Best position for membrane sweep
Membrane Sweep | 5 Important Facts Before You Have One
If you consider a labor induction with a membrane sweep, you’re probably feeling very pregnant.
Maybe your estimated due date has come and gone.
Or perhaps you’re uncomfortable and tempted to try to get things moving along.
Your healthcare provider might even have suggested having a sweep as a way of making labor start ‘naturally’. And if your midwife is suggesting it … surely it’s alright?
Although it might sound like a natural option, remember anything that starts labor before it begins on its own is intervening with the natural process.
Having said that, there might be a compelling need to get labor going, and if you’re trying to avoid more medical interventions, this could potentially help.
Keep reading to find out everything you need to know about to ‘sweep a membrane’.
What is a membrane sweep?
Sweeping the membranes is essentially stimulating your body to release prostaglandins. They act on the cervix to soften and ripen it, which then encourages dilation in response to contractions.
Often this stage is already happening ‘behind the scenes’. You might not notice or pay much attention to that low ache in your pelvis or lower back. During late pregnancy, everything hurts, so what’s one more ache to pay attention to? Often it’s only in hindsight you realize that it was probably your cervix getting ready for the main show.
If this process of ripening and softening of the cervix is already underway, membrane sweeping might be more effective at getting contractions started and established.
Unlike having artificial oxytocin (Pitocin or Syntocinon) for induction, a membrane sweep doesn’t involve medication. As with any labor induction method, however, the more favorable your cervix is, the more likely the method will be successful.
How is a membrane sweep done?
It doesn’t sound very glamorous, but membrane sweeping is similar to having a cervical check or a Pap smear.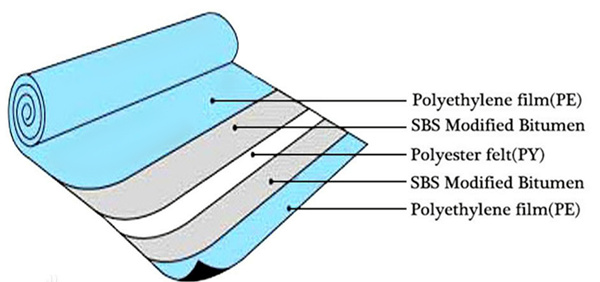
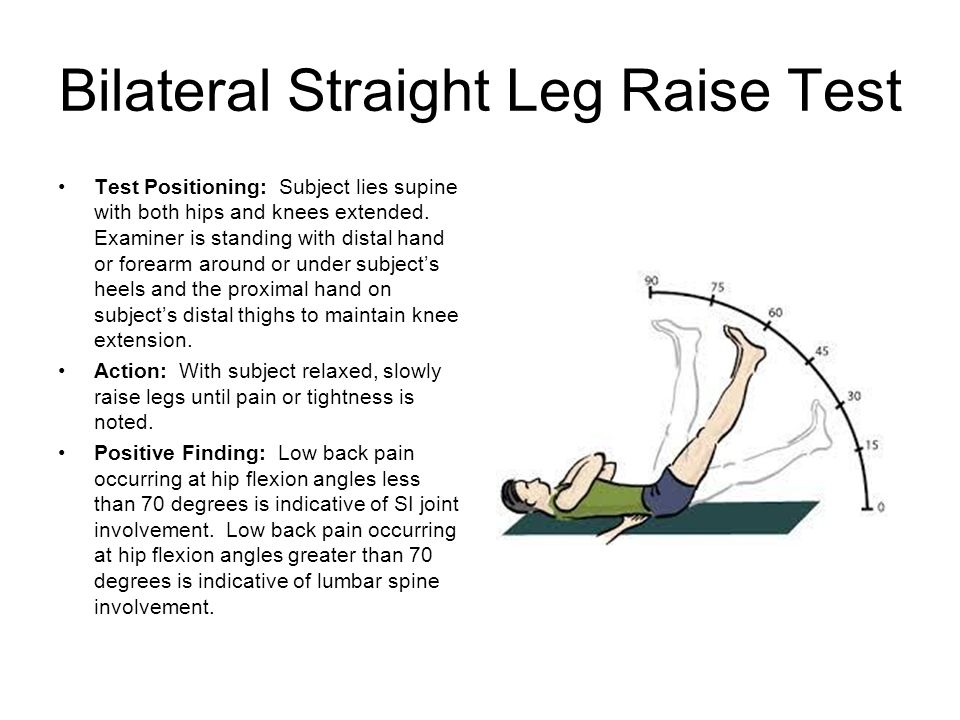
When you’re ready and have given your informed consent, you lie on your back with knees apart and ankles together.
The midwife or doctor separates your labia, inserts a gloved finger into your vagina and up to your cervix to feel for effacement and dilation.
This can determine how ‘ready’ your cervix is. This is called your Bishop’s score. Remember, the more favorable your cervix, the more likely membrane sweeps are to work.
Using a firm circular or sweeping motion, your midwife or doctor will sweep and separate the membrane of the amniotic sac from the cervix. Some providers also stretch the cervix to increase the chances of the membrane sweep working.
Should I have a membrane sweep?
Pregnancy isn’t considered overdue until after 42 completed weeks.
Many hospitals have a policy of inducing labor when you’ve reached 10 days past your due date.
A ‘stretch and sweep’ (stretching the cervix and membrane sweeping) is thought to be more natural than a medical or formal induction method because no medication is involved.
Keep in mind, though, membrane sweeping to induce labor is still an intervention, as it interferes with the normal and natural process of labor starting on its own.
Do membrane sweeps work at 38 weeks?
Commonly, pregnancy is considered a term after 37 weeks. This doesn’t mean, however, your baby is ready to be born yet. And reaching your due date doesn’t mean you’re overdue.
Only 3-5% of babies are born at 40 weeks and many pregnancies go beyond that –especially with first babies.
Leading health experts strongly recommend against inducing labor before 39 weeks, unless there’s a genuine and pressing medical reason.
Being born early puts babies at higher risk for health and developmental problems. Important brain and lung development occurs during those last weeks in the uterus.
If your body and your baby aren’t ready, trying to stimulate labor to begin at 38 weeks can lead to disappointment and an even greater risk of complications.
How effective are membrane sweeps to induce labor?
This is the million-dollar question. If labor begins after a sweep, the natural conclusion is the procedure has worked.
If labor begins after a sweep, the natural conclusion is the procedure has worked.
It’s possible, though, you were in prelabor and baby was ready to arrive.
The success of a membrane sweep really depends on whether you have a favorable or unfavorable cervix. This is explained above in the information about the Bishop’s score.
The general consensus is sweeping the membranes might kick-start labor within the first 7 days after the procedure, but generally no sooner than 24-48 hours.
- A study involving 190 women found a membrane sweep at 38 weeks reduced total gestation time. Of the membrane stripping group, only 10% went past 41 weeks, compared with 25% in the non-sweep group
- This study showed membrane sweeping every 48 hours, from 41 weeks, decreased the risk of post-term pregnancy. The membrane sweep group had 23% of pregnancies go to 42 weeks, compared with 41% of the non-sweep group
- This Cochrane review showed eight women would need to have membrane sweeping to avoid one medical induction.
 The authors concluded membrane sweeping didn’t produce a clinically important benefit.
The authors concluded membrane sweeping didn’t produce a clinically important benefit.
When taken overall, the difference in numbers is small and the length of pregnancy reduction is a matter of days, not weeks.
The outcome also depends on the number of weeks of pregnancy when the membrane stripping occurred.
Signs a membrane sweep for labor induction has worked
You might experience these symptoms a few hours after having the membranes swept. Be mindful this might be because the cervix is irritated and spontaneous labor might not occur.
Right after membrane stripping, you might experience:
- Cramping with mild discomfort
- Spotting
- Light bleeding, as blood vessels begin to break when the cervix starts to dilate
- Irregular contractions or tightenings
- Pain
- Broken sac or ‘waters breaking’
- Release of the mucous plug.
You shouldn’t have severe pain or heavy bleeding. If you experience heavy bleeding or any severe pain, or if you have any concerns, always contact your midwife or healthcare provider
5 important FAQs about membrane sweeping
#1: Is a membrane sweep natural?
You might hear membrane stripping to induce labor being referred to as a ‘natural’ method because there’s no medication used, just your natural prostaglandins.
Unfortunately, that simply isn’t true.
Any procedure that attempts to start labor before it begins on its own, is doing so by artificial means.
Find out more in our article What Causes Labour To Start?.
If membrane stripping doesn’t bring on labor, you’re more likely to have a medical induction. If your body and your baby aren’t ready for birth, you can end up having more interventions. These can include a forceps or vacuum birth, or even a c-section.
Your baby might also experience problems as a result of being born early.
However, if labor induction is necessary for medical reasons, a sweep could be a better option. You can prepare for further medical intervention and have a plan for making those decisions.
For more information check out Induction of Labour – What Are The Risks Of Being Induced?
#2: Is it painful to have a stretch and sweep?
The answer depends on each individual, as everyone is different. It helps to discuss your questions with your care providers at home or at your doctor’s office. They can provide medical advice that will help with your decision to have labor induced.
They can provide medical advice that will help with your decision to have labor induced.
During pregnancy, the cervix is closed and angled slightly back towards your tailbone (a posterior cervix).
As your body prepares for labor, the cervix shifts forward, softens, and even opens slightly.
These changes can happen in the weeks before or up to the time you notice signs of labor.
When your cervix hasn’t moved forward and is higher in the vagina, the vaginal examination can be a bit uncomfortable or even painful.
Your baby won’t feel the exam as the amniotic fluid acts as a cushion.
#3: Is a membrane sweep necessary?
All women need to give consent to any procedure, including a vaginal examination and a stretch and membrane sweeping.
No woman should have a medical or obstetric procedure performed, either during pregnancy or birth, without her consent.
Your healthcare provider must discuss the risks and benefits with you before the procedure.
This lets you make an informed choice, in the context of your health information, whether or not to have the membrane sweep.
It’s important to inform your healthcare provider of the procedures you do or don’t want.
You also have the right to refuse a vaginal exam if you don’t want one.
If you need to have an induction, here are 8 Tips For A Positive Induction Birth.
Are You Getting BellyBelly’s Pregnancy Week By Week Emails?
We think they’re the best on the internet!
Click to get the FREE weekly updates our fans are RAVING about.
#4: Can I sweep my own membranes?
When you’re 42 weeks pregnant, you’re thinking, ‘Come on uterus, do your thing!’
You might consider getting things started on your own.
In my practice, many women have used a finger to feel their own cervix, during pregnancy and even during labor.
And some women have tried to ‘stretch’ their cervix with their fingers. Or had their partner try a membrane stripping.
Or had their partner try a membrane stripping.
Midwife Dawn Reid agrees. She’s had many clients perform a self-sweep, in an effort to avoid medical induction methods.
Dawn says, ‘I would always recommend waiting for babies to choose their birthday and use the stretch and sweep as a last resort’.
She also found a self-sweep was ‘often not successful and imaginably quite tricky’.
Midwives and doctors have years of knowledge and experience, so wait and have them perform one.
Alternatively, just listen to your body and your baby, put your feet up and enjoy those final pregnant moments.
#5: Can membrane stripping break your waters?
There’s a small increased risk of your waters rupturing prematurely after a membrane sweep.
This means the protective sac surrounding your baby breaks and amniotic fluid can leak out.
If contractions don’t start within a certain time frame, there’s an increased risk of infection.
When the amniotic sac breaks, the baby’s head needs to be well engaged in the pelvis to avoid cord prolapse. This is when the cord flows out past the baby’s head; it is considered a medical emergency.
This is when the cord flows out past the baby’s head; it is considered a medical emergency.
Your baby’s position should be checked before a vaginal exam, to minimize the risk of this emergency.
Your healthcare professional might suggest other methods of induction, so it’s wise to be ready for this possibility before choosing to have a membrane sweeping.
If an induction is necessary for medical reasons, membrane stripping isn’t usually performed on its own; other induction methods are also used.
For more information read Methods Of Induction Of Labour – 4 Different Methods.
Stretch and sweep | Pregnancy Birth and Baby
Stretch and sweep | Pregnancy Birth and Baby beginning of content3-minute read
Listen
What is a stretch and sweep?
A stretch and sweep or membrane sweep is a relatively gentle way of trying to start labour. It is a simple procedure that sometimes initiates labour, reducing the risk of babies being born over the due date (42 weeks of pregnancy).
It is a simple procedure that sometimes initiates labour, reducing the risk of babies being born over the due date (42 weeks of pregnancy).
When is a stretch and sweep offered?
Your midwife or doctor may offer to perform a stretch and sweep when you have reached full term (38 weeks) to try to start labour. Some hospitals and doctors recommend the procedure only if you are 40 to 41 weeks pregnant in an attempt to prevent an overdue delivery, which may place the baby at risk.
What to expect from a stretch and sweep
The procedure is carried out by a midwife or doctor as part of an internal vaginal examination. They put a couple of lubricated, gloved fingers into your vagina and insert their index finger into the opening of the cervix or neck of your womb
They then use a circular movement to try to separate the membranes of the amniotic sac, containing the baby, from your cervix. This action, which releases hormones called prostaglandins, prepares the cervix for birth and may initiate labour.
Your midwife or doctor will:
- check the estimated date of delivery
- ask you to empty your bladder and lie down
- detect your baby’s position by feeling your abdomen
- do an internal examination to find if your cervix is closed or open
- massage around the neck of your womb if it is soft but closed — this 'stretch' can stimulate your body to release prostaglandins, which encourage the cervix to open
- insert an index finger into the neck of the womb, if it is open, and use circular motions to loosen or 'sweep' the amniotic sac membranes from the top of the cervix — this triggers the release of hormones and may start labour
If you find the procedure too uncomfortable, ask your midwife or doctor to stop.
Afterwards you might experience:
- discomfort
- mild or occasionally strong pain
- slight bleeding
- cramps or irregular contractions
Take paracetamol and have a warm bath if you are in pain. If your membranes rupture, the pain is bad or you have fresh, red bleeding, contact your midwife, doctor or maternity unit.
If your membranes rupture, the pain is bad or you have fresh, red bleeding, contact your midwife, doctor or maternity unit.
The process can be repeated if labour does not start over the next couple of days.
What are the benefits and risks of a stretch and sweep?
Stretch and sweep procedures at 41 weeks of pregnancy greatly reduce the percentage of women who deliver their babies beyond term. They may be offered as an alternative to inducing birth through medication or by rupturing the membranes.
The procedure is safe in a normal pregnancy. There is a slight chance the membranes may break during the procedure.
Ask your doctor or midwife about the benefits and risks before agreeing to a stretch and sweep procedure.
Sources:
BabyCenter (What is a membrane sweep?), Queensland Health (Queensland Clinical Guidelines, Induction of labour), The Royal Hospital for Women (Sweeping membranes to encourage spontaneous labour), Cochrane (Membrane sweeping for induction of labour), NSW Health (Pregnancy beyond 41 weeks)Learn more here about the development and quality assurance of healthdirect content.
Last reviewed: January 2021
Back To Top
Related pages
- Having the baby
Need more information?
Disclaimer
Pregnancy, Birth and Baby is not responsible for the content and advertising on the external website you are now entering.
OKNeed further advice or guidance from our maternal child health nurses?
1800 882 436
Video call
- Contact us
- About us
- A-Z topics
- Symptom Checker
- Service Finder
- Linking to us
- Information partners
- Terms of use
- Privacy
Pregnancy, Birth and Baby is funded by the Australian Government and operated by Healthdirect Australia.
Pregnancy, Birth and Baby is provided on behalf of the Department of Health
Pregnancy, Birth and Baby’s information and advice are developed and managed within a rigorous clinical governance framework. This website is certified by the Health On The Net (HON) foundation, the standard for trustworthy health information.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
This information is for your general information and use only and is not intended to be used as medical advice and should not be used to diagnose, treat, cure or prevent any medical condition, nor should it be used for therapeutic purposes.
The information is not a substitute for independent professional advice and should not be used as an alternative to professional health care. If you have a particular medical problem, please consult a healthcare professional.
Except as permitted under the Copyright Act 1968, this publication or any part of it may not be reproduced, altered, adapted, stored and/or distributed in any form or by any means without the prior written permission of Healthdirect Australia.
Support this browser is being discontinued for Pregnancy, Birth and Baby
Support for this browser is being discontinued for this site
- Internet Explorer 11 and lower
We currently support Microsoft Edge, Chrome, Firefox and Safari. For more information, please visit the links below:
- Chrome by Google
- Firefox by Mozilla
- Microsoft Edge
- Safari by Apple
You are welcome to continue browsing this site with this browser. Some features, tools or interaction may not work correctly.
Membrane methods for wastewater treatment.
Pollution water in the water consumption zone is serious factor worsening ecological state of cities. It produced both by resetting part untreated wastewater from cities and enterprises, located above the water intake zone of this city and water pollution by river transport, and due to getting into reservoirs of fertilizers and pesticides, entered into the fields. Moreover, if with the first types of pollution can be construction of treatment facilities fight effectively, then prevent pollution of the water basin produced agricultural activities, very difficult. In areas of high moistening about 20% of fertilizers and pesticides introduced into the soil, gets into watercourses. It is important to note that water treatment plumbing facilities are incapable of purify drinking water from solutions of these substances, so drinking water can contain them in high concentrations and adversely affect on human health. Growing chemicalization agriculture will inevitably lead to an increase in fertilizers and pesticides applied to soil, and accordingly with this their concentration in water will increase.
Struggle with this type of pollution requires use of fertilizers and pesticides in catchment areas exclusively in granular form, development and introduction of rapidly decomposing pesticides, as well as biological plant protection methods.
Cities are also powerful sources pollution of the water basin. In large cities per inhabitant (with taking into account contaminated surface wastewater) is daily discharged into water bodies about 1 m 3 polluted drains. Therefore, cities need powerful treatment facilities.
2. Membrane water treatment methods
Membrane water treatment systems, industrial development of which began around 1985, currently applied in almost all industries that consume purified water.
Fig.1. Cellulose acetate membrane (microscopic image)
First artificial membranes were made in the 19th century from processed in nitrogen cellulose acid (cellulose) - raw materials, which are nothing but shells plant cells, i.e. natural membranes. From cellulose nitrate learned how to make celluloid, and later cellophane, but with the actively fought against microporosity, so how would you like to receive in the first place protective materials impervious to air and moisture. And only at 1960 Loeb and Sourirajan invented the membrane from another type of modified cellulose - acetate, which was already suitable for practical use (Fig. 1).
And only at 1960 Loeb and Sourirajan invented the membrane from another type of modified cellulose - acetate, which was already suitable for practical use (Fig. 1).
wide introduction of membrane processes in practice was made possible by the development of polymer science and the use synthetic polymer membranes.
membranes, like other filter materials, can be considered as semi-permeable environments: they let water through, but not miss, more precisely, worse miss some impurities. However, if the usual filtration is used to remove from water relatively large formations - dispersed and large colloidal impurities, then membrane technologies - to extract small colloidal particles, as well as dissolved compounds. For this membrane must have pores very small size.
driving the force that causes the liquid to penetrate through an obstacle in the form of a thin partitions, maybe: a) attached pressure; b) concentration difference dissolved substances; c) difference temperatures on both sides of the partition; d) electromotive force. In this part we confine ourselves to considering baromembrane phenomena - separation processes under pressure action.
In this part we confine ourselves to considering baromembrane phenomena - separation processes under pressure action.
Main difference between membranes and conventional filters environments is that they are thin, and removed impurities are not retained in volume, but only on the surface of the membrane. The dirt holding capacity of the surface is obviously much less than volume. It seemed would, the membrane should because of this very quickly get clogged and stop skipping water. So it would be, if in the membrane filter did not occur permanently membrane self-cleaning. For this the so-called "tangential" scheme of water movement in the apparatus, with which collects water from both sides membranes: one part of the flow passes through the membrane and forms a filtrate (or permeate), that is, purified water, and the other directed along the surface of the membrane, to flush out trapped impurities and remove them from the filtration zone. This part flow is called concentrate or retentate, and usually it is either discarded into the drain, or (for example, when cleaning galvanic drains) are diverted for further processing and selection of the necessary components. Thus the node membrane filtration has one input and two exits, and part of the water is constantly used to clean the membrane. (AT two-stage membrane plants second stage concentrate can be much cleaner than raw water, so it can be used by giving back to the setup input. In this manner achieve a reduction in water consumption.)
Thus the node membrane filtration has one input and two exits, and part of the water is constantly used to clean the membrane. (AT two-stage membrane plants second stage concentrate can be much cleaner than raw water, so it can be used by giving back to the setup input. In this manner achieve a reduction in water consumption.)
2.1 Membrane classification by pore size
FROM in terms of technological capabilities distinguish membranes for ultrafiltration, nanofiltration and reverse osmosis. AT In this series, the pore size decreases, and working pressure increases.
Ultrafiltration membranes have the largest pores diameter from 1 to 0.05 microns (1 micron = 10-6 m) and usually operate at pressures of 2-5 bar. They are used, for example, for post-treatment drinking tap water from colloidal and high molecular weight contaminants, if no adjustment of its saline is required composition.
Nanofiltration elements (pores 5-50 nm, or 0. 05-0.005 µm) used to soften water with high hardness, to remove heavy ions metals and organochlorines. Monovalent ions such as Na, K, Cl, NO3 are retained weakly - on average no more than 10-30%. Working nanofiltration pressure is usually not exceeds 5-7 bar.
05-0.005 µm) used to soften water with high hardness, to remove heavy ions metals and organochlorines. Monovalent ions such as Na, K, Cl, NO3 are retained weakly - on average no more than 10-30%. Working nanofiltration pressure is usually not exceeds 5-7 bar.
Reverse osmosis membranes have pores less than 10 in diameter nanometers (less than 0.01 µm), operate at pressures up to 100 bar and allow deep desalination, or demineralization. Reverse osmosis is used to produce ultrapure water for industrial needs, as well as for desalination of marine and brackish groundwater, and degree of desalination (selectivity) is usually at least 92-97%.
2.2 Membrane element types
membranes may have different geometries. shape: tubular, hollow fiber and flat.
Tubular membranes are tubes several millimeters in diameter up to 1-2 cm, made of porous material such as ceramics. Wherein they can be symmetrical or asymmetrical. symmetrical membrane has the same porosity throughout the volume of the material. At the asymmetric tubes on one of the surfaces - outer or internal - during manufacture form a thin layer of the same or other material with much more density. This layer is working, since it is he who determines membrane holding capacity. The coarser material plays the role of the carrier substrate with drainage properties. Purified water supply carried out by the working surfaces.
symmetrical membrane has the same porosity throughout the volume of the material. At the asymmetric tubes on one of the surfaces - outer or internal - during manufacture form a thin layer of the same or other material with much more density. This layer is working, since it is he who determines membrane holding capacity. The coarser material plays the role of the carrier substrate with drainage properties. Purified water supply carried out by the working surfaces.
membranes in the form of hollow fibers (Hollow Fiber) also have tubular shape, but their diameter is usually 0.1 to 0.5 mm. Because of such a small size per unit volume of the filter apparatus can be placed huge the number of fibers, and their total the working surface will be in tens and even hundreds of times higher than that of tubular membranes of large diameter (see table). Having a developed work surface, hollow fiber filters have much larger than tubular performance, other things being equal conditions - pressure, pore size, etc. However, this advantage also has a downside. side: due to the fact that the movement is being cleaned liquid along the work surface each fiber is difficult to control and regulate, fiber membrane is prone to contamination, and cleaning its surface is extremely difficult. Therefore, hollow fiber filters create more problems in operation, require thorough pre-cleaning supplied for water treatment. Except in addition, having the highest density packaging, the fibers have and the thickest working layer of the membrane (relative to the entire thickness of the wall), so their throughput capacity per unit work surface may be inferior other membranes.
However, this advantage also has a downside. side: due to the fact that the movement is being cleaned liquid along the work surface each fiber is difficult to control and regulate, fiber membrane is prone to contamination, and cleaning its surface is extremely difficult. Therefore, hollow fiber filters create more problems in operation, require thorough pre-cleaning supplied for water treatment. Except in addition, having the highest density packaging, the fibers have and the thickest working layer of the membrane (relative to the entire thickness of the wall), so their throughput capacity per unit work surface may be inferior other membranes.
Fig.2. Composite RA-membrane: 1 - membrane; 2 - glue; 3 - drainage gasket 4 - tube for permeate 5 holes
Fig.3. Roll element, before assembly
flat membranes are produced in the form of films (thin film), which can be non-substrate (homogeneous substance), reinforced (with a fabric base and applied porous material) and substrate (with a substrate from large-porous material and applied working layer). Modern reverse osmosis membranes as a rule, thin-film composite, that is, multilayered, and each layer is made from different chemical connections. On fig. 2 such a membrane shown in section. As a basis (1) non-woven fabric is used polystyrene. Apply enough on top thick layer of microporous polysulfone (2), the purpose of which is that it should have good permeability, but while resisting deformation (compression) under pressure. Upper layer (3) - barrier - made of aromatic polyamide (RA, Nylon).
Modern reverse osmosis membranes as a rule, thin-film composite, that is, multilayered, and each layer is made from different chemical connections. On fig. 2 such a membrane shown in section. As a basis (1) non-woven fabric is used polystyrene. Apply enough on top thick layer of microporous polysulfone (2), the purpose of which is that it should have good permeability, but while resisting deformation (compression) under pressure. Upper layer (3) - barrier - made of aromatic polyamide (RA, Nylon).
Table 2
By packing method for flat membranes distinguish between flat-frame, disco-modular and roll (Spiral Wound) devices. Most roll filter elements are common, in which, as their name suggests, membranes together with drainage gaskets screwed onto the drain tube roll (see Fig. 3.4). When submitting the original water from the butt end, the filtrate moves in a spiral and collects in a drainage tube, and the concentrate exits from the opposite butt. Packing density elements occupy an intermediate position between tubular and hollow fiber membranes (see table), have a comfortable geometry and are characterized by extremely small thickness of the working layer, which in aggregate provides them with the best combination of high specific productivity and relatively low propensity to pollution.
Packing density elements occupy an intermediate position between tubular and hollow fiber membranes (see table), have a comfortable geometry and are characterized by extremely small thickness of the working layer, which in aggregate provides them with the best combination of high specific productivity and relatively low propensity to pollution.
world leaders in the production of membranes and membrane elements are firms Dow Chemical, Filmtec, Hydranautics, Osmonics (USA).
2.3 Basic laws of processes membrane separation
one. The flow of purified water is directly proportional membrane area.
2. The more water flows through the membrane, the higher the applied pressure.
3. Membrane performance is higher (ceteris paribus), the thinner membrane. For multilayer membranes take into account the thickness of the densest working layer.
four. An increase in water temperature reduces its viscosity and consequently increases membrane throughput. The flow increase is approx. 3% for every degree Celsius.
An increase in water temperature reduces its viscosity and consequently increases membrane throughput. The flow increase is approx. 3% for every degree Celsius.
5. Membrane performance decreases with an increase in the concentration of impurities.
6. Filtration of water through coarse membranes can be carried out at any pressure.
However, when the hole size of the membrane the septum becomes so small that approaching the size of molecules fundamentally changes. Because of dissolved salts can no longer pass freely through membrane, osmotic pressure directed towards operating pressure in the membrane element. The operating pressure should now exceed this is a counteraction, otherwise the water through membrane won't work at all. Wherein osmotic pressure is greater than higher concentration of dissolved salts: every 1000 mg/l=1 g/l of salts give an increase osmotic pressure by 0.6-0.8 bar.
For example, for sea water with salinity 35 g/l osmotic pressure is approx. 25 bar, and at lower pressure pump membrane desalination marine water is not possible.
25 bar, and at lower pressure pump membrane desalination marine water is not possible.
On the Indeed, osmotic pressure different for different salts and depends on radius, charge and structure their ions. Differences for the most common salts are 1.5-2 times.
At increase in liquid temperature osmotic pressure rises, which could would lead to a decrease in permeability membranes, if not for the simultaneous decrease in viscosity, the effect of which opposite and under normal conditions stronger (see item 4).
Fig.4. Roll element: 1 - shell; 2 - membranes; 3 - internal drainage gasket; four - outer gasket; 5 - tube for permeate; 6 - holes; 7 - ring seal gasket
7. The conversion (recovery) is the relationship the volume of the obtained filtrate to the volume source water, expressed in%. Because the part of the water is used for washing membranes, the conversion should be less 100% and is usually in the range of 40-80%. The conversion can be adjusted by changing parameters of the filtration process. High conversion means less dump water into the drain and more receive the final product. In some cases (but not always) such savings is very important and is accompanied by special efforts. But here in no way case, you can not overdo it: reducing share of the concentrate, can be provoked membrane fouling. Conversion depends on the quality of the source water, including from its preliminary cleaning, and on the required quality of the filtrate.
The conversion can be adjusted by changing parameters of the filtration process. High conversion means less dump water into the drain and more receive the final product. In some cases (but not always) such savings is very important and is accompanied by special efforts. But here in no way case, you can not overdo it: reducing share of the concentrate, can be provoked membrane fouling. Conversion depends on the quality of the source water, including from its preliminary cleaning, and on the required quality of the filtrate.
For nanofiltration and reverse osmosis established: by increasing the conversion, they receive higher salinity filtrate (reduce the selectivity of the process).
Except Moreover, an increase in conversion leads to an increase in osmotic pressure, which has to be overcome. it associated with the phenomenon of concentration polarizations: salts, retained membrane, accumulate in a thin layer water near its surface. As a result with an increase in conversion speed filtration drops.
eight. Change in pressure affects selectivity membrane separation (decrease impurity concentration in % vs. with source water). With increasing pressure the flow of water through the membrane increases, and the passage of impurities is practically not is changing. In addition, under the influence pressure polymer membrane multiple thickens and becomes less permeable for impurities. Therefore, in the region of small pressure selectivity increases linearly with increasing pressure.
Fig.5. Typical diagram of a membrane plant. one - pre-filter; 2 - manometers; 3 - solenoid valve; 4 - relay pressure; 5 - thermometer; 6 - high pump pressure; 7 - electric motor; 8 - pressure gauge; 9- membrane housing; 10 - membrane; 11 - sensor conductivity; 12 - control panel with conductometer; 13 - permeate rotameter; 14 - three-way valve; 15 - ball valve; 16 - concentrate rotameter; 17 - three-way tap; 18 - ball valve; 19 - recycle rotameter concentrate; 20 - cleaning tank solution; 21, 22 - ball valves.
However then growth slows down, and at some pressure selectivity reaches maximum determined by membrane type and the nature of the removed substances.
From impurity concentration selectivity does not depend (in the region of low concentrations).
Selectivity nanofiltration and reverse osmosis relation to various ions in the main coincides with the series of increasing their energy hydration:
H+ < NO3- < I- < Br- < Cl- < K+ < F- < Na+ < SO42- < Ba2+ < Ca2+ < Mg2+ < Cd2+< Zn2+ < Al3+
and in general, as we can see, it grows with increasing ion charge. This is probably related to the fact that each ion in solution is not free, and hydrated: it is electrostatically attracts surrounding molecules water, which form something like 'fur coats', and the size of such formation much larger than the free diameter ion and depend primarily on its charge.
2. 4 Representations about the separation mechanism on membranes
4 Representations about the separation mechanism on membranes
Easily explain why on membranes particulate matter is trapped and large colloids: they are simply more than pores. But for nano- and reverse osmosis membranes explanation their poor ion permeability dissolved salts is not at all obvious. Although the pore sizes are extremely small, the diameters most simple ions in a few more times less. Simplified representations that the passage of water molecules and solutes across the membrane happens the same way as sieving solid particles through the sieve, obviously not corresponds to reality. For such phenomena can no longer be neglected interaction between molecules and ions with a friend and with the atoms of a solid.
Exists a number of hypotheses for describing transport processes in membranes.
Diffusion theory: it is assumed that molecules water, and salt ions diffuse through membrane, but the diffusion coefficient y ions are much lower.
capillary theory: water passes through the membrane as through a system of capillaries, and inside the capillary it is in bound state due to education hydrogen bonds with surface atoms; the movement of water is accompanied severing some ties and education new. Since ions do not form hydrogen bonds, then for them this method passage of the capillary is impossible.
For hydrophilic membranes (surface which are well wetted by water) as a result of adsorption on the pore walls a layer of pure water appears, and if pore diameter does not exceed twice the thickness of such a layer, then the ions of the dissolved connections cannot pass through them.
Due to water structuring in fine pores its solubility decreases. and there is a kind of ejection of particles solute from the pore.
Apparently all these representations in one way or another fair and in the aggregate help to understand the observed patterns.
2.5 Contamination membranes and their washing
How more in the source water of substances with low solubility or interacting with the membrane material, the more the likelihood of its contamination (fouling).
home cause of this kind of problem is called concentration polarization, that is, a local increase in concentration impurities near the working surface membranes. Mechanical and colloidal particles under such conditions tend to to enlargement and formation of aggregates, which can be deposited on the membrane, blocking her. For salts with relatively low solubility enhancement concentrations can also cause sediment formation.
For to reduce the intensity pollution, optimize the design membrane elements and their scheme connections with each other if the installation multistage. At the same time, they achieve as much linear speed as possible movement of water along the surface of the membrane, including through recycling concentrate, and maximum turbulence flow.
However sedimentation of solid contaminants and colloidal (gel) film on the surface still happens, and to remove them it is necessary to carry out regeneration flushing. Flushing is assigned either when performance drops installation by 10-15%, or with an increase resistance of the membrane circuit to 2-2.5 bar, or after a certain time interval.
Apply as mechanical countercurrent washes, and chemical, using detergents. Mechanical flush usually short lived and carried out quite often, and it is easily automated and does not require attendant participation. So, in reverse osmosis devices ARO manufactured by AquaPro Industrial Co. with membranes manufactured by Osmonics or Hydranautics (Fig. 6), mechanical flushing with pulsating flow lasts 1.5 minutes and is carried out automatically every time you turn it on installation and every 12 hours of operation. This procedure does not, of course, solve all pollution problems, but allows carry out chemical cleaning less often.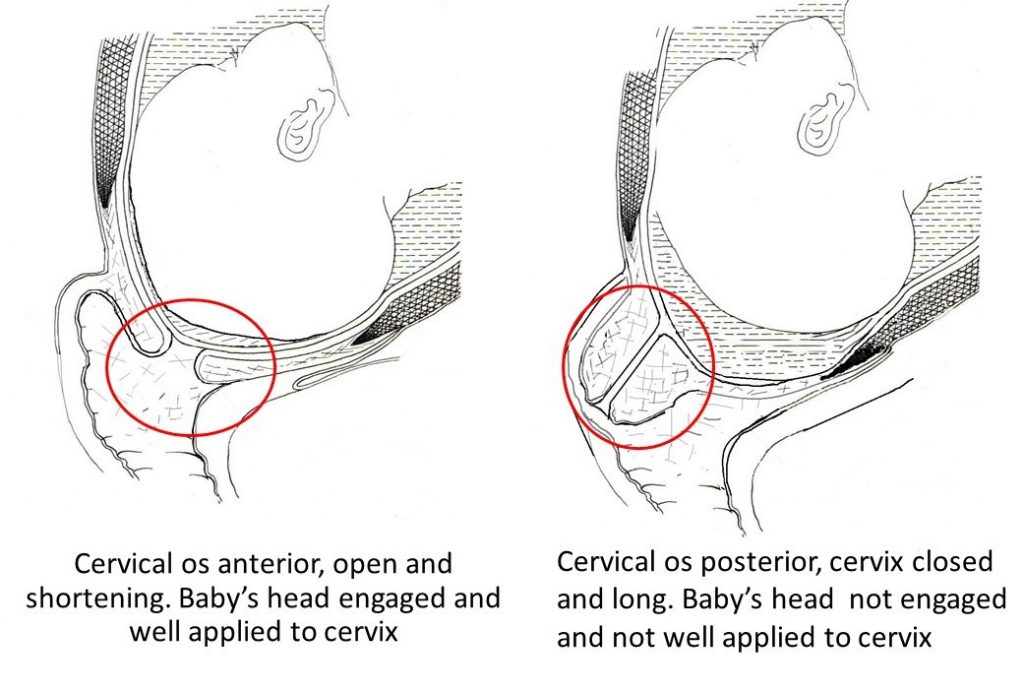
detergents chemical cleaning solutions chosen primarily on the basis of chemical resistance considerations membranes. Detergent formulations are divided into acidic and alkaline, which is associated with chemical properties of contaminants. Acidic are used to remove inorganic precipitates such as salts hardness (carbonates and sulfates of Ca and Mg), hydroxides of Fe and Al. Alkaline solutions used to remove biological and organic films, compounds silicon
For polymer membranes main components cleaning solutions are inorganic and organic acids (hydrochloric, phosphoric, sulfamic, citric, oxalic), as well as alkalis, organic and inorganic complexing agents.
Fig.6. Reverse osmosis unit ARO-800 (AquaPro)
sulfuric and nitric acid are not used: nitric acid can destroy the material membranes, and the use of sulfuric contributes to the formation of precipitation calcium sulfate, which can be removed without membrane damage is impossible.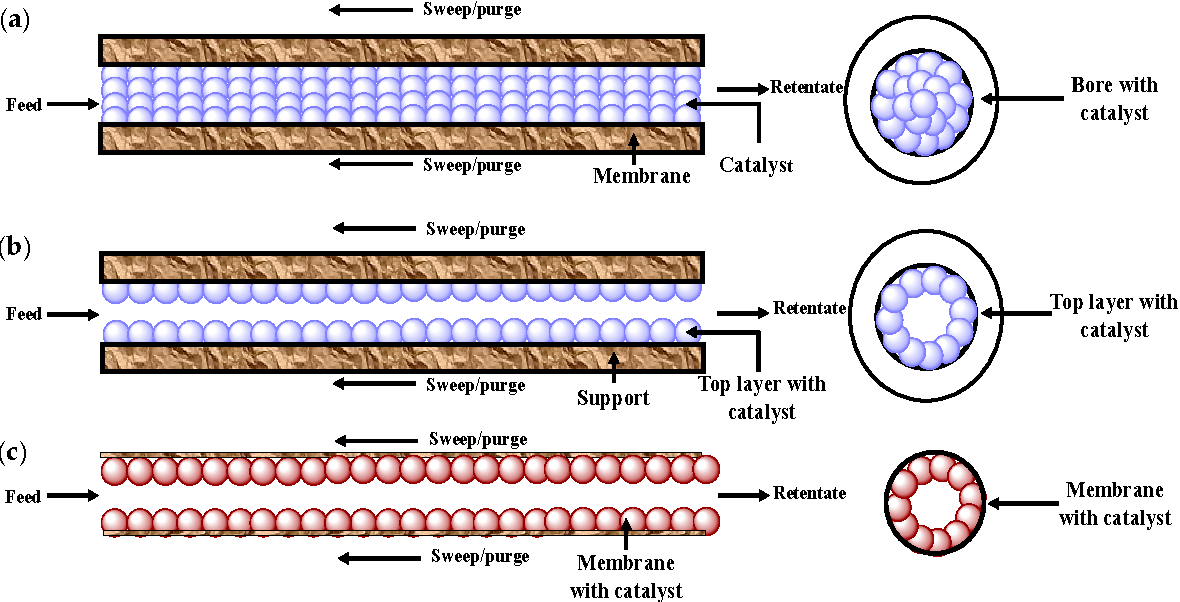
organic acids, in addition to acidic properties, are also good complexing agents with respect to metal cations, but lemon is better in this regard oxalic, as its complexes are better soluble.
Permissible concentration depends on the strength of the acid: in the case of polyamide membranes, the concentration HCl should not exceed 0.2-0.5%, weaker organic acids - 1-2%.
AT alkaline compounds are most often used caustic soda or phosphate solutions - trisodium phosphate or tripolyphosphate. In an alkaline environment, silicon-containing compounds become soluble silicates, and in colloidal, organic and biological films are weakened adsorption interaction with surface. Another effective remedy is to add to the solution surfactants (surfactants).
Optimal washing temperature - about +40°С, flow rate should be close to maximum for this type of membrane. The end criterion for flushing can be serve to stabilize the pH of the cleaning solution, but at the same time its value should not differ from the original by more than 30-40%.
2.6 Preliminary water treatment
Before by supplying water to the membrane, as a rule, cleaned of coarse impurities, as well as those solutes which can either damage the membrane (active chlorine), or cause deposits on its surface (salt rigidity). Such preliminary water treatment allows significantly increase membrane life and duration of work between chemical washes.
For removal of active chlorine usually using sorption filters activated carbon, and to reduce hardness water - ion-exchange filters with Na-cation exchanger.
Rice. 7. Membrane module
Exist also special methods of preliminary water treatment that allows significantly improve efficiency cleaning, exit to a more comfortable mode operation of the membrane plant. An example such an approach is the COUV method - complexation-ultrafiltration. The essence of the method is that additives are introduced into the solution, forming water-soluble complex compounds with the components you need delete. This leads to an increase sizes of newly formed particles, which allows for division into larger pore membranes. Then concentrated complex is destroyed, and the complexing agent returns for reuse.
This leads to an increase sizes of newly formed particles, which allows for division into larger pore membranes. Then concentrated complex is destroyed, and the complexing agent returns for reuse.
Method COUF has two important advantages. First, there is the possibility selection on large-pored ultrafiltration membrane low molecular weight compounds that under normal conditions, they do not linger on it. Through this, it can be achieved higher filtration rate than when using membranes with fine pores. Secondly, it is possible to achieve selective removal (concentration) selected component, while all the rest pass through the membrane.
About with the same purpose - enlargement of dissolved or colloidal impurities - apply water pretreatment solutions coagulants, flocculants, pH correction, etc. Note that such methods are widely used in conventional filtering to expand its boundaries applications.
3. Classification of membrane methods
Microfiltration - mechanical filtration of fine and colloidal impurities as large as usually above 0. 1 µm. Usually the elements microfiltrations are installed in as insurance for the last purification steps in complexes water treatment. Microfiltration used in medicine, food industry at enterprises producing alcoholic and non-alcoholic drinks, wine, beer, vegetable oil, other products for water purification water treatment systems, for filtering semi-finished products, ingredients, various technological media, finished product before bottling, for air purification and gases, etc.
1 µm. Usually the elements microfiltrations are installed in as insurance for the last purification steps in complexes water treatment. Microfiltration used in medicine, food industry at enterprises producing alcoholic and non-alcoholic drinks, wine, beer, vegetable oil, other products for water purification water treatment systems, for filtering semi-finished products, ingredients, various technological media, finished product before bottling, for air purification and gases, etc.
Ultrafiltration - according to the rating of water filtration takes intermediate position between nanofiltration and microfiltration. Ultrafiltration membranes have pore size from 20 to 1000 A (or 0.002-0.1 µm) and allow the retention of fine particles and colloidal impurities, macromolecules (lower limit of molecular weight is several thousand), algae, unicellular microorganisms, cysts, bacteria and viruses, etc.
Nanofiltration - used to obtain extra pure water purified from bacteria, viruses, microorganisms, colloidal particles organic compounds (including pesticides), heavy salt molecules metals, nitrates, nitrites and other harmful impurities. Big advantage nanofiltration before reverse osmosis in the production of drinking water - is preservation of vital human health salts and trace elements. Reverse osmosis - applied to produce two over pure water, pore sizes in reverse osmosis membranes are comparable in size water molecules. This way it happens purification of water from all soluble and insoluble impurities.
Big advantage nanofiltration before reverse osmosis in the production of drinking water - is preservation of vital human health salts and trace elements. Reverse osmosis - applied to produce two over pure water, pore sizes in reverse osmosis membranes are comparable in size water molecules. This way it happens purification of water from all soluble and insoluble impurities.
four. Practical application
For fine and ultra-fine wastewater treatment water is used reverse osmosis method and ultrafiltration. These methods implemented in the filtering process waste water through semi-permeable membranes at pressures exceeding osmotic. membranes leak solvent molecules, holding back solute molecules, sizes which are no more than solvent molecules (reverse osmosis at pressure up to 10 MPa) or an order of magnitude more (ultrafiltration at P \u003d 0.1 - 0.5 MPa).
Back osmosis is used to separate solutions containing particles with sizes 0. 0001--0.001 microns, and ultrafiltration for particles with sizes of 0.001--0.02 microns. Data methods are recommended for content in electrolytes:
0001--0.001 microns, and ultrafiltration for particles with sizes of 0.001--0.02 microns. Data methods are recommended for content in electrolytes:
monovalent salts -- not more than 10%,
divalent -- 15%,
multivalent -- twenty%.
Influence of parameters on the reverse osmosis process
Moscow
- home
- Support
- Useful
- What factors affect the reverse osmosis process?
The reverse osmosis membrane is a key, but not the only, component of a reverse osmosis filter. It does not operate in a vacuum: there are many factors that affect its performance. Let's consider the main ones.
Every reverse osmosis filter has a clean water tank. In conventional filters, this is the so-called water-air tank. It is divided into 2 chambers - with water and with air. Air pressure pushes clean water into the faucet. There is another pressure - tap pressure: due to it, tap water passes through the membrane. Both of them "press" on the membrane from opposite sides.
All reverse osmosis systems are equipped with pre-filter modules, carbon or polypropylene (or both).