Fragile x genetic
Fragile X syndrome: MedlinePlus Genetics
Description
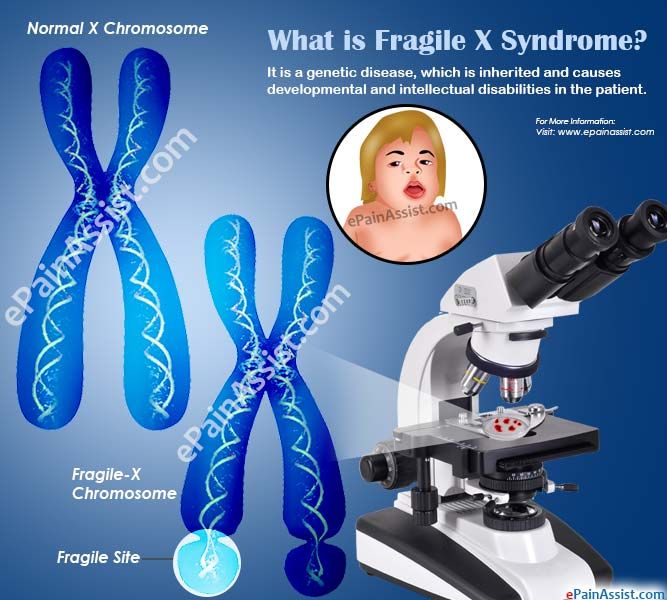
Fragile X syndrome is a genetic condition that causes a range of developmental problems including learning disabilities and cognitive impairment. Usually, males are more severely affected by this disorder than females.
Affected individuals usually have delayed development of speech and language by age 2. Most males with fragile X syndrome have mild to moderate intellectual disability, while about one-third of affected females are intellectually disabled. Children with fragile X syndrome may also have anxiety and hyperactive behavior such as fidgeting or impulsive actions. They may have attention deficit disorder (ADD), which includes an impaired ability to maintain attention and difficulty focusing on specific tasks. About one-third of individuals with fragile X syndrome have features of autism spectrum disorder that affect communication and social interaction. Seizures occur in about 15 percent of males and about 5 percent of females with fragile X syndrome.
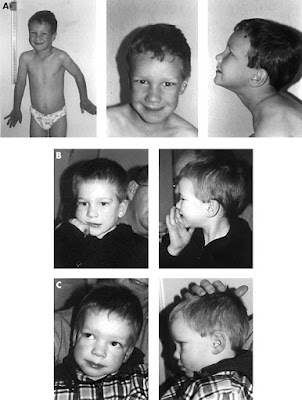
Most males and about half of females with fragile X syndrome have characteristic physical features that become more apparent with age. These features include a long and narrow face, large ears, a prominent jaw and forehead, unusually flexible fingers, flat feet, and in males, enlarged testicles (macroorchidism) after puberty.
Frequency
Fragile X syndrome occurs in approximately 1 in 4,000 males and 1 in 8,000 females.
Causes
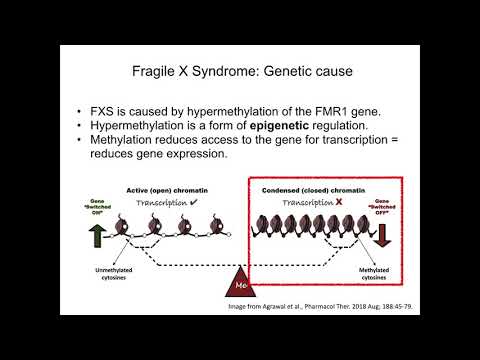
Mutations in the FMR1 gene cause fragile X syndrome. The FMR1 gene provides instructions for making a protein called FMRP. This protein helps regulate the production of other proteins and plays a role in the development of synapses, which are specialized connections between nerve cells. Synapses are critical for relaying nerve impulses.
Nearly all cases of fragile X syndrome are caused by a mutation in which a DNA segment, known as the CGG triplet repeat, is expanded within the FMR1 gene. Normally, this DNA segment is repeated from 5 to about 40 times.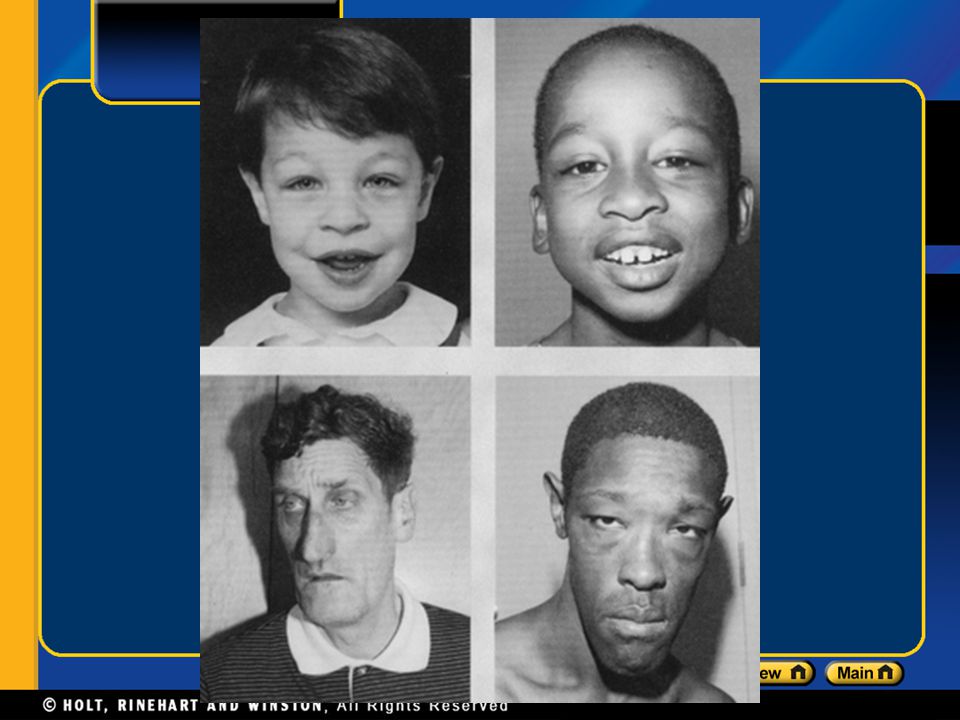 In people with fragile X syndrome, however, the CGG segment is repeated more than 200 times. The abnormally expanded CGG segment turns off (silences) the FMR1 gene, which prevents the gene from producing FMRP. Loss or a shortage (deficiency) of this protein disrupts nervous system functions and leads to the signs and symptoms of fragile X syndrome.
In people with fragile X syndrome, however, the CGG segment is repeated more than 200 times. The abnormally expanded CGG segment turns off (silences) the FMR1 gene, which prevents the gene from producing FMRP. Loss or a shortage (deficiency) of this protein disrupts nervous system functions and leads to the signs and symptoms of fragile X syndrome.
Males and females with 55 to 200 repeats of the CGG segment are said to have an FMR1 gene premutation. Most people with this premutation are intellectually normal. In some cases, however, individuals with a premutation have lower than normal amounts of FMRP. As a result, they may have mild versions of the physical features seen in fragile X syndrome (such as prominent ears) and may experience emotional problems such as anxiety or depression. Some children with an FMR1 premutation may have learning disabilities or autistic-like behavior. The premutation is also associated with an increased risk of disorders called fragile X-associated primary ovarian insufficiency (FXPOI) and fragile X-associated tremor/ataxia syndrome (FXTAS).
Inheritance
Fragile X syndrome is inherited in an X-linked dominant pattern. A condition is considered X-linked if the mutated gene that causes the disorder is located on the X chromosome, one of the two sex chromosomes. (The Y chromosome is the other sex chromosome.) The inheritance is dominant if one copy of the altered gene in each cell is sufficient to cause the condition. X-linked dominant means that in females (who have two X chromosomes), a mutation in one of the two copies of a gene in each cell is sufficient to cause the disorder. In males (who have only one X chromosome), a mutation in the only copy of a gene in each cell causes the disorder. In most cases, males experience more severe symptoms of the disorder than females.
In women, the FMR1 gene premutation on the X chromosome can expand to more than 200 CGG repeats in cells that develop into eggs. This means that women with the premutation have an increased risk of having a child with fragile X syndrome.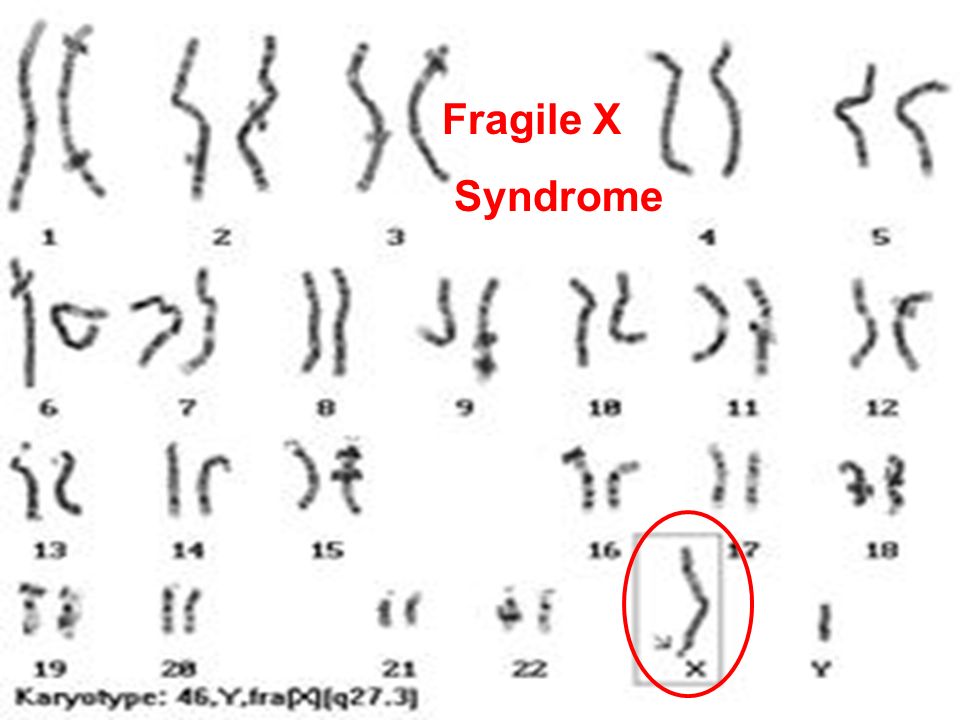 By contrast, the premutation in men does not expand to more than 200 repeats as it is passed to the next generation. Men pass the premutation only to their daughters. Their sons receive a Y chromosome, which does not include the FMR1 gene.
By contrast, the premutation in men does not expand to more than 200 repeats as it is passed to the next generation. Men pass the premutation only to their daughters. Their sons receive a Y chromosome, which does not include the FMR1 gene.
Other Names for This Condition
- Fra(X) syndrome
- FRAXA syndrome
- FXS
- Marker X syndrome
- Martin-Bell syndrome
- X-linked mental retardation and macroorchidism
Additional Information & Resources
Genetic Testing Information
- Genetic Testing Registry: Fragile X syndrome
Genetic and Rare Diseases Information Center
- Fragile X syndrome
Patient Support and Advocacy Resources
- Disease InfoSearch
- National Organization for Rare Disorders (NORD)
Research Studies from ClinicalTrials.gov
- ClinicalTrials.gov
Catalog of Genes and Diseases from OMIM
- FMRP TRANSLATIONAL REGULATOR 1
Scientific Articles on PubMed
- PubMed
References
- Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, Hagerman R.
 Annotation: Deconstructing the attention deficit in fragile X syndrome: a developmental neuropsychological approach. J Child Psychol Psychiatry. 2004 Sep;45(6):1042-53. Review. Citation on PubMed
Annotation: Deconstructing the attention deficit in fragile X syndrome: a developmental neuropsychological approach. J Child Psychol Psychiatry. 2004 Sep;45(6):1042-53. Review. Citation on PubMed - Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB Jr, Moine H, Kooy RF, Tassone F, Gantois I, Sonenberg N, Mandel JL, Hagerman PJ. Fragile X syndrome. Nat Rev Dis Primers. 2017 Sep 29;3:17065. doi: 10.1038/nrdp.2017.65. Review. Citation on PubMed
- Hunter JE, Berry-Kravis E, Hipp H, Todd PK. FMR1 Disorders. 1998 Jun 16 [updated 2019 Nov 21]. In: Adam MP, Everman DB, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022. Available from http://www.ncbi.nlm.nih.gov/books/NBK1384/ Citation on PubMed
- Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1.
 Lancet Neurol. 2007 Jan;6(1):45-55. Citation on PubMed
Lancet Neurol. 2007 Jan;6(1):45-55. Citation on PubMed - Koukoui SD, Chaudhuri A. Neuroanatomical, molecular genetic, and behavioral correlates of fragile X syndrome. Brain Res Rev. 2007 Jan;53(1):27-38. Epub 2006 Jul 17. Review. Citation on PubMed
- Salcedo-Arellano MJ, Dufour B, McLennan Y, Martinez-Cerdeno V, Hagerman R. Fragile X syndrome and associated disorders: Clinical aspects and pathology. Neurobiol Dis. 2020 Mar;136:104740. doi: 10.1016/j.nbd.2020.104740. Epub 2020 Jan 10. Review. Citation on PubMed or Free article on PubMed Central
- Sherman S, Pletcher BA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005 Oct;7(8):584-7. Citation on PubMed or Free article on PubMed Central
- Sullivan SD, Welt C, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011 Jul;29(4):299-307. doi: 10.1055/s-0031-1280915. Epub 2011 Oct 3.
 Review. Citation on PubMed
Review. Citation on PubMed - Terracciano A, Chiurazzi P, Neri G. Fragile X syndrome. Am J Med Genet C Semin Med Genet. 2005 Aug 15;137C(1):32-7. Review. Citation on PubMed
- Van Esch H. The Fragile X premutation: new insights and clinical consequences. Eur J Med Genet. 2006 Jan-Feb;49(1):1-8. Epub 2005 Dec 5. Review. Citation on PubMed
- Willemsen R, Oostra BA, Bassell GJ, Dictenberg J. The fragile X syndrome: from molecular genetics to neurobiology. Ment Retard Dev Disabil Res Rev. 2004;10(1):60-7. Review. Citation on PubMed
How Fragile X Syndrome is Inherited
Fragile X syndrome (FXS) is a genetic disorder. A genetic disorder means that there are changes to the person’s genes. FXS, or the risk for developing FXS, can be passed down from parents to children through genes.
Many people who have a family member with FXS may wonder what this means for their own health or for the health of other family members. If you are concerned or wish to learn more, you may want to talk to a healthcare provider or genetic counselor.
If you are concerned or wish to learn more, you may want to talk to a healthcare provider or genetic counselor.
Below is some information to help you understand how FXS and some related conditions can be passed down through genes.
Genes
Each cell in the human body contains thousands of genes. Genes determine many things about the person. For example, what the person will look like and whether he or she is likely to have certain illnesses. In addition, genes have instructions for making proteins in the cells. Proteins are needed for the body to work correctly.
FXS is caused by a change (mutation) in the Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene. The FMR1 gene makes a protein called FMRP that is needed for brain development.
Chromosomes
Genes are found on chromosomes. Every human cell contains 23 pairs of chromosomes. People get their chromosomes from their parents. People get one of each pair of chromosomes from their mother and one of each pair from their father. The chromosomes that form the 23rd pair are called the sex chromosomes. They decide if a person is male or female. Females have two X chromosomes (XX), and males have one X and one Y chromosome (XY).
The chromosomes that form the 23rd pair are called the sex chromosomes. They decide if a person is male or female. Females have two X chromosomes (XX), and males have one X and one Y chromosome (XY).
The FMR1 gene is on the X chromosome.
DNA
The chromosomes and genes have a special code called DNA. DNA has four chemical letters, called “bases”: A, C, T, and G. The order of the letters determines the information carried in each gene, like the way that a specific pattern of letters makes up the words in a sentence.
How FXS is Inherited
To understand how FXS is inherited, it helps to know about the changes in the FMR1 gene that cause FXS and other fragile X-associated disorders. There is a place in the FMR1 gene where the DNA pattern of the chemical letters, CGG, is repeated over and over again. Different people have different numbers of these CGG repeats, but most people have less than 45 repeats. People with FXS nearly always have more than 200 repeats, many more than normal. Having more than 200 repeats causes the FMR1 gene to “turn off” so that it can’t make FMRP (the protein made by the FMR1 gene). When a person’s FMR1 gene has more than 200 repeats, so that it can’t make FMRP, the person has FXS.
Having more than 200 repeats causes the FMR1 gene to “turn off” so that it can’t make FMRP (the protein made by the FMR1 gene). When a person’s FMR1 gene has more than 200 repeats, so that it can’t make FMRP, the person has FXS.
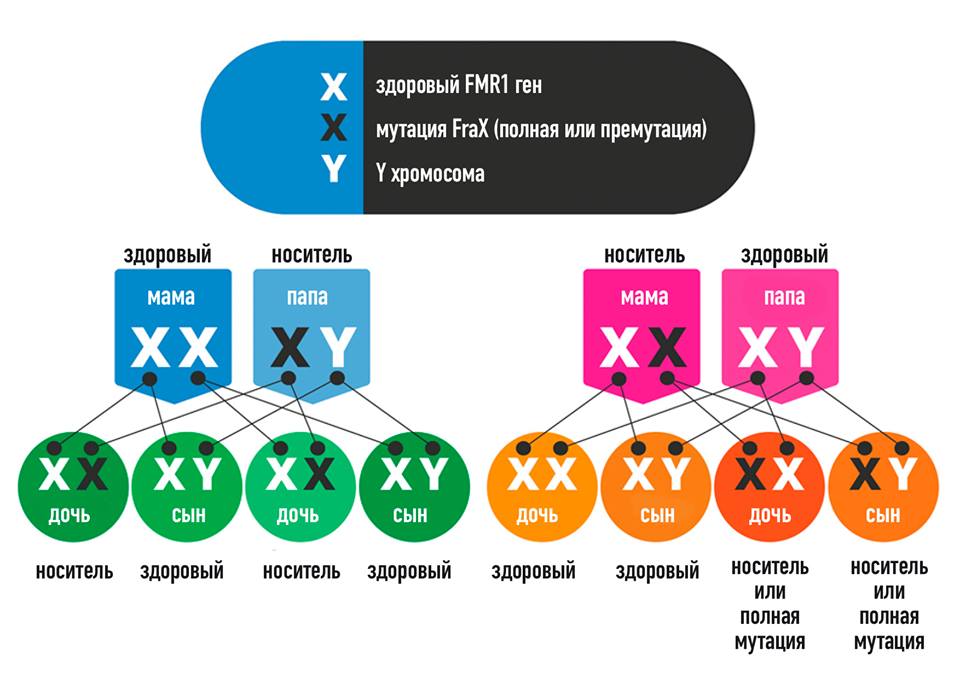
Each person is in one of the four groups shown below based on the number of CGG repeats in the person’s FMR1 gene. The number of CGG repeats that a person has can be determined by a blood test ordered by a healthcare provider or genetic counselor. People with different numbers of CGG repeats have different risks of developing fragile X-associated disorders and of having children with FXS.
A female has two copies of the FMR1 gene, one on each of her two X chromosomes. The number of CGG repeats on each copy of the FMR1 gene is usually different. For example, a female might have 30 CGG repeats on one copy of her FMR1 gene, but 70 CGG repeats on her other copy. The group a female is in (normal, intermediate, premutation, or full mutation, as shown below) is based on her FMR1 gene copy with the greatest number of CGG repeats. A male has only one copy of the FMR1 gene on his only X chromosome, so the group a male is in is based on the number of CGG repeats in that one copy.
A male has only one copy of the FMR1 gene on his only X chromosome, so the group a male is in is based on the number of CGG repeats in that one copy.
Normal: 5 to 44 Repeats
Most males have about 5 to 44 repeats of the chemical letters, CGG, in their FMR1 gene and most females also have 5 to 44 repeats in each of their FMR1 genes. This is considered a normal number of repeats. People with a normal number of repeats do not have FXS and do not pass a higher chance for having FXS to their children.
Intermediate: 45 to 54 Repeats
People who have an intermediate number of repeats (45 to 54) do not have FXS and are not at risk for having children with FXS. However, they may have a slightly higher chance of having some symptoms related to other fragile X-associated disorders and may pass the slightly higher chance of having these disorders to their children.
Premutation: 55 to 200 Repeats
People who have 55 to 200 repeats are said to have a “premutation” in the FMR1 gene.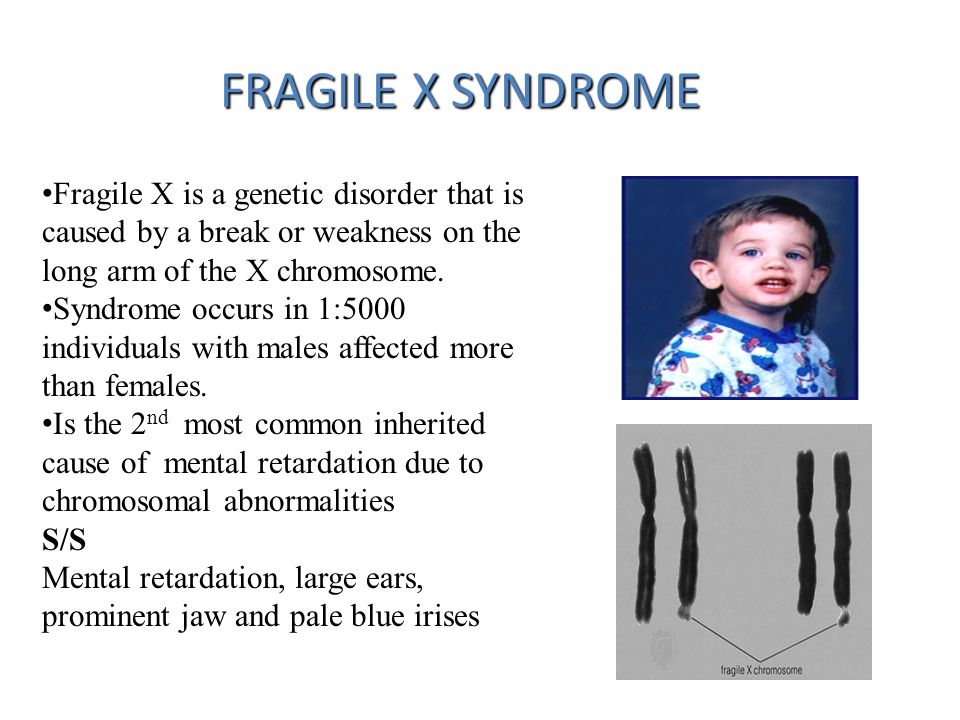 They do not have FXS but they might have, or may later develop, other fragile X-associated disorders. In addition, people with a premutation can have children with a premutation or full mutation (FXS). However, the chances of having a child with a premutation or a full mutation are different for women with a premutation than they are for men with a premutation, as described below.
They do not have FXS but they might have, or may later develop, other fragile X-associated disorders. In addition, people with a premutation can have children with a premutation or full mutation (FXS). However, the chances of having a child with a premutation or a full mutation are different for women with a premutation than they are for men with a premutation, as described below.
- The number of repeats in the egg cells of a woman with a premutation can increase from the premutation range (55 to 200 repeats) to the full mutation range (more than 200 repeats). Therefore, a woman with a premutation can pass on a full mutation. The more CGG repeats a woman with a premutation has, the more likely her child will inherit an FMR1 gene with a full mutation and, therefore, have FXS. With each pregnancy, a woman with a premutation in one of her FMR1 genes has a 50% chance of passing on either the premutation or a full mutation to her child (daughters or sons), and a 50% chance of not passing on either the premutation or the full mutation.

- A man with a premutation will pass on his premutation to his daughters, but not to his sons. A man with a premutation will not pass on a full mutation to any of his children.
Full Mutation (FXS): More than 200 Repeats
People with a full mutation (more than 200 repeats) have FXS.
- With each pregnancy, women have a 50% chance of passing fragile X on to their child (sons or daughters).
Learn more about the genetics of FXS
Genetic features of fragile X syndrome
March 16, 2017
Among the group of hereditary diseases, there are two diseases related to the most common causes of intellectual disability . The most famous and most common pathology is Down syndrome, associated with the presence of an extra 21st chromosome in the human genome. In this article, we will talk about the second most common hereditary disease that leads to mental retardation, and can also be accompanied by other clinical manifestations .
Fragile X syndrome or Martin-Bell syndrome is the result of a disorder in the FMR1 gene (fragile X mental retardation-1), which is located on the X chromosome and plays an important role in the appearance and development of nerve connections, learning and memorization. The frequency of this syndrome among boys is 1:4000.
The so-called “fragility” of the X chromosome is manifested in the fact that the chromosome looks atypical with special staining, as if one piece has separated, although physically it remains intact. The genetic basis of this phenomenon is an increase in the number of trinucleotide repeats CGG in the FMR1 gene located on the X chromosome.
In healthy people, the number of repeats in this gene ranges from 5 to 54 . If repeats are greater than 200 , then the production of protein from the FMR 1 gene is impaired, which leads to the development of Martin-Bell syndrome and the clinical manifestation of the disease.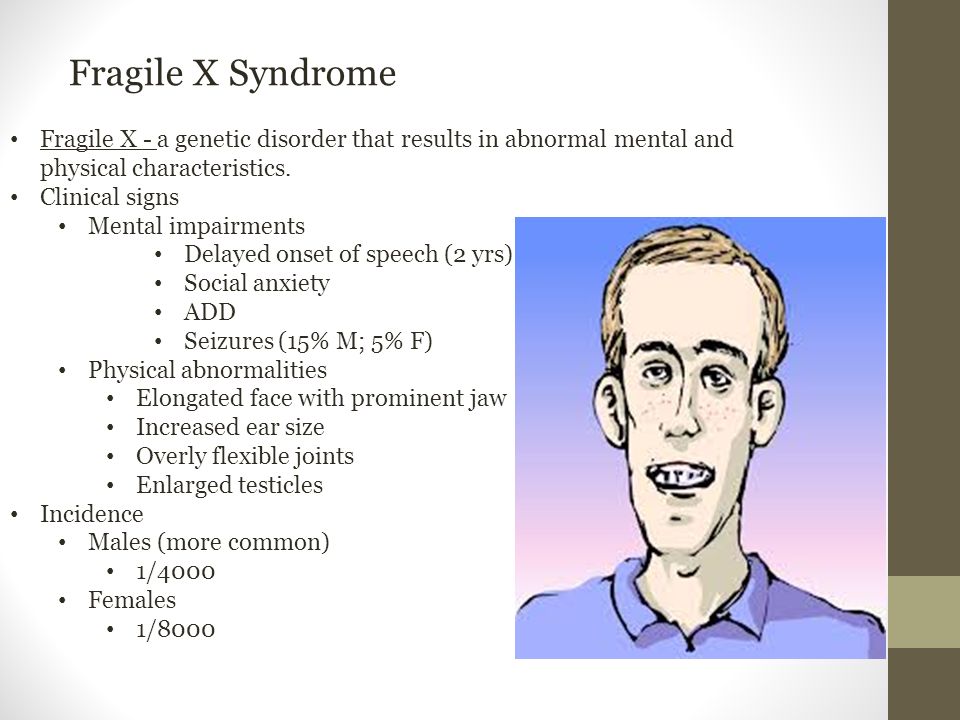 The premutational state is the number of CGG repeats from 55 to 200. In this state, the disease does not typically manifest in humans, but the more repeats a carrier has in this gene, the more likely it is that her or his children will have the number of repeats. more than 200 and the disease will develop. In the case of a carrier of a premutation during the formation of germ cells, the number of repeats can increase, therefore, if the parent has a number of repeats from 55 to 200, then the probability of having a child with a mutant gene is high FMR 1 and Martin-Bell syndrome. At the same time, the carriage of the premutational state by the future father and mother is not equivalent in terms of the probability of the occurrence of a mutant allele in their children: if the mother is the carrier, then the probability of a significant increase in the number of repeats is much higher. The number of repeats from 45 to 54 is an intermediate form that has no effect on human health, but can lead to problems in future generations, as in the case of a premutational state of the gene.
The premutational state is the number of CGG repeats from 55 to 200. In this state, the disease does not typically manifest in humans, but the more repeats a carrier has in this gene, the more likely it is that her or his children will have the number of repeats. more than 200 and the disease will develop. In the case of a carrier of a premutation during the formation of germ cells, the number of repeats can increase, therefore, if the parent has a number of repeats from 55 to 200, then the probability of having a child with a mutant gene is high FMR 1 and Martin-Bell syndrome. At the same time, the carriage of the premutational state by the future father and mother is not equivalent in terms of the probability of the occurrence of a mutant allele in their children: if the mother is the carrier, then the probability of a significant increase in the number of repeats is much higher. The number of repeats from 45 to 54 is an intermediate form that has no effect on human health, but can lead to problems in future generations, as in the case of a premutational state of the gene.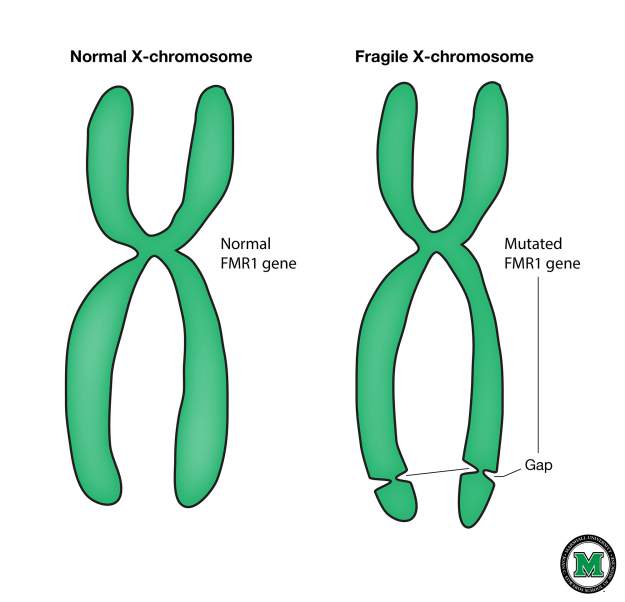
It is important to consider that the inheritance and development of the disease depends on the sex , since the FMR gene 1 is located on the X chromosome. Men have only one X chromosome, which they receive from their mother. Therefore, if this one chromosome turned out to be “fragile”, they have a disease. Women have two X chromosomes, but only one of them is active. Therefore, the presence of one X chromosome with the FMR 1 mutant gene may not manifest itself clinically, in case of inactivation of the “fragile” chromosome, or lead to the development of the disease in 30-50% of cases. A man with a fragile X chromosome can pass it on to all his daughters, but not to any of his sons. A woman with a mutant chromosome has a chance to pass it on to both sons and daughters with equal probability.
The premutational state of the gene affects both the fate of the descendants of the carrier of such a gene and directly on his health:
-
Development of primary ovarian insufficiency ( FXPOI ) (decrease in ovarian reserve and menopause before the age of 40).
 Mutation FMR 1 causes premature ovarian failure in 5% of women with this diagnosis. Among carriers of the premutation, about a quarter develop this condition. It affects not only the general reproductive possibilities, but also the selection of the stimulation protocol for ART, as it often causes a poor ovarian response to stimulation. Interestingly, according to Genetico data, although poor ovarian response to stimulation affects the number of embryos produced per cycle, it does not lead to an increase in the proportion of aneuploid embryos.
Mutation FMR 1 causes premature ovarian failure in 5% of women with this diagnosis. Among carriers of the premutation, about a quarter develop this condition. It affects not only the general reproductive possibilities, but also the selection of the stimulation protocol for ART, as it often causes a poor ovarian response to stimulation. Interestingly, according to Genetico data, although poor ovarian response to stimulation affects the number of embryos produced per cycle, it does not lead to an increase in the proportion of aneuploid embryos. -
Fragile X-associated tremor/ataxia ( FXTAS ). This condition develops more often in men: when the premutation is carried by a man, it manifests itself in 33% of cases, and when the premutation is carried by a woman - only in 5-10%. FXTAS syndrome begins to manifest in old age. There is a tremor, a wobbly gait, speech may suffer.
The diagnostic method used in the Genetico laboratory is based on the use of a polymerase chain reaction with a special set of primers that allows not only the detection of normal, premutational and mutational states, but also to accurately determine the number of repeats in cases where they are less than 200.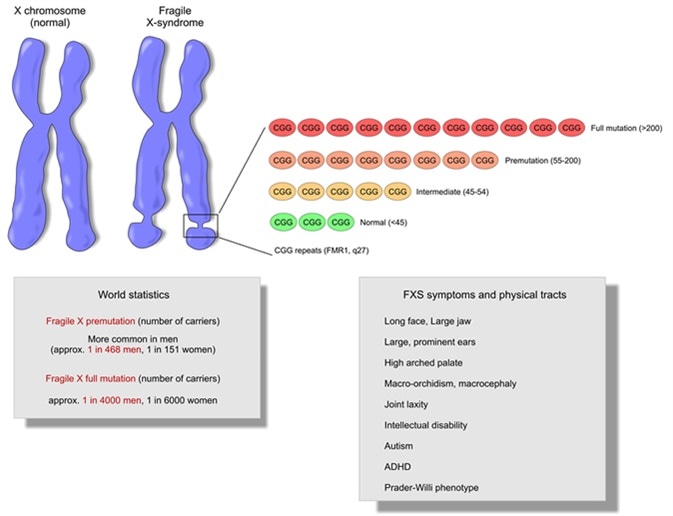 Such diagnostics make it possible to identify fragile X syndrome at the molecular level, as well as to assess the likelihood of having a child with this syndrome and the possibility of the patient developing disorders associated with an increased number of repeats in the gene FMR 1 . This diagnostic also allows the to detect the presence of AGG repeats among the CGG repeats. It is believed that AGG regions that interrupt a long sequence of CGG repeats confer DNA stability and reduce the risk of an increase in the number of repeats in the next generation.
Such diagnostics make it possible to identify fragile X syndrome at the molecular level, as well as to assess the likelihood of having a child with this syndrome and the possibility of the patient developing disorders associated with an increased number of repeats in the gene FMR 1 . This diagnostic also allows the to detect the presence of AGG repeats among the CGG repeats. It is believed that AGG regions that interrupt a long sequence of CGG repeats confer DNA stability and reduce the risk of an increase in the number of repeats in the next generation.
Genetic test that determines the number of repeats in the FMR gene 1 , recommended to undergo in the first place sons with intellectual disabilities. Also analysis of the state of the gene FMR 1 required :
1) women with reproductive problems or fertility problems associated with elevated levels of follicle stimulating hormone (FSH)
2) patients with intellectual disability and their relatives
X-chromosomes or mental retardation without a precise diagnosis
4) women whose relatives had disorders associated with the premutational state FMR1
5) patients with late onset tremor and cerebellar ataxia (muscle coordination disorders due to damage to the brain systems that control muscle movement).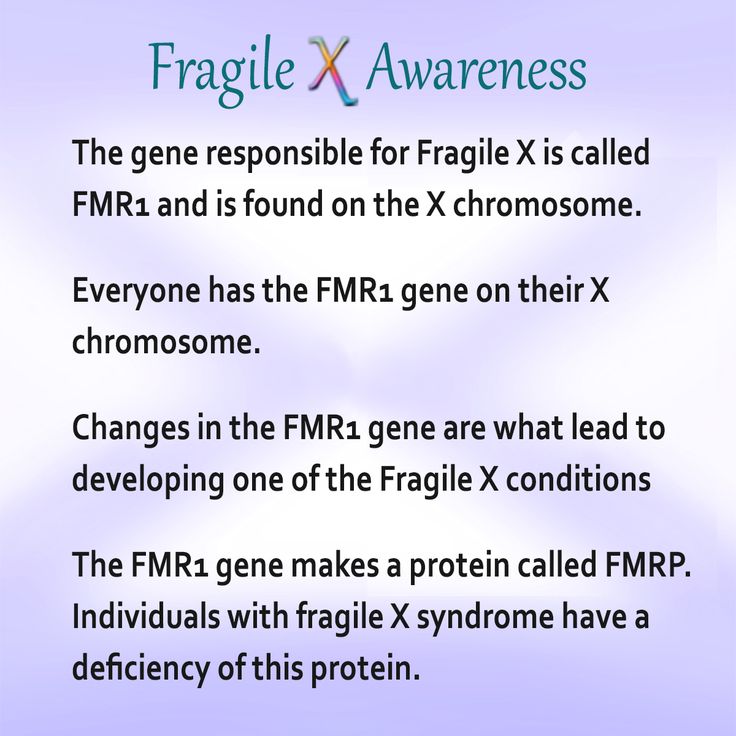
If an asymptomatic mutation in the FMR1 gene is detected in a woman, the use of donor oocytes or preimplantation genetic diagnosis (PGD) may be recommended to rule out the possibility of the syndrome in the child. It is also important to correctly assess the risk of having a sick child in the case of premutational state of the gene FMR 1 in future parents. In this case, according to the results of the test, it is recommended to consult a geneticist.
Author: Ochir Migyaev
Genetico laboratory intern
Martin-Bell syndrome. FMR1 gene. Analysis. interpretation, clinical manifestations of Martin-Bell syndrome
Site searchFORUM:
- Help me to understand.
- Uzi
- Pregnancy or not
- fibrinogen
- fibrinogen
- Pregnancy planning
- Metalobic block
- Marina
- Pregnancy with a donor egg
- CT scan of the kidneys for a man: impact on sperm quality during a planned pregnancy
- CIR experience in prescribing plaquenil
- Can food increase platelets?
- Question for Babak T.
 A._Genferon candles
A._Genferon candles - Biochemical abnormalities
- Histology after a frozen pregnancy 16 weeks
- Covid, APS and genotyping
- pregnancy consultation
- Results of 1 screening
- Growing follicles
- Pregnancy. Increase in blood flow indicators
-
- Pregnancy management
- Family infertility
- Inbeance of pregnancy
- Pregnancy after IVF
- Preparation for IVF
- Unsuccessful attempts to IVF
- Endocrine disorders in women
- Andrological problems
- Pregnancy High risk
- Laboratory
- Claus expert ultrasound
- For specialists
- Training
- Presentations
-
10/17/2022
Lyakherova O.
 V., Maryino
V., Maryino -
10/17/2022
Neat and attentive doctor
-
10/06/2022
Procedure for checking tubal patency
All reviews / Add
July 29, 2020
Martin-Bell syndrome. FMR1 gene. Analysis. interpretation, clinical manifestations of Martin-Bell syndrome Sign up for an online consultation at the CIR by clicking on the link https://vk.cc/9LJxc9
In this video, Dr. Guzov, creator of the CIR https://vk.cc/9z2aLK, talks about Martin-Bell syndrome: what is Martin-Bell syndrome, how to diagnose it. what are the clinical manifestations and how can people with Martin-Bell syndrome be helped. And also what is the FMR1 gene and fragile X syndrome.
00:00 Video start. FMR1 gene. Martin-Bell Syndrome.
01:55 Fragile X syndrome.
04:42 FMR1 gene. The gene for mental retardation associated with a fragile X chromosome.