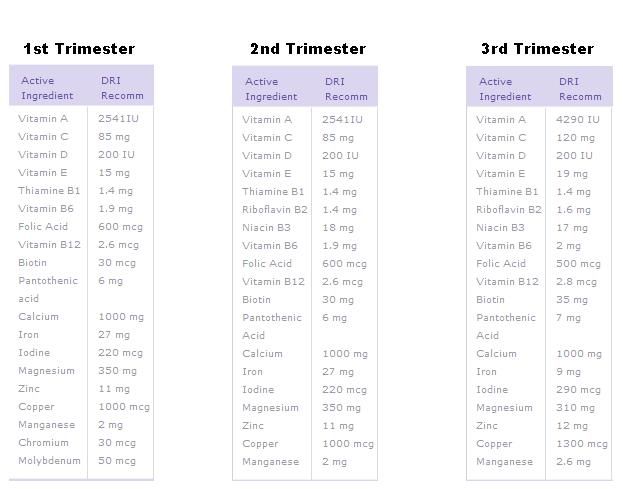
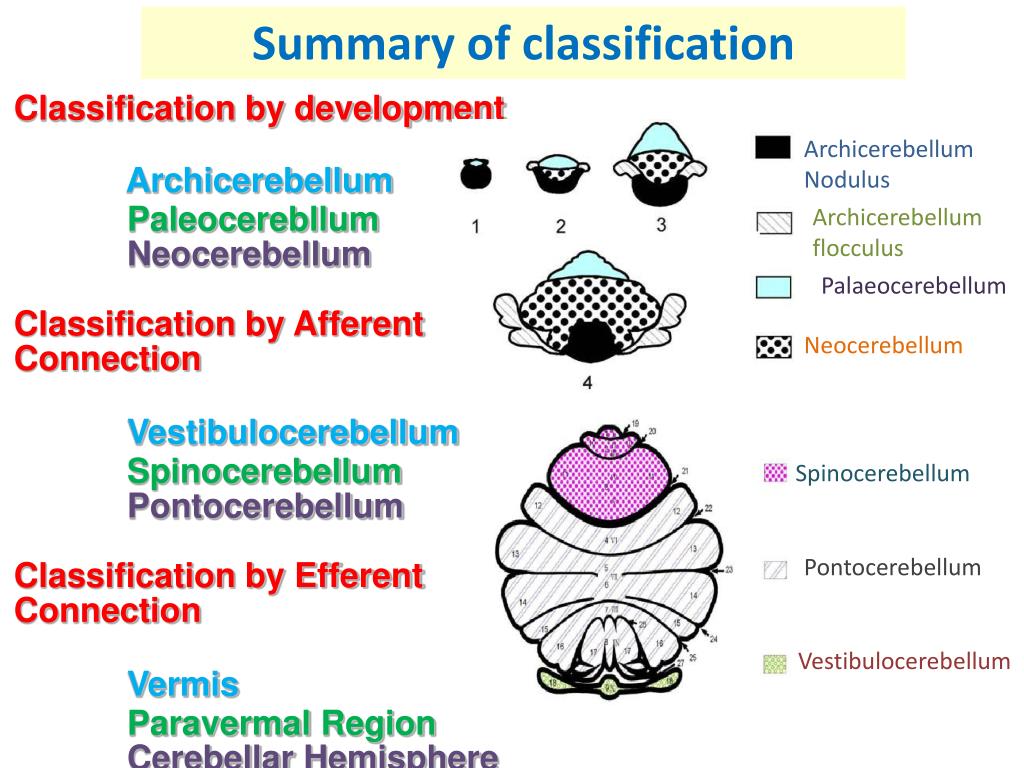
When does the cerebellum develop in a fetus
When Does a Fetus Develop a Brain?
Pregnancy is an exciting time full of rapid change and development for both you and your baby. While the growth happening on the outside is clear to everyone (hello, growing belly!), it’s the development we can’t see that is truly fascinating.
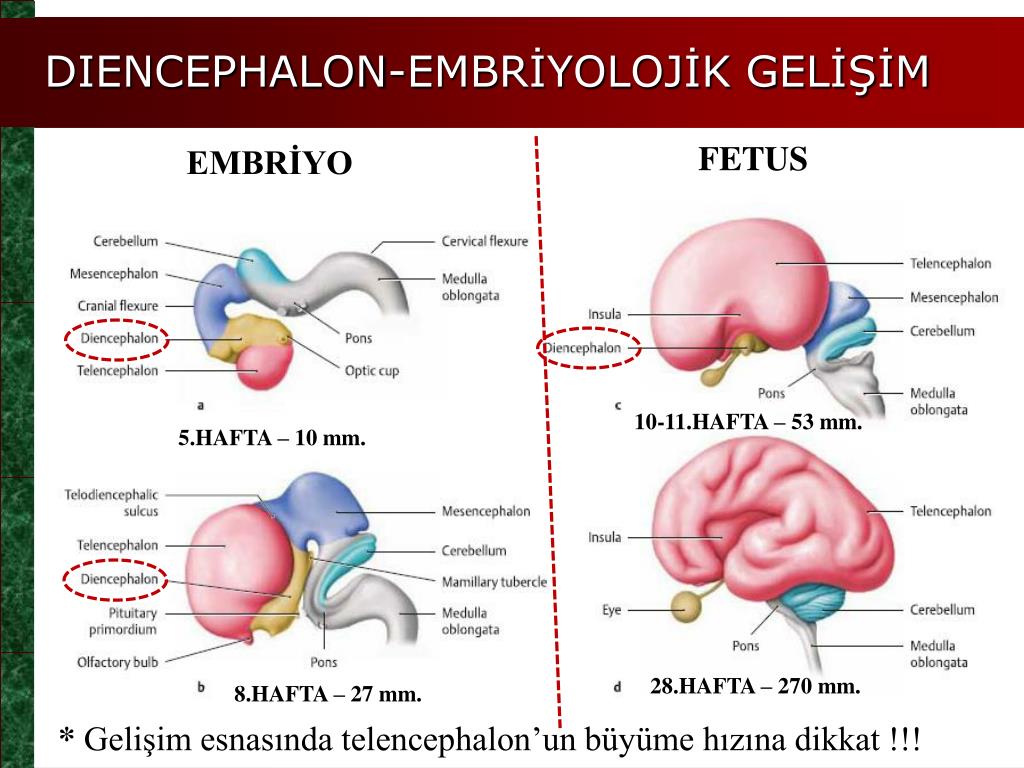
Your fetus will begin the process of developing a brain around week 5, but it isn’t until week 6 or 7 when the neural tube closes and the brain separates into three parts, that the real fun begins.
Around week 5, your baby’s brain, spinal cord, and heart begin to develop. Your baby’s brain is part of the central nervous system, which also houses the spinal cord. There are three key components of a baby’s brain to consider. These include:
- Cerebrum: Thinking, remembering, and feeling occurs in this part of the brain.
- Cerebellum: This part of the brain is responsible for motor control, which allows the baby to move their arms and legs, among other things.
- Brain stem: Keeping the body alive is the primary role of the brain stem. This includes breathing, heartbeat, and blood pressure.
The first trimester is a time of rapid development and separation of the various parts of the brain, according to Kecia Gaither, MD, MPH, double board certified in obstetrics and gynecology and maternal-fetal medicine, and director of perinatal services at NYC Health + Hospitals/Lincoln.
Within 4 weeks, the rudimentary structure known as the neural plate develops, which Gaither says is considered the precursor to the nervous system. “This plate elongates and folds on itself forming the neural tube — the cephalad portion of the tube becomes the brain, while the caudal portion elongates to eventually become the spinal cord,” she explains.
The neural tube continues to grow, but around week 6 or 7, Gaither says it closes, and the cephalad portion (aka the rudimentary brain) separates into three distinct parts: front brain, midbrain, and hindbrain.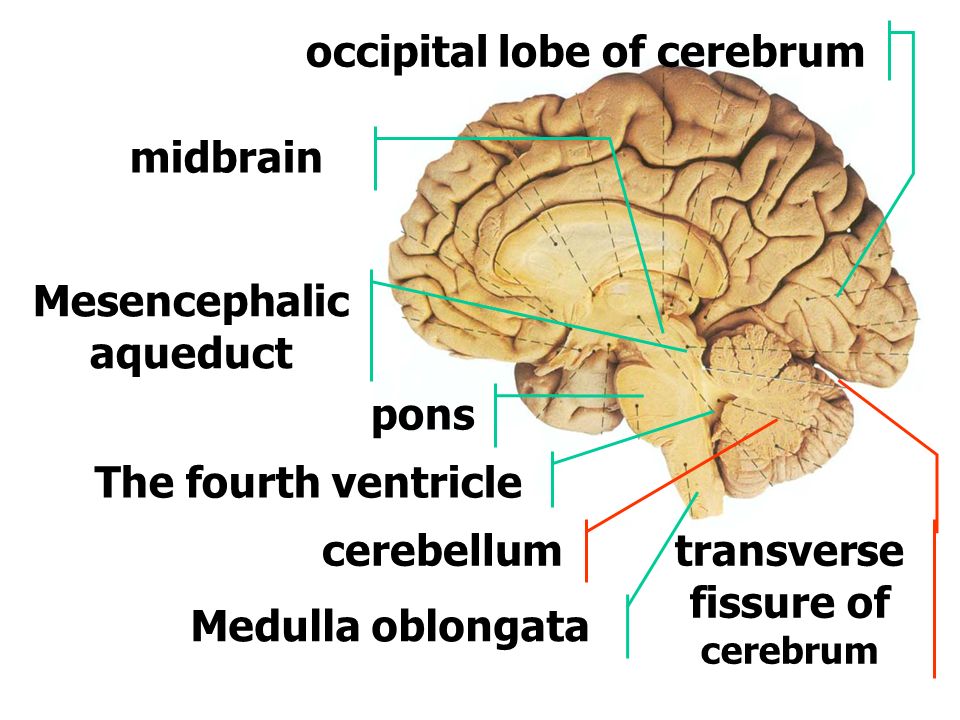
It’s also during this time that neurons and synapses (connections) begin to develop in the spinal cord. These early connections allow the fetus to make its first movements.
During the second trimester, Gaither says the brain begins to take command of bodily functions. This includes specific movements that come from the hindbrain, and more specifically, the cerebellum.
One of the first notable developments, sucking and swallowing, are detectable around 16 weeks. Fast-forward to 21 weeks, and Gaither says baby can swallow amniotic fluid.
It’s also during the second trimester that breathing movements begin as directed by the developing central nervous system. Experts call this “practice breathing” since the brain (and more specifically, the brain stem) is directing the diaphragm and chest muscles to contract.
And don’t be surprised if you feel some kicking during this trimester. Remember the cerebellum or the part of the brain responsible for motor control? Well, its directing the baby’s movements, including kicking and stretching.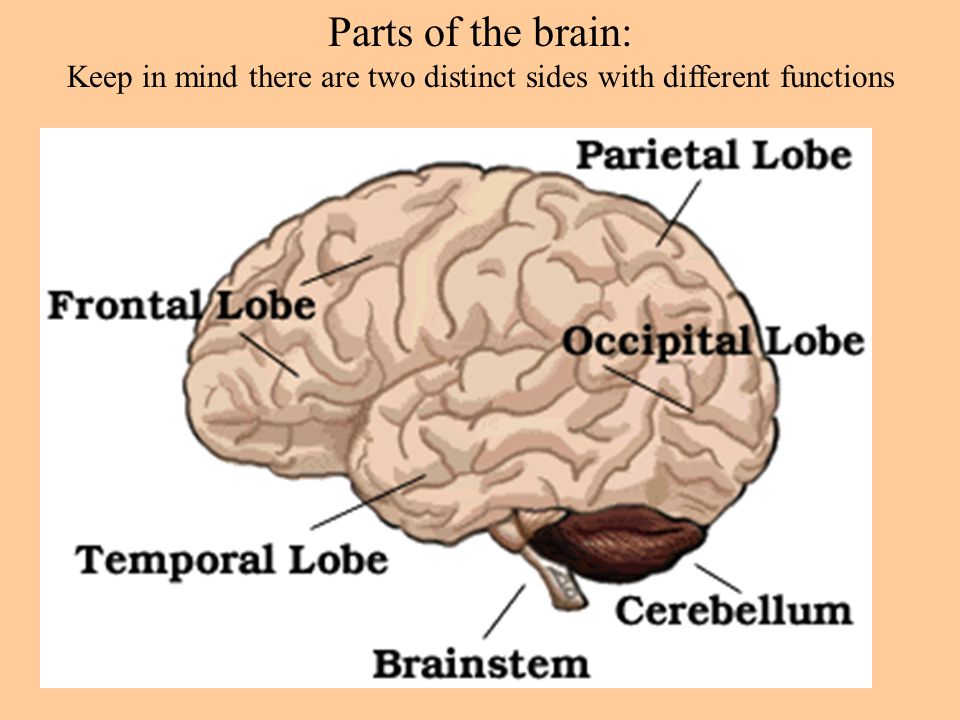
Gaither points out that a fetus can begin to hear during the late second trimester, and a sleep pattern emerges as the brainwaves from the developing hypothalamus become more mature.
By the end of the second trimester, Gaither says the fetal brain looks structurally much like the adult brain with the brain stem almost entirely developed.
The third trimester is full of rapid growth. In fact, as your baby continues to grow, so does the brain. “All the convoluted surfaces of the brain materialize, and the halves (right brain and left brain) will separate,” explains Gaither.
The most notable part of the brain during this final trimester is the cerebellum — hence, the kicking, punching, wiggling, stretching, and all of the other movements your baby is performing.
Share on PinterestIllustration by Alyssa Kiefer
While it may feel like you have control over nothing for the next 9 months, you do have a say in the foods you eat. Healthy brain development starts before pregnancy.
According to the Centers for Disease Control and Prevention, a healthy diet that includes folic acid, both from foods and dietary supplements, can promote a healthy nervous system.
“There are a number of defects along the baby’s brain and spinal cord that can occur when there is an abnormality occurring within the first weeks of brain development,” says Gaither. This may include anencephaly or spina bifida.
Gaither says two supplements in particular are involved with fetal brain development:
Folic acidFolic acid (vitamin B9, specifically) supports fetal brain and spinal development. Not only does it play a role forming the neural tube, but Gaither says it’s also involved in the production of DNA and neurotransmitters, and it’s important for the production of energy and red blood cells.
Gaither recommends taking at least 400 to 600 micrograms of folic acid daily while you’re trying to conceive, and then continue with 400 micrograms daily during pregnancy.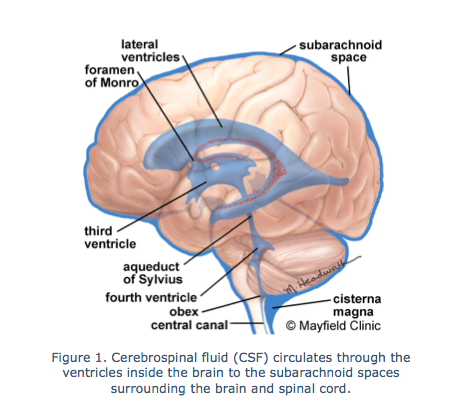
“If you’ve had a child with a neural tube defect, then 4 grams daily in the preconceptual period is advised,” says Gaither.
Foods rich in folate/folic acid include dark green leafy vegetables, flaxseed, and whole grains.
Omega-3 fatty acids
Also important for fetal brain development are omega-3 fatty acids. “The brain has a high fat content, and the omegas are helpful in the deposition of the fat in not only the brain, but the eyes as well,” explains Gaither.
Omegas are also helpful in the neural synapse development or nerve connections to each other.
Foods rich in omega 3-fatty acids include salmon, walnuts, and avocados.
Fetal brain development starts before you may even realize you’re pregnant. That’s why it’s important to start on a prenatal vitamin that contains folic acid right away. If you’re not pregnant, but thinking about having a baby, add a prenatal vitamin to your daily routine.
The brain begins to form early in the first trimester and continues until you give birth.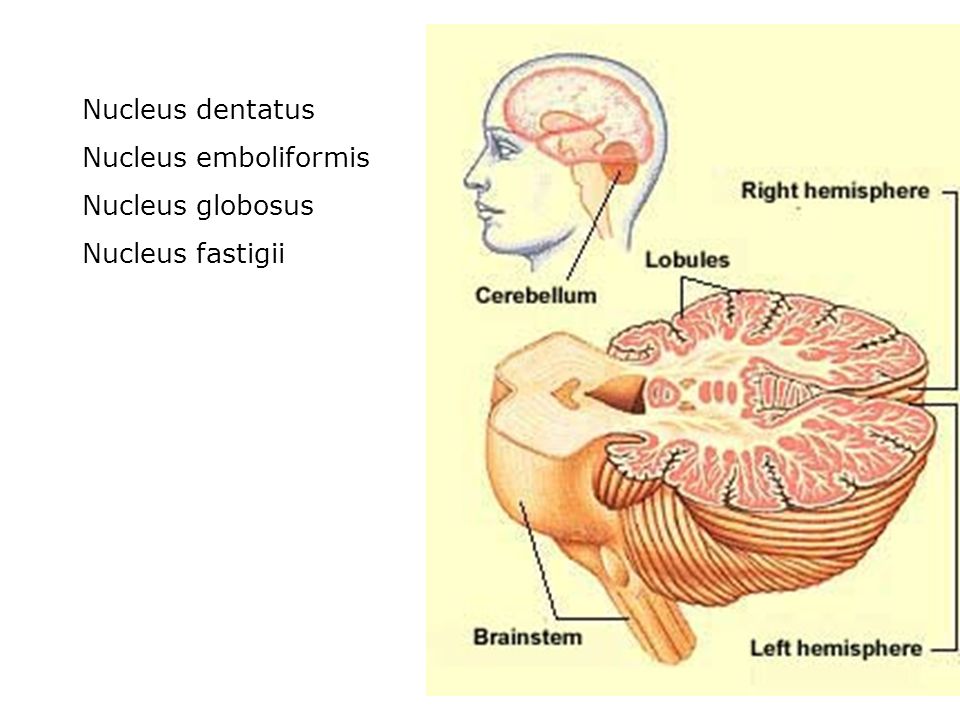 During pregnancy, fetal brain development will be responsible for certain actions like breathing, kicking, and the heartbeat.
During pregnancy, fetal brain development will be responsible for certain actions like breathing, kicking, and the heartbeat.
Talk to your doctor if you have any questions about your pregnancy, fetal brain development, or how to nurture the baby’s developing brain.
Development of the Fetal Cerebral Cortex in the Second Trimester: Assessment with 7T Postmortem MR Imaging
Research ArticlePediatrics
Open Access
Z. Zhang, Z. Hou, X. Lin, G. Teng, H. Meng, F. Zang, F. Fang and S. Liu
American Journal of Neuroradiology July 2013, 34 (7) 1462-1467; DOI: https://doi.org/10.3174/ajnr.A3406
- Article
- Figures & Data
- Supplemental
- Info & Metrics
- References
Abstract
BACKGROUND AND PURPOSE: Few investigators have analyzed the fetal cerebral cortex with MR imaging of high magnetic strength. Our purpose was to document the sulcal development and obtain quantitative measurements of the fetal brain in the second trimester.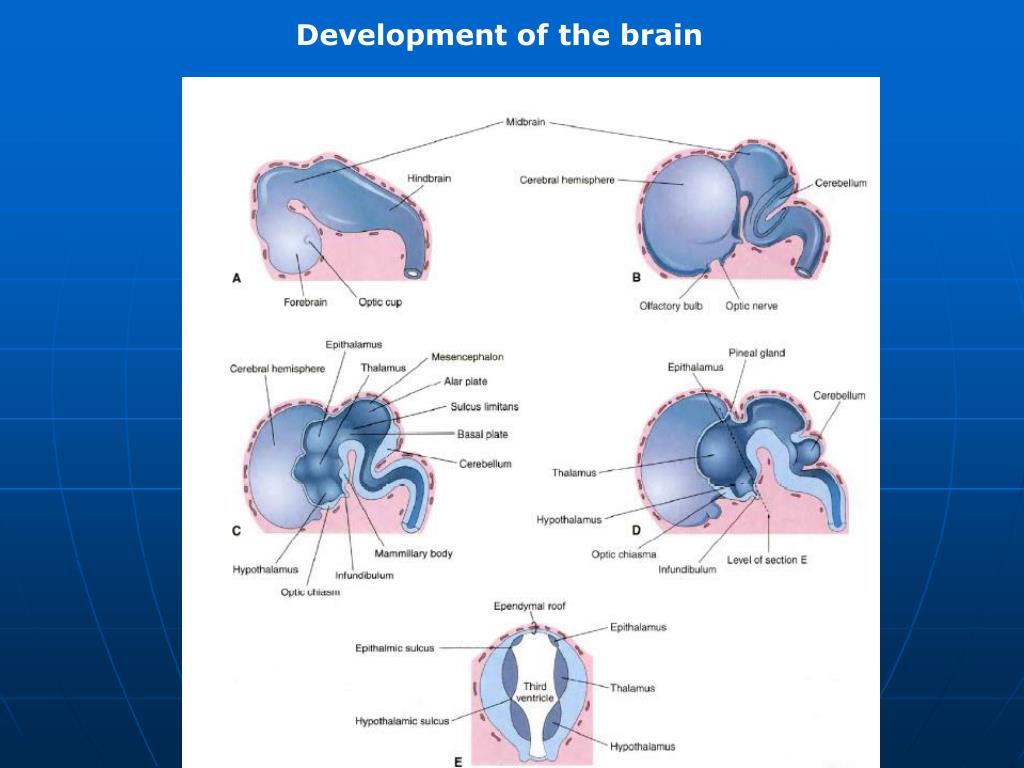
MATERIALS AND METHODS: The brains of 69 fetal specimens, with GA 12–22 weeks, were first scanned on a 7T MR imaging scanner. Then the sequential development of the different fissures and sulci was analyzed, and quantitative measurements of the cerebral cortex were obtained.
RESULTS: A new chronology of sulcal development during 12–22 weeks GA was summarized. Before 12 weeks, few sulci were present; by 16 weeks, many sulci were present. The 16th week could be considered the most intensive time point for sulcal emergence. Most sulci, except for the postcentral sulcus and intraparietal sulcus, were present by 22 weeks GA. Measurements of the fetal brains, each with different growth rates, linearly increased with GA, but no sexual dimorphisms or cerebral asymmetries were detected.
CONCLUSIONS: The second trimester is the most important phase, during which most sulci are present and can be clearly shown on 7T postmortem MR imaging.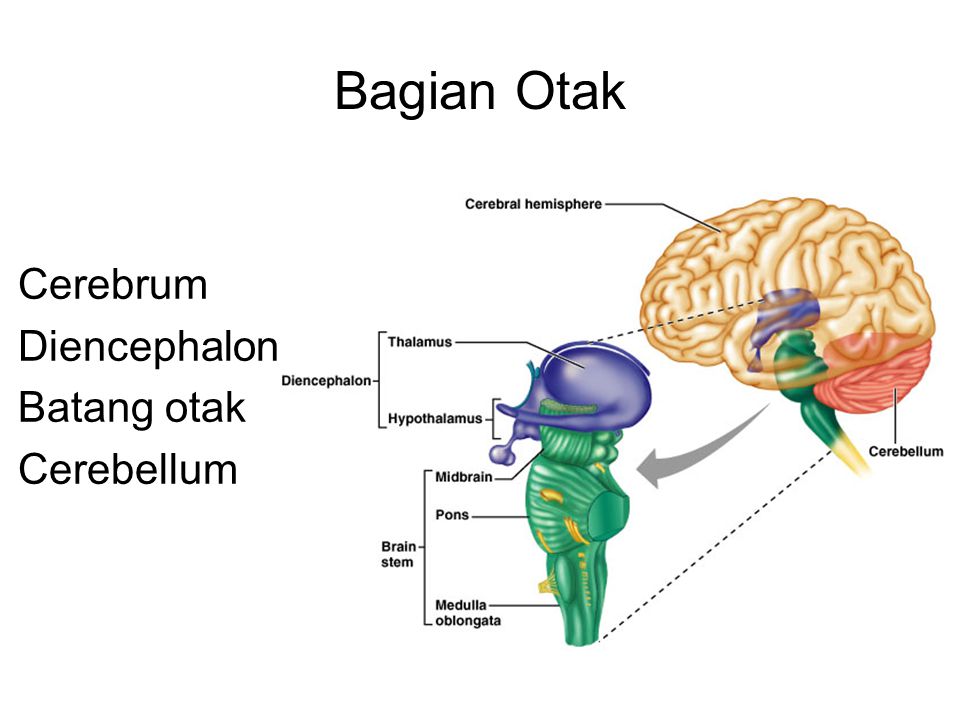 It is apparent that the specific time during which neuropathologic features of sulci appear, previously thought to be well understood, should be redefined. Quantitative data provide assistance in the precise understanding of the immature brain. The present results are valuable in anatomic education, research, and assessment of normal brain development in the uterus.
It is apparent that the specific time during which neuropathologic features of sulci appear, previously thought to be well understood, should be redefined. Quantitative data provide assistance in the precise understanding of the immature brain. The present results are valuable in anatomic education, research, and assessment of normal brain development in the uterus.
ABBREVIATIONS:
- GA
- gestational age
- US
- ultrasonography
The developmental process of the fetus in vivo is divided into the first, second, and third trimesters, which are closely associated with the different developmental stages of the CNS. The first and second trimesters correspond to the neurulation, differentiation of cerebral vesicles, and neurogenesis.1⇓–3 Disorders of migration are more likely to occur in the second trimester. Either undermigration or overmigration of neurons will lead to cortical abnormalities, such as gray matter heterotopia, agyria/pachygyria, heterotopia within the molecular layer (layer 1 of the cortex), and neuronal heterotopia.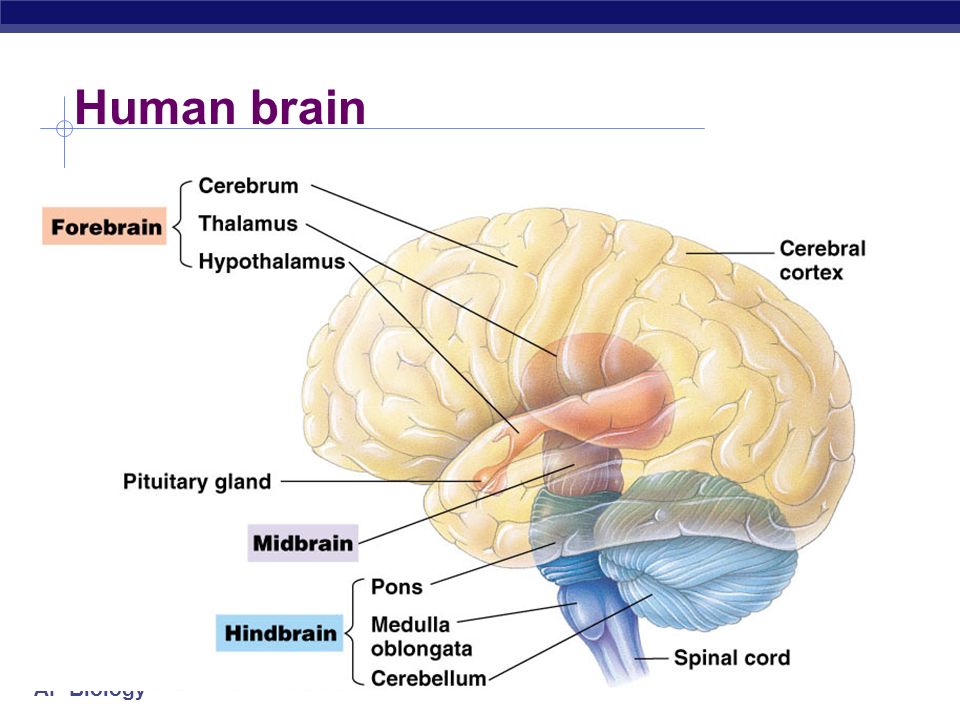 4 Therefore, it is important to study fetal brain development in the second trimester.
4 Therefore, it is important to study fetal brain development in the second trimester.
The sulci first appear as shallow fossa and then develop into a deeper and more curved pattern on the cerebral cortex.5,6 The timing of the appearance of these different types of sulci is so precise that neuropathologists consider sulcation to be a reliable estimation of GA and consequently a good marker of fetal brain maturation.6 Fetal sulcal development has been studied in neuropathology,5 with US7 and MR imaging in vitro8 and in vivo.6 However, few investigators have described the normal patterns of fetal sulcation with an MR imaging scanner of high magnetic strength.
The dimensions of the developing fetal brain change rapidly in early life, and morphometric normative data are crucial for the assessment of normal maturation and diagnosis of brain anomalies.9 Currently, prenatal morphometric studies have been focused as early as 17 weeks GA,10 but only the linear biometric values were obtained on 2D images without 3D reconstruction of the fetal brain. In addition, the existing MR imaging postprocessing software with automated tissue segmentation widely used for adults is not suitable for the fetal brain,11 and thus we still lack 3D reliable and consistent quantitative measurements of the fetal brain at the early developmental phase.
In addition, the existing MR imaging postprocessing software with automated tissue segmentation widely used for adults is not suitable for the fetal brain,11 and thus we still lack 3D reliable and consistent quantitative measurements of the fetal brain at the early developmental phase.
In our study, we present a normal trajectory of fetal sulci development and obtain quantitative data of the fetal cerebral cortex during 12–22 weeks GA.
Materials and Methods
Subjects
A total of 85 fetal specimens of 12–22 weeks GA were obtained from medically indicated or spontaneous abortions, and fetal deaths from hospitals in the Shandong Province of China.
The inclusion criteria were the same as those in our previous research12⇓⇓–15: 1) maternal pregnancy records showing an absence of documented fetal chromosomal abnormalities, stressful intrauterine conditions, maternal genetic disease in the families, or a history of seizures in the case of eclampsia; 2) results of US examination for the fetus during pregnancy and results of postmortem MR imaging examinations for the specimen indicating an anatomically normal and developmentally appropriate fetal CNS; and 3) further validated detailed autopsy combined with neuropathologic examinations also describing no detectable CNS malformations.
Finally, 69 specimens with an appropriately developed fetal brain were reserved for the study (GA dispositions and numbers of the chosen specimens are listed in Table 1). This study was conducted after approval of the Ethical Committee at Shandong University.
Table 1:
GA dispositions and numbers of chosen specimens (n = 69)
Image Acquisition
The brains were not removed from the calvaria and were scanned by a 7T micro-MR with a maximal gradient of 360 mT (70/16 PharmaScan; Bruker BioSpin, Bremen, Germany). A rat body coil with an inner diameter of 60 mm was selected to scan all of the fetuses. For T1-weighted images, section thickness was 0.8 mm; section interval, 0.8 mm; TR, 384.4 ms; TE, 15.8 ms; matrix size, 512 × 512; number of excitations, 1; and field of view, 6 × 6 cm. For T2-weighted images, section thickness was 0.5 mm; section interval, 0.5 mm; TR, 17,000 ms; TE, 50 ms; matrix size, 256 × 256; number of excitations, 4; and field of view, 6 × 6 cm.
Structure Annotation, 3D Reconstruction, and Quantitative Measurements
The brain structures were assigned and annotated based on a histology atlas of second-trimester fetal brains.16 The T2-weighted images were segmented and reconstructed by Amira 4.1 software (www.amira.com). The segmentation on the cortical surface was performed at the borderline between the cortical plate and the marginal zone (Fig 1A, C). The 3D reconstruction models were automatically obtained after segmentation. We simultaneously segmented all images twice using 2 anatomists to obtain a mean.
Fig 1.
Methods of image segmentation with Amira 4.1. A, The original image. B, Segmentation of the hemisphere. C, Different colors are filled in the structures after segmentation. D, 3D visualization model and measurements of brain length, width, and height.
After segmentation, a polygonal surface model was created.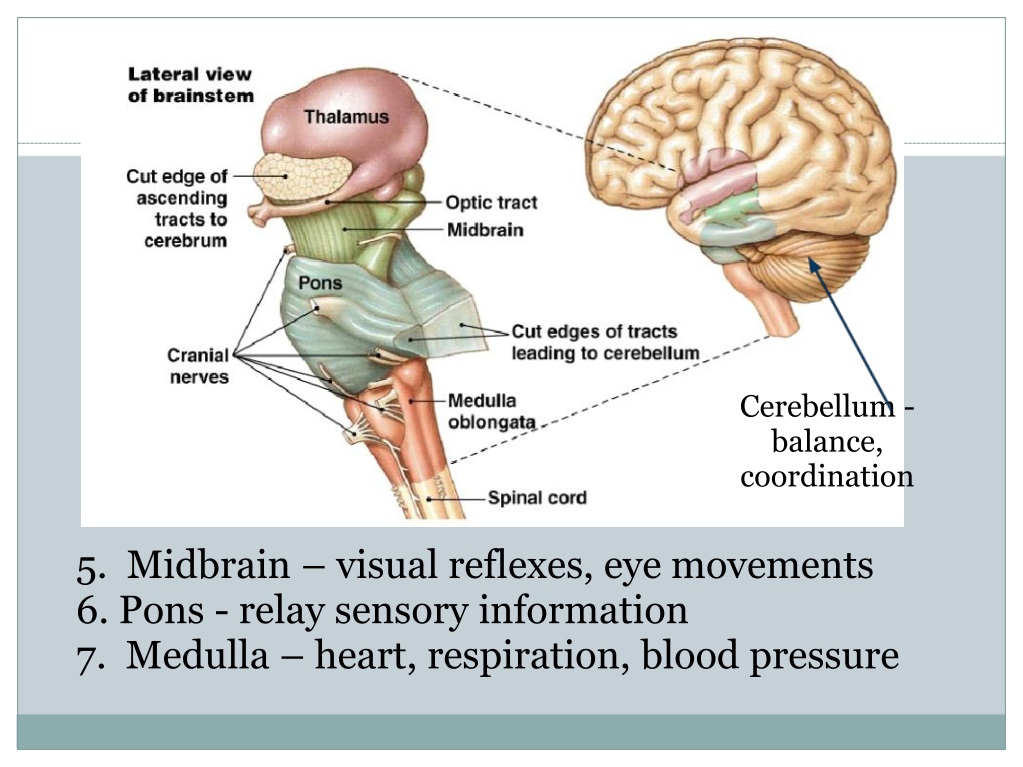 The Amira software was used to generate a triangular surface grid for the brain embedded in a voxel dataset. Then the surface area for each triangular grid was summed to obtain the surface area of the whole brain. A brain mask for the 3D volume was created by segmentation. All of the voxels in the mask were accumulated to obtain brain volume, and a linear interpolation method was used to get the voxels in the gap between 2 sections. The brain volume and surface area were just of the supratentorial brain, and the ventricles were included.
The Amira software was used to generate a triangular surface grid for the brain embedded in a voxel dataset. Then the surface area for each triangular grid was summed to obtain the surface area of the whole brain. A brain mask for the 3D volume was created by segmentation. All of the voxels in the mask were accumulated to obtain brain volume, and a linear interpolation method was used to get the voxels in the gap between 2 sections. The brain volume and surface area were just of the supratentorial brain, and the ventricles were included.
Brain length, width, and height were also calculated. Brain height was defined as the vertical distance between 2 horizontal planes, containing the most superior and inferior points of the brain, respectively. Brain length and width were defined in a similar fashion (Fig 1D).
Statistical Analysis
Our criterion to define a sulcus is the same as that used by Garel et al.6 A clear indentation at the surface of the brain was considered to be the earliest indication of a sulcus. One sulcus was considered to be present if it was observed in more than 75% of cases, detectable if observed in 25% to 75% of cases, and absent if observed in less than 25% of cases. A paired t test was used to detect significant differences in sex and hemispheres. All of the statistical work was done with SPSS version 17.0 (SPSS, Chicago, Illinois).
One sulcus was considered to be present if it was observed in more than 75% of cases, detectable if observed in 25% to 75% of cases, and absent if observed in less than 25% of cases. A paired t test was used to detect significant differences in sex and hemispheres. All of the statistical work was done with SPSS version 17.0 (SPSS, Chicago, Illinois).
Results
Of the 69 specimens, 31 were male and 38 were female. All were from the Chinese Han ethnic group with an average age of 18.348 ± 2.869 weeks GA. The GA span of male specimens was 13–22 weeks (mean ± SD, 18.129 ± 2.592 weeks GA), and the GA span of female specimens was 12–22 weeks (mean ± SD, 18.526 ± 3.100 weeks GA). There were no significant sex differences in GA (P = .571). There was no statistically significant interobserver variation (P = .810) at preliminary analysis.
Sulci
The chronology of sulcal development during 12–22 weeks GA is listed in Table 2. At 12 weeks GA, few sulci except the interhemispheric fissure and the lateral sulcus were present (Fig 2A, -E). By 16 weeks GA, many sulci were present, such as the central sulcus, the superior frontal sulcus, calcarine fissure, and parieto-occipital sulcus (Fig 2B, -F, and -J), and the 16th week GA could be considered to be the most intensive point of sulcal emergence. At 20 weeks GA, the present sulci became a little deeper and more obvious on the cortical surface (Fig 2C, -G, and -L). The collateral sulcus and the posterior part of the superior temporal sulcus emerged as well. Most sulci, except the postcentral sulcus and intraparietal sulcus, were not present until 22 weeks GA (Fig 2D, -H). At this point, all of the visible sulci were straight, immature, and had not developed the secondary branches (Fig 2). In addition, most appeared deeper and more distinguishable at the right hemisphere, especially the superior frontal sulcus and the central sulcus (Fig 2B, D).
By 16 weeks GA, many sulci were present, such as the central sulcus, the superior frontal sulcus, calcarine fissure, and parieto-occipital sulcus (Fig 2B, -F, and -J), and the 16th week GA could be considered to be the most intensive point of sulcal emergence. At 20 weeks GA, the present sulci became a little deeper and more obvious on the cortical surface (Fig 2C, -G, and -L). The collateral sulcus and the posterior part of the superior temporal sulcus emerged as well. Most sulci, except the postcentral sulcus and intraparietal sulcus, were not present until 22 weeks GA (Fig 2D, -H). At this point, all of the visible sulci were straight, immature, and had not developed the secondary branches (Fig 2). In addition, most appeared deeper and more distinguishable at the right hemisphere, especially the superior frontal sulcus and the central sulcus (Fig 2B, D).
Table 2:
Chronology of sulcal development during 12–22 weeks GAa
Fig 2.
Transverse T2-weighted 7T MR images of 12 (A, E), 16 (B, F), 20 (C, G), and 22 (D, H) weeks GA. Sagittal T2-weighted 7T MR images of 13 (I), 16 (J), 18 (K), and 20 (L) weeks GA. The lateral sulcus and the interhemispheric fissure can be distinguished at 12 weeks GA (A, E). At 16 weeks GA, more sulci can be observed (B, F), such as the central sulcus and the superior frontal sulcus. At 20–22 weeks GA, the sulci are more clearly delineated (E–H). On the sagittal images, development of the calcarine fissure, the parieto-occipital sulcus, the central sulcus, and the superior frontal sulcus can be clearly distinguished. The bar in each figure represents 1 cm; if, interhemisphaeric fissure; cas, callosal sulcus; cis, cingular sulcus; cf, calcarine fissure; pof, parieto-occipital sulcus; ots, occipitotemporal sulcus; las, lateral sulcus; sfs, superior frontal sulcus; its, inferior temporal sulcus; pcs, precentral sulcus; cns, central sulcus.
The lateral sulcus, which was the first to be distinguished, was the most obvious of all of the sulci. During 12–22 weeks GA, the lateral sulcus became deeper and wider, with the opercula insulae approaching each other. However, the opercula insulae did not fold until 22 weeks GA (Fig 2E–H).
The calcarine fissure and the parieto-occipital sulcus were the most prominent in the medial cerebral surface. The emergence time of the calcarine fissure was a little earlier than that of the parieto-occipital sulcus, and they gradually became separate by 20 weeks GA (Fig 2I–L).
The appearance of all of the other sulci, such as the central sulcus, the posterior part of the superior temporal sulcus, and the superior frontal sulcus, did not change much and remained shallow and tiny before 22 weeks GA (Fig 2).
3D Visualization Model of the Fetal Brain
The 3D visualization model demonstrates well the morphologic changes of the cortical surface (Fig 3). Most sulci, delineated as shallow fossae on the cerebral surface, could be observed (Fig 3), such as the lateral sulcus, central sulcus, and superior frontal sulcus. The length, depth, and location of the sulci could also be clearly demonstrated (Fig 3). The sulci were discontinuous and appeared as a cluster of separated shallow fossae, which merged together to form a single immature sulcus after 16 weeks GA (Fig 3B–D), such as the central sulcus and inferior temporal sulcus. The lateral sulcus was described as a wide fossa on the cortical surface (Fig 3). It became deeper as GA increased, and the operculum insulae did not fold until 22 weeks GA (Fig 3D).
Most sulci, delineated as shallow fossae on the cerebral surface, could be observed (Fig 3), such as the lateral sulcus, central sulcus, and superior frontal sulcus. The length, depth, and location of the sulci could also be clearly demonstrated (Fig 3). The sulci were discontinuous and appeared as a cluster of separated shallow fossae, which merged together to form a single immature sulcus after 16 weeks GA (Fig 3B–D), such as the central sulcus and inferior temporal sulcus. The lateral sulcus was described as a wide fossa on the cortical surface (Fig 3). It became deeper as GA increased, and the operculum insulae did not fold until 22 weeks GA (Fig 3D).
Fig 3.
3D visualization model of the telencephalon, cerebellum, and brain stem of 12 (A), 16 (B), 20 (C), and 22 (D) weeks GA. Sulci on the brain surface are visible. The tiny lateral sulcus can be observed at 12 weeks GA (A), then it becomes wider and deeper (B–D). A cluster of shallow “sulcal roots” is delineated at 16 weeks GA (B), then the roots merge together and become deeper (C–D). ifs indicates inferior frontal sulcus; psts, posterior part of the superior temporal sulcus.
A cluster of shallow “sulcal roots” is delineated at 16 weeks GA (B), then the roots merge together and become deeper (C–D). ifs indicates inferior frontal sulcus; psts, posterior part of the superior temporal sulcus.
Quantitative Assessment of the Fetal Brain
From the slope of the lines, it could be concluded that the total cerebrum was developing at a different speed. The brain surface area and volume increased the fastest (Fig 4), followed by its length (Fig. 5A). The height increased the slowest (Fig. 5C). There were no sexual dimorphisms or cerebral asymmetries in the measurements (P > .05).
Fig 4.
Quantitative measurements of fetal brain surface area and volume. The measurements increase linearly with GA. The surface area increases faster. Surface area is in square centimeters, and volume is in cubic centimeters. BA indicates brain surface area; BV, brain volume. The blue points represent the males, and the green represent the females.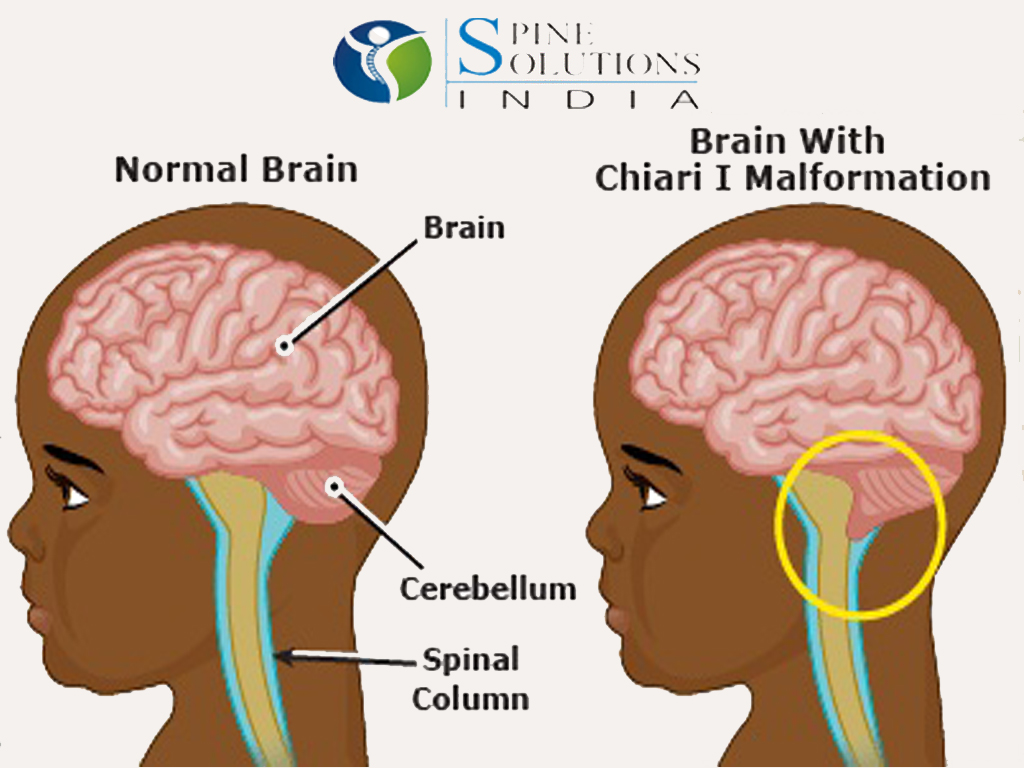
Fig 5.
Quantitative measurements of fetal brain length (A), width (B), and height (C). All of the measurements increase linearly with GA. Brain length increases the fastest, and height increases the slowest. Length, width, and height are in centimeters. BL indicates brain length; BW, brain width; BH, brain height.
Discussion
Sulcation in the Second Trimester
Development of the fetal cerebral cortex is defined by changes of the sulci, because they are indicators of brain maturation.5,6 Exploring the sequential development of sulci can greatly enrich our knowledge of prenatal radiology and will supply certain guidance for assessing normal cortical development in vivo.1⇓–3
Our results show that most of the sulci can be observed much earlier on 7T MR imaging than on in vivo fetal MR imaging. An example of this is the central sulcus, which is commonly observed on fetal MR imaging at 24–25 weeks GA,6 but can be clearly observed on 7T postmortem MR imaging at 16 weeks GA. Thus, we believe that MR imaging with higher magnetic strength can delineate small sulci earlier.
Thus, we believe that MR imaging with higher magnetic strength can delineate small sulci earlier.
There were inconsistencies between our results and those of previous research. For example, we found the central sulcus at 16 weeks GA, but Chi et al5 found it at 20 weeks GA on neuropathologic examinations. We found that the superior frontal sulcus and inferior temporal sulcus had already been present at 16 weeks GA in almost all of the specimens, but Chi et al5 described the neuropathologic appearance of the these 2 sulci at 25 weeks and 30 weeks GA, respectively. These inconsistencies need to be studied further. Genetic, environmental, nutritional, and other differences exist between our generation and past generations, as well as between white and Asian races,17 which may affect the developmental speed of the fetal brain. The emergence time of sulci, described in great detail in anatomic and embryologic books and in published studies, should be re-examined.
There are many possible reasons for the inconsistencies between our results and those described in past research,14 such as fluid loss of the fetal brain caused by fetal death or formalin fixation, method of describing the presence of a sulcus (being observed in more than 75% or 50% cases), method of examination (US or MR imaging of different magnetic strength), different races (Asian or Western), and other unknown reasons. It is thought that all of these variables cannot lead to such an appreciable difference between our chronology of sulcal development and that of Chi et al.5
In our study, the sulci are described as a cluster of separated shallow fossae at 16 weeks GA, termed “sulcal roots” in previous research.18 It can be concluded that sulcal development starts from several separated fossae on the cerebral surface, these fossae then join together, and a single complete sulcus is formed. The 16th week of GA may be considered as the original point for formation of the sulcal roots.
Quantitative Measurements of the Fetal Brain in the Second Trimester
Quantitative analysis in our study may provide valuable assistance in modeling normal fetal brain development. Currently, quantitative data on the fetal brain mainly come from research on neonates or premature infants,19,20 and very little is known about the range of normal values in the first and second trimesters. Therefore, our measurements, obtained from a large cohort, may be a valuable reference to determine whether the cortex is developing normally during the midtrimester. Although the fetuses in our study have undergone formalin fixation, which slightly reduces the volume and increases the width of the sulci, previous studies have confirmed the clinical application of these measurements.8,12⇓–14,21
Our measurements of the surface area and volume of the brain at 13–21 weeks GA were approximately 10 cm2 and 5 cm3 greater than those of Huang et al,22 obtained with 11. 7T and 4.7T postmortem MR imaging, respectively. However, our results might be more accurate because they were obtained with 7T MR imaging from a greater amount of specimens, and all of the specimens were scanned in situ, which may have avoided deformations caused by human factors or gravity when the brain is removed from the cranial cavity.
7T and 4.7T postmortem MR imaging, respectively. However, our results might be more accurate because they were obtained with 7T MR imaging from a greater amount of specimens, and all of the specimens were scanned in situ, which may have avoided deformations caused by human factors or gravity when the brain is removed from the cranial cavity.
Brain length in our research was roughly 0.5 cm smaller than the fronto-occipital diameter of 20–22 weeks GA, described by Parazzini et al,9 and was roughly 1 cm smaller than the fronto-occipital diameter of 17–22 weeks GA, described by Moreira et al.10 For brain width, our research demonstrates a width of approximately 0.5 cm greater than the cerebral biparietal diameter described by Parazzini et al9 and Moreira et al.10 These inconsistencies are mainly the result of different definitions of brain measurements found in our research and in theirs. Our measurements may be more convincing because they were obtained from 3D visualization models based on MR imaging of high magnetic strength instead of 2D in vivo fetal MR imaging.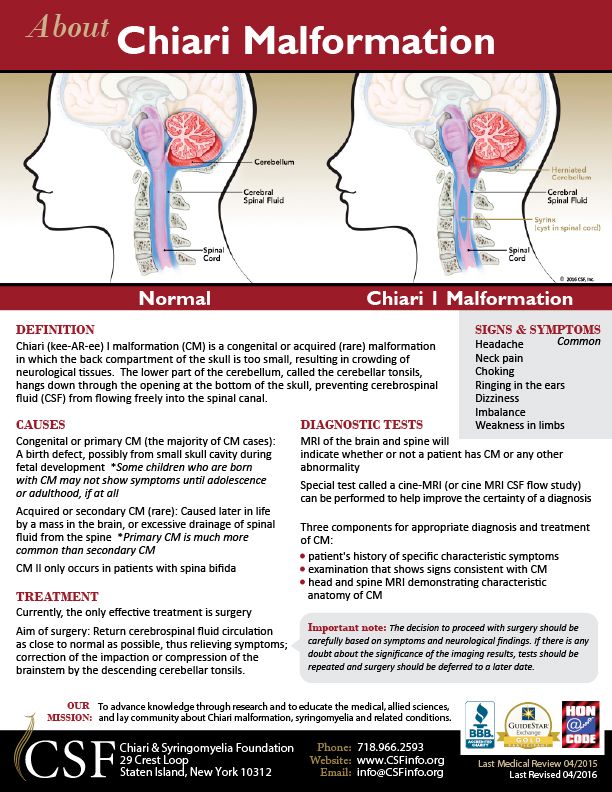
There have been 2 opposing views in US research about whether sexual dimorphisms and cerebral asymmetries are present in fetal brains in the second half of gestation,23⇓–25 but MR imaging studies have discovered these features in premature and neonatal brains.19,24 Our results, however, indicate that no significant sexual dimorphisms and cerebral asymmetries are present during the second trimester. Because the total number of subjects and their distribution in each GA group are relatively small, it can only be suspected that brain sexual differentiation starts in the third trimester, when the brain has finished the initial developmental phase and will step into an accelerated and complicated developmental period.
Superiority of 7T Postmortem MR Imaging in Demonstration of the Fetal Brain in the Second Trimester
The low-image contrast of in vivo fetal MR imaging precludes the application of postprocessing tools dedicated to the adult brain.11 However, postmortem MR imaging obtained with high magnetic strength is of high quality and is sufficient for segmentation, reconstruction, and quantitative analysis. The consistency in demonstrating the cortical surface between the gross anatomy and the 3D visualization model has been proved.14 Manual segmentation, which may be both tedious and time-consuming for large imaging, is much more precise than automatic segmentation, especially for the immature fetal brain. It is thought that these 3D visualization models, with dynamic demonstration, can exactly delineate developmental changes of the fetal brain during the second trimester, and supply quantitative data about the cortical surface area and volume that is hard to obtain by US, in vivo MR imaging, or histology studies. Our results may benefit the comprehension of immature structures in the second trimester.
The consistency in demonstrating the cortical surface between the gross anatomy and the 3D visualization model has been proved.14 Manual segmentation, which may be both tedious and time-consuming for large imaging, is much more precise than automatic segmentation, especially for the immature fetal brain. It is thought that these 3D visualization models, with dynamic demonstration, can exactly delineate developmental changes of the fetal brain during the second trimester, and supply quantitative data about the cortical surface area and volume that is hard to obtain by US, in vivo MR imaging, or histology studies. Our results may benefit the comprehension of immature structures in the second trimester.
Conclusions
The second trimester is the most important phase, during which most sulci are present and can be clearly shown on 7T postmortem MR imaging. Perhaps the specific time during which neuropathologic features of sulci emerge, previously thought to be well understood, should be redefined. Quantitative data assist greatly in the precise understanding of the immature brain. Measurements of fetal brains with different growth rates increase linearly with GA. Fetal brain sexual dimorphisms and asymmetries may arise and develop during the third trimester. Our results are valuable in anatomic education, research, and assessment of normal brain development in the uterus.
Acknowledgments
We thank Hamed Haghnazar, Sam Hobel, and Xuntao Yin for linguistic advice during the revision.
Footnotes
This work was supported by the National Natural Science Foundation of China (No. 31071050; No. 81001223), Natural Science Foundation and Doctoral Foundation of Shandong Province (No. ZR2009CM091; No. BS2010YY048), and Promotive Research Fund for Excellent Young and Middle-Aged Scientists of Shandong Province (No. BS2010YY042).
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- 1.↵
- Encha-Razavi F,
- Sonigo P
.
 Features of the developing brain. Childs Nerv Syst 2003;19:426–28
Features of the developing brain. Childs Nerv Syst 2003;19:426–28 - 2.↵
- Prayer D,
- Kasprian G,
- Krampl E,
- et al
. MRI of normal fetal brain development. Eur J Radiol 2006;57:199–216
- 3.↵
- Glenn OA
. MR imaging of the fetal brain. Pediatr Radiol 2010;40:68–81
- 4.↵
- Fogliarini C,
- Chaumoitre K,
- Chapon F,
- et al
. Assessment of cortical maturation with prenatal MRI: part II: abnormalities of cortical maturation. Eur Radiol 2005;15:1781–89
- 5.↵
- Chi JG,
- Dooling EC,
- Gilles FH
. Gyral development of the human brain. Ann Neurol 1977;1:86–93
- 6.
 ↵
↵ - Garel C,
- Chantrel E,
- Brisse H,
- et al
. Fetal cerebral cortex: normal gestational landmarks identified using prenatal MR imaging. AJNR Am J Neuroradiol 2001;22:184–89
- 7.↵
- Monteagudo A,
- Timor-Tritsch IE
. Development of fetal gyri, sulci and fissures: a transvaginal sonography study. Ultrasound Obstet Gynecol 1997;9:222–28
- 8.↵
- Hansen PE,
- Ballesteros MC,
- Soila K,
- et al
. MR imaging of the developing human brain. Part 1. Prenatal development. Radiographics 1993;13:21–36
- 9.↵
- Parazzini C,
- Righini A,
- Rustico M,
- et al
. Prenatal magnetic resonance imaging: brain normal linear biometric values below 24 gestational weeks.
 Neuroradiology 2008;50:877–83
Neuroradiology 2008;50:877–83 - 10.↵
- Moreira NC,
- Teixeira J,
- Themudo R,
- et al
. Measurements of the normal fetal brain at gestation weeks 17 to 23: a MRI study. Neuroradiology 2011;53:43–48
- 11.↵
- Habas PA,
- Kim K,
- Rousseau F,
- et al
. Atlas-based segmentation of developing tissues in the human brain with quantitative validation in young fetuses. Hum Brain Mapp 2010;31:1348–58
- 12.↵
- Zhang Z,
- Liu S,
- Lin X,
- et al
. Development of fetal cerebral cortex: assessment of the folding conditions with post-mortem magnetic resonance imaging. Int J Dev Neurosci 2010;28:537–43
- 13.↵
- Zhang Z,
- Liu S,
- Lin X,
- et al
.
 Development of laminar organization of the fetal cerebrum at 3.0T and 7.0T: a postmortem MRI study. Neuroradiology 2011;53:177–84
Development of laminar organization of the fetal cerebrum at 3.0T and 7.0T: a postmortem MRI study. Neuroradiology 2011;53:177–84 - 14.↵
- Zhang Z,
- Liu S,
- Lin X,
- et al
. Development of fetal brain of 20 weeks gestational age: Assessment with post-mortem magnetic resonance imaging. Eur J Radiol 2011;80:e432–39
- 15.↵
- Meng H,
- Zhang Z,
- Geng H,
- et al
. Development of the subcortical brain structures in the second trimester: assessment with 7.0-T MRI. Neuroradiology 2012;54:1153–59
- 16.↵
- Bayer SA,
- Altman J
. The human brain during the second trimester. In: Atlas of Human Central Nervous System Development, Indianapolis: CRC Press; 2005
- 17.↵
- Tang Y,
- Hojatkashani C,
- Dinov ID,
- et al
.
The construction of a Chinese MRI brain atlas: a morphometric comparison study between Chinese and Caucasian cohorts. Neuroimage 2010;51:33–41
- 18.↵
- Régis J,
- Mangin JF,
- Ochiai T,
- et al
. “Sulcal root” generic model: a hypothesis to overcome the variability of the human cortex folding patterns. Neurol Med Chir (Tokyo) 2005;45:1–17
- 19.↵
- Dubois J,
- Benders M,
- Borradori-Tolsa C,
- et al
. Primary cortical folding in the human newborn: an early marker of later functional development. Brain 2008;131:2028–41
- 20.↵
- Dubois J,
- Benders M,
- Cachia A,
- et al
. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 2008;18:1444–54
- 21.
↵
- Torkildsen A
. The gross anatomy of the lateral ventricles. J Anat 1934;68:480–91
- 22.↵
- Huang H,
- Xue R,
- Zhang J,
- et al
. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 2009;29:4263–73
- 23.↵
- Tilea B,
- Alberti C,
- Adamsbaum C,
- et al
. Cerebral biometry in fetal magnetic resonance imaging: new reference data. Ultrasound Obstet Gynecol 2009;33:173–81
- 24.↵
- Kivilevitch Z,
- Achiron R,
- Zalel Y
. Fetal brain asymmetry: in utero sonographic study of normal fetuses. Am Obstet Gynecol 2010;202:359.e1–8
- 25.
 ↵
↵ - Gilmore JH,
- Lin W,
- Prastawa MW,
- et al
. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci 2007;27:1255–60
- Received August 19, 2012.
- Accepted after revision October 2, 2012.
View Abstract
PreviousNext
Back to top
Congenital malformations
What is hydrocephalus? An imbalance between the formation, circulation and resorption of cerebrospinal fluid (CSF) can lead to the development of a pathological condition - hydrocephalus.
The term hydrocephalus from the Greek. Hydro - water and cephalon - the brain, literally translated as "dropsy of the brain."
Depending on the time of development of this condition, congenital and acquired hydrocephalus are distinguished. Hydrocephalus that occurs during fetal development is called congenital, fetal or fetal hydrocephalus. On average, congenital hydrocephalus is diagnosed in 0.5-3 cases per 1000 newborn babies.
Hydrocephalus that occurs during fetal development is called congenital, fetal or fetal hydrocephalus. On average, congenital hydrocephalus is diagnosed in 0.5-3 cases per 1000 newborn babies.
Why is hydrocephalus dangerous? Excessive accumulation of cerebrospinal fluid leads to expansion of the cerebrospinal fluid spaces of the brain, compression and dislocation of the brain tissue, which as a result leads to disruption of the processes of metabolism and trophism of brain structures. These changes in general have an extremely adverse effect on the development of the fetus, and, subsequently, on the condition of the child.
Causes of congenital hydrocephalus. All the variety of causes of congenital hydrocephalus can be divided into three groups:
1. Malformations of the central nervous system and liquorodynamic pathways
2. Intrauterine infections
3. Chromosomal abnormalities
Causes of congenital hydrocephalus:
- growth or intraventricular hemorrhage.
- X-linked hydrocephalus with stenosis of the aqueduct of Sylvius (genetically determined X-linked disease). Quite a rare disease. However, among newborns with hydrocephalus occurs in 5% of cases.
- Other chromosomal abnormalities: trisomy 18, 21 pairs of chromosomes, etc.
- Malformations of Chiari I, II and III types. This group includes malformations of brain structures, in which there is an incorrect position of the cerebellum and a violation of the structure of the brain stem.
- Myelomeningocele (neural tube defect). A defect in the spinal canal through which the membranes of the brain, the unformed spinal cord itself, and the roots of the spinal cord exit. Another name for this condition is spinal hernia.
- Tumors of the posterior cranial fossa and tumors of the ventricles of the brain.
- Arachnoid cyst.
- Dandy-Walker Syndrome. Refers to malformations of the posterior cranial fossa. It can develop both against the background of genetic diseases, and under the influence of toxic and infectious factors.
 The syndrome includes hydrocephalus, a cyst of the posterior cranial fossa, a violation of the structure and development of the cerebellum.
The syndrome includes hydrocephalus, a cyst of the posterior cranial fossa, a violation of the structure and development of the cerebellum. - Intrauterine infections: cytomegalovirus infection, rubella, parainfluenza, chicken pox. As a result of a viral infection, arachnoiditis (inflammation of the arachnoid membrane of the brain) is formed with impaired liquorodynamics.
Diagnosis of congenital hydrocephalus . The expansion of the ventricles of the brain (as one of the signs of hydrocephalus) can be detected during a fetal ultrasound (ultrasound). The diagnosis of hydrocephalus can be suspected at the end of the first trimester (at week 13). However, it is possible to speak more clearly about the expansion of the ventricles of the brain at 20-24 weeks of intrauterine development.
Further tactics of doctors. Enlargement of the cerebral ventricles in the fetus is a signal for a specialist to conduct a more detailed study: search for ultrasound signs of combined anomalies in the development of the central nervous system and other possible causes of hydrocephalus. In some cases, additional invasive studies are carried out, such as amniocentesis (puncture of the membranes), with the collection of material to determine the level of alpha-fetoprotein and study the chromosome set of the fetus. The condition of a pregnant woman and a fetus with hydrocephalus is monitored by a team of specialists: an obstetrician-gynecologist, a geneticist and a pediatric neurosurgeon.
In some cases, additional invasive studies are carried out, such as amniocentesis (puncture of the membranes), with the collection of material to determine the level of alpha-fetoprotein and study the chromosome set of the fetus. The condition of a pregnant woman and a fetus with hydrocephalus is monitored by a team of specialists: an obstetrician-gynecologist, a geneticist and a pediatric neurosurgeon.
Treatment options. Treating a fetus and child with hydrocephalus is always associated with certain risks and difficulties. In making a decision and choosing tactics, the underlying disease or syndrome, against which the formation of hydrocephalus occurred, plays an important role. Thus, gross malformations of the brain may be an indication for termination of pregnancy.
All methods of treatment can be divided into conservative and surgical. Depending on the cause of congenital hydrocephalus, its severity and the condition of the child, treatment can begin with conservative methods: the appointment of diuretics and drugs that reduce the production of cerebrospinal fluid, drugs that improve brain metabolism, antihypoxants, etc. One of the surgical treatments for hydrocephalus is bypass surgery. The essence of the method is the imposition of a shunt (a system of tubes and valves), through which "excessive" cerebrospinal fluid is discharged into the natural cavities of the body: into the abdominal cavity, the cavity of the right atrium, etc. The timing of such operations is determined by the neurosurgeon based on the condition of the child and the cause of hydrocephalus. According to experts, the earlier such treatment is carried out, the better the results of treatment. In most cases, the shunt is installed for life, does not solve the problem of the underlying disease, but allows the brain structures to develop.
One of the surgical treatments for hydrocephalus is bypass surgery. The essence of the method is the imposition of a shunt (a system of tubes and valves), through which "excessive" cerebrospinal fluid is discharged into the natural cavities of the body: into the abdominal cavity, the cavity of the right atrium, etc. The timing of such operations is determined by the neurosurgeon based on the condition of the child and the cause of hydrocephalus. According to experts, the earlier such treatment is carried out, the better the results of treatment. In most cases, the shunt is installed for life, does not solve the problem of the underlying disease, but allows the brain structures to develop.
FIND OUT THE COST OF TREATMENT!
Operations performed in utero. Fetal bypass is currently under investigation. However, these operations are still experimental. Potential complications of intrauterine intervention in most cases outweigh the possible benefits of such treatment.
Medical tactics. Since the first detection of cerebral ventricular expansion in the fetus, specialists have been conducting dynamic monitoring of the state of the fetal brain structures. If hydrocephalus does not progress and the fetus develops normally, specialists wait until the baby is born and, if necessary, perform bypass surgery. With progressive ventriculomegaly (expansion of the ventricles of the brain), delivery is performed by caesarean section at a gestational age of 35 weeks and postnatally (after birth) bypass surgery is performed.
Question-answer
- At 30 weeks of gestation, ultrasound revealed cerebral ventricular enlargement and fetal hydrocephalus. What should we do?
- Mom needs to be registered with a perinatal geneticist for further research (search for the cause of hydrocephalus, possible genetic and chromosomal diseases). A detailed ultrasound examination should be carried out by an ultrasound diagnostician with a specialization in perinatal genetics. You may need repeated ultrasound in dynamics. Mom can contact the leading clinics in Germany and Israel for a more accurate examination and determine further treatment tactics. Doctors with vast experience in working with children suffering from hydrocephalus will diagnose as soon as possible, as safely as possible for the child and mother.
You may need repeated ultrasound in dynamics. Mom can contact the leading clinics in Germany and Israel for a more accurate examination and determine further treatment tactics. Doctors with vast experience in working with children suffering from hydrocephalus will diagnose as soon as possible, as safely as possible for the child and mother.
- At 29 weeks, the brain ventricles were enlarged and a preliminary conclusion was made - fetal hydrocephalus. We are currently undergoing further examination aimed at finding the cause of this condition. What threatens our child with hydrocephalus?
- It is difficult to answer the question unequivocally, since there are no complete data. The very state of hydrocephalus leads to compression of brain structures and disruption of metabolic processes in it. Children with hydrocephalus may have certain neurological symptoms (hypertonicity, regurgitation, restlessness), in severe cases, seizures may occur in children. Such babies learn motor and mental skills worse, and there may be an intellectual deficit. In order to help the child as much as possible and neutralize the possible consequences of hydrocephalus, it is recommended that the mother be registered with a perinatal geneticist and consult with a pediatric neurosurgeon. Diagnosis and treatment of congenital hydrocephalus is carried out by qualified specialists from leading clinics in Germany and Israel. Parents can receive a remote consultation service (according to the results of ultrasound and tests), as well as a “second opinion” of a specialist.
Such babies learn motor and mental skills worse, and there may be an intellectual deficit. In order to help the child as much as possible and neutralize the possible consequences of hydrocephalus, it is recommended that the mother be registered with a perinatal geneticist and consult with a pediatric neurosurgeon. Diagnosis and treatment of congenital hydrocephalus is carried out by qualified specialists from leading clinics in Germany and Israel. Parents can receive a remote consultation service (according to the results of ultrasound and tests), as well as a “second opinion” of a specialist.
Questions about fetometry: page 4
Do fetal measurements correspond to gestational age? I was recently at an obstetric ultrasound, they issued a printout, I can’t understand what it all means: BPR, LZR and OG? Doctors of medical clinics "Art-Med" answer the questions of patients about the results of ultrasound of the fetus.
22.6 weeks of pregnancy, IVF, obstetric term 23. 1. The size of the cerebellum is 24 mm (22.1 weeks of pregnancy). According to ultrasound: BPR 55, OG 208, OJ 179, LZR 74, DBC 41, DKG 39, weight 557, lateral ventricles 4 mm each, large cistern 6 mm. Ultrasound at 18.1 weeks of gestation: cerebellum 18 mm (17.6 weeks of gestation), lateral ventricles 4 mm each, cisterna magna 6 mm. Is it possible to talk about a lag in the development of the brain?
1. The size of the cerebellum is 24 mm (22.1 weeks of pregnancy). According to ultrasound: BPR 55, OG 208, OJ 179, LZR 74, DBC 41, DKG 39, weight 557, lateral ventricles 4 mm each, large cistern 6 mm. Ultrasound at 18.1 weeks of gestation: cerebellum 18 mm (17.6 weeks of gestation), lateral ventricles 4 mm each, cisterna magna 6 mm. Is it possible to talk about a lag in the development of the brain?
There is no cause for concern at this stage. The state of the fetal brain should be observed in dynamics. Repeat ultrasound in 2-3 weeks.
20 weeks of pregnancy, according to ultrasound: BDP 48.3; PDM 20.8; coefficient 2.3221. Is there any reason for concern?
No cause for concern at this stage. But to clarify the situation, repeat the ultrasound in 2-4 weeks.
24 weeks and 4 days pregnant. Ultrasound results: BPR - 63 mm, LZR - 81 mm, OG - 231 mm, coolant - 190 mm, DB - 43 mm, DP - 37 mm. I am worried about DB and DP, is it below normal? What about head size? At 20 weeks it was normal.
The fetus is of normal size. Look at the baby's relatives - for sure there are people among them with not too long legs.
32 weeks of pregnancy, according to the results of obstetric ultrasound: BDP 7.8; LZR 9.9; femur length 5.8; leg bone length 5.1; humerus length 5; forearm 4.6; OG 281; coolant 262; weight 1700. Conclusion: the size of the fetus corresponds to 30 weeks 4 days. Is this normal or is there a developmental delay? Do I need to do another ultrasound?
Judging by the given data, there is intrauterine growth retardation. It is necessary to do ultrasound in dynamics, according to the results, treatment can be prescribed.
37 weeks pregnant. Last period August 9, BPD 96, HC 335, AC 334, FL 73, fetal weight 3300, placenta 38, amniotic index 27. What is the gestational age for the size of the fetus? On ultrasound they said that the head is large. How true is this? How much should be normal?
The size of the fetus corresponds to the gestational age. The baby's head is just round. Look at his immediate family, most likely the family has this feature.
The baby's head is just round. Look at his immediate family, most likely the family has this feature.
23 weeks of pregnancy according to the last menstruation, according to ultrasound - 21 weeks. BPR 51, LZR 70, OG 191, OB 184, DB 44, CG 39, duodenum 40, CP 37, weight 496, cerebellum 20, lateral ventricles of the brain 5, lateral cistern 4, umbilical cord has 3 vessels. Are there any developmental delays?
Judging by what you wrote, there is intrauterine growth retardation of the fetus. It is necessary to do an ultrasound in dynamics and see how the fetus develops.
Pregnancy 32 weeks and 3 days, fetometry results: BDP - 79 mm, head circumference - 288 mm, fronto-occipital head size - 102 mm, transverse diameter of the cerebellum - 39 mm, abdominal circumference - 271 mm, femur length - 60 mm, the length of the bones of the lower leg is 51 mm, the length of the humerus is 53 mm, the length of the bones of the forearm is 50 mm. Estimated fetal weight: 1707. Diagnosis: tendency to fetal malnutrition. Are the sizes correct?
Estimated fetal weight: 1707. Diagnosis: tendency to fetal malnutrition. Are the sizes correct?
No cause for concern at this stage. But it is worth repeating the ultrasound of the fetus in 2 weeks. If the baby grows up, then there is no question of any lag in growth.
31 - 32 weeks pregnant. BPR - 80 mm, LZR - 105 mm, cerebellum - 30 mm. Conclusion - no fetal malformations were found, but I read that at this time the normal size of the cerebellum is 35 - 41 mm. Why is it dangerous for the fetus?
The rates in different tables may differ. If in doubt, sign up for an expert ultrasound.
Pregnancy 21.4 weeks, diagnosis - cerebellar hypoplasia. According to the results of fetal ultrasound: BDP - 45.7 mm, OH - 176.4 mm, OB - 167.0 mm, DB - 35.0 mm, cerebellar size - 20.3 mm. Does the size of the cerebellum correspond to the gestational age?
The dimensions of the cerebellum fit within the normative indicators, there is a slight lag in the size of the fetus from the specified gestational age.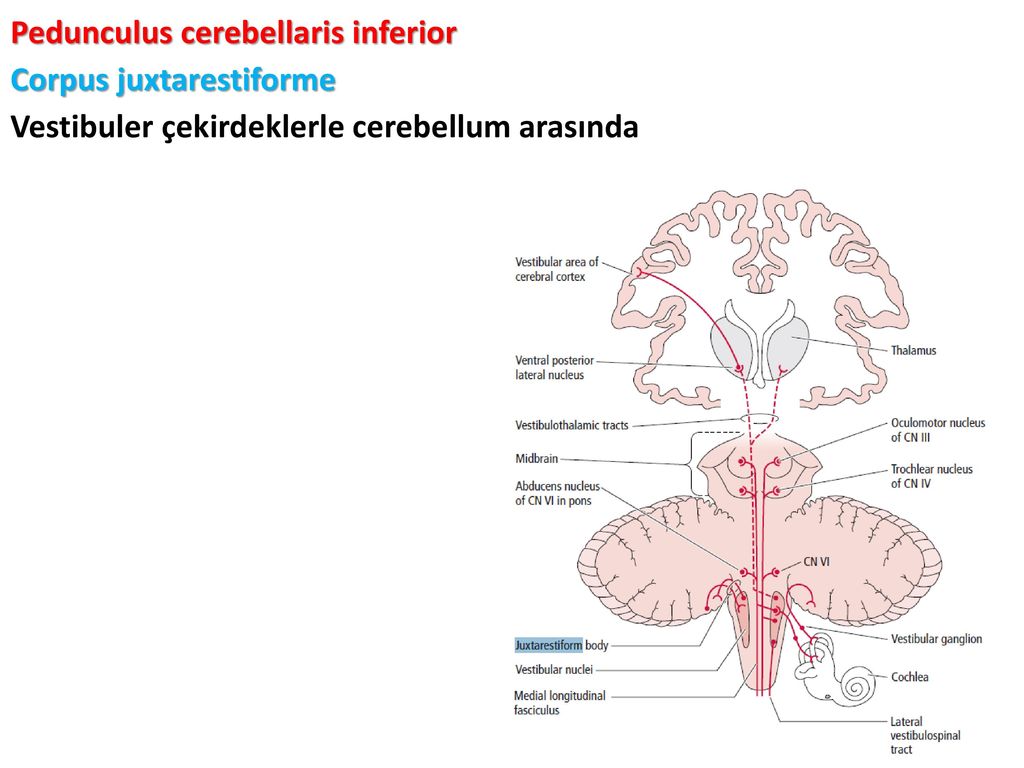 We recommend a retest after 3 weeks.
We recommend a retest after 3 weeks.
Pregnancy 28 weeks 3 days, results of fetal ultrasound: BPR - 66 mm, LZR - 99 mm, exhaust gas - 261 mm, coolant - 233 mm, DB - 51 mm. Fruit weight: 1092 gr. + 159 gr. No developmental defects were found. Placenta - 28 mm, degree of maturity - 0, IAI - 14, heart rate - 142 beats / min. Doppler is normal. Ultrasound at 20 weeks showed no abnormalities. Is there a fetal growth retardation? Does BPD indicate some pathology of the brain in the fetus?
When assessing the size of the head, first of all, the head circumference (CG) is taken into account. In your situation, it corresponds to the term of 28 weeks of pregnancy. To assess the rate of fetal growth, it is necessary to take into account data on the last menstruation and data from previous ultrasounds.
Pregnancy 29.4 weeks. Fetometry: bpr 78 mm, lzr 99 mm, og 280 mm, og 263 mm, db 55 mm. Anatomy: cerebellum 34 mm, lateral ventricles of the brain 9.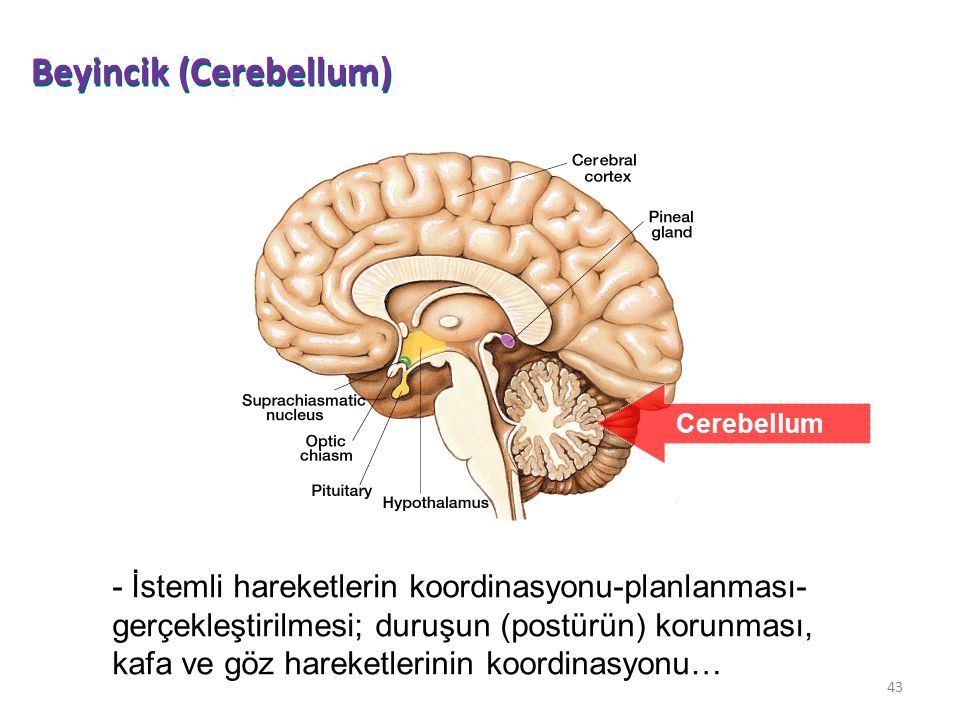 9 mm, p.p.p. 6.8 mm, Sylvian furrow 12 mm, cistern major 9 mm. Kidneys: pelvis on the right up to 3.7 mm, on the left - 3.3 mm. The rest of the parameters are normal. Are these normal readings? Particularly interested in the structure of the brain.
9 mm, p.p.p. 6.8 mm, Sylvian furrow 12 mm, cistern major 9 mm. Kidneys: pelvis on the right up to 3.7 mm, on the left - 3.3 mm. The rest of the parameters are normal. Are these normal readings? Particularly interested in the structure of the brain.
Size according to gestational age. The dimensions of the lateral ventricles of the brain attract attention: 9.9 mm is the upper limit of the norm. In this situation, repeat the obstetric ultrasound in 3-4 weeks to clarify the tactics of pregnancy management.
Pregnancy 25-26 weeks, according to fetal ultrasound: BDP 62.9; DB 50.1; OG 243.3; coolant 220.7. Are these data up to date?
Yes, the fetus can have such dimensions at 25-26 weeks of gestation, there is a tendency to develop a large fetus.
20 weeks 4 days pregnant. According to ultrasound: BPR 49 mm, LZR 64 mm, head circumference 179 mm, abdominal circumference 149 mm, length of the femur left 28 mm, right 28 mm, length of the left leg bones 26 mm, right 26 mm, length of the left humerus 27 mm, right 27 mm, length of the forearm bones of the left 23, right 23 mm, fetal weight 303 g.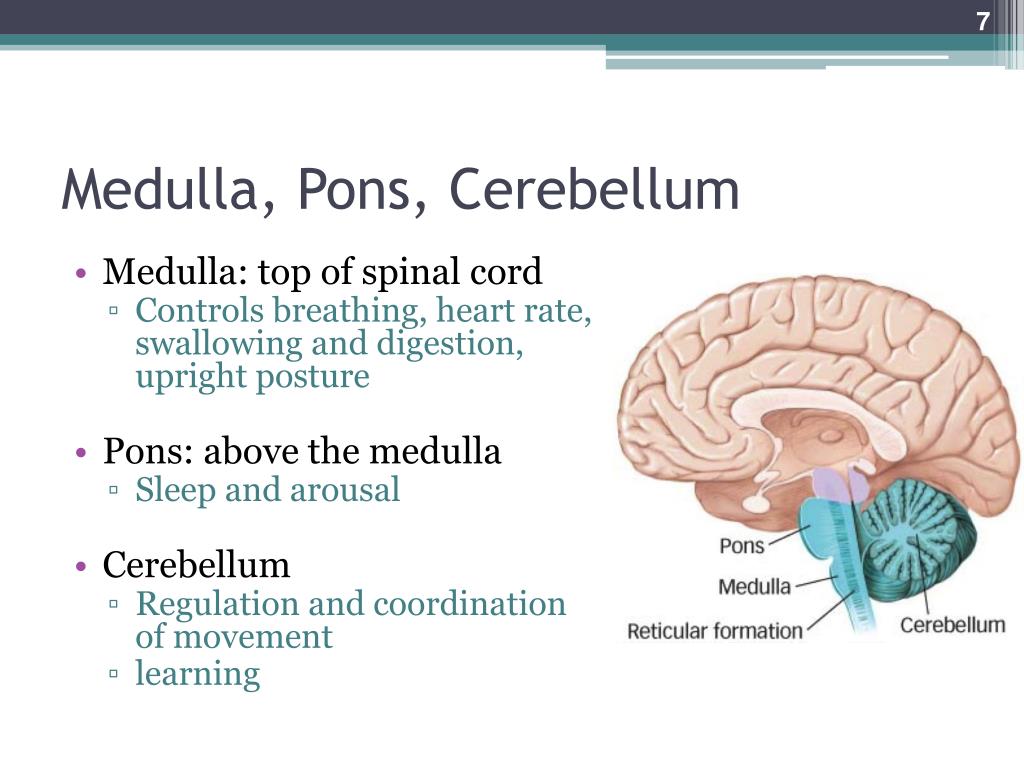 Fruit sizes correspond to 19weeks 4 days. Conclusion: relative shortening of tubular bones, cardiomegaly. What might this lead to in the future?
Fruit sizes correspond to 19weeks 4 days. Conclusion: relative shortening of tubular bones, cardiomegaly. What might this lead to in the future?
In your case, attention is drawn to the shortening of the tubular bones, which increases the likelihood of fetal chromosomal pathology. Invasive diagnostics is carried out to identify the chromosomal pathology of the fetus.
Results of obstetric ultrasound (term 32 weeks 3 days): BPD of the head - 82.4 (corresponding to 33.1 weeks) OGK - 296.5 (corresponding to 32.6 weeks) OB - 275.8 (corresponding to 31.5 weeks), DBD - 62.9(corresponding to 32.4 weeks), the gestational age according to ultrasound is 32.4 weeks. The doctor said that all indicators are normal. Only here the circumference of the abdomen is slightly out of date, but the discrepancy is only 1 week. Prescribed chimes 1 tablet 3 times a day. Repeat ultrasound after 3 weeks. Doppler parameters are within the normal range. Why does this discrepancy depend, how dangerous is it and what to do?
The situation is not dangerous, this may be a variant of the norm. It is necessary to follow the doctor's prescription, followed by ultrasound control.
It is necessary to follow the doctor's prescription, followed by ultrasound control.
On ultrasound (pregnancy - 11 days 5 days) - KTR - 53 mm, BDP - 15 mm. The term turns out to be almost 12 weeks, and the BDP is small. According to the tables, it should be approximately 17mm - 18mm. Does this indicate microcephaly or other abnormalities?
It is premature to talk about microcephaly at this time. We need dynamic monitoring.
What is the term for ultrasound: obstetric or fetal? In consultation put 24 weeks, and ultrasound - 26 weeks.
In the conclusion of the ultrasound at this time, the obstetric gestational age is indicated. In this situation, it is worth recalculating the gestational age, focusing on all known data. Read more about this in the Medical Publications section.
Gestational age - 33.2 weeks. Ultrasound result on December 10, 2013: BPR - 91 mm, LZR - 106 mm, lower leg - 54 mm, thigh - 65 mm, shoulder - 58 mm, forearm - 52 mm, OG - 322 mm, OB - 304 mm.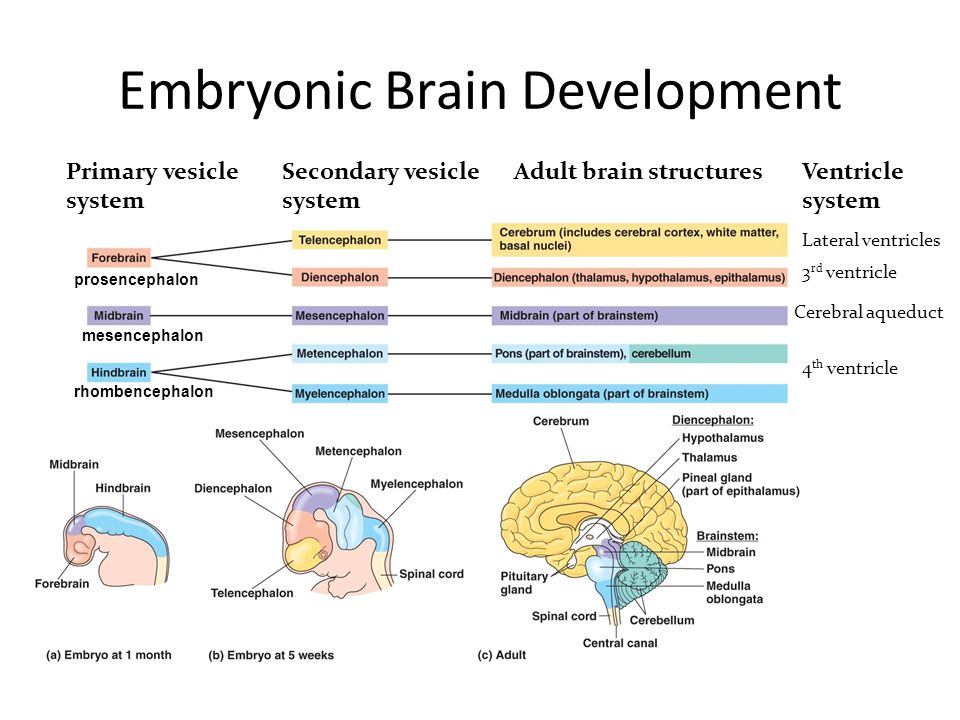 Such a big difference between the terms of development of the head and lower leg is frightening - 5 weeks. On previous ultrasounds everything was normal. Does this signal intrauterine growth retardation?
Such a big difference between the terms of development of the head and lower leg is frightening - 5 weeks. On previous ultrasounds everything was normal. Does this signal intrauterine growth retardation?
This indicates more likely an anomaly in the development of the fetus, first of all, skeletal dysplasia, if the ultrasound is done correctly. In any case, you need to wait for the birth, and then show the child's genetics.
Pregnancy 32 weeks, indicators of the third U3I: BR - 80, LZR - 99, OG - 291, OB - 277, thigh length - 59, lower leg - 55, shoulder - 54, forearm - 50, WEIGHT - 1900 gr. Weight corresponds to 31-32 weeks, they said our baby will be small. Is it true?
What does "small" mean? Everything in the world is relative. You will have a good baby. But to make the answer more specific, repeat the ultrasound after 3-4 weeks. It is important to measure correctly.
Ultrasound at 31 weeks and 5 days, BDP - 82, OG - 298, LZR - 106, OB - 273, BC - 62, lower leg - 51, humerus - 55, forearm - 48. Doppler - umbilical artery - 0.55, uterine arteries: left - 0.43, right - 0.44 . Fruit weight - 1900 gr. Is the fetus appropriate for the gestational age?
Doppler - umbilical artery - 0.55, uterine arteries: left - 0.43, right - 0.44 . Fruit weight - 1900 gr. Is the fetus appropriate for the gestational age?
The length of the bones of the lower leg, femur and humerus corresponds to a period of 28-29 weeks + - 2 weeks, at the moment there is no developmental delay. It is necessary to repeat the ultrasound in 2-3 weeks to see the dynamics of growth.
CTE of the fetus at 12 weeks 41 mm - is this normal?
At 12 weeks of gestation, CTE is normally between 42 and 59 mm. If your last menstrual period is 12 weeks, then with late ovulation, the gestational age may differ, that is, be less. It is very important whether there were more ultrasounds and whether the CTE was measured correctly (during the measurement, the fetus should be in a neutral position). You can come to us for an ultrasound and we will determine the timing.
The last menstruation was on August 7, 2013, ovulation on August 23, on September 30, the CTE of the fetus was 7. 7 mm. Is there a lag in the development of the fetus?
7 mm. Is there a lag in the development of the fetus?
No developmental delay. If there is any doubt, repeat the ultrasound after 2-3 weeks.
Is there a suspicion of chromosomal pathology or developmental delay if the BDP is below normal and the cephalic index is 69%, and the rest of the parameters are normal and in the conclusion the ultrasound doctor writes that no congenital malformations were found. Ultrasound at 21-22 weeks: BDP of the fetus - 46 mm, fronto-occipital size - 66 mm, lateral ventricles of the brain - 6 mm, cerebellum - 22 mm, a large cistern is not expanded, nasolabial triangle, nasal bone and orbits without features, head circumference - 188 mm, abdominal circumference - 166 mm, femur length - 38 mm, leg bones length - 34 mm, humerus length - 34 mm, forearm bones length - 30 mm, estimated fetal weight - 458 g, spine, intestines, lungs without features, the stomach, kidneys are visualized, the heart: a four-chamber section, a section through 3 vessels, sections through the main vessels were obtained, heart rate 147 bpm. Ultrasound indicators at 12-13 weeks are normal, a low-risk group for chromosomal pathologies.
Ultrasound indicators at 12-13 weeks are normal, a low-risk group for chromosomal pathologies.
If the biparietal size is below normal, this may indicate an emerging microcephaly in the fetus. But not always - it can be a variant of the norm. Dynamic monitoring is required.
I am 35 weeks and 5 days pregnant, according to ultrasound the length of the femur is 62 mm. Is there a backlog?
Thigh length 62 mm corresponds to 33 weeks of pregnancy. I would recommend repeating the measurement of the fetal bones. If the lag is confirmed, it is worth going to an appointment with a geneticist.
At the 20th week of pregnancy I had an ultrasound scan. BPR heads - 96, OK heads - 171, these sizes correspond to the period of 19.6 weeks of pregnancy?
The circumference of the fetal head corresponds to the gestational age. BPR is incorrect, should be about 46 mm.
Pregnancy 34 weeks.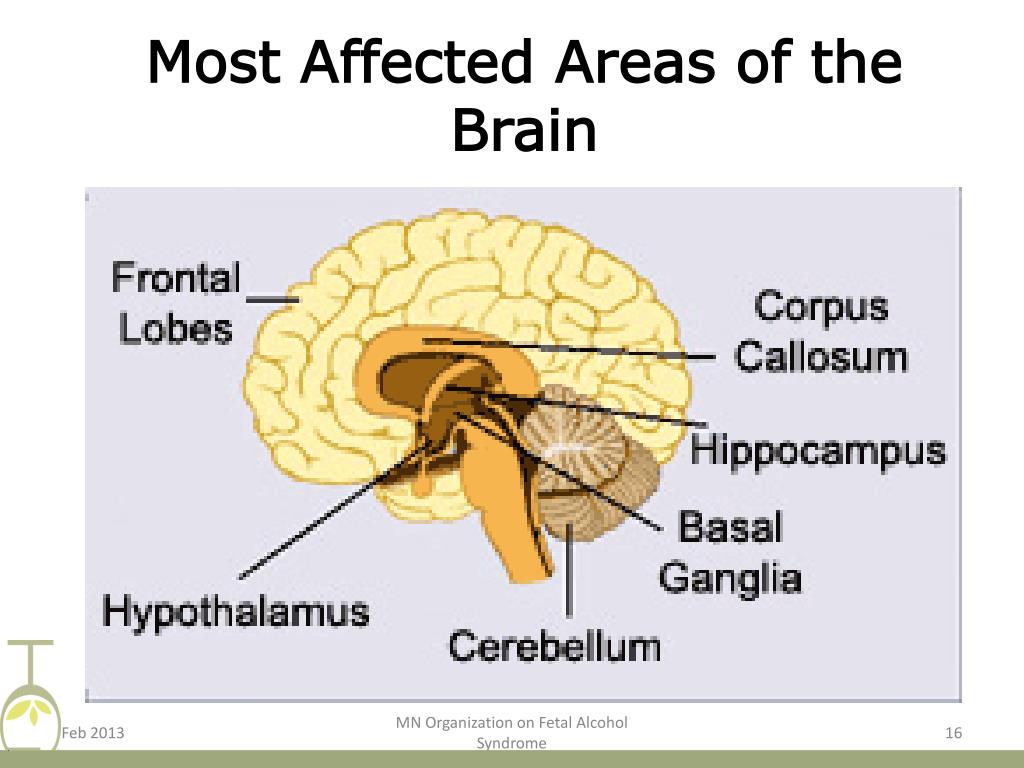 The last ultrasound was at 32.4: BPD 93mm (development at 38w2d). HC 319mm (development on 35w3d). AC 303mm (on 34w3d). FL 65mm (for 33w2d), weight 2460 g. Now I'm in Spain, I can't understand the details of what the doctor says. She warned that we will have a large child, since I am 169 talland husband 189. But I was scared by the size of the head. She said everything is fine, but her head circumference is now at 38 weeks. He says there is nothing to be afraid of, just a large fruit. How can an advance of almost 6 weeks be normal? Another ultrasound done one month early at 28 weeks and 4 days: BPD 79mm (development at 31w 6d). HC 279mm (30w 2d). AC 251mm (for 29w 2d). FL 54mm (for 28w1d), weight 1380 g.
The last ultrasound was at 32.4: BPD 93mm (development at 38w2d). HC 319mm (development on 35w3d). AC 303mm (on 34w3d). FL 65mm (for 33w2d), weight 2460 g. Now I'm in Spain, I can't understand the details of what the doctor says. She warned that we will have a large child, since I am 169 talland husband 189. But I was scared by the size of the head. She said everything is fine, but her head circumference is now at 38 weeks. He says there is nothing to be afraid of, just a large fruit. How can an advance of almost 6 weeks be normal? Another ultrasound done one month early at 28 weeks and 4 days: BPD 79mm (development at 31w 6d). HC 279mm (30w 2d). AC 251mm (for 29w 2d). FL 54mm (for 28w1d), weight 1380 g.
Repeat OB in a couple of weeks. If there are no anomalies and malformations of the fetal brain, take a closer look at the baby's relatives: maybe there are large-headed ones among them?
At 29-30 weeks of pregnancy, an ultrasound scan was done - the dimensions of the fetus: og-268, BPR-77, LZR-92, OD-216, DB-48. Two weeks later, at a period of 32 weeks of pregnancy: OG-256, BPR-73, LZR-89, OZH-233, DB-53. How is it possible that the size of the head has decreased in 2 weeks. Could this be the case, or is it a hardware error?
Two weeks later, at a period of 32 weeks of pregnancy: OG-256, BPR-73, LZR-89, OZH-233, DB-53. How is it possible that the size of the head has decreased in 2 weeks. Could this be the case, or is it a hardware error?
Bone dimensions could not decrease. It is recommended to repeat the study. First of all, in this situation it is necessary to clarify the presence of placental insufficiency and intrauterine growth retardation of the fetus.
Fourth pregnancy, second birth, first caesarean section, first two miscarriages at 5 weeks. According to ultrasound at 32 weeks, the size of the fetus corresponds to 30-31 weeks, she underwent treatment. After 2 weeks, repeated ultrasound - BDP - 82, LZR - 105, OG - 302, coolant - 292, hips - 63, lower leg - 55, shoulder - 57, forearm - 49. After 2 weeks, I also did an ultrasound of BDP - 85.7, LZR - 110, OG - 316, OB - 306, hips - 65, lower leg - 60, shoulder - 59, forearm - 52, cerebellum - MRM - 43. At the same time, doppler is normal, CTG is normal, movements are normal. I've been to several doctors, they just shrug. What could it be?
I've been to several doctors, they just shrug. What could it be?
This may be a feature of your baby. Take a closer look at his relatives - maybe there are medium-sized ones with a small head among them? If not, it is worth showing the child's genetics after childbirth.
Pregnancy 28.6 weeks. She did an ultrasound scan: BPR 70, LZR 91, OG 261, OG 228, DBC 56, DG 46, DPC 49, DPR 46, cerebellum 34, placenta on the back wall 8.1 cm above the internal os, placenta thickness up to 2.8 cm , structure without features, degree of maturity 1, amniotic fluid index 12.7 cm, umbilical cord has 3 vessels, cervix 3.6, internal os is closed. Doppler: umbilical artery IR 0.70, uterine artery: right IR 0.45; on the left IR 0.47. Correspond to the values of the term 29weeks? And is there a problem with blood flow?
The child begins to lag behind in growth. Apparently, we are talking about placental insufficiency.
I am expecting a second child (the first girl was born large: 3870 g, 56 cm, with my height 158). Doctors say that the second children during full-term pregnancy in most cases are larger than the first. At 32-33 weeks, she did an ultrasound scan: BPRG 88, LZRG 111, DlBK 60, DlKG 62, DlPK 62, OG 310, OZH 88/270, weight 2200. Do the dimensions of the fetus correspond to the gestational age? When compared with the ultrasound data of the first daughter (conducted exactly at the same time), the child already seems larger.
Doctors say that the second children during full-term pregnancy in most cases are larger than the first. At 32-33 weeks, she did an ultrasound scan: BPRG 88, LZRG 111, DlBK 60, DlKG 62, DlPK 62, OG 310, OZH 88/270, weight 2200. Do the dimensions of the fetus correspond to the gestational age? When compared with the ultrasound data of the first daughter (conducted exactly at the same time), the child already seems larger.
Your baby's bone size is ahead of the average.
I am 32 years old, 31-32 weeks pregnant. The results of fetometry: Biparietal head size 78 mm, Fronto-occipital size 100 mm, Head circumference 286 mm, Abdominal circumference 272 mm, Length of the femur and left and right 58 mm, length of the bones of the leg and left and right 51 mm, length of the humerus and left and right 52 mm, the length of the bones of the forearm and left and right 48 mm. Fruit weight 1674 gr. Heart rate 143 beats / min. The ultrasound doctor said that the length of the femur, the bones of the lower leg, the humerus and the bones of the forearm do not correspond to the gestational age.