12 week scan nuchal
12 Week Scan | SO + GI Scan
At 12 Weeks: Nuchal Translucency ScanThe nuchal translucency (NT) scan, or “12-week scan,” is an ultrasound performed in the first trimester between 11.5 weeks and 13 weeks, six days. This scan is combined with a blood test that looks at two specific hormones of pregnancy: the free-Beta hCG and PAPP-A (pregnancy associated plasma protein A). This combined test is an extremely accurate non-invasive screening test available to help identify a fetus at risk for Down syndrome as well as other chromosomal abnormalities and some major structural abnormalities. The sensitivity of this only recently eclipsed by NIPT. An ultrasound screening test is non-invasive and does not have any side effects or complications.
However, it will not give a yes/no answer to whether a problem such as Down syndrome is present. The only way to diagnose Down syndrome or other chromosomal abnormalities is by having a diagnostic test — either a CVS or an amniocentesis — and testing the fetal cells. These tests are invasive and require a needle to be passed into the maternal abdomen and uterus and therefore carry a small risk of miscarriage.
Many patients do not wish to have the diagnostic test because of the small risk of miscarriage and prefer to have the ultrasound screening test, the NT scan, to help them decide if they wish to proceed to testing the fetus. Unfortunately, while NT combined with the first trimester biochemical blood test is a very accurate screening test available for chromosomal abnormalities, it will not detect all fetuses affected with Down syndrome or other chromosomal abnormalities.
Chromosomal abnormalities occur when there is a change in the number or structure of the chromosomes. Normally we have 46 chromosomes, 23 pairs numbered 1-22 and a pair of sex chromosomes.
Boys have XY and girls have XX. The most common chromosomal abnormality seen at birth is Down syndrome. In Down syndrome, an extra copy of chromosome 21 is present, giving a total of 47 chromosomes.
Accredited practices:
To be certain your NT scan is performed correctly, it is important that you have your scan performed at an accredited practice. A NT computer package was developed by the London Fetal Medicine Foundation in the 1990s and was based on more than 100.000 pregnancies.
Strict auditing of the NT ultrasound work performed by every accredited practice in the world is undertaken several times a year. This ensures that the NT scan test is being performed correctly. Recently, the Royal Australian College of Obstetricians and Gynaecologists has taken over regular auditing of Australian practices.
Advantages of the 12 week NT scanThere are several advantages to undergoing a 12-week NT scan, including:
- Estimate individual risk for Down syndrome and other chromosomal abnormality
- Screen for spina bifida
- More accurately date the pregnancy
- Diagnose multiple fetuses
- Diagnose early pregnancy failure
- Allow early detection of some major abnormalities
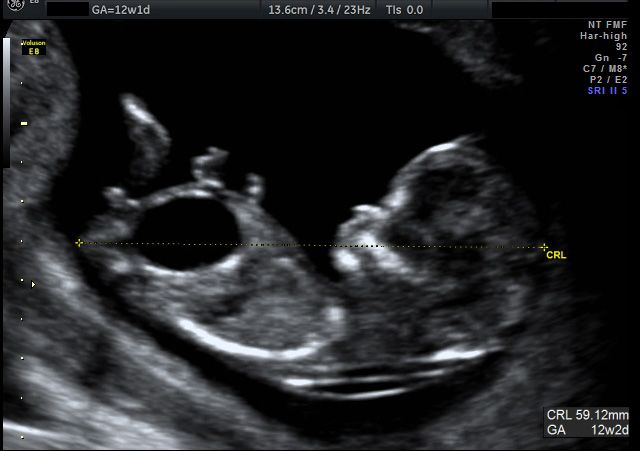
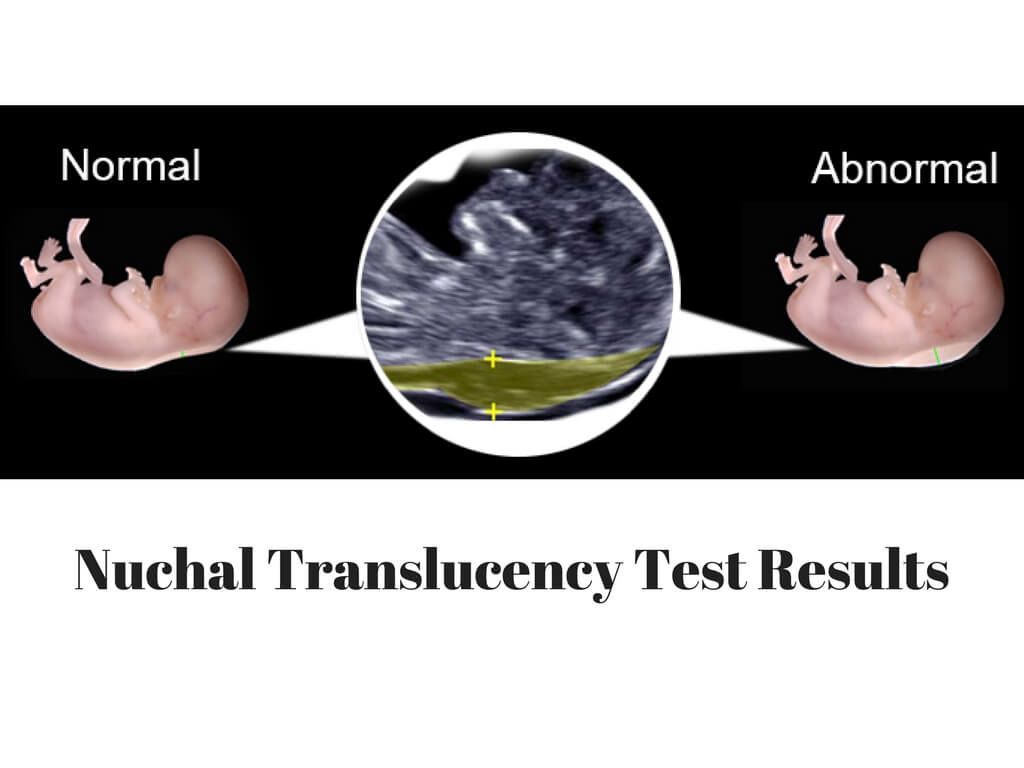
At the end of the first trimester, there is usually a small amount of fluid beneath the skin of the fetus at the back of the head and neck. This fluid is called the nuchal translucency (NT), and can be easily and accurately measured to within a tenth of a millimetre.
This fluid is called the nuchal translucency (NT), and can be easily and accurately measured to within a tenth of a millimetre.
When there is extra fluid and the NT measurement is thicker than normal, there is an association with chromosomal anomalies such as Down syndrome and some structural abnormalities in the fetus. Not all babies with an increased NT measurement have Down syndrome or any structural abnormality.
The extra fluid at the back of the head and neck usually disappears by 18 weeks. The fluid in itself is not an abnormality and does not harm the baby but may simply be the sign of a potential problem. The baby is measured from head to bottom to determine the crown-rump length and the nuchal translucency is measured. These measurements are entered into the computer program with the patient’s date of birth and the first trimester biochemistry blood test. The computer then calculates the patient’s age-related risk for Down syndrome and the new individual risk for this pregnancy.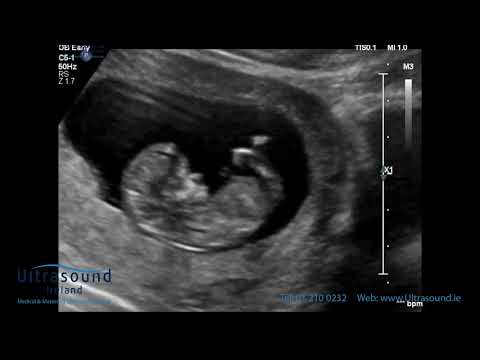 The findings are then discussed with the patient. The combined NT result will provide the patient with a risk assessment. This will either be a high risk (risk is greater than 1 in 300) or a low risk (risk less than 1 in 300). As this is a screening test and not a diagnostic test, even with a low calculated risk, Down syndrome is not completely excluded and can still occur occasionally.
The findings are then discussed with the patient. The combined NT result will provide the patient with a risk assessment. This will either be a high risk (risk is greater than 1 in 300) or a low risk (risk less than 1 in 300). As this is a screening test and not a diagnostic test, even with a low calculated risk, Down syndrome is not completely excluded and can still occur occasionally.
Free- BhCG (Beta human chorionic gonadotrophin) and PAPP-A (Pregnancy associated plasma protein-A) levels can be assessed from a blood test. This is best done at 10 weeks, some two weeks prior to the NT scan but it can also be done just before or on the same day as the NT scan. The PAPP-A level tends to be lower and the BhCG level tends to be higher in pregnancies affected by Down syndrome.
Accuracy of Nuchal TranslucencyWhen the ultrasound is combined with the first trimester blood test (BhCG and PAPPA) and nasal bone, the detection rate increases to 95%.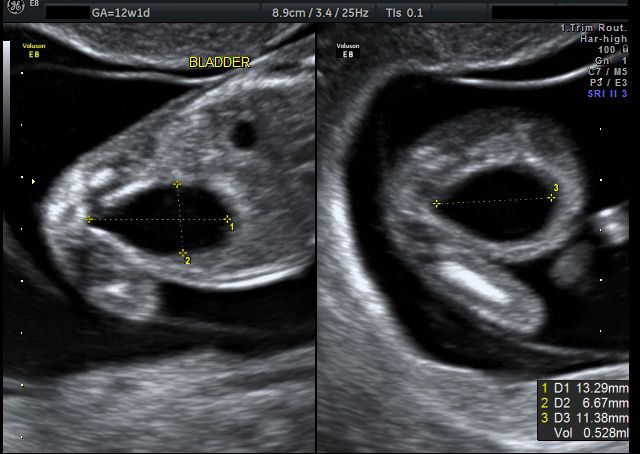 None of these screening tests can give 100% detection. If a pregnant woman wants to completely exclude a chromosome abnormality, then she should consider having prenatal testing with either a CVS or an amniocentesis. Genetic counselling is also recommended. The results of the examination will be available to you shortly after the examination. One of our genetic counsellors or doctors will be available to discuss the result and the options for further testing.
None of these screening tests can give 100% detection. If a pregnant woman wants to completely exclude a chromosome abnormality, then she should consider having prenatal testing with either a CVS or an amniocentesis. Genetic counselling is also recommended. The results of the examination will be available to you shortly after the examination. One of our genetic counsellors or doctors will be available to discuss the result and the options for further testing.
Nuchal translucency scan - 12 week scan, down's syndrome screening.
This is often parents’ favourite scan – you will be amazed at just how much detail you can see and if everything looks normal, the risk of miscarriage and major abnormality becomes very small. If you haven’t had a scan yet, this scan will:
- Make sure that the pregnancy is in the right place
- Count how many babies there are!
- Accurately date your pregnancy and decide on a definite due date for you
- Look at the basic structure of the baby
But the main purpose of this particular scan is to screen for chromosomal abnormalities such as Down’s syndrome. Down’s syndrome is something that affects about 1 in 700 pregnancies overall, but it becomes more common as a mother gets older. It happens because the egg that is released at conception has an extra copy of chromosome 21 and this in turn leads to every cell in the baby having an extra copy of chromosome 21.
Down’s syndrome is something that affects about 1 in 700 pregnancies overall, but it becomes more common as a mother gets older. It happens because the egg that is released at conception has an extra copy of chromosome 21 and this in turn leads to every cell in the baby having an extra copy of chromosome 21.
This causes a wide range of both physical disability and learning difficulties. At the moment there still isn’t a completely safe test that will tell you that your baby definitely does or doesn’t have Down’s syndrome, but the NHS offers everyone combined first trimester screening, which is a test performed at around 12 weeks using a combination of ultrasound scan findings and a basic blood test to assess the likelihood of whether your baby is or isn’t affected. The key ultrasound marker at this stage is the nuchal translucency measurement, or the space at the back of the baby’s neck. At this stage in the baby’s development it is normal for some fluid to build up in this space at the back of the baby’s neck – it happens to every baby so a little bit of fluid is entirely normal, but a baby with problems will often retain more fluid and the nuchal translucency measurement is increased. As well as being a good marker for babies with Down’s syndrome, an increased nuchal translucency measurement can also pick up other genetic conditions, such as Edwards’ syndrome (where the baby has an extra copy of chromosome 18) and Patau’s syndrome (an extra copy of chromosome 13), and some other structural problems, including heart abnormalities.
As well as being a good marker for babies with Down’s syndrome, an increased nuchal translucency measurement can also pick up other genetic conditions, such as Edwards’ syndrome (where the baby has an extra copy of chromosome 18) and Patau’s syndrome (an extra copy of chromosome 13), and some other structural problems, including heart abnormalities.
The nuchal translucency scan is best done during the 12th week, but it can be done from 11 weeks and 3 days up until 14 weeks and your local NHS hospital will offer you an appointment to have this done at around this time. Nevertheless, some parents may opt to have this done privately instead.
Reasons why patients choose to have it done at Beard Mill Clinic include:
Plenty Of Time
Each appointment at Beard Mill Clinic is allocated one hour to make sure that there is plenty of time to do the scan and discuss the results with you.
Wealth Of Experience
Victoria has spent over 20 years working with Professor Kypros Nicolaides who has been the leading pioneer in developing the nuchal translucency scan and screening for abnormalities at this stage of pregnancy. During this time, she has been directly involved in developing the risk calculation software and in teaching other people how to perform the nuchal scan. In doing this, she has come to understand the screening process inside out and can use her expertise to give you the best possible advice.
During this time, she has been directly involved in developing the risk calculation software and in teaching other people how to perform the nuchal scan. In doing this, she has come to understand the screening process inside out and can use her expertise to give you the best possible advice.
Extra Markers
Victoria is trained and certified to look at the additional markers for Down’s syndrome that are not routinely offered at most other scanning clinics. These additional markers include:
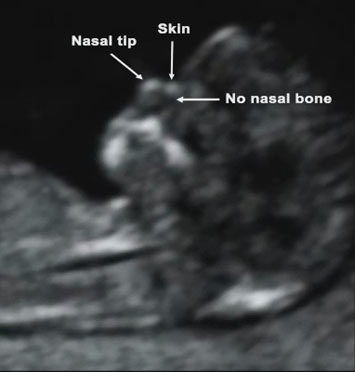
- Looking at the baby’s nose bone and its profile
- Listening to the flow of blood across a valve in the baby’s heart (the tricuspid valve)
- Measuring the resistance in the vessel that takes blood into the baby’s heart (the ductus venosus)
Babies with Down’s syndrome are more likely to have a small or absent nose bone, with a flat profile. They often have leakage across the tricuspid valve and reverse flow in the ductus venosus. So adding in these additional markers will take the average detection rate of the standard technique of 80% up to 95%.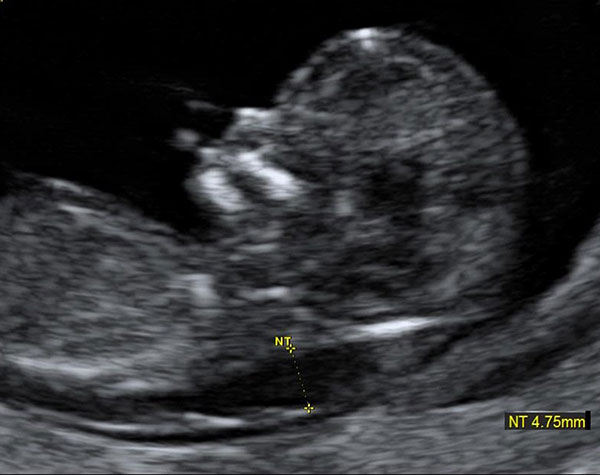
Same Day Results
When the blood has been taken in advance of the scan, Victoria is able to process all the results straight away and explain both the scan findings and blood results to you, showing you how your measurements fall within the context of the “normal” range and how this then affects your own specific risk for Down’s syndrome.
The risk calculation software used at Beard Mill Clinic displays simple graphs which really help you understand what the risk means and Victoria gives you plenty of time to ask questions and clarify anything you are not sure about. You then take away a comprehensive report with all the results clearly documented.
Even if the blood results are not available, Victoria will give you as much explanation as she can based on the scan findings and help you to understand how the blood results fit into the risk assessment. She is able to process blood samples within 24 hours, so will ring you the following day with the final result and then e-mail you your report.
Early Blood Tests
The blood test that is used to screen for Down’s syndrome is usually taken at the time of the nuchal translucency scan, but the research data suggests that the results are actually more accurate if the blood is taken at 9 weeks, rather than 12 weeks. So wherever possible, Victoria will try to arrange for you to have your blood test before your scan. This has the double advantage of giving you the best possible result and ensuring that the blood results are ready when you come for your scan, so that your risk can be discussed with you face-to-face, rather than given to you by letter a week or two later, or over the phone.
Of course this isn’t always possible to arrange, in which case, it can be done at the same time as the scan. But as an added incentive, Victoria will offer to do a quick scan if you come to Beard Mill Clinic early to have the bloods taken, allowing you to hear the heart beat and to check your dates. There is no additional charge for this.
Awkward Babies
Some of the patients who contact Beard Mill Clinic about the nuchal translucency scan do so because it hasn’t been possible to measure the nuchal when they went for their routine NHS appointment. This is often because the baby wasn’t in the right position, but Victoria has the luxury of much more time and has not yet failed to get a nuchal measurement. So if you find yourself in this position, do ring to make an appointment.
Referral For Further Tests
In most cases, patients will be reassured by their result, but if your risk of Down’s syndrome is high, or a problem is suspected, Victoria will arrange a direct referral to your own NHS consultant and ensure you receive the right follow-up.
Full Explanation
Taking the measurements is the easy bit, interpreting the results and communicating these to the parents can sometimes be the more challenging part of screening. And this is where Victoria’s expertise comes into its own.
Through her training, she has acquired a deep understanding of how the individual components of the screening tests work and endeavours to explain this as fully as she can.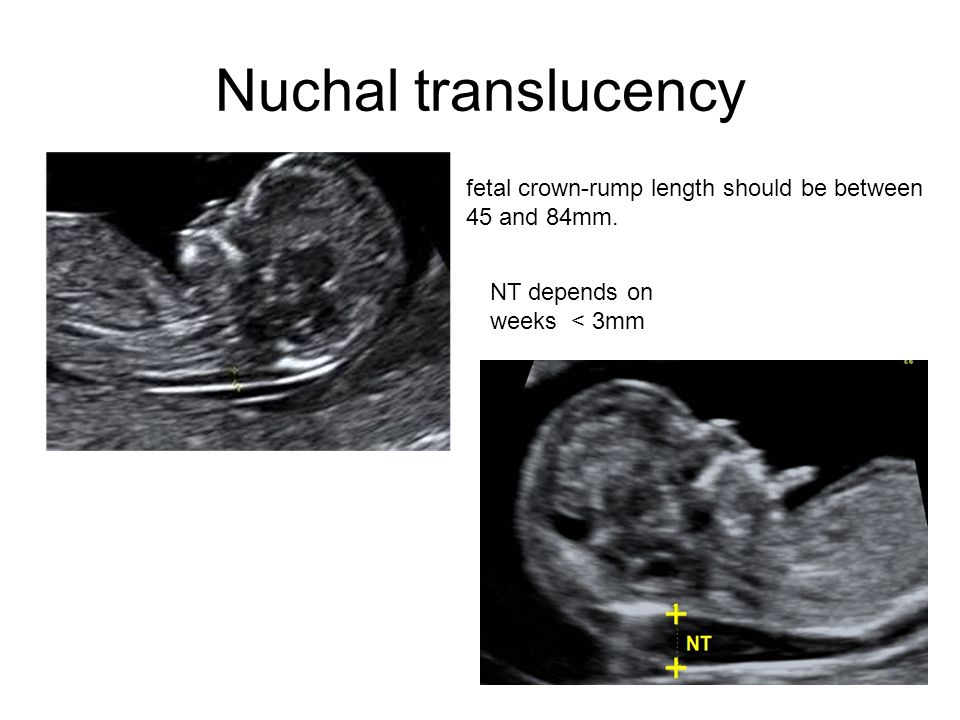
She is passionate about providing each and every patient with the best possible standard of screening and then equipping them with the knowledge and understanding to use this information appropriately.
D. Nemescu • Three-dimensional ultrasound in the diagnosis of iniencephaly
RS80 ultrasound scanner
The benchmark of new standards! Unprecedented clarity, resolution, ultra-fast data processing, and a comprehensive suite of state-of-the-art ultrasound technologies to meet the most demanding diagnostic challenges.
Introduction
Iniencephaly is a rare neural tube pathology characterized by a triad: occipital bone defect, congenital spina bifida in the cervicothoracic region, and severe retroflexion of the head [1]. The name of this defect comes from the fusion of two roots - inion (external occipital protuberance) and encephalosis (enkephalos, brain). This phenomenon was first described by J. Saint-Hilaire [2]. H.Lewis identified two main groups: gap iniencephaly, with the formation of cerebral herniation, and closed iniencephaly, in which there is damage to the spine in the absence of cerebral herniation. The frequency of pathology varies: 0.1-10:10,000 [3] and higher, depending on the possibilities of diagnosing the syndrome. Iniencephaly is more common among female fetuses - M:W=1:10 4 .
The frequency of pathology varies: 0.1-10:10,000 [3] and higher, depending on the possibilities of diagnosing the syndrome. Iniencephaly is more common among female fetuses - M:W=1:10 4 .
The etiology of the disease is unknown. There is seasonality and age fluctuations in the frequency of detection of the syndrome. The association of iniencephaly with the intake of substances such as sleeping pills, vinblastine, estreptonigrin, triparanol and with diseases such as syphilis (in the mother) and in the case of closely related relationships in parents has been described [5-7]. Other risk factors are low fertility and low socioeconomic status [8]. For example, the prevalence of this pathology in Northern China is 25 times higher than in the United States.
Clinical observation
A 35-year-old woman with her 8th pregnancy at 13.5 weeks (Fig. 1) was referred for examination to assess fetal pathology (Fig. 2). Ultrasound scanning revealed pathology of the fetal shape in the form of a lack of a clear separation between the head and body with the face turned upwards, excessive lordosis in the cervical and thoracic spine, significant shortening of the spine, and complete underdevelopment of the anterior cerebral bladder (Fig. 2, 3).
2, 3).
Fig. 1. Fetal thigh of normal length for a given gestational age.
Fig. 2. Pathology of the shape of the fetus in the form of a lack of a clear separation between the head and body. Excessive lordosis in the cervicothoracic spine. An abnormal position of the face in relation to the body is noted. 3D reconstruction of the posterior surface of the head (lower right) shows a defect - the absence of the occiput.
Fig. 3. Pathology of the cervical spine, absence of the neck and complete underdevelopment of the anterior cerebral bladder. Longitudinal scan.
Primary superficial 3D reconstruction showed the absence (defect) of the occiput, especially in the high threshold mode (Fig. 4). At the same time, the longitudinal sections (Fig. 2, 3) showed the continuity of the contour of the posterior surface of the head. When using the maximum mode, the 3D volumetric reconstruction in longitudinal section also shows retroflexion with lordosis of the cervical spine, but with a clearly identifiable undeveloped vertebra (Fig.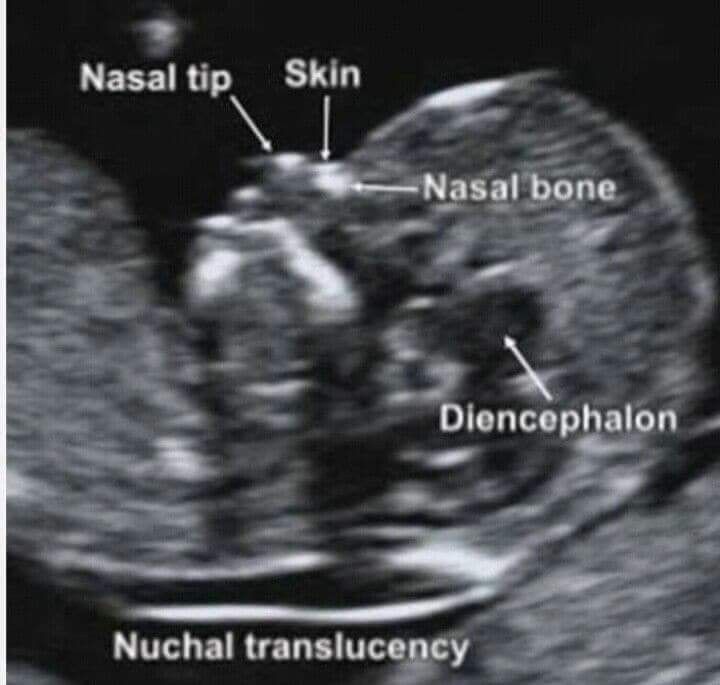 5).
5).
Fig. 4. Dorsal view of the fetus showing the absence of the occiput. 3D reconstruction, surface mode.
Fig. 5. Pathology of the spine - undeveloped cervical vertebra (arrow). 3D reconstruction, maximum mode.
A high axial scan at the level of the lateral ventricles did not reveal a bilateral prominent intrahemispheric echogenic choroid plexus that normally almost completely fills the lumens of the lateral ventricles at this gestational age. Instead, a wide single ventricle filled with fluid was observed, in which there was no median echo, which displaced the cerebral cortex laterally (Fig. 2). When examining the face from the front, hypotelorism was noted (Fig. 6).
Fig. 6. Hypothelorism, fetal face when viewed from the left and front.
a) B-mode.
b) 3D surface reconstruction.
The above signs characterize the complete underdevelopment of the anterior cerebral bladder and correspond to the diagnosis of iniencephaly.
The pregnancy was terminated, and the results of the post-mortem examination fully coincided with the ultrasound findings (Fig. 7). A leading lesion of the spine was noted, which is very characteristic of iniencephaly.
Fig. 7. Iniencephaly. Pathological study. Different types (a-c).
Discussion
Iniencephaly is a pathology of the neural tube. Probably, during embryogenesis, it manifests itself several days later than anencephaly. Pathological development of the coracoid segment of the brain tube and impaired segmentation of the cervical-occipital region cause incomplete development of the base of the skull, excessive stretching of the head and shortening of the spine with its congenital splitting.
This pathology differs from anencephaly in that the anterior part of the brain tube is closed in iniencephaly. Microscopic examination of the brain reveals a number of anomalies, "including microencephaly, polymicrogyria, heterotopic glial tissue in the meninges, atresia of the ventricular system, marked disorganization of the brainstem and spinal cord tissue, and large cerebellar cysts" [9].
There are a number of hypotheses trying to explain the appearance of iniencephaly. The latter was associated with dilatation and rupture of the neural tube [10], non-closure of the neural tube during embryogenesis [11], early vascular disorders [12] with a restructuring of the normal angiogenesis of the vessels responsible for perfusion in the neural tube, followed by their malformation. M.Jones et al. obtained clinical evidence supporting the hypothesis that axial dysraphic disorders may be the result of primary rearrangement of the embryonic mesoderm. This may explain the presence of mesodermal lesions observed in iniencephaly [13].
This anomaly is inherited in isolated cases. I. Kjaer et al. argue that iniencephaly may be the result of "gene changes in the embryonic period that disrupt the dorsoventral orientation of the body axis, anatomically manifested by the incorrect position of the notochord" [14].
Diagnostic criteria for iniencephaly [15,16] - deficiency of the occipital bones of varying severity, manifested in an enlarged foramen magnum; partial or complete absence of the cervical and thoracic vertebrae, accompanied by incomplete closure of the vertebral arches and (or) bodies; a significant shortening of the spinal column due to pronounced lordosis with overstretching of the malformed spinal cord in the cervicothoracic region, which is determined by medial-sagittal scanning [17]; excessive dorsal flexion of the head, upward turning of the face, and a direct transition of the mandibular skin to the thorax due to the absence of a neck.
Other ultrasound findings include: open cervical spine with meningocele, often anencephaly, lumbosacral myelomeningocele, or caudal regression.
Diagnosis is based on excessive dorsal head flexion, very short and deformed cervical and thoracic spine, and general shortening of the fetus. Many cases diagnosed prenatally had high AFP levels and/or polyhydramnios. There is evidence that the earliest diagnosis was made at 13 weeks [18].
Iniencephaly is manifested by various anomalies: it is a defect of the occiput, prolapse of the brain through the enlarged occipital foramen, sharp lordosis in the cervical spine, microencephaly, polymicrogyria, ectopic glial tissue in the meninges, atresia of the ventricular system of the brain, cerebellar cysts, disorganization of the brainstem brain, circulatory disorders in the vessels of the neck and spine, common carotid trunk.
Iniencephaly may be accompanied by a number of other anomalies: anencephaly, cerebral herniation, complete underdevelopment of the anterior cerebral bladder, congenital absence of the jaw, microstomia, synmyelia, hydrocephalus, spina bifida. May present with cleft palate, cardiac malformations (right-sided heart, common carotid artery, transposition of large arteries), hiatal hernia or diaphragmatic agenesis, pulmonary hypoplasia or hyperplasia, umbilical artery agenesis, umbilical hernia, polycystic kidney disease, horseshoe kidney, overgrowth arms comparable to legs, backward bending of the knees, arthrogryposis, clubfoot and polyhydramnios [1,15,16,19,twenty].
May present with cleft palate, cardiac malformations (right-sided heart, common carotid artery, transposition of large arteries), hiatal hernia or diaphragmatic agenesis, pulmonary hypoplasia or hyperplasia, umbilical artery agenesis, umbilical hernia, polycystic kidney disease, horseshoe kidney, overgrowth arms comparable to legs, backward bending of the knees, arthrogryposis, clubfoot and polyhydramnios [1,15,16,19,twenty].
The differential diagnosis is made with Klippel-Feil syndrome (shortening of the neck with fusion of the cervical vertebrae), anencephaly with cervical retroversion, cervical spinal hernia and cervical teratoma [3]. The differential diagnosis between iniencephaly and Klippel-Feil syndrome is difficult and controversial. Some authors believe that Klippel-Feil syndrome may be a mild form of iniencephaly [21]. The distinction between slit iniencephaly and anencephaly with dorsal retroflexion appears from the outset [19]. Anencephaly appears before the closure of the neural folds on the 24th day of pregnancy [22].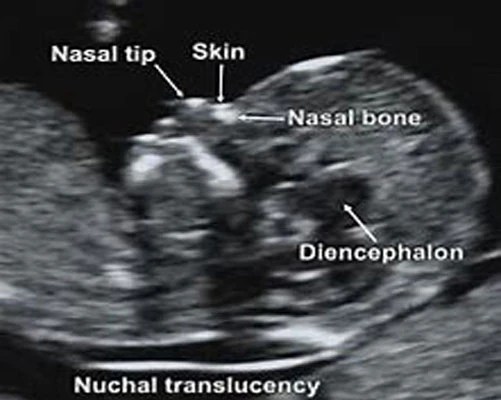 On the other hand, iniencephaly develops after the brain tube has closed [19].
On the other hand, iniencephaly develops after the brain tube has closed [19].
Neonatal iniencephaly is always fatal. Four cases of long-term survival have been described for very mild slit iniencephaly [23], but in these cases the deformity was minimal and could probably be regarded as Klippel-Feil syndrome. In all cases of detection of this anomaly, termination of pregnancy is recommended. In subsequent pregnancies, some experts prescribe folic acid.
Iniencephaly was previously regarded as a rare pathology of the neural tube. Nowadays, due to the improved diagnosis of iniencephaly by ultrasound criteria using 3D ultrasonography, an increasing number of cases are detected prenatally.
Literature
- Lewis HL. Iniencephalus. Am J Obstet Gynecol 1897; 35:11-53
- SaintxHilaire IG. Iniencephalus. In Histoire des Anomalies de l'Organisation. Paris: J.B. Baillier. 1986: Vol.2:308-10
- Romero R, Pilu G, Jeanty P, et al.
 Prenatal Diagnosis of Congenital Anomalies. Norwalk, CT: Appleton and Lange, 1988:64-7
Prenatal Diagnosis of Congenital Anomalies. Norwalk, CT: Appleton and Lange, 1988:64-7 - Morocz I, Szeifert T, Molnar P, et al. Prenatal diagnosis and pathoanatomy of iniencephaly. Clin Genet 1986:30:81-6
- Garofalo R, Simosa V, Morean F. Defectos del cierre del tubo neural. Arch Venez Pueric Pediat 1990;53:85-9.
- Sosa Olavarria A. Ultrasonografia y Clinica Embriofetal. Valencia, Venezuela: Editorial Tatum, 1994
- Bermudez A, Sosa Olavaria A, Rivas M, Mira M. Iniencefalia: serie de seis casos. Rev Obstet Ginecol Venez. 1995:55:161-5
- Nyberg DA, Mahomy BS, Pretorius DH: Diagnostic Ultrasound of FetalAnomalies - Text and atlas. Littleton, MA: Year Book Medical Publishers, 1990:146.
- Morocz I, Molnar P, Toth Z, et al. Diagnostic criteria of iniencephaly based on the authors own cases and the review of the literature. Morphol Igazsagugyi Orv Sz 1985; 25:58-65.
- Gardner WJ. Klippel Feil syndrome, iniencephalus, anencephalus, hindbrain, hernia and mirror movement. Overdistention of the neural tube. Child's Brain 1990;5:361-79.
- Scherrer C, Hammer F, Schmzel A, Briner J. Brain stem cervical cord dysrraphic lesion in iniencephaly. Pediafr Pathol 1992;12:469-76
- Stevenson RE, Kely JC, et al. Vascular basis for neural tube defects: a hypothesis. Pediatrics 1987;80:102-6
- Jones MC, Jones KL, Chernoff GF. Possible mesodermal origin for axial dysraphic disorders. J Pediatr 1982; 101:845-9
- Kjaer I, Mygind H, Fischer Hansen B. Notochordal remnants in human iniencephaly suggest disturbed dorsoventral axis signaling. Am J Med Genet 1999;84:425-32
- Cimmino CV, Painter JW. Iniencephaly. Radiology 1962;84:425-32
- Bose S, Makhani JS, Thaker SV. Iniencephaly. Ind J Med Sci 1964;18:590-4
- Morocz I, Szeifert T, Molnar P, et al.
 Prenatal diagnosis and pathoanatomy of iniencephaly. Clin Genet 1986;30:81-6
Prenatal diagnosis and pathoanatomy of iniencephaly. Clin Genet 1986;30:81-6 - Sherer DM. Endovaginal sonographic diagnosis of iniencephaly apertus and craniorachischisis at 13 weeks, menstrual age. J Clin Ultrasound 1993;2:127
- Lemire, RJ, Beckwith, B, Shepard TH. Iniencephaly and anencephaly with spinal retroflexion, a comparative study of eight human specimens. Teratology 1972;6:27-36
- Schram JHN, Krenning RA, Olthof R, et al. A case of iniencephaly. Ned Tijdschr Geneeskd 1980; 124:1108-11
- Gilmour JR. The essential identity of Klippel-Feil syndrome and iniencephaly. J Pathol 1941 ;53:117-31
- Streeter GL. developmental horizons in human embryos. Age groups XI to XXIII. Embryology reprint Vol. II, Carnegie Inst. Wash., Washington DC, 1951
- Katz VL, Aylsworth AS, Albright SG. Iniencephaly is not uniformly fatal. Prenat Diagn 1989;9:595-9
RS80 ultrasound scanner
Setting new standards! Unprecedented clarity, resolution, ultra-fast data processing, and a comprehensive suite of state-of-the-art ultrasound technologies to meet the most demanding diagnostic challenges.
Microcephaly
Microcephaly- Popular Topics
- Air pollution
- Coronavirus disease (COVID-19)
- Hepatitis
- Data and statistics »
- News bulletin
- The facts are clear
- Publications
- Find the country »
- A
- B
- in
- g
- D
- ё С
- and
- I 93 К
- Л
- М
- Н
- О
- П
- Р
- С
- Т
- У
- Ф
- Х
- Ц
- Ч
- Ш
- Щ
- b
- s
- b
- e
- y
- i
- WHO in countries »
- Reporting
- Regions »
- Africa
- America 9009four
- Southeast Asia
- Europe
- Eastern Mediterranean
- Western Pacific
- Media Center
- Press releases
- Statements
- Media messages
- Comments
- Reporting
- Online Q&A
- Developments
- Photo reports
- Questions and answers
- Update
- Emergencies "
- News "
- Disease Outbreak News
- WHO Data »
- Dashboards »
- COVID-19 Monitoring Dashboard
- Basic moments "
- About WHO »
- CEO
- About WHO
- WHO activities
- Where does WHO work?
- Governing Bodies »
- World Health Assembly
- Executive committee
- Main page/
- Media Center /
- Newsletters/
- Read more/
- Microcephaly
\n
Magnitude of the problem
\n
\nMicrocephaly is a rare condition.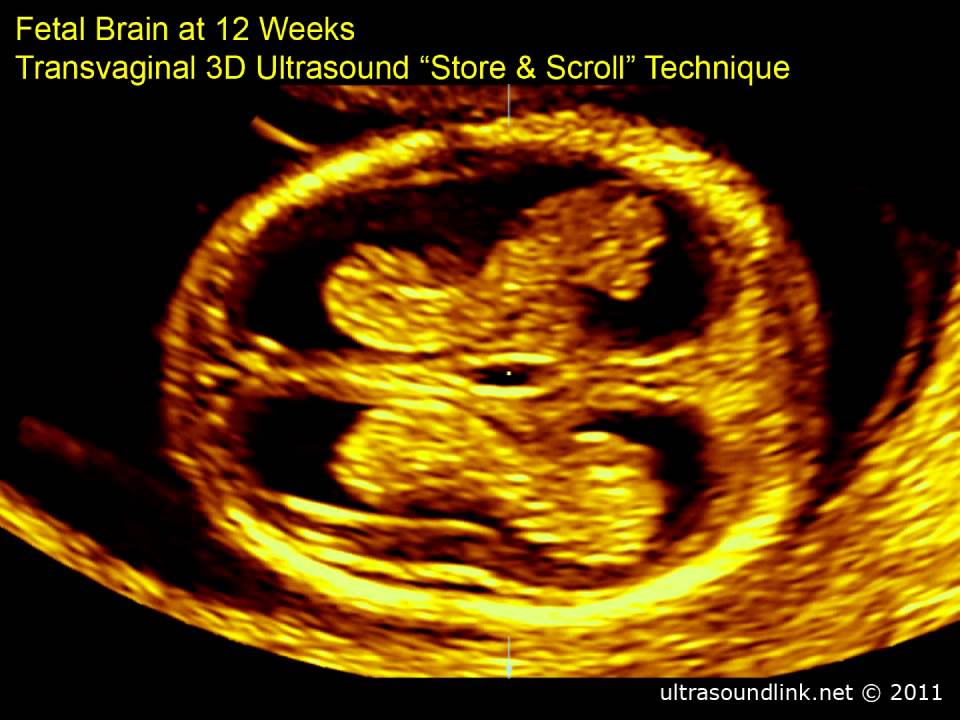 The prevalence of microcephaly is estimated to vary considerably due to different definitions and depending on target populations. Scientists are investigating a potential, though unproven, link between an increase in microcephaly cases and Zika virus infection.
The prevalence of microcephaly is estimated to vary considerably due to different definitions and depending on target populations. Scientists are investigating a potential, though unproven, link between an increase in microcephaly cases and Zika virus infection.
\n
Diagnosis
\n
\nMicrocephaly can sometimes be diagnosed by fetal ultrasound. The most appropriate period for diagnosis is the end of the second trimester (about 28 weeks) or the third trimester of pregnancy.
\n
\nChild circumference of newborns should be measured at least 24 hours after birth and compared with the WHO standard child development indicators. The results are interpreted taking into account the gestational age of the child, as well as his height and weight. If there is suspicion, the child is sent for examination to the pediatrician and for a brain scan, the circumference of his head is measured once a month in early infancy and compared with standard indicators.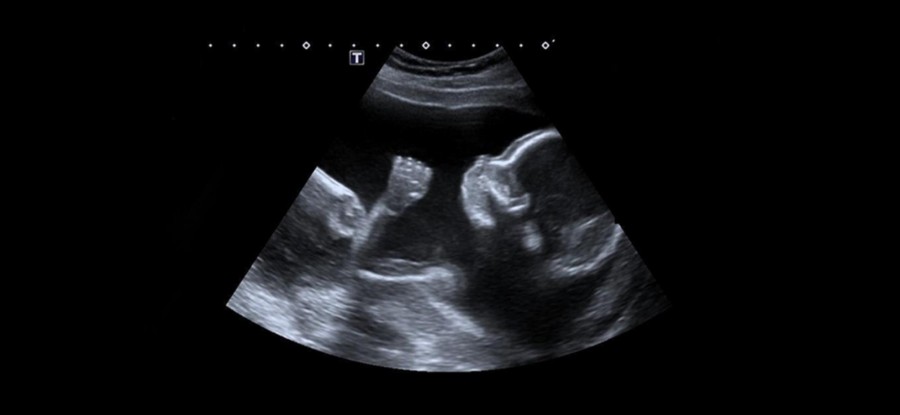 Doctors should also test for known causes of microcephaly.
Doctors should also test for known causes of microcephaly.
\n
Causes of microcephaly
\n
\nMicrocephaly has many potential causes, but often the cause remains unknown. The most common causes include:
\n
- \n
- intrauterine infections: toxoplasmosis (caused by a parasite found in meat that has not been properly cooked), rubella, herpes, syphilis, cytomegalovirus, and HIV; \n
- exposure to toxic chemicals: maternal exposure to heavy metals such as arsenic and mercury, alcohol, radiation, and smoking; \n
- genetic pathologies such as Down syndrome; and \n
- severe malnutrition during fetal development. Signs and symptoms with vision. Some children with microcephaly develop quite normally.
\n
Treatment and care
\n
\nThere is no specific treatment for microcephaly.
 A multidisciplinary team is needed to evaluate and treat newborns and children with microcephaly. Early promotional activities and play programs can have a positive impact on development. Family counseling and parental support are also very important.
A multidisciplinary team is needed to evaluate and treat newborns and children with microcephaly. Early promotional activities and play programs can have a positive impact on development. Family counseling and parental support are also very important. \n
WHO activities
\n
\nSince mid-2015, WHO has been working closely with the Americas to investigate and respond to the outbreak.
\n
\nThe Strategic Response Program and Joint Action Plan outlines steps being taken by WHO and partners in response to Zika virus and potential complications:
\n
- \n
- Working closely with affected countries to conduct outbreak investigations caused by the Zika virus and responding to an unusual rise in microcephaly cases. \n
- Engage with local communities to communicate the risks of Zika virus disease and how they can protect themselves.
 \n
\n - Provide guidance and mitigate the potential impact on women of childbearing age and pregnant women and families affected by the Zika virus. \n
- Assist affected countries to improve care for pregnant women and families with children born with microcephaly. \n
- Investigation of reported increases in microcephaly cases and possible association with Zika virus infection, with the participation of experts and partners. \n
\n
","datePublished":"2018-02-16T09:06:00.0000000+00:00","image":"https://cdn.who.int/media/images/default -source/imported/measure-microcephaly475-jpg.jpg?sfvrsn=7be7eab_0","publisher":{"@type":"Organization","name":"World Health Organization: WHO","logo":{" @type":"ImageObject","url":"https://www.who.int/Images/SchemaOrg/schemaOrgLogo.jpg","width":250,"height":60}},"dateModified": "2018-02-16T09:06:00.0000000+00:00","mainEntityOfPage":"https://www.
 who.int/ru/news-room/fact-sheets/detail/microcephaly","@context" :"http://schema.org","@type":"Article"};
who.int/ru/news-room/fact-sheets/detail/microcephaly","@context" :"http://schema.org","@type":"Article"}; Basic Facts
- Microcephaly is a condition in which a baby is born with a small head or the head stops growing after birth.
- Microcephaly is a rare condition. One child out of several thousand children is born with microcephaly.
- The most reliable way to detect microcephaly in a baby is to measure the circumference of the baby's head 24 hours after birth, compare it with the WHO standard child development indicators, and then measure the head growth in early infancy.
- Children born with microcephaly may experience seizures as they grow, as well as physical and learning disabilities.
- There is no specific test to detect microcephaly in a fetus, but an ultrasound scan in the third trimester of pregnancy can sometimes reveal the problem.
- There is no specific treatment for microcephaly.
Microcephaly is a neonatal malformation in which the baby's head is much smaller than other babies of the same age and sex.
 Combined with improper brain development, children with microcephaly may develop developmental disabilities. The severity of microcephaly varies from mild to severe.
Combined with improper brain development, children with microcephaly may develop developmental disabilities. The severity of microcephaly varies from mild to severe. Magnitude of the problem
Microcephaly is a rare condition. The prevalence of microcephaly is estimated to vary considerably due to different definitions and depending on target populations. Scientists are investigating a potential, though unproven, link between an increase in microcephaly cases and Zika virus infection.
Diagnosis
Microcephaly can sometimes be diagnosed with a fetal ultrasound. The most appropriate period for diagnosis is the end of the second trimester (about 28 weeks) or the third trimester of pregnancy.
Head circumference of newborns should be measured at least 24 hours after birth and compared with WHO standard child development indicators. The results are interpreted taking into account the gestational age of the child, as well as his height and weight. If there is suspicion, the child is sent for examination to the pediatrician and for a brain scan, the circumference of his head is measured once a month in early infancy and compared with standard indicators.
 Doctors should also test for known causes of microcephaly.
Doctors should also test for known causes of microcephaly. Causes of microcephaly
Microcephaly has many potential causes, but often the cause remains unknown. The most common causes include:
- intrauterine infections: toxoplasmosis (caused by a parasite found in undercooked meat), rubella, herpes, syphilis, cytomegalovirus and HIV;
- exposure to toxic chemicals: maternal exposure to heavy metals such as arsenic and mercury, alcohol, radiation and smoking;
- genetic pathologies such as Down syndrome; and
- severe malnutrition during fetal development.
Signs and symptoms
Many babies born with microcephaly may have no other symptoms at birth, but may later develop epilepsy, cerebral palsy, learning disabilities, hearing loss, and vision problems. Some children with microcephaly develop quite normally.
Treatment and care
There is no specific treatment for microcephaly. A multidisciplinary team is needed to evaluate and treat newborns and children with microcephaly.
 Early promotional activities and play programs can have a positive impact on development. Family counseling and parental support are also very important.
Early promotional activities and play programs can have a positive impact on development. Family counseling and parental support are also very important. WHO activities
Since mid-2015, WHO has been working closely with the Americas to investigate and respond to the outbreak.
The Strategic Response Program and Joint Action Plan outlines steps being taken by WHO and partners in response to Zika virus and potential complications:
- Working closely with affected countries to investigate the Zika virus outbreak and respond to unusual an increase in the incidence of microcephaly.
- Engage with local communities to communicate the risks of Zika virus disease and how they can protect themselves.
- Provide guidance and mitigate the potential impact on women of childbearing age and pregnant women and families affected by the Zika virus.
- Assistance to affected countries to improve care for pregnant women and families with children born with microcephaly.