Immunity from breast milk
Breastfeeding Benefits Your Baby’s Immune System
Log in | Register
Ages & Stages
Ages & Stages
Listen
Español
Text Size
By: Claire McCarthy, MD, FAAP
Breast milk is the food naturally designed to best meet the needs of human babies. It has all the necessary nutrients, in just the right amounts, and is easy to digest. Beyond the nutritional benefits, here's a great bonus: Breast milk also helps build and support your baby's immune system. Read on to learn how.
Breast milk: food & infection fighter
Breast milk contains antibodies that can fight infection. Those antibodies are present in high amounts in
colostrum, the first milk that comes out of the breasts after birth. However, there are antibodies in breastmilk the entire time a mother continues to nurse. Through these antibodies, the mother can pass on some protection from infectious illness she had in the past, and those she gets while breastfeeding. Breast milk can literally give babies a head start in preventing and fighting infections.
Breast milk also is made up of other proteins, fats, sugars and even white blood cells that work to fight infection in many different ways. They are especially helpful in fighting gastrointestinal infections, since breast milk heads right to the stomach and intestine when your baby eats. The different factors in breast milk work directly within the intestine before being absorbed and reaching the entire body. This also sets the stage for a protective and balanced immune system that helps recognize and fight infections and other diseases even after breastfeeding ends.
Other factors in breast milk directly stimulate and support the immune system. These include lactoferrin and interleukin-6, -8 and -10. These proteins help to balance the immune system inflammatory response, which is needed for immune function but can be damaging in excess.
These proteins help to balance the immune system inflammatory response, which is needed for immune function but can be damaging in excess.
There's even evidence that nursing mothers who are vaccinated against COVID-19 can pass along antibodies to the virus through breast milk. Although it's not proven, these antibodies may help protect babies too young for the vaccine. (See Breastfeeding During the COVID-19 Pandemic.")
Is breastmilk probiotic?
Breast milk has "probiotic" factors, too. Some support the immune system and others serve as a nutrient source for healthy bacteria in the body, called the human microbiome. The healthy microbiome can play a lifelong role in not only preventing infection, but also in decreasing the risk of allergies, asthma, obesity and other chronic diseases.
With all these immunity-boosting factors in breast milk, it is not surprising that breastfed babies are less likely to suffer from ear infections, vomiting, diarrhea, pneumonia, urinary tract infections, and certain types of
meningitis. Research also shows that children who nurse for more than six months are less likely to develop childhood
leukemia and lymphoma than those who receive formula. This may be in part because these types of cancer are affected by disruptions to the immune system.
Research also shows that children who nurse for more than six months are less likely to develop childhood
leukemia and lymphoma than those who receive formula. This may be in part because these types of cancer are affected by disruptions to the immune system.
Remember
To help keep babies healthy, communities can take steps to support mothers who choose to breastfeed their babies. This can include offering paid leave and giving employees places and time to pump breast milk. If you're breastfeeding your baby or have any questions, never hesitate to talk with your pediatrician. If you can't breastfeed, or for personal reasons choose not to, talk to your pediatrician about the many other ways to support your baby's health.
More information
- Breastfeeding: AAP Policy Explained
- Breastfeeding During the COVID-19 Pandemic
About Dr. McCarthy
Claire McCarthy, MD, FAAP is a primary care pediatrician at Boston Children's Hospital, an Assistant Professor of Pediatrics at Harvard Medical School, a senior editor for Harvard Health Publications, and an official spokesperson for the American Academy of Pediatrics. She writes about health and parenting for the
Harvard Health Blog, Huffington Post and many other online and print publications.
She writes about health and parenting for the
Harvard Health Blog, Huffington Post and many other online and print publications.
- Last Updated
- 7/19/2022
- Source
- American Academy of Pediatrics (Copyright © 2020)
The information contained on this Web site should not be used as a substitute for the medical care and advice of your pediatrician. There may be variations in treatment that your pediatrician may recommend based on individual facts and circumstances.
Breastfeeding provides passive and likely long-lasting active immunity
Review
. 1998 Dec;81(6):523-33; quiz 533-4, 537.
doi: 10.1016/S1081-1206(10)62704-4.
L A Hanson 1
Affiliations
Affiliation
- 1 Department of Clinical Immunology, Göteborg University, Sweden. [email protected]
- PMID: 9892025
- DOI: 10.1016/S1081-1206(10)62704-4
Review
L A Hanson. Ann Allergy Asthma Immunol. 1998 Dec.
. 1998 Dec;81(6):523-33; quiz 533-4, 537.
doi: 10.1016/S1081-1206(10)62704-4.
Author
L A Hanson 1
Affiliation
- 1 Department of Clinical Immunology, Göteborg University, Sweden.
 [email protected]
[email protected]
- PMID: 9892025
- DOI: 10.1016/S1081-1206(10)62704-4
Abstract
Objectives: The reader of this review will learn about the mechanisms through which breastfeeding protects against infections during and most likely after lactation, as well as possibly against certain immunologic diseases, including allergy.
Data sources: I have followed the literature in the area closely for the last 30 to 40 years and have made repeated literature searches through MEDLINE, most recently in 1998. Textbooks and peer-reviewed journals have been sought for, as well as books representing meeting reports in English, French, German, and Spanish.
Results: Human milk protects against infections in the breastfed offspring mainly via the secretory IgA antibodies, but also most likely via several other factors like the bactericidal lactoferrin. It is striking that the defense factors of human milk function without causing inflammation, some components are even directly anti-inflammatory. Protection against infections has been well evidenced during lactation against, e.g., acute and prolonged diarrhea, respiratory tract infections, otitis media, urinary tract infection, neonatal septicemia, and necrotizing enterocolitis. There is also interesting evidence for an enhanced protection remaining for years after lactation against diarrhea, respiratory tract infections, otitis media, Haemophilus influenzae type b infections, and wheezing illness. In several instances the protection seems to improve with the duration of breastfeeding. Some, but not all studies have shown better vaccine responses among breastfed than non-breastfed infants. A few factors in milk like anti-antibodies (anti-idiotypic antibodies) and T and B lymphocytes have in some experimental models been able to transfer priming of the breastfed offspring. This together with transfer of numerous cytokines and growth factors via milk may add to an active stimulation of the infant's immune system. Consequently, the infant might respond better to both infections and vaccines. Such an enhanced function could also explain why breastfeeding may protect against immunologic diseases like coeliac disease and possibly allergy. Suggestions of protection against autoimmune diseases and tumors have also been published, but need confirmation.
A few factors in milk like anti-antibodies (anti-idiotypic antibodies) and T and B lymphocytes have in some experimental models been able to transfer priming of the breastfed offspring. This together with transfer of numerous cytokines and growth factors via milk may add to an active stimulation of the infant's immune system. Consequently, the infant might respond better to both infections and vaccines. Such an enhanced function could also explain why breastfeeding may protect against immunologic diseases like coeliac disease and possibly allergy. Suggestions of protection against autoimmune diseases and tumors have also been published, but need confirmation.
Conclusions: Breastfeeding may, in addition to the well-known passive protection against infections during lactation, have a unique capacity to stimulate the immune system of the offspring possibly with several long-term positive effects.
Similar articles
-
Session 1: Feeding and infant development breast-feeding and immune function.

Hanson LA. Hanson LA. Proc Nutr Soc. 2007 Aug;66(3):384-96. doi: 10.1017/S0029665107005654. Proc Nutr Soc. 2007. PMID: 17637091 Review.
-
Breastfeeding protects against illness and infection in infants and children: a review of the evidence.
Oddy WH. Oddy WH. Breastfeed Rev. 2001 Jul;9(2):11-8. Breastfeed Rev. 2001. PMID: 11550600 Review.
-
The impact of breastmilk on infant and child health.
Oddy WH. Oddy WH. Breastfeed Rev. 2002 Nov;10(3):5-18. Breastfeed Rev. 2002. PMID: 12592775 Review.
-
Human milk: Defense against infection.
Hanson LA, Söderström T.
Hanson LA, et al. Prog Clin Biol Res. 1981;61:147-59. Prog Clin Biol Res. 1981. PMID: 6798576 Review.
-
Immunology of breast milk.
Palmeira P, Carneiro-Sampaio M. Palmeira P, et al. Rev Assoc Med Bras (1992). 2016 Sep;62(6):584-593. doi: 10.1590/1806-9282.62.06.584. Rev Assoc Med Bras (1992). 2016. PMID: 27849237 Review.
See all similar articles
Cited by
-
A cross-sectional study evidences regulations of leukocytes in the colostrum of mothers with obesity.
Piñeiro-Salvador R, Vazquez-Garza E, Cruz-Cardenas JA, Licona-Cassani C, García-Rivas G, Moreno-Vásquez J, Alcorta-García MR, Lara-Diaz VJ, Brunck MEG. Piñeiro-Salvador R, et al. BMC Med.
2022 Nov 1;20(1):388. doi: 10.1186/s12916-022-02575-y. BMC Med. 2022. PMID: 36316769 Free PMC article.
-
Impact of the COVID-19 Pandemic on Breastfeeding Support Services and Women's Experiences of Breastfeeding: A Review.
Lubbe W, Niela-Vilén H, Thomson G, Botha E. Lubbe W, et al. Int J Womens Health. 2022 Oct 6;14:1447-1457. doi: 10.2147/IJWH.S342754. eCollection 2022. Int J Womens Health. 2022. PMID: 36225180 Free PMC article. Review.
-
Effects of Vaccination Against Influenza, Pertussis, and COVID-19 on Human Milk Antibodies: Current Evidence and Implications for Health Equity.
Hunagund S, Golan Y, Asiodu IV, Prahl M, Gaw SL. Hunagund S, et al. Front Immunol. 2022 Jul 12;13:910383. doi: 10.3389/fimmu.
2022.910383. eCollection 2022. Front Immunol. 2022. PMID: 35903100 Free PMC article. Review.
-
Maternal Stress and Human Milk Antibodies During the COVID-19 Pandemic.
Juncker HG, Ruhé EJM, Korosi A, van Goudoever JB, van Gils MJ, van Keulen BJ. Juncker HG, et al. Front Nutr. 2022 Jun 30;9:923501. doi: 10.3389/fnut.2022.923501. eCollection 2022. Front Nutr. 2022. PMID: 35845768 Free PMC article.
-
Quantification and Progress Over Time of Specific Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 in Breast Milk of Lactating Women Vaccinated With BNT162b2 Pfizer-BioNTech Coronavirus Disease 2019 Vaccine (LacCOVID).
Esteve-Palau E, Gonzalez-Cuevas A, Guerrero ME, Garcia-Terol C, Alvarez MC, Garcia G, Moreno E, Medina F, Casadevall D, Diaz-Brito V.
Esteve-Palau E, et al. Open Forum Infect Dis. 2022 May 11;9(6):ofac239. doi: 10.1093/ofid/ofac239. eCollection 2022 Jun. Open Forum Infect Dis. 2022. PMID: 35783685 Free PMC article.
See all "Cited by" articles
Publication types
MeSH terms
How breastfeeding affects the development of the child's immune system
Summary. With breast milk, cells of the mother's immune system enter the child's body
For a long time, scientists believed that breast milk provides immune protection against pathogens of infectious pathologies due to the presence of specific antibodies in it, forming the so-called passive immunity. In new work, researchers at the University of California, USA, have shown that breastfeeding, among other things, promotes the development of a child's own immune system through a process called "maternal learning immunity" by the team of scientists. The results of the work are presented in the Journal of Immunology.
Specific maternal immune cells found in breast milk pass through the baby's intestinal wall and travel to the thymus, an organ of the immune system. There, they "train" the developing cells to resist the pathogens that the mother encountered. In the course of the study, which used a specific model of feeding laboratory mice, scientists received important information about the vaccination of newborns. So, they noted that vaccination of a nursing mother has a significant impact on the state of the immune system of her child. The number of immune cells transferred from mother to child depends on what infectious agents and how often the woman has encountered. If her immunity against some pathogens is strained, she will pass on many immune defense cells through breast milk. Scientists have suggested that such mechanisms in the past contributed to the survival of children from royal families, since the nurses who were assigned to them were usually from the lower social strata and faced a large number of pathogens of infectious pathologies.
One of the infectious agents studied in the course of this work was the causative agent of tuberculosis, a widespread disease in many countries of the world, the incidence of which is constantly growing due to the emergence of antibiotic-resistant strains. In general, children vaccinated against tuberculosis rarely have a good immunological response - vaccination prevents the development of severe complications, but does not reduce the risk of the most common pulmonary form of the disease. The authors of the work hope that vaccination of nursing mothers can improve the level of immune protection of their children against tuberculosis. In this work, they obtained evidence that immunity created in this way is more effective than direct vaccination of a child. Undoubtedly, additional clinical studies are needed to make any recommendations.
The author of the work, Ameae Walker, emphasized that the identified mechanism for the transmission of immunological information is fundamentally new, it consists in copying maternal immune cells by the child's body for further protection against pathogens of infectious pathologies. It is known that some vaccines are unsafe for newborns or their administration does not provide the necessary level of immunological protection. Now, based on the results obtained, it is possible to recommend vaccination to women planning a pregnancy so that their future children receive the necessary level of immunity. The researchers acknowledge that at present such research has only been done in laboratory animals, but it is known that in humans, immune cells are also transferred from mother to child during breastfeeding.
It is known that some vaccines are unsafe for newborns or their administration does not provide the necessary level of immunological protection. Now, based on the results obtained, it is possible to recommend vaccination to women planning a pregnancy so that their future children receive the necessary level of immunity. The researchers acknowledge that at present such research has only been done in laboratory animals, but it is known that in humans, immune cells are also transferred from mother to child during breastfeeding.
Yulia Kotikovich
Immunity, breast milk and milk formula: finding the optimal balance
1. Bode L, McGuire M, Rodriguez JM, et al. It's alive: microbes and cells in human milk and their potential benefits to mother and infant. Ad Nutr. 2014;5(5):571-3.
2. Zakharova I.N., Machneva E.B., Oblogina I.S. Breast milk is living tissue! How to keep breastfeeding? Medical advice. 2017;19:24-9 [Zakharova IN, Machneva EB, Oblogina IS. Grudnoe moloko - zhivaia tkan! How sokhranit grudnoe vskarmlivanie? Meditsinskii sovet. 2017;19:24-9 (in Russian)].
Grudnoe moloko - zhivaia tkan! How sokhranit grudnoe vskarmlivanie? Meditsinskii sovet. 2017;19:24-9 (in Russian)].
DOI:10.21518/2079-701X-2017-19-24-29
3. Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci. 2013;7:79. DOI:10.3389/fnins.2013.00079
4. Khaertynov Kh.S., Anokhin V.A., Mustafin I.G., et al. Features of immunity in newborns with localized and generalized forms of bacterial infections. Ros. vestn. perinatol. and pediatrician. 2015;5:168-73 [Khaertynov KhS, Anokhin VA, Mustafin IG, et al. Osobennosti immuniteta u novorozhdennykh detei s lokalizovannymi i generalizovannymi formami bakterial'nykh infektsii. Ros. vestn. perinatol. i pediatrician. 2015;5:168-73 (in Russian)].
5. Ustyantseva L.S., Chistyakova G.N., Remizova I.I., et al. Features of innate and adaptive immunity in premature infants with severe hypoxic-ischemic lesions of the central nervous system. Ros. vestn. perinatol. and pediatrician. 2017;62:(3):59-65 [Ustiantseva LS, Chistiakova GN, Remizova II, et al.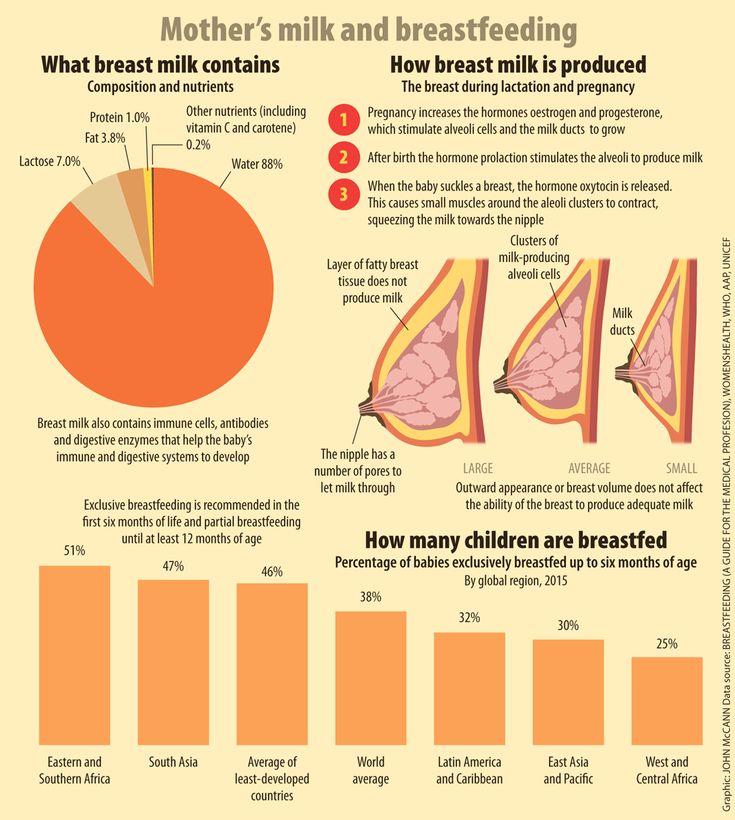 Osobennosti vrozhdennogo i adaptivnogo immuniteta nedonoshennykh detei s tiazhelym gipoksicheski-ishemicheskim porazheniem tsentral'noi nervnoi sistemy. Ros. vestn. perinatol. i pediatrician. 2017;62:(3):59-65 (in Russian)]. DOI:10.21508/1027-4065-2017-62-3-59-65
Osobennosti vrozhdennogo i adaptivnogo immuniteta nedonoshennykh detei s tiazhelym gipoksicheski-ishemicheskim porazheniem tsentral'noi nervnoi sistemy. Ros. vestn. perinatol. i pediatrician. 2017;62:(3):59-65 (in Russian)]. DOI:10.21508/1027-4065-2017-62-3-59-65
6. Victora CG, Alufsio RB, Barros JD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-90.
7. Ivanova I.E. The role of breast milk in the immunological protection of the child and the formation of his immune system. Public health of Chuvashia. 2015;4:63-71 [Ivanova I.E. Rol grudnogo moloka v immunologicheskoi zashchite rebenka i formirovanii ego immunnoi sistemy. Zdravookhranenie Chuvashii. 2015;4:63-71 (in Russian)].
8. Geppe N.A., Meleshkina A.V., Yablokova E.A., Chebysheva S.N. Advantages of adapted formulas based on goat's milk for functional disorders of the gastrointestinal tract in formula-fed infants. Attending doctor. 2020;3:43-9 [Geppe NA, Meleshkina AV, Iablokova EA, Chebysheva SN. Dostoinstva adaptirovannykh smesei na osnove kozego moloka pri funktsionalnykh narusheniiakh zheludochno-kishechnogo trakta u detei rannego vozrasta na iskusstvennom vskarmlivanii. Lechashchii vrach. 2020;3:43-9(in Russian)]. DOI:10.26295/OS.2020.72.94.007
Dostoinstva adaptirovannykh smesei na osnove kozego moloka pri funktsionalnykh narusheniiakh zheludochno-kishechnogo trakta u detei rannego vozrasta na iskusstvennom vskarmlivanii. Lechashchii vrach. 2020;3:43-9(in Russian)]. DOI:10.26295/OS.2020.72.94.007
9. Kazyukova T.V., Il'enko L.I., Kotlukov V.K. Goat milk in the nutrition of infants and young children. Pediatrics. 2017;96(1):75-82 [Kaziukova TV, Ilenko LI, Kotlukov VK. Koze moloko v pitanii detei grudnogo i earlygo vozrasta. Pediatricia. 2017;96(1):75-82 (in Russian)].
10. Donnet-Hughes A, Duc N, Serrant P, et al. Bioactive molecules in milk and their role in health and disease: the role of transforming growth factor-beta. Immunol Cell Biol. 2000;78:74-9.
11. Miles EA, Childs CE, Calder PC. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients. 2021;13(1):247.
DOI:10.3390/nu13010247
12. Plaza-Díaz J, Fontana L, Gil A. Human Milk Oligosaccharides and Immune System Development.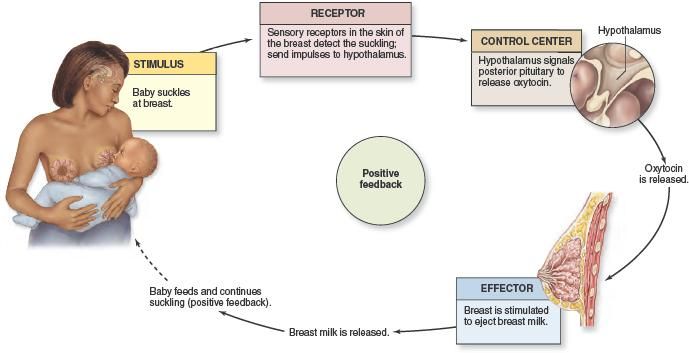 Nutrients. 2018;10(8):1038. DOI:10.3390/nu10081038
Nutrients. 2018;10(8):1038. DOI:10.3390/nu10081038
13. Gutierrez S, Svahn SL, Johansson ME. Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci. 2019;20(20):5028. DOI:10.3390/ijms20205028
14. Vahn SL, Ulleryd MA, Grahnemo L, et al. Dietary Omega-3 Fatty Acids Increase Survival and Decrease Bacterial Load in Mice Subjected to Staphylococcus aureus-Induced Sepsis. Infect Immun. 2016;84(4):1205-13. DOI:10.1128/IAI.01391-15
15. Husson MO, Ley D, Portal C, et al. Modulation of host defense against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect. 2016;73(6):523-35. pmid:27746159.
16. Russel FD, Burgin-Maunder CS. Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. MarDrugs. 2012;10(11):2535-59.
17. Oh SF, Vickery TW, Serhan ChN. Chiral Lipidomics of E-Series Resolvins: Aspirin and the Biosynthesis of Novel Mediators. Biochim Biophys Acta. 2011;1811(11):737-47.
18. Field C, Van Aerde J, Robinson LE, Clandinin MT. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br J Nutr. 2008;99:91-9.
Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br J Nutr. 2008;99:91-9.
19. Nikolaeva S.V., Usenko D.V., Shushakova E.K., et al. Importance of omega-3 polyunsaturated fatty acids for children. breast cancer. 2020;2:28-32 [Nikolaeva SV, Usenko DV, Shushakova EK, et al. Znachenie omega-3 polinenasyshchennykh zhirnykh kislot dlia detei. RMZh. 2020;2:28-32 (in Russian)].
20. Miles EA, Childs CE, Calder PC. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients. 2021;13(1):247.
DOI:10.3390/nu13010247
21. Letifov G.M., Polyanskaya F.I., Panova I.V. Adapted milk formulas and dry milk drinks based on goat's milk in the nutrition of healthy children. Pediatric practice. 2016;6:20-4 [Letifov GM, Polianskaia FI, Panova IV. Adaptirovannye molochnye smesi i sukhie molochnye napitki na osnove koz'ego moloka v pitanii zdorovykh detei. Praktika pediatra. 2016;6:20-4 (in Russian)].
2016;6:20-4 (in Russian)].
22. Komarova O.N. The influence of the fat component of mixtures on the development of the child. Attending doctor. 2013;7:76 [Komarova ON. Vliianie zhirovogo komponenta smesei na razvitie rebenka. Lechashchii vrach. 2013;7:76 (in Russian)].
23. Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: A review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2009;81:151-8.
24. Havlicekova Z, Jesenak M, Banovcin P, Kuchta M. Beta-palmitate – a natural component of human milk in supplemental milk formulas. Nutr J. 2016;15:28.
25. Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother's Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front Immunol. 2018;9:361. DOI:10.3389/fimmu.2018.00361
26. Borovik T.E., Semyonova N.N., Lukoyanova O.L., et al. Efficacy of using an adapted goat milk-based formula in the nutrition of healthy children in the first six months of life: results of a multicenter prospective comparative study. Questions of modern pediatrics. 2017;16(3):226-34 [Borovik TE, Semenova NN, Lukoianova OL, et al. Effektivnost ispolzovaniia adaptirovannoi smesi na osnove kozego moloka v pitanii zdorovykh detei pervogo polugodiia zhizni: rezultaty mnogotsentrovogo prospektivnogo sravnitelnogo issledovaniia. Voprosy sovremennoi pediatrii. 2017;16(3):226-34 (in Russian)].
Questions of modern pediatrics. 2017;16(3):226-34 [Borovik TE, Semenova NN, Lukoianova OL, et al. Effektivnost ispolzovaniia adaptirovannoi smesi na osnove kozego moloka v pitanii zdorovykh detei pervogo polugodiia zhizni: rezultaty mnogotsentrovogo prospektivnogo sravnitelnogo issledovaniia. Voprosy sovremennoi pediatrii. 2017;16(3):226-34 (in Russian)].
27. Litmanovitz I, Bar-Yoseph F, Lifshitz Y, et al. Reduced crying in term infants fed high beta-palmitate formula: a double-blind randomized clinical trial. BMC Pediatr. 2014;14:152. DOI:10.1186/1471-2431-14-152
28. Kiseleva E.S., Mokhova Yu.A. Breast milk and its components: impact on the child's immunity. Pediatrics. 2010;89(6):62-9 [Kiseleva ES, Mokhova IuA. Grudnoe moloko i ego komponenty: vliianie na immunitet rebenka. Pediatricia. 2010;89(6):62-9 (in Russian)].
29. Lukoyanova O.L. Breast milk as a reference model for the creation of infant formula. Questions of modern pediatrics. 2012;11(4):111-5 [Lukoianova OL. Grudnoe moloko kak etalonnaia model dlia sozdaniia detskikh molochnykh smesei. Voprosy sovremennoi pediatrii. 2012;11(4):111-5 (in Russian)].
Grudnoe moloko kak etalonnaia model dlia sozdaniia detskikh molochnykh smesei. Voprosy sovremennoi pediatrii. 2012;11(4):111-5 (in Russian)].
30. Garwolińska D, Namieśnik J, Kot-Wasik A, Hewelt-Belka W. Chemistry of Human Breast Milk-A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J Agric Food Chem. 2018;66(45):11881-96. DOI:10.1021/acs.jafc.8b04031
31. Keshishyan E.S., Berdnikova E.K. Nucleotides in the nutrition of young children. Attending doctor. 2004;1:53-4 [Keshishian ES, Berdnikova EK. Nukleotidy v pitanii detei rannego vozrasta. Lechashchii vrach. 2004;1:53-4 (in Russian)].
32. Baranov A.A., Tutelyan V.A., Chumakova O.V., et al. Nutrition optimization program for children aged 1 to 3 years in the Russian Federation. Guidelines. M., 2019 [Baranov AA, Tutelian VA, Chumakova OV, et al. Nutrition optimization program for children aged 1 to 3 years in the Russian Federation. Metodicheskie rekomendatsii. Moscow, 2019(in Russian)].
33. Gribakin S.G., Bokovskaya O.A., Davydovskaya A.A. Child nutrition and immunity: in pursuit of the ideal. Attending doctor. 2013;8:72-6 [Gribakin SG, Bokovskaia OA, Davydovskaia AA. Nutrition rebenka i immunitet: v pogone za idealom. Lechashchii vrach. 2013;8:72-6 (in Russian)].
34. Dementieva Yu.N. Immunological aspects of breastfeeding. Ros. vestn. perinatol. and pediatrician. 2015;4:19-24 [Dementeva IuN. Immunologicheskie aspekty grudnogo vskarmlivaniia. Ros. vestn. perinatol. i pediatrician. 2015;4:19-24 (in Russian)].
35. Merenkova S.P. Physiological significance of the nutrient composition of adapted milk formulas. Bulletin of SUSU. 2013;1(1):56-62 [Merenkova SP. Fiziologicheskoe znachenie nutrient composition sostava adaptirovannykh molochnykh smesei. Vestnik IuUrGU. 2013;1(1):56-62 (in Russian)].
36. Linette P, Tao H, Hanneke B. Naturally high content of nucleotides in goat milk based infant formula. Abstracts of ESPGHAN 51st Annual Meeting, 2018; p. 1091.
1091.
37. Zakharova I.N., Sugyan N.G., Glotova A.P. Goat milk in the nutrition of children with functional disorders of the gastrointestinal tract. Medical advice. 2020;(18):103-9[Zakharova IN, Sugian NG, Glotova AP. Koze moloko v pitanii detei s funktsionalnymi narusheniiami zheludochno-kishechnogo trakta. Meditsinskii sovet. 2020;(18):103-9 (in Russian)]. DOI:10.21518/2079-701X-2020-18-103-109
38. Zakharova I.N., Dmitrieva Yu.A., Yagodkin M.V. Human milk oligosaccharides: another step towards bringing infant formula closer to the "gold standard" of infant feeding. Medical advice. 2018;7:30-7 [Zakharova IN, Dmitrieva IuA, Iagodkin MV. Oligosakharidy grudnogo moloka: eshche odin shag na puti priblizheniia detskikh molochnykh smesei k "zolotomu standartu' vskarmlivaniia rebenka. Meditsinskii sovet. 2018;7:30-7 (in Russian)]. DOI:10.21518/2079-701X-2018-17-30-37
39. Donovan SM, Comstock SS. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann Nutr Metab. 2016;69 Suppl. 2(Suppl. 2):42-51.
Ann Nutr Metab. 2016;69 Suppl. 2(Suppl. 2):42-51.
DOI:10.1159/000452818
40. Johannesen SA, Beeren SR, Blank D, et al. Glycan analysis via derivatization with a fluorogenic pyrylium dye. Carbohydr Res. 2012;352:94-100. DOI:10.1016/j.carres.2012.02.016
41. Oganezova I.A. Gut microbiota and immunity: immunomodulatory effects of Lactobacillus rhamnosus GG. breast cancer. 2018;26(9):39-44 [Oganezova IA. Kishechnaia mikrobiota i immunitet: immunomoduliruiushchie effekty Lactobacillus rhamnosus GG. RMZh. 2018;26(9):39-44 (in Russian)].
42. Pakhomovskaya N.L., Venediktova M.M. Influence of the microbiota of a child of the first year of life on his development. Medical advice. 2018;2:200-5 [Pakhomovskaia NL, Venediktova MM. Vliianie mikrobioty rebenka pervogo goda zhizni na ego razvitie. Meditsinskii sovet. 2018;2:200-5 (in Russian)]. DOI:10.21518/2079-701X-2018-2-200-205
43 Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatric Res. 2015;77(1-2):229-35.
Pediatric Res. 2015;77(1-2):229-35.
44. Mezoff EA, Hawkins JA, Ollberding NJ, et al. The human milk oligosaccharide 2'‑fucosyllactose augments the adaptive response to extensive intestinal resection. Am J Physiol Gastrointest Liver Physiol. 2016;310(6):G427-38.
45. Wickramasinghe S, Pacheco AR, Lemay DG, Mills DA. Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiol. 2015;15:172.
46. Goehring KC, Kennedy AD, Prieto PA, Buck RH. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLOS One. 2014;9(7):e101692.
47. Steenhout P, Sperisen P, Martin F-P, et al. Term infant formula supplemented with human milk oligosaccharides (2'‑fucosyllactose and lacto-N-neotetraose) shifts stool microbiota and metabolic signatures closer to that of breastfed infants. FASEB J. 2016;30(Suppl. 1):275-7.
48. Zuurveld M, van Witzenburg NP, Garssen J, et al. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Frontiers in Immunology. 2020;11:801. DOI:10.3389/fimmu.2020.00801
Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Frontiers in Immunology. 2020;11:801. DOI:10.3389/fimmu.2020.00801
49. Meyrand M, Dallas DC, Caillat H, et al. Comparison of milk oligosaccharides between goats with and without the genetic ability to synthesize as1-casein. Small Rumin Res. 2013;113(2‑3):411-20. DOI:10.1016/j.smallrumres.2013.03.014
50. Leong A, Liu Z, Almshawit H, et al. Oligosaccharides in goats' milk-based infant formula and their prebiotic and anti-infection properties. Br J Nutr. 2019;122(4):441-9.
51. Daddaoua A, Puerta V, Requena P, et al. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2006;136(3):672-6. DOI:10.1093/jn/136.3.672
52. Chen YL, Liao FH, Lin SH, Chien YW. A Prebiotic Formula Improves the Gastrointestinal Bacterial Flora in Toddlers. Gastroenterol Res Pract. 2016;2016:3504282.
53. Abramova T.V., Pyrieva E.A., Toboleva M. A., et al. Probiotic BB-12 in children's nutrition. Pharmateka. 2016;15:90-5 [Abramova TV, Pyreva EA, Toboleva MA, et al. Probiotik VV-12 v pitanii detei. Farmateka. 2016;15:90-5 (in Russian)].
A., et al. Probiotic BB-12 in children's nutrition. Pharmateka. 2016;15:90-5 [Abramova TV, Pyreva EA, Toboleva MA, et al. Probiotik VV-12 v pitanii detei. Farmateka. 2016;15:90-5 (in Russian)].
54. Ursova N.I. Importance of breastfeeding for infant growth and development. Almanac of Clinical Medicine. 2015;42:23-37 [Ursova N.I. Significance grudnogo vskarmlivaniia dlia rosta i razvitiia mladentsa. Al'manakh klinicheskoi meditsiny. 2015;42:23-37 (in Russian)].
55. Tretyakova O.S. The physiological role of iron in the human body. Child doctor. 2013;1(22):14-8 [Tretiakova OS. Physiologicheskaia rol zheleza v organizme cheloveka. Ditiachii likar. 2013;1(22):14-8 (in Russian)].
56. Haschke F, Haiden N, Thakkar SK. Nutritive and Bioactive Proteins in Breast Milk by Ferdinand Haschke et al. Ann Nutr Metab. 2016;69(Suppl. 2):17-26.
57. Baqui AH, Black RE, El Arifeen S, et al. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: community randomized trial.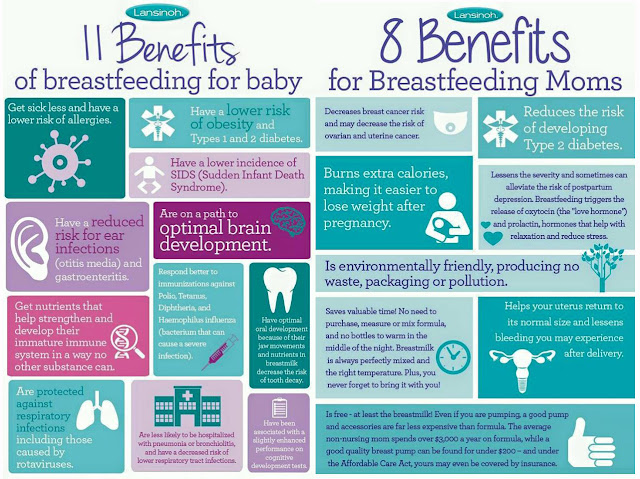 BMJ. 2002;325(7372):1059.
BMJ. 2002;325(7372):1059.
58 Bahl R, Bhandari N, Saksena M, et al. Efficacy of zinc-fortified oral rehydration solution in 6- to 35-month-old children with acute diarrhea. J Pediatr. 2002;141(5):677-82.
59. Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigenpresenting cells. J Nutr. 2002;132(12):3736-9.
60. Geissmann F, Revy P, Brousse N, et al. Retinoids regulate survival and antigen presentation by immature dendritic cells. J Exp Med. 2003;198(4):623-34.
61. Arthur JR, McKenzie RC, Beckett GJ. Selenium in the immune system. J Nutr. 2003;133(5 Suppl. 1):1457S-9S.
62. Stuss M, Michalska-Kasiczak M, Sewerynek E. The role of selenium in thyroid gland pathophysiology. Endocrynol Pol. 2017;68(4):440-65. DOI:10.5603/EP.2017.0051
63. Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12(1):236. DOI:10.3390/nu12010236
Nutrients. 2020;12(1):236. DOI:10.3390/nu12010236
64. Kondratyeva E.I., Barabash N.A., Stankevich S.S., et al. The effect of trace elements on the health status of children who are on different types of feeding. Ros. vestn. perinatol. and pediatrician. 2008;2:24-9 [Kondrateva EI, Barabash NA, Stankevich SS, et al. Vliianie mikroelementov na sostoianie zdorovia detei, nakhodiashchikhsia na razlichnykh vidakh vskarmlivaniia. Ros. vestn. perinatol. i pediatrician. 2008;2:24-9 (in Russian)].
65. Ryumina I.I. Mixtures based on goat's milk when choosing artificial feeding of a newborn and a child of the first year of life. Medical Council. 2021;1:30-6 [Riumina II. Smesi na osnove koz'ego moloka pri vybore iskusstvennogo vskarmlivaniia novorozhdennogo i rebenka pervogo goda zhizni. Meditsinskii sovet. 2021;1:30-6 (in Russian)]. DOI:10.21518/2079-701X-2021-1-30-35
________________________________________________
1. Bode L, McGuire M, Rodriguez JM, et al. It's alive: microbes and cells in human milk and their potential benefits to mother and infant. Ad Nutr. 2014;5(5):571-3.
It's alive: microbes and cells in human milk and their potential benefits to mother and infant. Ad Nutr. 2014;5(5):571-3.
2. Zakharova IN, Machneva EB, Oblogina IS. Grudnoe moloko - zhivaia tkan! How sokhranit grudnoe vskarmlivanie? Meditsinskii sovet. 2017;19:24-9 (in Russian)
DOI:10.21518/2079-701X-2017-19-24-29
3. Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci. 2013;7:79. DOI:10.3389/fnins.2013.00079
4. Khaertynov KhS, Anokhin VA, Mustafin IG, et al. Osobennosti immuniteta u novorozhdennykh detei s lokalizovannymi i generalizovannymi formami bakterial'nykh infektsii. Ros. vestn. perinatol. i pediatrician. 2015;5:168-73 (in Russian)
5. Ustiantseva LS, Chistiakova GN, Remizova II, et al. Osobennosti vrozhdennogo i adaptivnogo immuniteta nedonoshennykh detei s tiazhelym gipoksicheski-ishemicheskim porazheniem tsentral'noi nervnoi sistemy. Ros. vestn. perinatol. i pediatrician. 2017;62:(3):59-65 (in Russian) DOI:10. 21508/1027-4065-2017-62-3-59-65
21508/1027-4065-2017-62-3-59-65
6. Victora CG, Alufsio RB, Barros JD, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475-90.
7. Ivanova IE. Rol grudnogo moloka v immunologicheskoi zashchite rebenka i formirovanii ego immunnoi sistemy. Zdravookhranenie Chuvashii. 2015;4:63-71 (in Russian)
8. Geppe NA, Meleshkina AV, Iablokova EA, Chebysheva SN. Dostoinstva adaptirovannykh smesei na osnove kozego moloka pri funktsionalnykh narusheniiakh zheludochno-kishechnogo trakta u detei rannego vozrasta na iskusstvennom vskarmlivanii. Lechashchii vrach. 2020;3:43-9(in Russian) DOI:10.26295/OS.2020.72.94.007
9. Kaziukova TV, Ilenko LI, Kotlukov VK. Koze moloko v pitanii detei grudnogo i earlygo vozrasta. Pediatricia. 2017;96(1):75-82 (in Russian)
10. Donnet-Hughes A, Duc N, Serrant P, et al. Bioactive molecules in milk and their role in health and disease: the role of transforming growth factor-beta. Immunol Cell Biol. 2000;78:74-9.
2000;78:74-9.
11. Miles EA, Childs CE, Calder PC. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients. 2021;13(1):247.
DOI:10.3390/nu13010247
12. Plaza-Díaz J, Fontana L, Gil A. Human Milk Oligosaccharides and Immune System Development. Nutrients. 2018;10(8):1038. DOI:10.3390/nu10081038
13. Gutierrez S, Svahn SL, Johansson ME. Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci. 2019;20(20):5028. DOI:10.3390/ijms20205028
14. Vahn SL, Ulleryd MA, Grahnemo L, et al. Dietary Omega-3 Fatty Acids Increase Survival and Decrease Bacterial Load in Mice Subjected to Staphylococcus aureus-Induced Sepsis. Infect Immun. 2016;84(4):1205-13. DOI:10.1128/IAI.01391-15
15. Husson MO, Ley D, Portal C, et al. Modulation of host defense against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect. 2016;73(6):523-35. pmid:27746159.
16. Russel FD, Burgin-Maunder CS.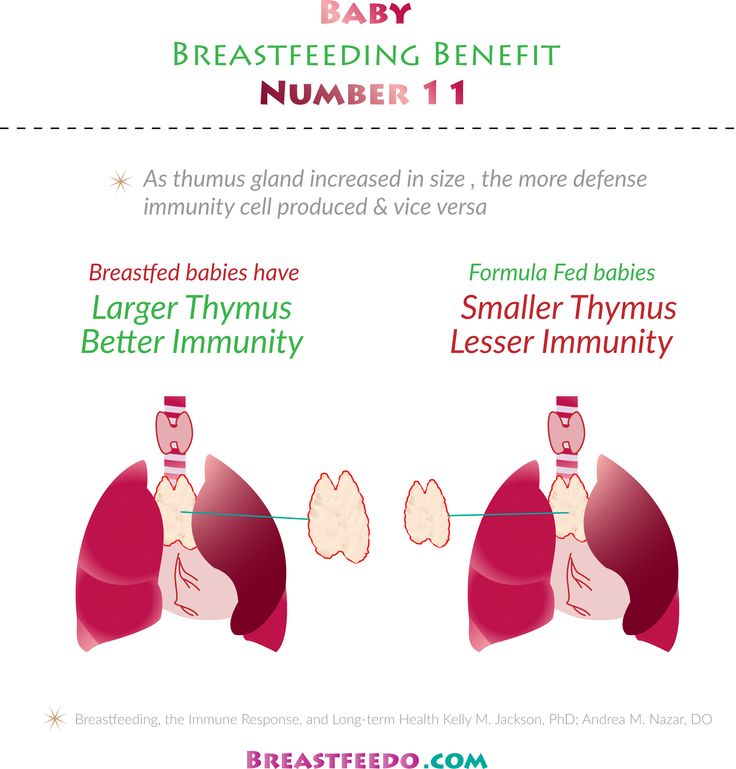 Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. MarDrugs. 2012;10(11):2535-59.
Distinguishing health benefits of eicosapentaenoic and docosahexaenoic acids. MarDrugs. 2012;10(11):2535-59.
17. Oh SF, Vickery TW, Serhan ChN. Chiral Lipidomics of E-Series Resolvins: Aspirin and the Biosynthesis of Novel Mediators. Biochim Biophys Acta. 2011;1811(11):737-47.
18. Field C, Van Aerde J, Robinson LE, Clandinin MT. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br J Nutr. 2008;99:91-9.
19. Nikolaeva SV, Usenko DV, Shushakova EK, et al. Znachenie omega-3 polinenasyshchennykh zhirnykh kislot dlia detei. RMZh. 2020;2:28-32 (in Russian)
20. Miles EA, Childs CE, Calder PC. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients. 2021;13(1):247.
DOI:10.3390/nu13010247
21. Letifov GM, Polianskaia FI, Panova IV. Adaptirovannye molochnye smesi i sukhie molochnye napitki na osnove koz'ego moloka v pitanii zdorovykh detei. Praktika pediatra. 2016;6:20-4 (in Russian)
Praktika pediatra. 2016;6:20-4 (in Russian)
22. Komarova ON. Vliianie zhirovogo komponenta smesei na razvitie rebenka. Lechashchii vrach. 2013;7:76 (in Russian)
23. Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: A review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2009;81:151-8.
24. Havlicekova Z, Jesenak M, Banovcin P, Kuchta M. Beta-palmitate – a natural component of human milk in supplemental milk formulas. Nutr J. 2016;15:28.
25. Le Doare K, Holder B, Bassett A, Pannaraj PS. Mother's Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front Immunol. 2018;9:361. DOI:10.3389/fimmu.2018.00361
26. Borovik TE, Semenova NN, Lukoianova OL, et al. Effektivnost ispolzovaniia adaptirovannoi smesi na osnove kozego moloka v pitanii zdorovykh detei pervogo polugodiia zhizni: rezultaty mnogotsentrovogo prospektivnogo sravnitelnogo issledovaniia. Voprosy sovremennoi pediatrii. 2017;16(3):226-34 (in Russian)
Voprosy sovremennoi pediatrii. 2017;16(3):226-34 (in Russian)
27. Litmanovitz I, Bar-Yoseph F, Lifshitz Y, et al. Reduced crying in term infants fed high beta-palmitate formula: a double-blind randomized clinical trial. BMC Pediatr. 2014;14:152. DOI:10.1186/1471-2431-14-152
28. Kiseleva ES, Mokhova IuA. Grudnoe moloko i ego komponenty: vliianie na immunitet rebenka. Pediatricia. 2010;89(6):62-9 (in Russian)
29. Lukoianova OL. Grudnoe moloko kak etalonnaia model dlia sozdaniia detskikh molochnykh smesei. Voprosy sovremennoi pediatrii. 2012;11(4):111-5 (in Russian)
30. Garwolińska D, Namieśnik J, Kot-Wasik A, Hewelt-Belka W. Chemistry of Human Breast Milk-A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J Agric Food Chem. 2018;66(45):11881-96. DOI:10.1021/acs.jafc.8b04031
31. Keshishian ES, Berdnikova EK. Nukleotidy v pitanii detei rannego vozrasta. Lechashchii vrach. 2004;1:53-4 (in Russian)
32. Baranov AA, Tutelian VA, Chumakova OV, et al. Nutrition optimization program for children aged 1 to 3 years in the Russian Federation. Metodicheskie rekomendatsii. Moscow, 2019(in Russian)
Nutrition optimization program for children aged 1 to 3 years in the Russian Federation. Metodicheskie rekomendatsii. Moscow, 2019(in Russian)
33. Gribakin SG, Bokovskaia OA, Davydovskaia AA. Nutrition rebenka i immunitet: v pogone za idealom. Lechashchii vrach. 2013;8:72-6 (in Russian)
34. Dementeva Iun. Immunologicheskie aspekty grudnogo vskarmlivaniia. Ros. vestn. perinatol. i pediatrician. 2015;4:19-24 (in Russian)
35. Merenkova S. P. Fiziologicheskoe znachenie nutrient composition sostava adaptirovannykh molochnykh smesei. Vestnik IuUrGU. 2013;1(1):56-62 (in Russian)
36. Linette P, Tao H, Hanneke B. Naturally high content of nucleotides in goat milk based infant formula. Abstracts of ESPGHAN 51st Annual Meeting, 2018; p. 1091.
37. Zakharova IN, Sugian NG, Glotova AP. Koze moloko v pitanii detei s funktsionalnymi narusheniiami zheludochno-kishechnogo trakta. Meditsinskii sovet. 2020;(18):103-9 (in Russian) DOI:10.21518/2079-701X-2020-18-103-109
38. Zakharova IN, Dmitrieva IuA, Iagodkin MV. Oligosakharidy grudnogo moloka: eshche odin shag na puti priblizheniia detskikh molochnykh smesei k "zolotomu standartu' vskarmlivaniia rebenka. Meditsinskii sovet. 2018;7:30-7 (in Russian) DOI:10.21518/2079-701X-2018-17-30-37
Zakharova IN, Dmitrieva IuA, Iagodkin MV. Oligosakharidy grudnogo moloka: eshche odin shag na puti priblizheniia detskikh molochnykh smesei k "zolotomu standartu' vskarmlivaniia rebenka. Meditsinskii sovet. 2018;7:30-7 (in Russian) DOI:10.21518/2079-701X-2018-17-30-37
39. Donovan SM, Comstock SS. Human Milk Oligosaccharides Influence Neonatal Mucosal and Systemic Immunity. Ann Nutr Metab. 2016;69 Suppl. 2(Suppl. 2):42-51.
DOI:10.1159/000452818
40. Johannesen SA, Beeren SR, Blank D, et al. Glycan analysis via derivatization with a fluorogenic pyrylium dye. Carbohydr Res. 2012;352:94-100. DOI:10.1016/j.carres.2012.02.016
41. Oganezova IA. Kishechnaia mikrobiota i immunitet: immunomoduliruiushchie effekty Lactobacillus rhamnosus GG. RMZh. 2018;26(9):39-44 (in Russian)
42. Pakhomovskaia NL, Venediktova MM. Vliianie mikrobioty rebenka pervogo goda zhizni na ego razvitie. Meditsinskii sovet. 2018;2:200-5 (in Russian) DOI:10.21518/2079-701X-2018-2-200-205
43 Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatric Res. 2015;77(1-2):229-35.
Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatric Res. 2015;77(1-2):229-35.
44. Mezoff EA, Hawkins JA, Ollberding NJ, et al. The human milk oligosaccharide 2'‑fucosyllactose augments the adaptive response to extensive intestinal resection. Am J Physiol Gastrointest Liver Physiol. 2016;310(6):G427-38.
45. Wickramasinghe S, Pacheco AR, Lemay DG, Mills DA. Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiol. 2015;15:172.
46. Goehring KC, Kennedy AD, Prieto PA, Buck RH. Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PLOS One. 2014;9(7):e101692.
47. Steenhout P, Sperisen P, Martin F-P, et al. Term infant formula supplemented with human milk oligosaccharides (2'‑fucosyllactose and lacto-N-neotetraose) shifts stool microbiota and metabolic signatures closer to that of breastfed infants. FASEB J. 2016;30(Suppl. 1):275-7.
FASEB J. 2016;30(Suppl. 1):275-7.
48. Zuurveld M, van Witzenburg NP, Garssen J, et al. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Frontiers in Immunology. 2020;11:801. DOI:10.3389/fimmu.2020.00801
49. Meyrand M, Dallas DC, Caillat H, et al. Comparison of milk oligosaccharides between goats with and without the genetic ability to synthesize as1-casein. Small Rumin Res. 2013;113(2‑3):411-20. DOI:10.1016/j.smallrumres.2013.03.014
50. Leong A, Liu Z, Almshawit H, et al. Oligosaccharides in goats' milk-based infant formula and their prebiotic and anti-infection properties. Br J Nutr. 2019;122(4):441-9.
51. Daddaoua A, Puerta V, Requena P, et al. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2006;136(3):672-6. DOI:10.1093/jn/136.3.672
52. Chen YL, Liao FH, Lin SH, Chien YW. A Prebiotic Formula Improves the Gastrointestinal Bacterial Flora in Toddlers.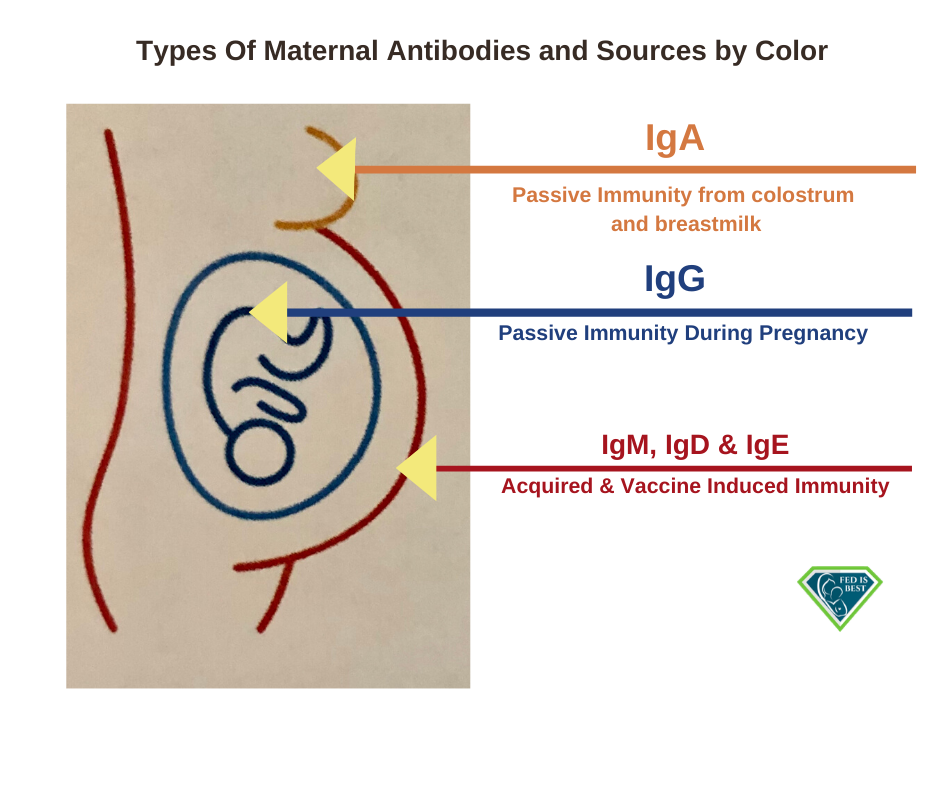 Gastroenterol Res Pract. 2016;2016:3504282.
Gastroenterol Res Pract. 2016;2016:3504282.
53. Abramova TV, Pyreva EA, Toboleva MA, et al. Probiotik VV-12 v pitanii detei. Farmateka. 2016;15:90-5 (in Russian)
54. Ursova N.I. Significance grudnogo vskarmlivaniia dlia rosta i razvitiia mladentsa. Al'manakh klinicheskoi meditsiny. 2015;42:23-37 (in Russian)
55. Tretiakova OS. Physiologicheskaia rol zheleza v organizme cheloveka. Ditiachii likar. 2013;1(22):14-8 (in Russian)
56. Haschke F, Haiden N, Thakkar SK. Nutritive and Bioactive Proteins in Breast Milk by Ferdinand Haschke et al. Ann Nutr Metab. 2016;69(Suppl. 2):17-26.
57. Baqui AH, Black RE, El Arifeen S, et al. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: community randomized trial. BMJ. 2002;325(7372):1059.
58 Bahl R, Bhandari N, Saksena M, et al. Efficacy of zinc-fortified oral rehydration solution in 6- to 35-month-old children with acute diarrhea. J Pediatr. 2002;141(5):677-82.












