Fetal nt test
Nuchal translucency test Information | Mount Sinai
Nuchal translucency screening; NT; Nuchal fold test; Nuchal fold scan; Prenatal genetic screening; Down syndrome - nuchal translucency
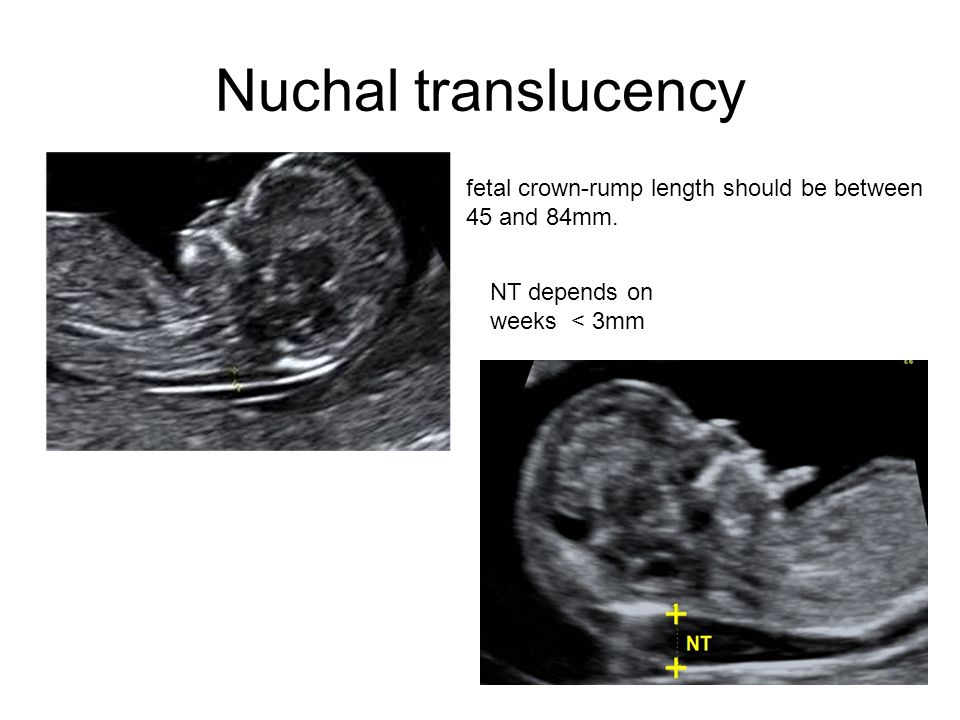
The nuchal translucency test measures the nuchal fold thickness. This is an area of tissue at the back of an unborn baby's neck. Measuring this thickness helps assess the risk for Down syndrome and other genetic problems in the baby.
How the Test is Performed
Your health care provider uses abdominal ultrasound (not vaginal) to measure the nuchal fold. All unborn babies have some fluid at the back of their neck. In a baby with Down syndrome or other genetic disorders, there is more fluid than normal. This makes the space look thicker.
A blood test of the mother is also done. Together, these two tests will tell if the baby could have Down syndrome or another genetic disorder.
How to Prepare for the Test
Having a full bladder will give the best ultrasound picture. You may be asked to drink 2 to 3 glasses of liquid an hour before the test. DO NOT urinate before your ultrasound.
How the Test will Feel
You may have some discomfort from pressure on your bladder during the ultrasound. The gel used during the test may feel slightly cold and wet. You will not feel the ultrasound waves.
The gel used during the test may feel slightly cold and wet. You will not feel the ultrasound waves.
Why the Test is Performed
Your provider may advise this test to screen your baby for Down syndrome. Many pregnant women decide to have this test.
Nuchal translucency is usually done between the 11th and 14th week of pregnancy. It can be done earlier in pregnancy than amniocentesis. Amniocentesis is another test that checks for birth defects.
Normal Results
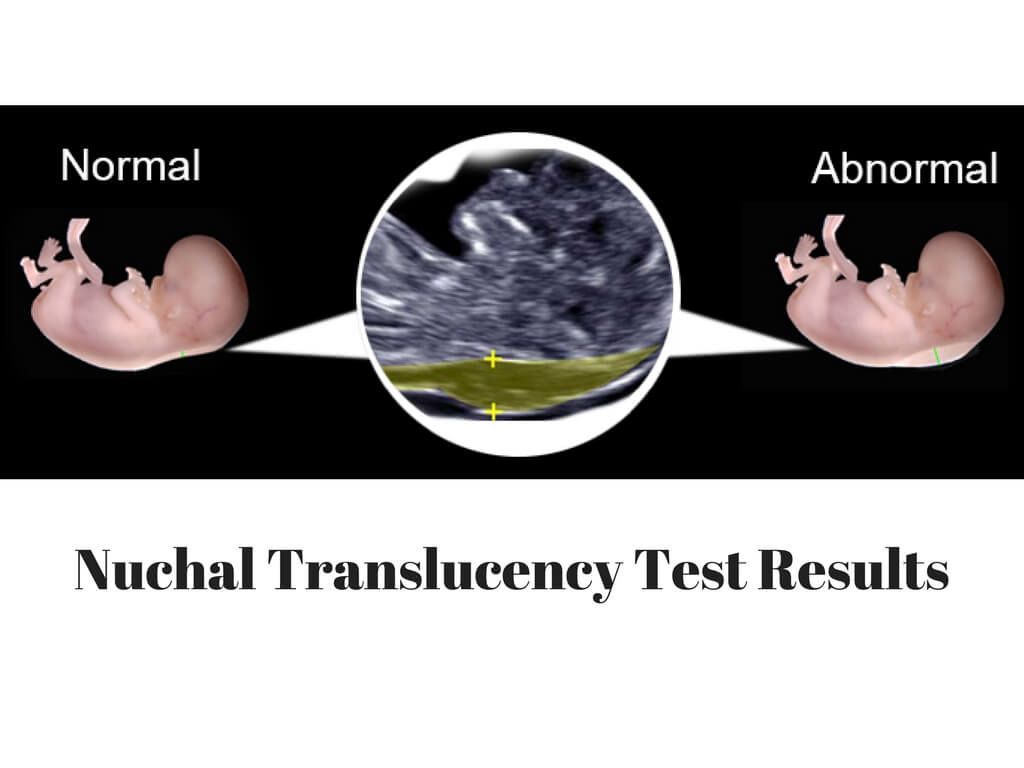
A normal amount of fluid in the back of the neck during ultrasound means it is very unlikely your baby has Down syndrome or another genetic disorder.
Nuchal translucency measurement increases with gestational age. This is the period between conception and birth. The higher the measurement compared to babies the same gestational age, the higher the risk is for certain genetic disorders.
The measurements below are considered low risk for genetic disorders:
- At 11 weeks -- up to 2 mm
- At 13 weeks, 6 days -- up to 2.8 mm
What Abnormal Results Mean
More fluid than normal in the back of the neck means there is a higher risk for Down syndrome, trisomy 18, trisomy 13, Turner syndrome, or congenital heart disease. But it does not tell for certain that the baby has Down syndrome or another genetic disorder.
If the result is abnormal, other tests can be done. Most of the time, the other test done is amniocentesis.
Risks
There are no known risks from ultrasound.
Driscoll DA, Simpson JL. Genetic screening and diagnosis. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe's Obstetrics: Normal and Problem Pregnancies. 8th ed. Philadelphia, PA: Elsevier; 2021:chap 10.
Walsh JM, D'Alton ME. Nuchal translucency. In: Copel JA, D'Alton ME, Feltovich H, et al, eds. Obstetric Imaging: Fetal Diagnosis and Care. 2nd ed. Philadelphia, PA: Elsevier; 2018:chap 45.
2nd ed. Philadelphia, PA: Elsevier; 2018:chap 45.
Wapner RJ, Dugoff L. Prenatal diagnosis of congenital disorders. In: Resnik R, Lockwood CJ, Moore TR, Greene MF, Copel JA, Silver RM, eds. Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice. 8th ed. Philadelphia, PA: Elsevier; 2019:chap 32.
Last reviewed on: 4/19/2022
Reviewed by: John D. Jacobson, MD, Department of Obstetrics and Gynecology, Loma Linda University School of Medicine, Loma Linda, CA. Also reviewed by David C. Dugdale, MD, Medical Director, Brenda Conaway, Editorial Director, and the A.D.A.M. Editorial team.
Nuchal Translucency Ultrasound Screening Test In 1st Trimester
Written by R. Morgan Griffin
Reviewed by Traci C. Johnson, MD on December 15, 2020
Who Gets the Test?
The first trimester screening is a safe, optional test for all pregnant women. It's a way of checking your baby's risk of certain birth defects, such as Down syndrome, Edward's syndrome (trisomy 18), trisomy 13, and many other chromosomal abnormalities, as well as heart problems.
Women carrying twins may be less likely to get the full first trimester screening. The results aren't as accurate as they are with single babies.
What the Test Does
The screening involves two steps. A blood test checks for levels of two substances -- pregnancy-associated plasma protein-A (PAPP-A) and human chorionic gonadotropin. A special ultrasound, called a nuchal translucency screening, measures the back of the baby's neck. At times, the nuchal translucency test may add on ultrasound markers, such as measuring a baby's nasal bone.
The combined result of the blood tests and the ultrasound gives you a sense of your baby's risk. However, it's not a diagnosis. Most women who have an abnormal first trimester screening go on to have healthy babies.
Whether you get this test is your choice. Some women want the test so they can prepare. Others don't. They may decide that knowing the results wouldn't change anything. Or they feel that the test could result in unnecessary stress and invasive testing.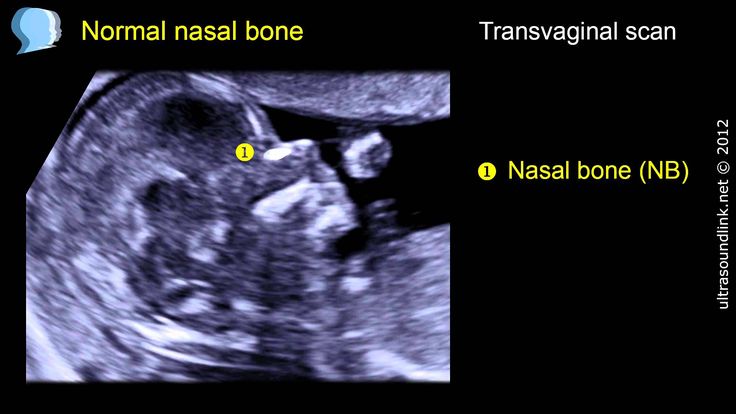 However knowing of possible risks would allow for increased monitoring during your pregnancy as well as giving you delivery options (special hospital, pediatric surgeon availability).
However knowing of possible risks would allow for increased monitoring during your pregnancy as well as giving you delivery options (special hospital, pediatric surgeon availability).
How the Test Is Done
The first trimester screen won't harm you or your baby. A technician will take a quick blood sample from your arm or fingertip. The nuchal translucency screening is a normal ultrasound. You'll lie on your back while a technician holds a probe against your belly. It will take between 20 to 40 minutes.
What to Know About Test Results
You should have the results in a few days. If your results are normal, your baby has a low risk of these birth defects. If they're abnormal, your doctor may suggest further tests to rule out problems. These could include ultrasounds or invasive procedures, like CVS or amniocentesis.
Try not to worry if your results are abnormal. Remember: This test can't diagnose birth defects. It only shows if your baby has a greater risk than average.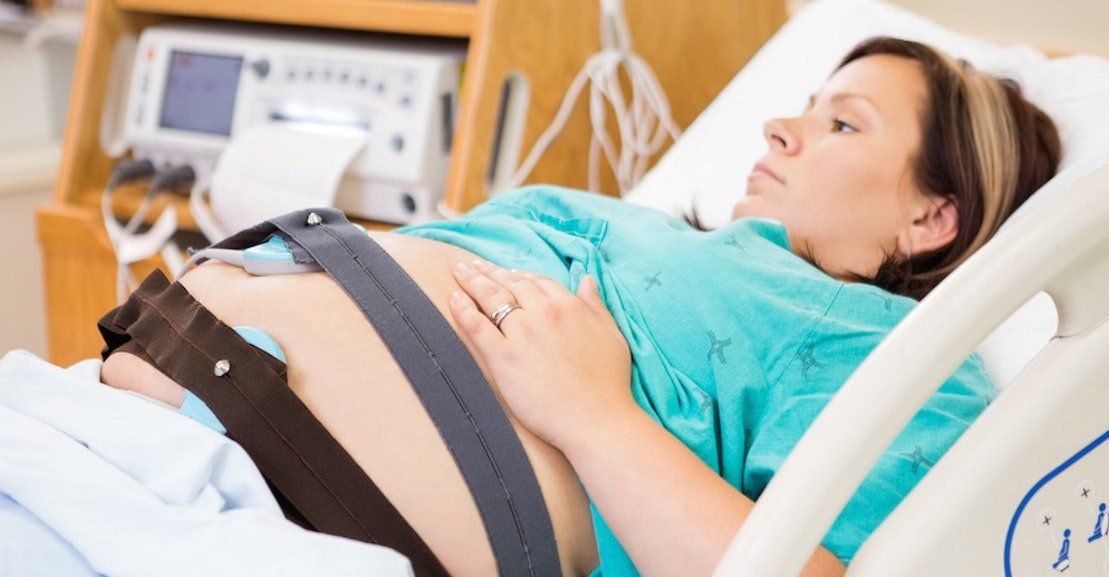
Sometimes your test results are combined with a second trimester screening. In that case, you may not get test results until your second trimester. Or you may get the results, and then get combined results after the second test.
How Often the Test Is Done During Your Pregnancy
You would get the first trimester screen once between the 11th and 13th week.
Other Names for This Test
Nuchal test, integrated screening
Tests Similar to This One
Triple screen, quad screen, MSAFP, sequential screening
Non-invasive prenatal test (NIPT): what are the studies and analyzes
Views: 21396 Published: / Updated:
- Pregnancy
Prenatal screening
Prenatal screening are activities that provide information about the health of the baby in the womb.
Screening during pregnancy helps to clarify the duration of pregnancy, as well as to determine whether there are any chromosomal abnormalities in the fetus or gross, absolutely lethal malformations.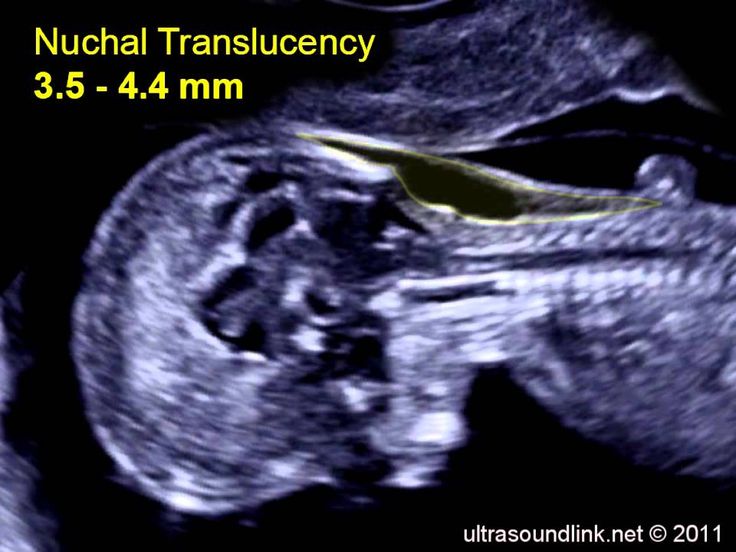
Chromosomal abnormalities are changes in the number of chromosomes or their structure. In humans, in every cell of the body, except for the sex cells, there are 46 chromosomes, 22 of them are paired: one chromosome is transmitted from the mother, the other from the father. Chromosomes 45 and 46 determine the sex of a person, so the karyotype of a normal healthy male is 46,XY; women - 46, xx.
In Russia, prenatal screening is performed three times during pregnancy: in the first, second and third trimesters. Screening of the first and second trimester includes the measurement of blood biochemical parameters and the performance of an ultrasound examination, therefore it is called combined.
In the third trimester, only ultrasound is performed for pregnant women.
The combined screening method is an indirect method for assessing the number of chromosomes of an unborn child.
From 11 to 13 weeks, the first prenatal screening is performed. During the ultrasound, the doctor determines the exact gestational age, the size of the fetus, its proportions.
During the ultrasound, the doctor determines the exact gestational age, the size of the fetus, its proportions.
For biochemical screening, two blood hormones are examined: b-hCG and PAPP-A. Ultrasound data and laboratory results are evaluated together with a program that calculates genetic risk.
For each ultrasound parameter and biochemical indicator, there are reference intervals, the limits of the norm. If there are deviations in the results, the woman receives a referral to perform clarifying procedures, including invasive tests: amniocentesis or chorionic villus biopsy. However, their appointment is not always justified, since in biochemical screening, false positive results occur with a frequency of 4-5%. Of the 20 women who were identified as high-risk and had an invasive confirmatory test, only one would be confirmed and 19out of 20, the procedure will not be justified.
The dangers of invasive prenatal diagnostics
Invasive methods of prenatal diagnosis are diagnostic procedures that are performed in the presence of certain indications. For genetic studies, analysis is performed from placental tissue (chorionic villus biopsy), amniotic fluid (amniocentesis), or cord blood (cordocentesis). In 99%, the analysis will give an accurate answer whether there is a pathology in the number of chromosomes in the fetus or not. But invasive manipulations pose a danger to the health of the baby and bear the mother's worries. Performance is associated with a 2–10% risk of pregnancy loss.
For genetic studies, analysis is performed from placental tissue (chorionic villus biopsy), amniotic fluid (amniocentesis), or cord blood (cordocentesis). In 99%, the analysis will give an accurate answer whether there is a pathology in the number of chromosomes in the fetus or not. But invasive manipulations pose a danger to the health of the baby and bear the mother's worries. Performance is associated with a 2–10% risk of pregnancy loss.
NIPT should be done before an invasive procedure
NIPT provides an alternative to biochemical screening. Its appointment is advisable after the detection of a high risk of Down syndrome, Edwards syndrome or other genetic disorder according to biochemical screening. If desired, NIPT can replace biochemical analysis. NIPT justifies invasive methods and allows a pregnant woman to maintain her health and the health of her unborn child.
Opportunities for NIPT
NIPT determines the presence of an extra chromosome in 21, 13, 18 pairs of chromosomes, as well as a non-standard number of chromosomes - 69 instead of 46 (triploidy).
NIPT assesses the risk of sex chromosome abnormalities. The appearance of an additional X or Y chromosome leads to the development of persistent genetic syndromes.
In exceptional cases, NIPT detects microdeletion syndromes in which a small amount of genetic information is missing.
As an additional option, the analysis determines the sex of the child.
Principle of NIPT
NIPT analyzes the child's genetic information from the mother's blood. The subject of the study is extracellular DNA. DNA from the trophoblast, the outer layer of the embryo from which the placenta is formed, enters the mother's blood. During the development of the embryo, trophoblast cells are renewed. Some of the cells die and enter the bloodstream. As the fetus grows and develops, the number of placental cells increases, and the percentage of fetal DNA increases accordingly. By 9th week of embryo development, the amount of fetal-placental DNA is 10% of the maternal one.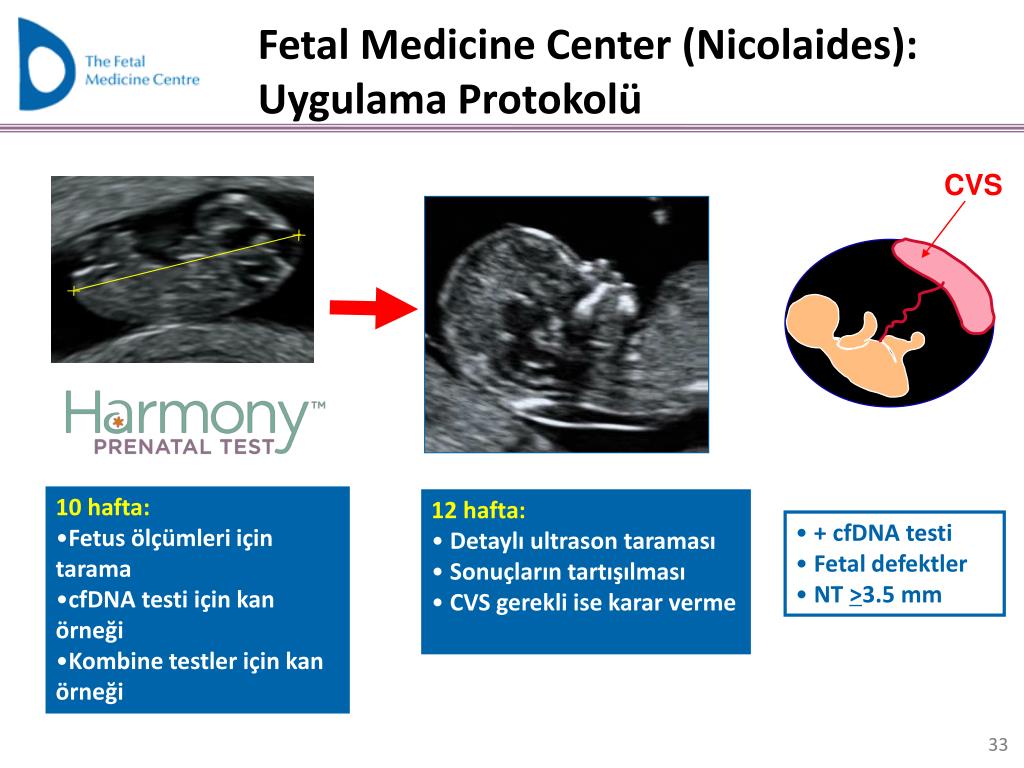
Stages of analysis
At the first stage, the blood is centrifuged and DNA is isolated. The second step is to increase the number of DNA copies and create genomic libraries for each woman. There is a decoding of the genome of the mother and child and comparison. The third stage is data analysis and processing using a mathematical algorithm. The final step is getting the report.
If the principle is the same, what are the differences?
The main problem when working with the fetal DNA fraction is the separation of the genetic information of the mother and fetus.
Differences in analysis methodology and data processing led to the existence of several NIPTs: Panorama, Arioza, Veracity, etc.
To analyze the extracellular DNA sequence, two bioinformatic approaches are used: quantitative and single nucleotide polymorphism (SNP) counting technologies.
The Quantitative scoring method compares the relative number of sequences of a chromosome of interest with a reference chromosome. The set of chromosomes for which euploidy is assumed can be used as a comparison. The method has a drawback - it is effective in identifying the risk of aneuploidy 21 and 18, but not 13 chromosomes.
The set of chromosomes for which euploidy is assumed can be used as a comparison. The method has a drawback - it is effective in identifying the risk of aneuploidy 21 and 18, but not 13 chromosomes.
The Panorama prenatal test uses a method to compare maternal and fetal DNA SNPs. The genotype of maternal DNA obtained from blood leukocytes is "subtracted" from the plasma sample. Thanks to the technology, the sensitivity of the method is 99%.
10 reasons why DNACOM does NIPT Panorama
In the DNACOM laboratory, we chose the Panorama test for the following reasons:
- Possibility of early risk assessment (from 9 completed weeks of gestation).
- The test operates at 2.8% fetal DNA, so the % indeterminate response is kept to a minimum.
- The test is suitable for screening pregnant women of all ages.
- The Panorama test has been validated as a screening test for microdeletion syndromes.
- Analysis performed at an accredited Natera laboratory, USA.
- 0% errors in determining the sex of the unborn baby.
- The analysis can be used for egg donation, surrogacy.
- The test is used during twin pregnancy and determines the zygosity for twins: monozygotic or dizygotic.
- Fetal fraction and fetal sex determination is determined for each twin fetus.
- Analytical data set:
- Kit sensitivity 98-99%.
- Kit specificity 99.5-100%.
- PPV (Positive Predictive Value).
- NPV (negative predictive value).
Restrictions
It is important to remember the limitations of the test:
- Specimens will not be accepted for testing if twins are known to be missing.
- Specimens from three or more pregnancies are not accepted for testing.
- Pregnancy blood transfusion, bone marrow transplantation, oncological diseases are contraindications to the test.
Result
The Panorama test result contains a report showing individual risks for each chromosome, PPV and NPV, % of fetal DNA and sex of the child. The analysis is issued on two forms, one of which is an American original.
Be sure to consult with your obstetrician-gynecologist and geneticist after receiving the results.
Knowing the features of the NIPT methodology and advantages will help you make the right decision in choosing a test for yourself and your unborn child.
- 40.190 Non-invasive prenatal test (Panorama, diagnosis of aneuploidy).
- 40.191 Non-invasive prenatal test (Panorama, diagnosis of aneuploidy + microdeletions).
NACE Non-invasive prenatal test
Non-invasive fetal chromosomal risk assessment
Interested?
To learn more
Our email: support. [email protected]
[email protected]
How does it work?
- NACE
- Benefits
- Indications
What is the NACE test?
- NACE is a non-invasive prenatal screening for the most common chromosomal abnormalities. Since the test is non-invasive, it does not cause any harm to the fetus.
- A simple maternal blood test detects fetal DNA circulating in the maternal circulation using Next Generation Sequencing (NGS) technology and our patented bioinformatic analysis tool.
How is the procedure?
Who needs a NACE test?
- High risk of chromosomal abnormality on first trimester screening.
- Previous pregnancy with a fetus with Down syndrome
- Ultrasound markers of chromosomal pathology
NACE test can be done:
- Single pregnancy
- When pregnant with twins
- Obtaining a result when the level of free fetal DNA in the mother's blood is less than 4%
- When conceived naturally
- When conceived under the ART program
- In programs using donor oocytes
- Women of all ages
- Women of all ethnicities
- Women with any BMI of
- Suitable for cases of consanguinity
(Gregg, et al. Genet Med. 2016)
Genet Med. 2016)
Study Limitations:
- Less than 10 weeks gestation
- Maternal chromosomal abnormalities
- Bone marrow or stem cell transplant, history of recent blood transfusion
- Malignant tumors
- Pregnancy with three or more fetuses
- Ultrasound markers and/or fetal malformations
- NACE is a screening test and is not a diagnostic test.
- Obtaining an unreliable result of non-invasive screening is possible in cases of: fetoplacental mosaicism, maternal chromosomal mosaicism, with the “vanishing twin” phenomenon or research errors.
- Shows the effect of low molecular weight heparin on the accuracy of the analysis. If heparin is prescribed during pregnancy, NACE blood sampling should be performed prior to heparin administration.
Documents and resources
- NACE documents for professionals
Scientific publications
Related research:
Milan M, Mateu E, Blesa D, Clemente-Ciscar M, Simon C.