was represented by a formation with even contours, uniformly increased echogenicity, with single cystic inclusions. In the center is a formation with zones of vascularization and blood flow (IR 0.82, speed 22 cm/s). The left ovary, 57 × 40 × 35 mm, is represented by a formation with thickened walls and septa, with papillary growths and CFD zones with an IR of 0.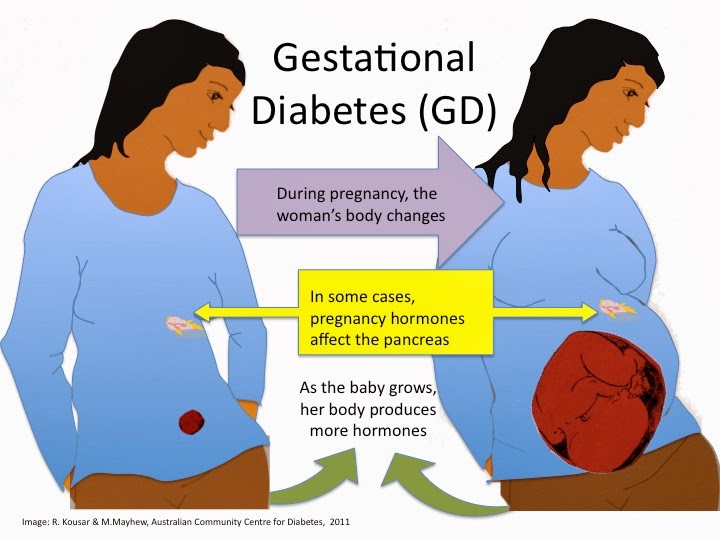 65 and a velocity of 8 cm/s (Fig. 1). nine0003
65 and a velocity of 8 cm/s (Fig. 1). nine0003
At 12-13 weeks. The patient underwent diagnostic laparoscopy, lower median laparotomy, right adnexectomy, resection of the left ovary, resection of the omentum, biopsy of the peritoneum. Histological examination confirmed the development of serous papillary cystadenocarcinoma, intravascular tumor emboli in the omentum, and peritoneal metastases.
An oncological consultation was held. Diagnosis “Pregnancy 14-15 weeks, associated with oncopathology. Ovarian cancer T3cNxM0. Condition after diagnostic laparoscopy, lower median laparotomy, right-sided adnexectomy, resection of the left ovary, resection of the omentum, biopsy of the peritoneum. According to the order of the Ministry of Health of Russia dated 03.12.2007 No. 736 “On approval of the list of medical indications for artificial termination of pregnancy”, abortion is indicated, followed by induction courses of chemotherapy, with the decision to perform a cytoreductive operation. nine0003
nine0003
2. Patient S., 28 years old. She was admitted to the hospital with an acute pain syndrome. Diagnosis at admission: “Pregnancy 30–31 weeks. Head presentation. Renal colic?". The patient was observed in the antenatal clinic from 6 weeks. and was examined in accordance with the order of the Ministry of Health of Russia No. 572n. Upon admission to the hospital, attention was drawn to the pronounced displacement of the pregnant uterus to the left and a palpable soft-elastic formation occupying the entire right lateral part of the abdomen. A comprehensive examination has been carried out. Ultrasound of the abdominal organs: the echographic picture may correspond to a retroperitoneal cyst on the right. MRI: under the liver anterior to the right kidney, a huge cystic formation with clear, even contours, size 19 is determined2 × 136 × 175 cm. Conclusion: MRI picture of a huge cystic formation in the subhepatic region, possibly a cyst of the right ovary (Fig. 2).
Due to the lack of indications for urgent delivery, it was decided to prolong the pregnancy up to 37 weeks.
At 37 weeks the patient was hospitalized in a planned manner, delivery was performed by caesarean section. Intraoperatively: a mass formation of a soft elastic consistency up to 20 cm in diameter was found to the right of the uterus. The tissue of the right ovary was not visualized, a right-sided adnexectomy was performed. The macropreparation is a thin-walled formation with a diameter of 20 cm and a weight of 3120 g, gray-blue in color, with a wall thickness of 0.5 cm. The inner surface is gray-yellow in color, a transparent yellowish liquid in the lumen (Fig. 3). nine0003
Postoperative diagnosis: “First emergency cephalic delivery at 37 weeks. Large tumor in the right ovary. Inferior median laparotomy. Caesarean section in the lower uterine segment. Adnexectomy on the right. A live full-term immature girl was born weighing 2680 g, 48 cm tall, with an Apgar score of 8/8 points. The puerperal woman was discharged in a satisfactory condition on the 5th day after the operation.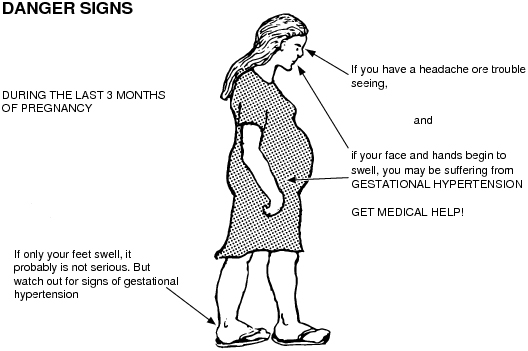
Histological conclusion: fragment of the fallopian tube wall, serous cystoadenoma of the right ovary. nine0003
3. Patient A., aged 22, was admitted to the hospital with a diagnosis of "Pregnancy 26-27 weeks, associated with breast cancer. Head presentation. Placental insufficiency, compensated form. Stage IV breast cancer. T4bN3M1. Metastases in the ovary, lymph nodes of the neck on the right. Right-sided metastatic pleurisy. Condition after 1 course of polychemotherapy.
This pregnancy is the first, registered in the antenatal clinic was from 7 weeks. AT 9weeks the patient independently discovered a painless induration in the right mammary gland, she did not seek medical help. From 22 weeks notes the appearance of compaction and soreness in the neck on the right. With the complaints described above, she was hospitalized in the department of general oncology of the Central Union of the Russian Federation, where a tumor of the right breast (infiltrative-edematous form), metastases to the axillary, sub-, supraclavicular and cervical lymph nodes on the right, metastases to the ovaries, ascites were diagnosed. A trepanobiopsy of a tumor of the right mammary gland, axillary lymph node on the right, tumor of the left ovary was performed. The result of histological examination: invasive cancer of the right breast, special type, clear cell carcinoma, rich in glycogen, grade 3, metastases in the axillary lymph node and left ovary. Molecular biological subtype of metastatic breast carcinoma to the ovary. Triple negative ( triple-negative ) basal-like type of tumor. RE neg., RP neg., HER2neu neg., Ki65 — 35%.
A trepanobiopsy of a tumor of the right mammary gland, axillary lymph node on the right, tumor of the left ovary was performed. The result of histological examination: invasive cancer of the right breast, special type, clear cell carcinoma, rich in glycogen, grade 3, metastases in the axillary lymph node and left ovary. Molecular biological subtype of metastatic breast carcinoma to the ovary. Triple negative ( triple-negative ) basal-like type of tumor. RE neg., RP neg., HER2neu neg., Ki65 — 35%.
According to CT scan of the chest revealed an increase in the lymph nodes of the mediastinum, the roots of the lungs, the right axillary region, bilateral exudative pleurisy. Ultrasound of the pelvic organs: in the projection of the left ovary, a formation with uneven tuberous contours 137 × 90 × 116 mm, a heterogeneous echostructure with anechoic inclusions, radially penetrating vessels with high-speed (22 cm/s) and low-resistance (IR - 0.25) blood flow characteristics is visualized (Fig. 4). The area of the right appendages is not available for inspection. No free fluid was found in the pelvis and abdominal cavity. nine0003
4). The area of the right appendages is not available for inspection. No free fluid was found in the pelvis and abdominal cavity. nine0003
Taking into account the stage and prevalence of the process, the patient underwent 2 courses of polychemotherapy with an interval of 3 weeks. according to the scheme: doxorubicin 80 mg + cyclophosphamide 80 mg. After the second course of polychemotherapy, progression of the disease was noted. Diagnosis: cancer of the right breast cT4bN3M1 (mts in the pleura, l / nodes of the neck on the right, ovaries). 2 courses of PCT. Progression (mts to left breast). Pregnancy 30 weeks. IHCI: Pgr — 0 points, Her2-neu — 0, Ki65 — 35%. Triple negative type. T4bN2M1. Stage IV nine0003
An oncoconsilium was held, and according to its conclusion, a decision was made to conduct early delivery by caesarean section with bilateral adnexectomy. The patient was prepared for operative delivery, prophylaxis of fetal respiratory distress syndrome (dexamethasone 24 mg) was carried out. In a planned manner, a team consisting of obstetrician-gynecologists and oncologists performed a lower median laparotomy, caesarean section in the lower uterine segment, bilateral adnexectomy, subtotal resection of the omentum, resection of the peritoneum. nine0003
In a planned manner, a team consisting of obstetrician-gynecologists and oncologists performed a lower median laparotomy, caesarean section in the lower uterine segment, bilateral adnexectomy, subtotal resection of the omentum, resection of the peritoneum. nine0003
Intraoperatively, the left ovary was found to be a cystic-tuberous tumor with a predominance of a solid structure, 20 × 15 × 10 cm in size, richly vascularized, easily injured on contact (Fig. 5). On the right, an ovary of a similar macroscopic structure, 6 × 4 × 4 cm in size. Intact ovarian tissue is not visualized.
According to the results of the histological conclusion: the right ovary is represented by a tumor tissue built from polymorphic cells with anisochromic nuclei, forming alveolar, trabecular structures and solid fields. There is no vascular invasion. nine0271 The left ovary is represented by a tumor tissue built from polymorphic cells with anisochromic nuclei, forming alveolar, trabecular structures and solid fields.