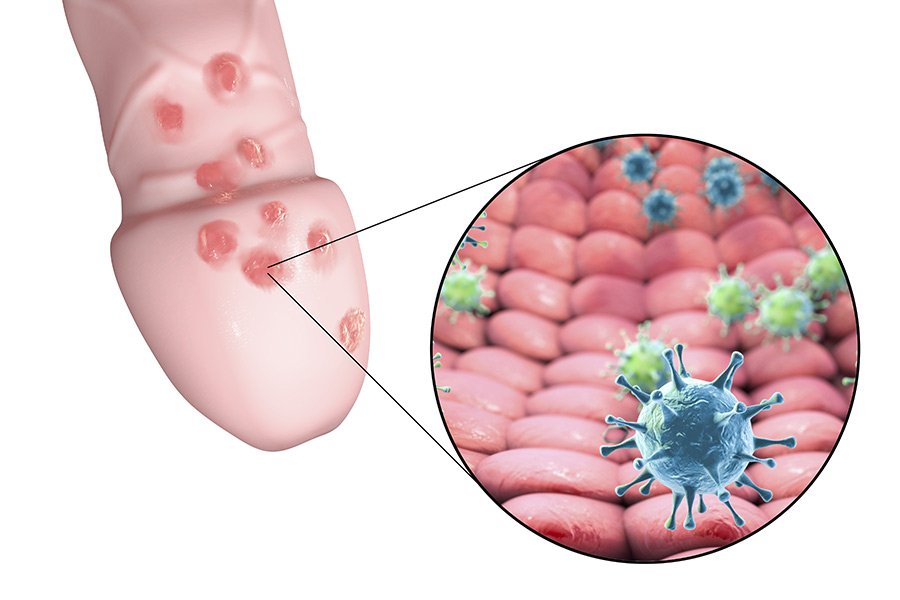
Chlamydia in toddlers
Chlamydia (for Parents) - Nemours KidsHealth
en español: Clamidia
Reviewed by: Amy W. Anzilotti, MD
Primary Care Pediatrics at Nemours Children's Health
What Is Chlamydia?
Chlamydia (kluh-MID-ee-uh) is a common, curable sexually transmitted disease (STD). Treatment can stop the spread of the infection and help prevent long-lasting problems.
What Are STDs?
STDs (also called sexually transmitted infections or STIs) are infections that spread through sex (vaginal, oral, or anal). Some STDs can spread through close contact with the genitals or body fluids.
How Do People Get Chlamydia?
Chlamydia spreads through sex (vaginal, oral, or anal) with someone who has the infection. Most people with chlamydia don’t have symptoms, so they may spread the infection without realizing it.
What Are the Signs & Symptoms of Chlamydia?
Chlamydia usually doesn't cause symptoms. If it does, they can include:
- discharge from the vagina, penis, or anus
- pain in the lower belly
- fever
- pain when peeing
What Causes Chlamydia?
A type of bacteria, Chlamydia trachomatis, causes chlamydia.
How Is Chlamydia Diagnosed?
To find out if someone has chlamydia, health care providers do tests on:
- fluid or discharge from the vagina, urethra, eye, or anus
- urine (pee)
How Is Chlamydia Treated?
Health care providers treat chlamydia with antibiotics. It is important to get tested again 3 months after treatment to make sure the infection is cured (even if there are no symptoms).
For the infection to go away, someone needs to:
- Take the antibiotics exactly as prescribed. They should not stop taking the medicine early because the infection could come back.
- Tell all partners from the last 2 months to get tested and treated (even if they don’t have symptoms).
- Not have sex until they and their partners are treated and any symptoms of chlamydia are gone (such as pain in the lower belly; fever; unusual discharge from the vagina, penis, or anus; and pain when peeing).
People can get chlamydia again if:
- Their partners aren't treated with antibiotics.

- They get treated but then have sex with someone else who has chlamydia.
What Problems Can Happen?
If it's not treated, chlamydia can lead to:
- in girls: pelvic inflammatory disease (PID), which can damage the reproductive system
- in guys: swelling in the testicles and tubes at the back of the testicles
- infertility: trouble getting pregnant (for girls) or getting someone pregnant (for guys)
Can Chlamydia Be Prevented?
The only way to prevent chlamydia and other STDs is to not have sex (oral, vaginal, or anal).
If someone decides to have sex, they can lower their risk of getting an STD by:
- using a latex condom every time they have sex (vaginal, oral, or anal)
- getting tested with any new partners before having sex
- only having sex with one partner (who doesn’t have sex with other people)
Anyone who is sexually active should get tested for STDs every year (or more often if recommended by their health care provider).
Reviewed by: Amy W. Anzilotti, MD
Date reviewed: January 2022
Share:
/content/kidshealth/misc/medicalcodes/parents/articles/chlamydia
Pediatric and Adolescent Chlamydia
Pediatric and adolescent chlamydia is a common sexually transmitted infection (STI) that impacts both males and females. If left untreated, it can cause permanent damage to a woman’s reproductive system, and difficulty getting pregnant later in life.
What is Pediatric and Adolescent Chlamydia?
Chlamydia is a commonly occurring STI that can be difficult to diagnose. Not everyone develops symptoms, but chlamydia can still be passed with or without obvious warning signs.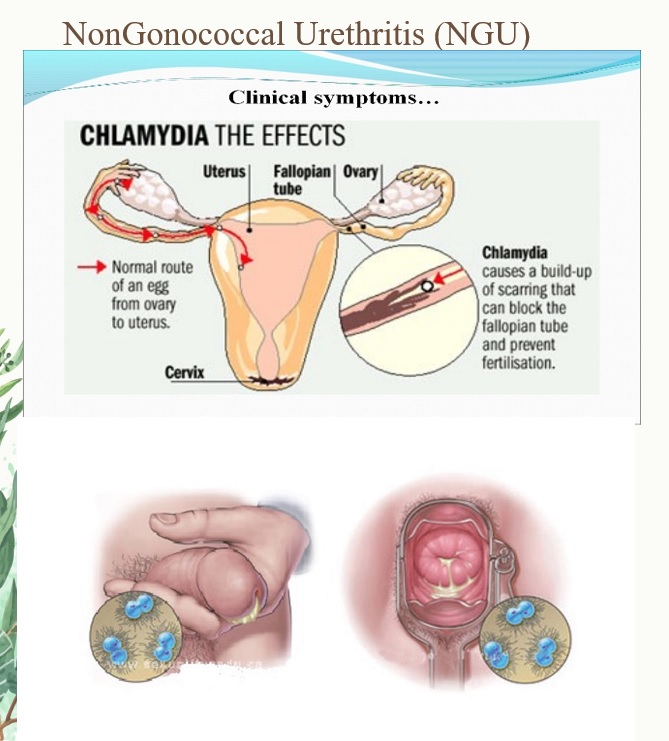 If left untreated, females can develop pelvic inflammatory disease (PID), which can damage the reproductive system and cause infertility.
If left untreated, females can develop pelvic inflammatory disease (PID), which can damage the reproductive system and cause infertility.
What are the signs and symptoms of Pediatric and Adolescent Chlamydia?
Most people with chlamydia will not experience symptoms. If symptoms are present, they will appear one to three weeks after a patient is exposed. Symptoms can include:
- Vaginal discharge (more than normal)
- Vaginal Bleeding / unusually heavy and long periods
- Burning during urination
- Lower abdomen pain (typically only with females) or pain during intercourse
How is Pediatric and Adolescent Chlamydia diagnosed?
Chlamydia is diagnosed with testing done in your health care provider’s office. Sometime doctors can get the information they need for the test from your urine, while others they may have to do a vaginal swab (Q tip).
What are the causes of Pediatric and Adolescent Chlamydia?
Chlamydia is an infection caused by the bacteria Chlamydia trachomatis that is found in the cervix (the passage forming the lower part of the uterus), throat, urethra (tube that carries urine from the bladder), vagina and rectum.
Risk factors
There are several risk factors that will increase the chance of chlamydia:
- Being sexually active and not using condoms– All persons that are sexually active have a greater risk of contracting chlamydia. Being sexually active and not using condoms increases that risk. Further, the more sexual partners you have, the more chances you have to get chlamydia. Being with a partner that has had multiple partners also increases your risk.
- Weakened immune system – Immune systems can be weakened by HIV/AIDS, immune-suppressing medications (organ transplants) or other reasons can increase being susceptible to getting chlamydia if exposed.
- Having a mother with chlamydia – It is possible for a mother to infect her child during vaginal childbirth.
How is Pediatric and Adolescent Chlamydia treated?
Chlamydia is treated with antibiotics that your health care provider can prescribe for you. It is important that the partner(s) of the person infected also get treated with antibiotics.
It is important that the partner(s) of the person infected also get treated with antibiotics.
Pediatric and Adolescent Chlamydia Doctors and Providers
-
M. Brett Cooper, MD Adolescent Medicine Specialist
-
Nirupama De Silva, MD Pediatric Gynecologist
-
Jenny Francis, MD Adolescent Medicine Specialist
-
Jason Jarin, MD Pediatric Gynecologist
Chlamydia of the central nervous system. Laboratory diagnostics and clinical and morphological features
In the classifications of diseases of the nervous system, infections play a rather modest role. In many guidelines, chlamydial lesions of the CNS are not even considered, but recently there is evidence of their possible involvement in a whole spectrum of various CNS diseases [1].
In many guidelines, chlamydial lesions of the CNS are not even considered, but recently there is evidence of their possible involvement in a whole spectrum of various CNS diseases [1].
All members of the Chlamydiaceae family dangerous to humans: Chlamydia trachomatis (Ch. trachomatis), Chlamydophila pneumoniae (Ch. pneumoniae), Chlamydophila psittaci (Ch. psittaci) are widespread and highly contagious. Their biological properties are of the same type and are covered in the literature; they serve as a prerequisite for the variability of the clinical manifestations of the infectious process and the probable diagnostic difficulties. In this regard, it should be noted the variety of interactions of an infectious agent with a host cell (reproduction, persistence with the development of aberrant reticular bodies and reactivation, L-transformation is also distinguished in the domestic literature) and inhibition or activation of apoptosis of the host cell, depending on the conditions and form of existence. pathogen. Chlamydiaceae are immunotropic: they are reserved in monocytes, lymphocytes, and macrophages;
pathogen. Chlamydiaceae are immunotropic: they are reserved in monocytes, lymphocytes, and macrophages;
Modeling of neurochlamydia in the experiment showed the affinity of the pathogen to the nervous system, and the morphological manifestations of the infectious process in the CNS or peripheral nervous system did not fundamentally differ depending on the route of infection and the type of pathogen [4, 5]. In the meninges, ventricular ependyma, and brain substance, the pathogen was detected predominantly paravasally, which was accompanied by inflammatory changes of a lymphocytic-macrophage nature [2, 4, 5]. In a study on 600 Balb/c mice, the pathogen ( Ch. trachomatis, Ch. pneumoniae, Ch. psittaci ) was detected regardless of the route of infection in all the studied structures of the nervous system (neurons, glial cells, histiocytes, myelinated and non-myelinated fibers, vessels of the microvasculature, ependyma of the cerebral ventricles and pia mater) in the form of elementary, transitional and reticular bodies.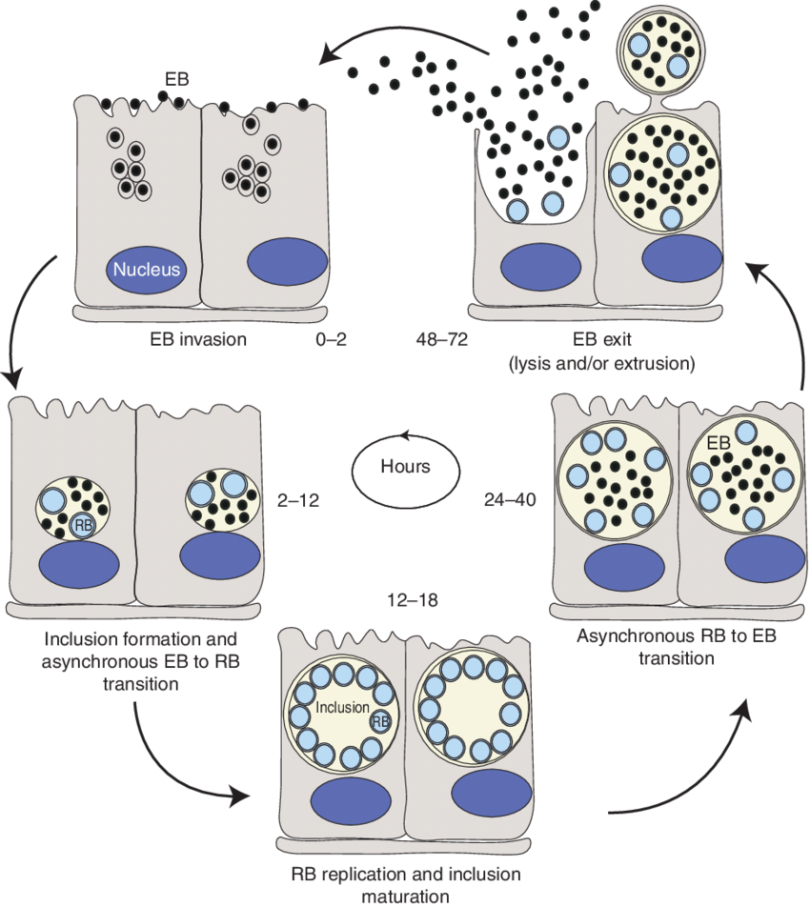 Ultrastructural signs of dystrophy and apoptosis, axonopathy and myelinopathy were revealed [5]. Common inflammatory and vascular disorders were also identified.
Ultrastructural signs of dystrophy and apoptosis, axonopathy and myelinopathy were revealed [5]. Common inflammatory and vascular disorders were also identified.
The fact of passage of chlamydia and chlamydophilus through the blood-brain (BBB) and blood-brain barrier, the ability of pathogens to persist and reproduce in the cells of the nervous system were also shown in other experimental studies [2, 3, 6, 7]. The passage of the pathogen through the BBB is associated with endocytosis, since in this way the pathogen penetrated into all types of cells, and migration through the endothelial cells of cerebral vessels as part of monocytes (shown for Ch. pneumoniae ) [3]. Dissemination from the primary focus of infection occurs hematogenously [2, 4], as well as from cell to cell from the cells of the olfactory epithelium of the nose to the olfactory bulbs and further to brain structures [8, 9].
Pathological changes in chlamydia are described by only a few authors, priority works belong to A. V. Zinzerling [10]. Pathological changes in the CNS in neurochlamydia are described only in single and targeted studies with intrauterine lesions [1, 11].
V. Zinzerling [10]. Pathological changes in the CNS in neurochlamydia are described only in single and targeted studies with intrauterine lesions [1, 11].
Chlamydial infection is discussed in connection with a wide range of neurological diseases. Intrauterine infection with Ch. trachomatis is associated with a generalized CNS disease in young children, but is almost not considered as a cause of neurological disorders in older children and adults. Ch. pneumoniae is more often discussed in connection with adult diseases: the prevalence of antibodies to Ch. pneumoniae in children under 5 years of age is minimal, by the age of 20 it reaches 50%, by the age of 70 it is 80% [2, 3]. Ch. psittaci is detected in the presence of severe respiratory symptoms and suspicion of psittacosis.
Intrauterine infection (IUI) as a cause of perinatal damage to the nervous system is "usually" diagnosed in the presence of a pronounced clinical picture of the disease in a newborn, occurring with the involvement of the central nervous system [1, 2, 11]. According to V.A. Zinserling, there is an insufficient diagnosis of IUI [11].
According to V.A. Zinserling, there is an insufficient diagnosis of IUI [11].
In the structure of the incidence of children in St. Petersburg, chlamydia accounts for about 1/3-1/4 of the deciphered cases of IUI, of which 13-17% are dead fetuses and premature babies [1, 11].
Chlamydia (as well as mycoplasmosis) is often not accompanied by severe symptoms in the neonatal period [1, 2, 11]. In the 1st year of life, psychomotor developmental delay, respiratory and intestinal infections are diagnosed [2, 11]. At an older age, the infection is masked by other diseases of the central nervous system, aggravating or imitating their course. In some children, the inflammatory process from birth has a sluggish character, in others, the onset of clinical manifestations is observed only a few years after “recovery” from perinatal encephalopathy, probably under the influence of an intercurrent process or superinfection [12, 13].
During the examination of 42 children (3-15 years old) with various non-infectious neurological and psycho-neurological diagnoses, who had a diagnosis of "perinatal encephalopathy" in infancy, in 93% (39) of them, causative agents of low-manifest infections (MMI) were detected (by culture and PCR) in different combinations and in various biological materials, including Ch. spp. was determined in 71% (30) of children [12, 13].
spp. was determined in 71% (30) of children [12, 13].
Etiotropic antibiotic therapy confirmed the participation of the identified IUI in the CNS lesion. Retrospectively (based on the regression of neurological symptoms), clinical manifestations of MMI were identified: asthenia and neuropsychiatric disorders, brainstem symptoms, extrapyramidal, less often pyramidal and cerebellar syndromes, as well as epileptic, diencephalic syndromes, and headache of the membrane-vascular nature [13]. Based on neuroimaging data, sluggish perinatal encephalitis had features of an inflammatory-degenerative process [13].
Infection with IUI pathogens ( Ch. spp., Mycoplasma spp., Ureaplasma spp. ) was accompanied by a complex of signs of connective tissue dysplasia (arachnoid cysts, dysplasia of the craniovertebral region in combination with dysplasia of cerebral vessels or internal organs) [13], which confirms the assumption V.A. Zinzerling about the disruption of embryogenesis by these IUIs [11].
According to pathomorphological studies in the Ural region in generalized intrauterine chlamydia, lesions of the pia mater were detected with the formation of peculiar granulomas, determined on the convexital surface of the brain in the form of cotton-like formations; inside the cells of these granulomas, chlamydia was determined (electron microscopy). However, in St. Petersburg, according to V.A. Zinzerling, these changes are not detected. According to his data, in all cases with verified generalized chlamydia, there was a slight increase in the number of fibroblasts with the formation of granuloma-like structures around the vessels in half of the cases [1, 11].
A large group of vascular diseases of the brain is usually associated with atherosclerosis of extra- and intracranial vessels. According to a prospective study (504 patients were examined and 85% of them after 2.5 years), IgA antibodies to Ch. pneumoniae correlated with the progression of carotid atherosclerosis [14]. As a result of a population study, Ch. pneumoniae (IgA to Ch. pneumoniae ) were identified as a probable risk factor for ischemic stroke in the presence of atherosclerosis after adjusting for risk factors [15].
As a result of a population study, Ch. pneumoniae (IgA to Ch. pneumoniae ) were identified as a probable risk factor for ischemic stroke in the presence of atherosclerosis after adjusting for risk factors [15].
Frequency of detection Ch. pneumoniae in atheromatous plaques (AP) of extra- and intracranial vessels is different. M. Cochrane et al. [16] found DNA Ch. pneumoniae in all patients according to 10 endarteriectomy data. According to other researchers, Ch. pneumoniae was detected by PCR in 15.8% (9 out of 57) of patients with asymptomatic cerebrovascular disease and only in 13.5% (5 out of 37) of patients with neurological symptoms. Identification of the pathogen in AB by PCR did not correlate with the presence of antibodies to Ch. pneumoniae in the blood of patients taken during surgery [17]. A high IgA titer (≥1/128) corresponded to a clinically manifest disease. After adjusting for other risk factors, IgA to Ch. pneumoniae as a sign of chronic infection was associated with the development of ischemic stroke in patients of different age groups [15, 18]. The combination of elevated IgA titer and specific IgG has also been associated with stroke and transient ischemic attack [3, 18, 19].
pneumoniae as a sign of chronic infection was associated with the development of ischemic stroke in patients of different age groups [15, 18]. The combination of elevated IgA titer and specific IgG has also been associated with stroke and transient ischemic attack [3, 18, 19].
At the same time, prospective multicenter studies did not reveal the expected effect of the use of macrolides due to the possible involvement of Ch. pneumoniae in the development of atherogenic cerebral stroke and coronary pathology [3, 19].
Three cases of vasculitis induced by Ch. have been published. pneumoniae , which proceeded as aseptic meningitis, cerebral arteritis resembling giant cell arteritis (GCA), polymyalgia rheumatica. All patients had high levels of antichlamydial IgG and IgA, other pathogens were not identified [20]. In two cases, positive clinical and serological dynamics against the background of antichlamydial therapy confirmed participation in the development of chlamydia disease [22].
Ch. pneumoniae was detected in the temporal arteries in 8 out of 9 patients with GCA [23]. According to this and prospective studies (18 biopsies with Ch. pneumoniae from 14 patients), it was found that Ch. pneumoniae (immunohistochemical method) were determined in granulomatous infiltrates and adventitia of the temporal arteries, where a significant number of dendritic cells were also noted. The presence of intercellular contact of dendritic cells, activated T cells and Ch. pneumoniae , which may be a mechanism for the formation of an immunopathological reaction involving Ch. pneumoniae in GCA [24].
However, in two other studies by PCR on a large number of temporal artery biopsies in GCA (from 30 patients) [25] and temporal arteritis (from 90 patients) [26] Ch. pneumoniae were found only in one case.
Most studies of Ch. pneumoniae is devoted to another autoimmune and demyelinating disease of the central nervous system - multiple sclerosis (MS). Research on a possible connection Ch. pneumoniae with MS began after the identification of Ch. pneumoniae in the cerebrospinal fluid of patients with rapidly progressive MS and symptom reduction after antibiotic therapy by S. Sriram et al. According to them, Ch. pneumoniae were detected in CSF samples by culture in 65% (24 of 37) of MS patients and in 11% (3 of 27) of patients with other neurological diagnoses, while by PCR using primers corresponding to the major outer membrane protein gene (MOMP ), - at 97% (36 out of 37) and 18% (5 out of 27) respectively. In the cerebrospinal fluid IgG antibodies to Ch. pneumoniae was present in 86% (32 out of 37) of MS patients and was absent in the control group (27 patients) [27]. In a pathoanatomical study of brain samples of patients with MS Ch. pneumoniae was found in the ependyma and periventricular area [28].
Research on a possible connection Ch. pneumoniae with MS began after the identification of Ch. pneumoniae in the cerebrospinal fluid of patients with rapidly progressive MS and symptom reduction after antibiotic therapy by S. Sriram et al. According to them, Ch. pneumoniae were detected in CSF samples by culture in 65% (24 of 37) of MS patients and in 11% (3 of 27) of patients with other neurological diagnoses, while by PCR using primers corresponding to the major outer membrane protein gene (MOMP ), - at 97% (36 out of 37) and 18% (5 out of 27) respectively. In the cerebrospinal fluid IgG antibodies to Ch. pneumoniae was present in 86% (32 out of 37) of MS patients and was absent in the control group (27 patients) [27]. In a pathoanatomical study of brain samples of patients with MS Ch. pneumoniae was found in the ependyma and periventricular area [28].
According to other data, in patients with MS Ch. pneumoniae was determined in the liquor in 2.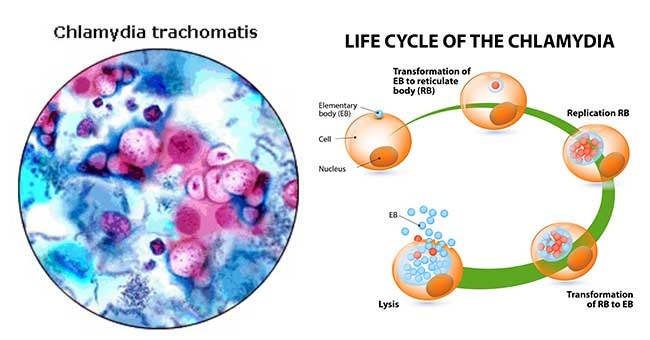 9-69% of cases [3], while the detection rate was Ch. pneumoniae in MS patients was always higher than in the control group. However, in a number of studies Ch. pneumoniae has not been diagnosed in MS patients [3, 19]. Frequency of detection of anti- Ch. pneumoniae -IgG differed variably from the results obtained in PCR diagnostics in MS patients and when compared with the control group [3, 19].
9-69% of cases [3], while the detection rate was Ch. pneumoniae in MS patients was always higher than in the control group. However, in a number of studies Ch. pneumoniae has not been diagnosed in MS patients [3, 19]. Frequency of detection of anti- Ch. pneumoniae -IgG differed variably from the results obtained in PCR diagnostics in MS patients and when compared with the control group [3, 19].
There is a perception that Ch. pneumoniae is associated with immune response activity in the CNS. When MOMP, 16S rRNA, and HSP-70 were simultaneously determined by PCR in three groups of patients (MS, other CNS inflammatory diseases, and the control group), MOMP and 16S rRNA were diagnosed with the same frequency in all groups. At the same time, among MS patients, they were more common in relapsing than in progressive course of the disease, and more often in clinically and MRI-active forms than in the stable course of the disease. HSP-70 was found only in three patients with a relapsing course in the exacerbation phase.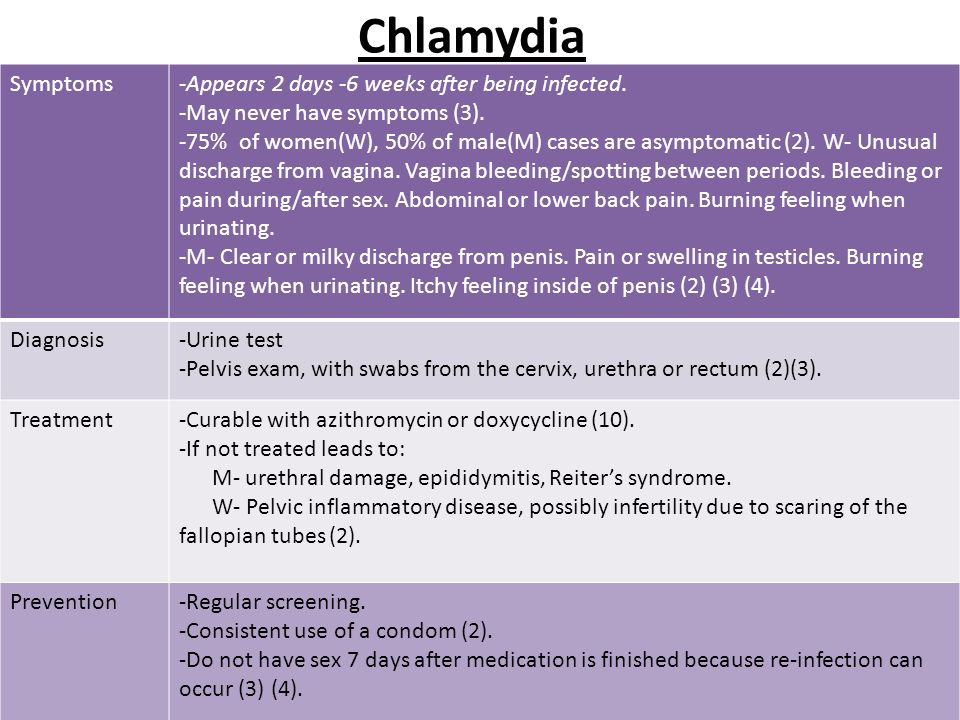 Higher detection rate Ch. pneumoniae also corresponded to an increase in the lesion according to MRI [3, 29].
Higher detection rate Ch. pneumoniae also corresponded to an increase in the lesion according to MRI [3, 29].
However, according to the antibody specificity index (ASI), anti- Ch. pneumoniae -IgG prevailed in patients with MS (16.9%) and inflammatory diseases of the CNS (21.6%) compared with the rest of the patients (1.9%). At the same time, among the group of patients with MS, they prevailed in patients with a progressive form of the disease [30]. A similar result was noted in the detection of specific high-affinity antibodies: in seropositive cerebrospinal fluid samples, they were more often detected in the progressive course of MS [3].
Clinical trial reports on the effects of antichlamydial therapy on MS are inconsistent and incomparable, also due to the lack of standards. According to some data, treatment did not affect the number of active lesions diagnosed by MRI, according to others, it contributed to MRI remission [3].
The result of a retrospective analysis of 26 studies in which Ch.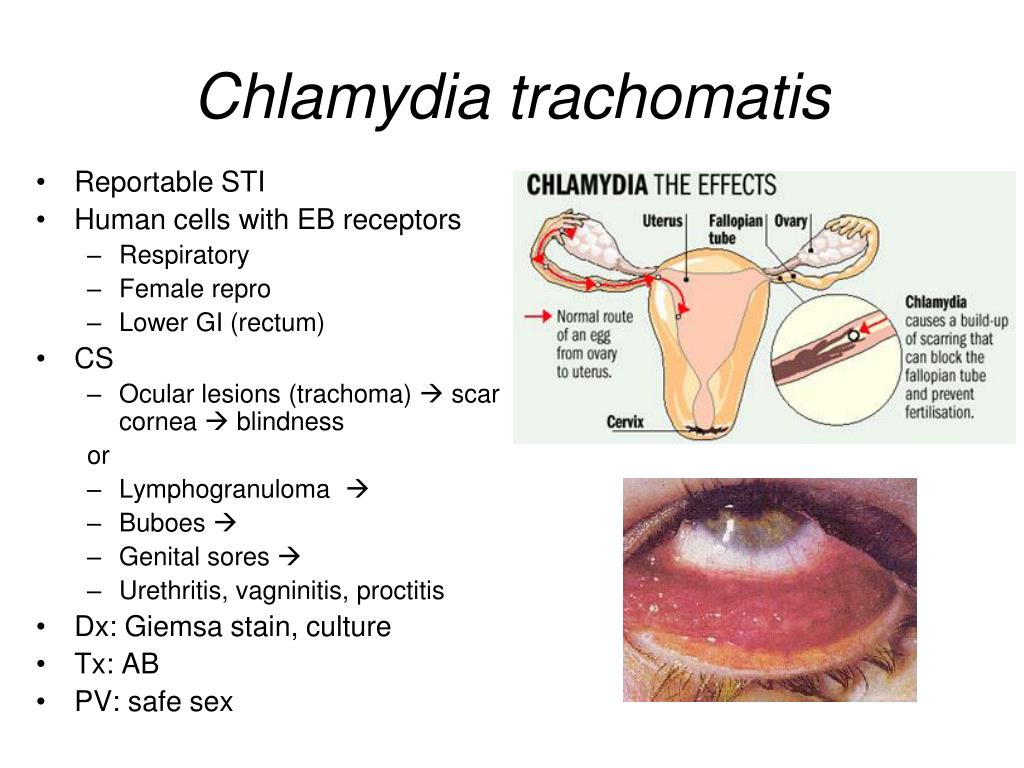 pneumoniae (1332 patients with MS and 1464 controls), obtained by statistical processing adjusted for gender and other differences, was insufficient to establish an etiological relationship between infection Ch. pneumoniae and the incidence of MS [3].
pneumoniae (1332 patients with MS and 1464 controls), obtained by statistical processing adjusted for gender and other differences, was insufficient to establish an etiological relationship between infection Ch. pneumoniae and the incidence of MS [3].
The involvement of infection in the development of degenerative diseases is rarely discussed. However, the presence of pro-inflammatory cytokines, the activation of microglial cells, and the formation of microglial nodules in the area of neuronal death in the brain prompted the search for possible microorganisms as cofactors in the development of genetically indeterminate Alzheimer's disease (AD) [19]. Only a few studies have been published (since 1998) that have found Ch. pneumoniae in the brain of AD patients. Most of them are held by B.J. Balin et al. and only two comparable results were obtained by independent researchers. In other works, attempts to identify Ch. pneumoniae in the brain in AD were unsuccessful.
First time Ch. pneumoniae was identified by B.J. Balin et al. by PCR in patients with AD in various areas of the brain (hippocampus, cerebellum, temporal cortex and prefrontal cortex) inside neurons, neuroglia, endothelial and paraendothelial cells and extracellularly [9, 31-33]. Ch. pneumoniae were located near amyloid deposits and neurofibrillary glomeruli, but did not directly contact amyloid plaques [32–34].
In 58% (11 of 19) patients with BA, an association of the APOE-ε4 gene with Ch was found. pneumoniae , found in brain tissue. Since the APOE-ε4 allele of the gene affects the deposition of the β-A4 amyloid component (present in senile plaques, neurofibrillary glomeruli, and cerebral vessel walls in AD), it is assumed that the identified symbiosis is involved in the pathobiology of AD [31].
In the experiment, intranasal infection with Ch. pneumoniae non-transgenic young female BALB/c mice directly contributed to the production of amyloid (Aβ1-42) in the mouse brain, which may be a model of the primary trigger for the development of the AD pathological process in sporadic cases of the disease [35].
When mice were infected intranasally with Ch. pneumoniae was found in the olfactory bulbs, which correlated with the results of postmortem studies in AD [8]. When conducting a randomized placebo-controlled multicenter clinical trial to determine the effectiveness of antichlamydial therapy (doxycycline and rifampicin) on the clinical course of the disease, it was found that the progressive decline in cognitive functions in patients treated with antibiotics was less intense than in the control group [36].
In recent years, there have been reports suggesting an "inflammatory", including infectious-inflammatory concept of the development of a number of other diseases of the central nervous system, manifested by altered consciousness, mental and behavioral disorders.
B. Fellerhoff et al. [37] hypothesized that infection with Chlamydiaceae is one of the pathogenetic factors in the development of schizophrenia. In the study (PCR) of blood samples Ch.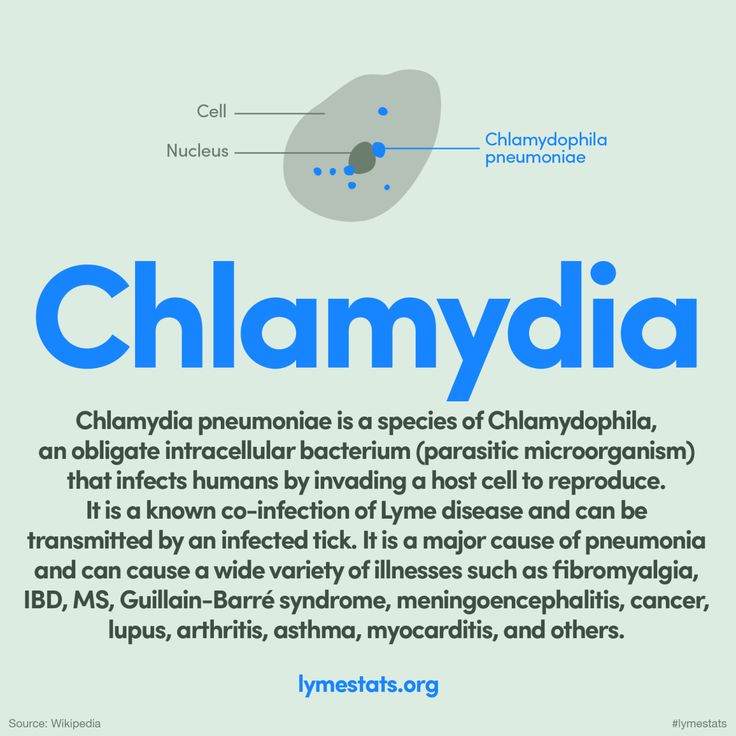 spp. were found in 50% (9out of 18) patients with schizophrenia, and in the control group - only in 6.97% (8 out of 115). Treatment (immunocorrection and antibiotics) has shown a steady improvement in mental status in patients with schizophrenia [37]. In a larger group of patients, the data were confirmed. A significant relationship was found between the incidence of schizophrenia and infection with Ch. spp., especially with Ch. psittaci. Schizophrenia in combination with the HLA-A genotype was also more often observed in Ch. spp. and Ch. psittaci [38]. Chlamydiaceae was detected by PCR in samples of the cortex of the frontal lobes of the brain in schizophrenia significantly more often (23.5% of 34) than in control samples (5.7% of 35). It is assumed that the development of mental disorders is caused by a change in the functional activity of infected microglial cells and neurons with impaired interneuronal connections [39].
spp. were found in 50% (9out of 18) patients with schizophrenia, and in the control group - only in 6.97% (8 out of 115). Treatment (immunocorrection and antibiotics) has shown a steady improvement in mental status in patients with schizophrenia [37]. In a larger group of patients, the data were confirmed. A significant relationship was found between the incidence of schizophrenia and infection with Ch. spp., especially with Ch. psittaci. Schizophrenia in combination with the HLA-A genotype was also more often observed in Ch. spp. and Ch. psittaci [38]. Chlamydiaceae was detected by PCR in samples of the cortex of the frontal lobes of the brain in schizophrenia significantly more often (23.5% of 34) than in control samples (5.7% of 35). It is assumed that the development of mental disorders is caused by a change in the functional activity of infected microglial cells and neurons with impaired interneuronal connections [39].
However, according to other data, the relationship of schizophrenia and other mental disorders with Ch. trachomatis and other IUI have not been determined [40].
trachomatis and other IUI have not been determined [40].
It has been suggested that infections may be involved in the formation of the autistic syndrome, including modulating the immunopathological state of the CNS. In all examined patients with autism in the blood serum (ELISA) elevated titers of antibodies to various neurospecific peptides were determined, which indicated an immunopathological process in the CNS with a violation of the BBB, and antibodies to Ch. pneumoniae and streptococcus [41].
According to other sources, in autistic syndrome Mycoplasma spp. was detected (PCR) more often than other microorganisms - in 58.3% (28 out of 48) compared with the control group of the same age - in 4.7% (2 out of 45), however, Ch. pneumoniae and HHV-6 were also likely cofactors of the disease. In autism, a significant predominance of Ch was determined. pneumoniae [42].
In children and adolescents with neuropsychiatric diagnoses and identified "asymptomatic" infection with Ch. spp., Mycoplasma spp., Ureaplasma spp. as a result of etiotropic treatment (selection of antimicrobial drugs on a transplanted cell culture), improvement was noted in 94% (17 out of 18) of cases. With cerebral palsy with intellectual mnestic and speech disorders (in 3 out of 5 patients), with autistic syndrome (in 4 out of 5), there was an improvement in learning, speech, social response, contact with others. With attention deficit hyperactivity disorder, all (8 patients) showed a significant improvement in attention, emotional background and behavior [13].
spp., Mycoplasma spp., Ureaplasma spp. as a result of etiotropic treatment (selection of antimicrobial drugs on a transplanted cell culture), improvement was noted in 94% (17 out of 18) of cases. With cerebral palsy with intellectual mnestic and speech disorders (in 3 out of 5 patients), with autistic syndrome (in 4 out of 5), there was an improvement in learning, speech, social response, contact with others. With attention deficit hyperactivity disorder, all (8 patients) showed a significant improvement in attention, emotional background and behavior [13].
Taking into account the biological properties of MMI pathogens (in particular, Ch. pneumoniae ), these data are consistent with modern ideas that the disruption of intersynaptic connections in autism may be the result of the activated state of microglia in different areas of the brain [43].
Role Ch. spp. has been shown in post-coma vegetative states (VS). During the examination (culturally, PCR, ELISA of samples of liquor, blood, scrapings from the mucous membranes of the nasopharynx and conjunctiva) of 32 patients in the VS (a consequence of traumatic brain injury and hypoxia), all MMI pathogens were identified, among which 9 prevailed0005 Ch. spp. - in 72% (23 out of 32) of patients in the cerebrospinal fluid and in 81% (26 out of 32) in the blood [1, 44]. Antichlamydial antibodies in cerebrospinal fluid and blood serum were detected in 84% (27 out of 32) of patients: 25% of them had antibodies to Ch. psittaci, in the rest to Ch. trachomatis and to Ch. pneumoniae, in half of the observations there were antibodies to 2-3 species Ch. spp.
spp. - in 72% (23 out of 32) of patients in the cerebrospinal fluid and in 81% (26 out of 32) in the blood [1, 44]. Antichlamydial antibodies in cerebrospinal fluid and blood serum were detected in 84% (27 out of 32) of patients: 25% of them had antibodies to Ch. psittaci, in the rest to Ch. trachomatis and to Ch. pneumoniae, in half of the observations there were antibodies to 2-3 species Ch. spp.
There were no inflammatory changes in the cerebrospinal fluid, as well as specific inflammatory changes during MRI in patients with chlamydial (and other MMI pathogens) lesions of the central nervous system. Post-mortem examination of 4 patients confirmed the presence of intra- and extracellular forms of Ch. spp., glial proliferation, demyelination, and vasculitis [44]. Chlamydia spp. in most cases were associated with pathogenic capsular forms Bacteroides fragilis , HHV-6, VEB, Cytomegalovirus (CMV). A significant relationship was found for Ch. spp. with a demyelinating process and a tendency to be associated with vasculitis [1, 44, 45].
spp. with a demyelinating process and a tendency to be associated with vasculitis [1, 44, 45].
Similar data were found in patients with chronic CNS diseases (epilepsy, vascular, degenerative diseases), in whom Ch. spp. [1].
In the treatment of MMI (primarily caused by Ch. spp. ), patients in the VS showed a significant improvement in psychoneurological status and brain metabolism (PET), as well as better long-term results compared with the control group, in which MMI treatment was not performed. This indicates the clinical significance of the identified, primarily chlamydial, CNS lesions in patients with VS [45].
Probably MMI (primarily caused by Ch. spp. ) contribute significantly to the progressive brain atrophy and Wallerian degeneration seen in VS.
In HIV, the factors that determine the course, progression, and prognosis of primary (HIV-related) neurological disorders have not been established [1]. Despite the wide distribution of Ch. spp., similarity of target cells for HIV and for Ch. spp. jointly neurochlamydia and neuroAIDS are almost not considered. It is believed that Ch. pneumoniae is involved in the progression of primary neurological disorders [46]. With AIDS-dementia complex Ch. pneumoniae was detected (PCR using primers of the MOMP and 16S rRNA genes) in the cerebrospinal fluid and brain tissue samples in 17.4% (4 of 23) patients, in addition, a high level of specific antibodies was detected in the cerebrospinal fluid [3]. Two in vivo examined patients with HIV in the stage of AIDS in the cerebrospinal fluid revealed (culturally) Ch. spp., as well as antibodies: with AIDS-dementia complex to Ch. trachomatis and Ch. pneumoniae, with aseptic meningitis to Ch. pneumoniae, no other opportunistic infections were found. In a targeted morphological study of the brain of patients with HIV, various types of Ch. spp. [47].
spp., similarity of target cells for HIV and for Ch. spp. jointly neurochlamydia and neuroAIDS are almost not considered. It is believed that Ch. pneumoniae is involved in the progression of primary neurological disorders [46]. With AIDS-dementia complex Ch. pneumoniae was detected (PCR using primers of the MOMP and 16S rRNA genes) in the cerebrospinal fluid and brain tissue samples in 17.4% (4 of 23) patients, in addition, a high level of specific antibodies was detected in the cerebrospinal fluid [3]. Two in vivo examined patients with HIV in the stage of AIDS in the cerebrospinal fluid revealed (culturally) Ch. spp., as well as antibodies: with AIDS-dementia complex to Ch. trachomatis and Ch. pneumoniae, with aseptic meningitis to Ch. pneumoniae, no other opportunistic infections were found. In a targeted morphological study of the brain of patients with HIV, various types of Ch. spp. [47].
Separate observations of people of different ages with acute meningoencephalitis, acute disseminated encephalomyelitis, cerebellar ataxia, as well as a number of lesions of the peripheral nervous system, in which a causal relationship of diseases with chlamydial infection was retrospectively suggested and acute chlamydial CNS disease were diagnosed, were published. It is possible that chlamydial infection only exacerbated the course of another, including neuroinfectious, disease and increased residual manifestations [3, 48].
No specific clinical signs of acute chlamydial lesions have been identified. As with perinatal infection, the onset or activation of the disease was often associated with a respiratory or intercurrent infection, sometimes it was possible to identify clinical signs of damage to other organs. Antichlamydial therapy (often in combination with antiviral and/or hormonal therapy) was effective in most cases [3, 19].
It should be noted the possibility of manifestation of chlamydial encephalitis in the form of acute psychosis. In one case, acute psychosis developed after an episode of headache with fever and for 3 weeks remained the only clinical manifestation of encephalitis caused by Ch. pneumoniae. There were no neurological symptoms and inflammatory changes in the CSF [49]. In another case, psychosis due to Ch. psittaci was accompanied by minimal neurological symptoms and proceeded against the background of pneumonia [50].
In one case, acute psychosis developed after an episode of headache with fever and for 3 weeks remained the only clinical manifestation of encephalitis caused by Ch. pneumoniae. There were no neurological symptoms and inflammatory changes in the CSF [49]. In another case, psychosis due to Ch. psittaci was accompanied by minimal neurological symptoms and proceeded against the background of pneumonia [50].
The lack of standardization of laboratory diagnostic methods, and sometimes an insufficient number of studies, do not allow us to finally judge the role of Ch. spp. in the development of demyelinating, degenerative, vascular diseases of the central nervous system, schizophrenia, autism and AIDS-dementia complex. Role Ch. spp. as a cofactor is likely but not proven. The role of other infectious pathogens in the diseases under consideration is also not specified. Significance of infection Ch. spp. in the formation of neurological symptoms was established for certain nosological groups (a consequence of perinatal brain damage, post-coma VS), in which the clinical significance of chlamydial infection in the central nervous system is shown. For chlamydial lesions of the central nervous system, the absence of inflammatory changes in the cerebrospinal fluid is characteristic, a possible false-negative result if Ch. spp. "gold standards" of diagnostics in cerebrospinal fluid and blood. At the same time, an indirect sign of the presence of Ch. spp. can serve as HHV-6, VEB, CMV, Bacteroides fragilis and "multiple autoimmune syndrome". With IUI - the presence of a number of manifestations of connective tissue dysplasia; the possibility of visualization of CNS lesions only with PET. It should be emphasized that there are no obvious macroscopic changes in the CNS even with generalized intrauterine chlamydia and the need for a targeted pathomorphological study using the entire spectrum of modern molecular biological, immunological and microbiological methods.
For chlamydial lesions of the central nervous system, the absence of inflammatory changes in the cerebrospinal fluid is characteristic, a possible false-negative result if Ch. spp. "gold standards" of diagnostics in cerebrospinal fluid and blood. At the same time, an indirect sign of the presence of Ch. spp. can serve as HHV-6, VEB, CMV, Bacteroides fragilis and "multiple autoimmune syndrome". With IUI - the presence of a number of manifestations of connective tissue dysplasia; the possibility of visualization of CNS lesions only with PET. It should be emphasized that there are no obvious macroscopic changes in the CNS even with generalized intrauterine chlamydia and the need for a targeted pathomorphological study using the entire spectrum of modern molecular biological, immunological and microbiological methods.
Diagnosis and treatment of respiratory atypical diseases in outpatient practice
Siginevich L.F.
The problem of acute respiratory diseases of the upper and lower respiratory tract is very relevant today and is of great social importance. In recent years, atypical pathogens: the smallest bacteria of the mycoplasma and chlamydia families, are considered as one of the leading etiological factors in relation to respiratory infections.
In recent years, atypical pathogens: the smallest bacteria of the mycoplasma and chlamydia families, are considered as one of the leading etiological factors in relation to respiratory infections.
Clinically acute respiratory disease caused by mycoplasmas and chlamydia does not differ from acute respiratory infections of other etiologies.
Mycoplasma and chlamydia are intracellular pathogens and can persist for a long time in epithelial cells, lymphopharyngeal ring, contribute to allergies, cause a more severe course of nonspecific lung diseases. These pathogens can colonize the human respiratory tract without causing any pathological picture.
There are data confirming the cytopathic effect of mycoplasmas on the ciliated epithelium of the bronchial mucosa and the ability of chlamydia to block the motor activity of cilia, disrupting the mechanisms of bronchial self-purification.
These microorganisms are characterized by a two-phase developmental cycle, consisting of non-infectious intracellular and infectious extracellular stages.
The active ability of mycoplasmas and chlamydia to provoke allergic reactivity of the body is of particular importance for people with a genetic predisposition to hypersensitivity and with dysfunction of the immune system. Since the immune system is not fully formed in early childhood, and the protective function of the body is not sufficiently developed, conditions are created for easier infection of the child's body. This is confirmed by data from surveys of different age groups of the population. The percentage of detection of mycoplasmas and chlamydia in the group of children under 5 years of age ranges from 50 to 74%.
The source of infections is a patient or a carrier.
Infection with mycoplasmas and chlamydia occurs by contact or airborne droplets.
The portal of entry for the airborne route is the mucous membrane of the upper respiratory tract and the lungs. Further service of the infectious process depends on the state of the immune system. The respiratory form of the disease can end in recovery within a week. With insufficient immune protection, a picture of nasopharyngitis, tracheitis, bronchitis or interstitial pneumonia develops.
With insufficient immune protection, a picture of nasopharyngitis, tracheitis, bronchitis or interstitial pneumonia develops.
It is practically impossible to diagnose mycoplasma and chlamydial infections of the upper and lower respiratory tract in everyday clinical practice in the acute period of the disease due to the lack of a mandatory specific examination of patients with symptoms of acute respiratory viral infections. It is possible to suspect these infections only in some cases, focusing on the known clinical peculiarity of the disease and individual details of the epidemiological history.
In our polyclinic, serological tests are used to detect pathogen antigens and specific antibodies.
Using ELISA in the blood of patients, it is possible to determine antibodies (AT) belonging to different classes of immunoglobulins (Ig A, M, G).
There is a specific sequence of different classes of immunoglobulins.
In the initial periods of the disease, the body produces mainly class M antibodies (Ig M).
Later, after 7-10 days, the synthesis of class M immunoglobulins decreases, and the body begins to produce a large amount of class G antibodies (Ig G), which perform the most effective protective function.
Detection of Ig G is equally effective and informative both in acute inflammatory process and in sluggish processes. 3-4 weeks after infection, the level of Ig G becomes constant and persists for a long time even after the complete elimination of bacteria. Since these infections do not cause persistent immunity, then, with successful treatment, approximately 3 months after the end of antibiotics, the determined Ig G titer should be below diagnostic indicators. Ig G may be at a low level for many years.
There are certain difficulties in the treatment of respiratory diseases caused by atypical pathogens. Mycoplasmas and chlamydia at different stages of development are located both intracellularly and extracellularly. And most antimicrobial agents act mainly on the extracellular form of pathogens, without acting on the intracellular form. Thus, conditions are created for the persistence of the pathogen, the chronization of the process, and even the formation of complications.
Thus, conditions are created for the persistence of the pathogen, the chronization of the process, and even the formation of complications.
The antibiotics erythromycin and doxycycline are now becoming less effective.
At the present stage, we successfully use fluoroquinolones and macrolides. These drugs are able to create higher concentrations of antibiotics in the foci of inflammation than in the blood serum. Macrolides accumulate most intensively in the tonsils, lymph nodes, paranasal sinuses, lungs, bronchial secretions, and pleural fluid.
A familial case of atypical respiratory infection in February 2011 is of interest.
A 40-year-old man came to the appointment with symptoms of a prolonged respiratory disease for 9sick day.
Complaints of general weakness, increased fatigue, evening subfebrile condition, dry cough, sore throat. Treatment: gargling and irrigation of the throat, synecod with little improvement.
Epid. Anamnesis: In the family, the first child to fall ill was 5 years old, attending a kindergarten, with similar symptoms, a week later his wife fell ill, and after another 2 days the patient himself. The child currently has a dry cough.
The child currently has a dry cough.
On the first day of illness, he noted body aches, joints, febrile temperature, which lasted 3 days, then decreased. Later, pain and sore throat joined, and a frequent dry cough appeared.
In a clinical blood test: leukocytosis 9.3 thousand/µl ESR 23 mm/hour.
X-ray of the lungs: pattern of increased lung pattern. Single dry rales are heard in the lungs during forced exhalation. Taking into account the epidemiological situation and persistent respiratory manifestations, a serological blood test for atypical infections was prescribed, which revealed antibodies to chlamydia trachomatis 8 times higher than the norm.
The next day, the wife came to the reception with a deterioration in her condition (after a short-term apparent improvement): progression of weakness, perspiration and sore throat, dry cough, subfebrile condition on the 11th day of the disease.
On auscultation: vesicular breathing, dry muffled rales on the left. An x-ray of the lungs was taken: the lungs were enphysimatous. Strengthened bronchovascular pattern in the lower basal sections.
An x-ray of the lungs was taken: the lungs were enphysimatous. Strengthened bronchovascular pattern in the lower basal sections.
In a clinical blood test: leukocytosis up to 9.8 thousand/µl, ESR - 35 mm/hour. He was prescribed antibacterial therapy with chemomycin, expectorants, and a serological blood test was taken.
Telephone contact with the patient after 3 days revealed a worsening of the condition: the presence of paroxysmal dry cough at night, increased sweating, low-grade fever. The patient was re-invited for an appointment, a CT scan of the lungs was made, which revealed 2-sided polysegmental pneumonia of the interstitial type with an increase in mediastinal lymph nodes up to 20 mm.
The patient was admitted to the hospital in the pulmonology department. In the blood serum taken in our clinic, antibodies to chlamydia trachomatis class G were found with an increase in the norm by 10 times. In the dynamics in the hospital, antibodies increased by 13 times. Class M antibodies were not detected. C-reactive protein 48 mg/l. Antibacterial therapy was carried out with rovamycin and moxifloxacin (avelox). Against the background of the complex therapy carried out in the hospital, the phenomena of pneumonia regressed, interstitial changes in the lungs almost completely disappeared, and the size of the lymph nodes in the mediastinum decreased. 3 weeks after discharge from the hospital, both spouses underwent a control serological blood test for chlamydia in the clinic: they revealed a significant decrease in Ig G. After another 3 weeks, the Ig G titer was slightly increased in both.
Class M antibodies were not detected. C-reactive protein 48 mg/l. Antibacterial therapy was carried out with rovamycin and moxifloxacin (avelox). Against the background of the complex therapy carried out in the hospital, the phenomena of pneumonia regressed, interstitial changes in the lungs almost completely disappeared, and the size of the lymph nodes in the mediastinum decreased. 3 weeks after discharge from the hospital, both spouses underwent a control serological blood test for chlamydia in the clinic: they revealed a significant decrease in Ig G. After another 3 weeks, the Ig G titer was slightly increased in both.
The analysis of these cases is interesting.
Firstly, it clearly shows the etiological relationship with atypical chlamydial infection of acute bronchitis in the husband, and chlamydial pneumonia in the wife.
Secondly, it was possible to detect serological signs of active chlamydial infection by repeated sampling of blood serum in the acute period of the disease and during convalescence.