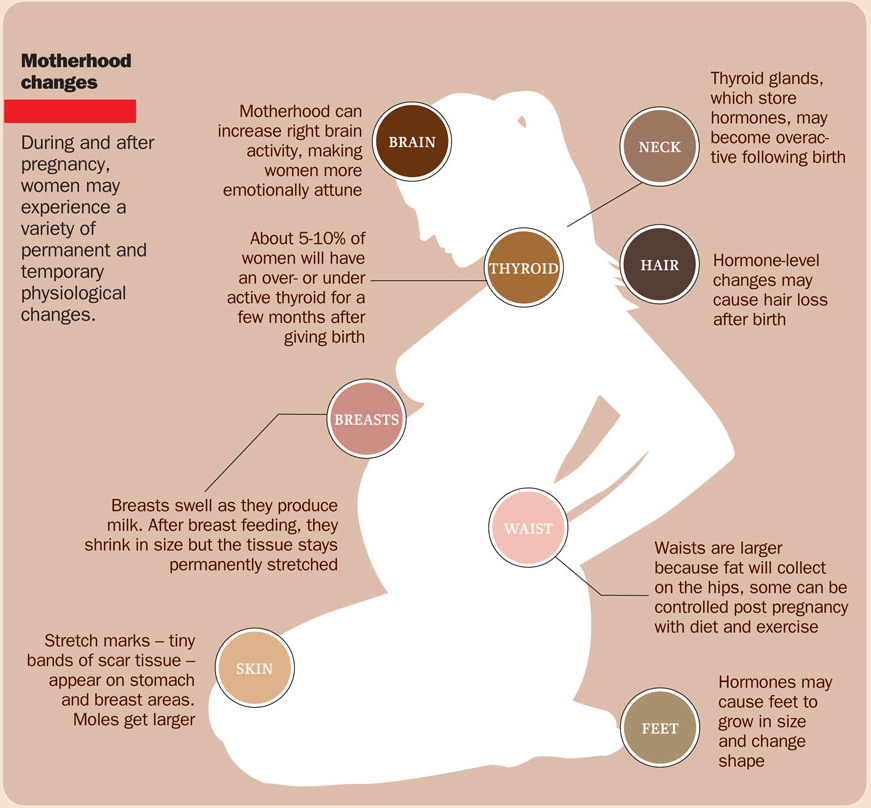
Thyroid levels high during pregnancy
Hypothyroidism in Pregnancy | American Thyroid Association
Leer en Español
WHAT ARE THE NORMAL CHANGES IN THYROID FUNCTION ASSOCIATED WITH PREGNANCY?
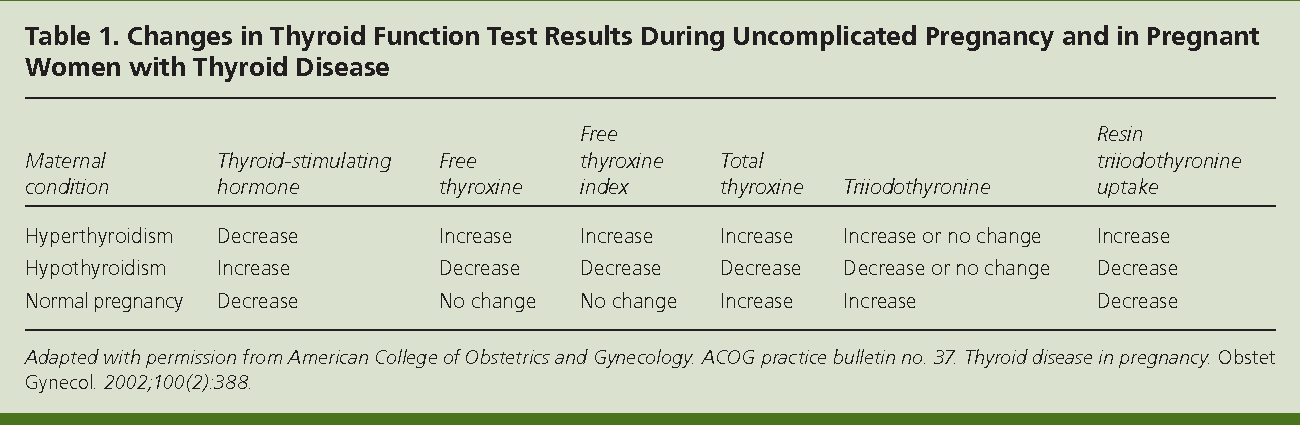
HORMONE CHANGES. Thyroid function tests change during normal pregnancy due to the influence of two main hormones: human chorionic gonadotropin (hCG) and estrogen. Because hCG can weakly stimulate the thyroid, the high circulating hCG levels in the first trimester may result in a low TSH that returns to normal throughout the duration of pregnancy. Estrogen increases the amount of thyroid hormone binding proteins, and this increases the total thyroid hormone levels but the “Free” hormone (the amount that is not bound and can be active for use) usually remains normal. The thyroid is functioning normally if the TSH and Free T4 remain in the trimester-specific normal ranges throughout pregnancy.
THYROID SIZE CHANGES. The thyroid gland can increase in size during pregnancy (enlarged thyroid = goiter). However, pregnancy-associated goiters occur much more frequently in iodine-deficient areas of the world. It is relatively uncommon in the United States. If very sensitive imaging techniques (ultrasound) are used, it is possible to detect an increase in thyroid volume in some women. This is usually only a 10-15% increase in size and is not typically apparent on physical examination by the physician. However, sometimes a significant goiter may develop and prompt the doctor to measure tests of thyroid function (see Thyroid Function Test Brochure).
WHAT IS THE THYROID GLAND?
The thyroid gland is a butterfly-shaped endocrine gland that is normally located in the lower front of the neck. The thyroid’s job is to make thyroid hormones, which are secreted into the blood and then carried to every tissue in the body. Thyroid hormones help the body use energy, stay warm and keep the brain, heart, muscles, and other organs working as they should.
WHAT IS THE INTERACTION BETWEEN THE THYROID FUNCTION OF THE MOTHER AND THE BABY?
For the first 18-20 weeks of pregnancy, the baby is completely dependent on the mother for the production of thyroid hormone. By mid-pregnancy, the baby’s thyroid begins to produce thyroid hormone on its own. The baby, however, remains dependent on the mother for ingestion of adequate amounts of iodine, which is essential to make the thyroid hormones. The World Health Organization recommends iodine intake of 250 micrograms/day during pregnancy to maintain adequate thyroid hormone production. Because iodine intakes in pregnancy are currently low in the United States, the ATA recommends that US women who are planning to become pregnant, who are pregnant, or breastfeeding, should take a daily supplement containing 150 mcg of iodine.
By mid-pregnancy, the baby’s thyroid begins to produce thyroid hormone on its own. The baby, however, remains dependent on the mother for ingestion of adequate amounts of iodine, which is essential to make the thyroid hormones. The World Health Organization recommends iodine intake of 250 micrograms/day during pregnancy to maintain adequate thyroid hormone production. Because iodine intakes in pregnancy are currently low in the United States, the ATA recommends that US women who are planning to become pregnant, who are pregnant, or breastfeeding, should take a daily supplement containing 150 mcg of iodine.
HYPOTHYROIDISM & PREGNANCY
WHAT ARE THE MOST COMMON CAUSES OF HYPOTHYROIDISM DURING PREGNANCY?
WHAT ARE THE MOST COMMON CAUSES OF HYPOTHYROIDISM DURING PREGNANCY?
Overall, the most common cause of hypothyroidism is the autoimmune disorder known as Hashimoto’s thyroiditis (see Hypothyroidism brochure). Hypothyroidism can occur during pregnancy due to the initial presentation of Hashimoto’s thyroiditis, inadequate treatment of a woman already known to have hypothyroidism from a variety of causes, or over-treatment of a hyperthyroid woman with anti-thyroid medications.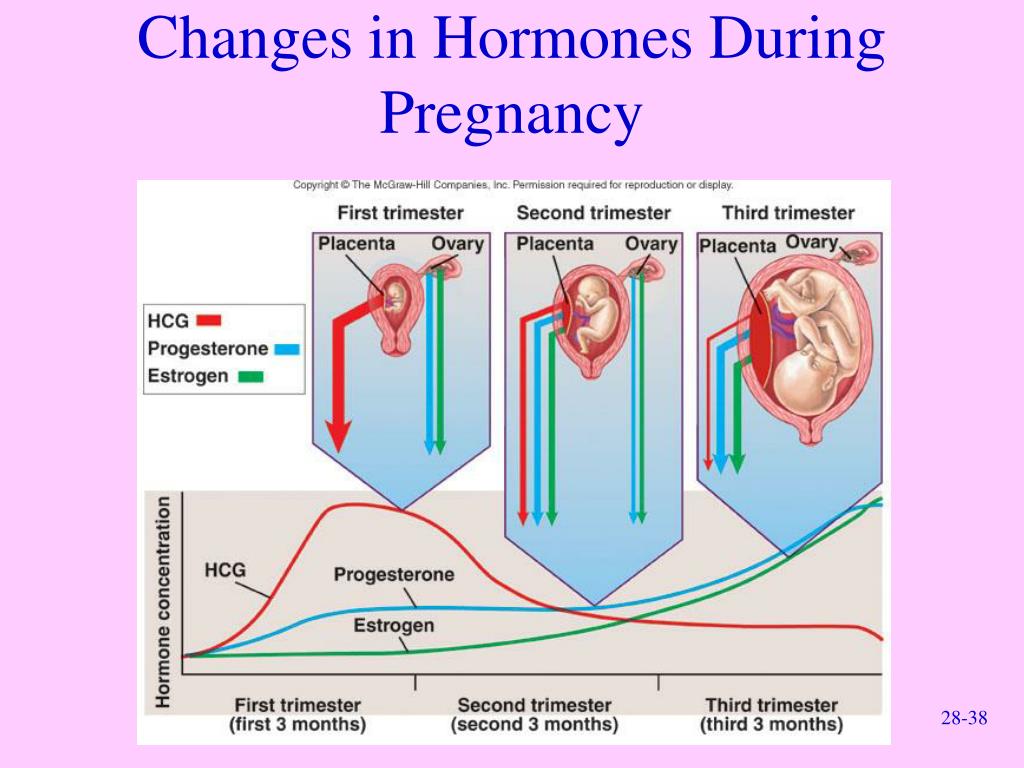 Approximately, 2.5% of women will have a TSH of greater than 6 mIU/L (slightly elevated) and 0.4% will have a TSH greater than 10 mIU/L during pregnancy.
Approximately, 2.5% of women will have a TSH of greater than 6 mIU/L (slightly elevated) and 0.4% will have a TSH greater than 10 mIU/L during pregnancy.
WHAT ARE THE RISKS OF HYPOTHYROIDISM TO THE MOTHER?
Untreated, or inadequately treated, hypothyroidism has increased risk of miscarriage, and has been associated with maternal anemia, myopathy (muscle pain, weakness), congestive heart failure, pre-eclampsia, placental abnormalities, and postpartum hemorrhage (bleeding). These complications are more likely to occur in women with severe hypothyroidism. Some risks also appear to be higher in women with antibodies against thyroid peroxidase (TPO). Women with mild hypothyroidism may have no symptoms or attribute symptoms they have to the pregnancy.
WHAT ARE THE RISKS OF MATERNAL HYPOTHYROIDISM TO THE BABY?
Thyroid hormone is critical for brain development in the baby. Children born with congenital hypothyroidism (no thyroid function at birth) can have severe cognitive, neurological and developmental abnormalities if the condition is not recognized and treated promptly. With early treatment, these developmental abnormalities largely can be prevented. Consequently, all newborn babies in the United States are screened for congenital hypothyroidism so they can be treated with thyroid hormone replacement therapy as soon as possible.
With early treatment, these developmental abnormalities largely can be prevented. Consequently, all newborn babies in the United States are screened for congenital hypothyroidism so they can be treated with thyroid hormone replacement therapy as soon as possible.
Untreated severe hypothyroidism in the mother can lead to impaired brain development in the baby. Recent studies have suggested that mild developmental brain abnormalities also may be present in children born to women who had mild untreated hypothyroidism during pregnancy. At this time, there is no general consensus of opinion regarding screening all women for hypothyroidism during pregnancy. However, the ATA recommends checking a woman’s TSH as soon as pregnancy is confirmed in women at high risk for thyroid disease, such as those with prior treatment for hyper- or hypothyroidism, a family history of thyroid disease, a personal history of autoimmune disease, and those with a goiter.
Women with established hypothyroidism should have a TSH test as soon as pregnancy is confirmed. They also should immediately increase their levothyroxine dose, because thyroid hormone requirements increase during pregnancy. (See below for specific dosing recommendations.) If new onset hypothyroidism has been detected, the woman should be treated with levothyroxine to normalize her TSH values (see Hypothyroidism brochure).
They also should immediately increase their levothyroxine dose, because thyroid hormone requirements increase during pregnancy. (See below for specific dosing recommendations.) If new onset hypothyroidism has been detected, the woman should be treated with levothyroxine to normalize her TSH values (see Hypothyroidism brochure).
WHO SHOULD BE TREATED FOR HYPOTHYROIDISM DURING PREGNANCY?
Women found to have a TSH level greater than 10 mIU/L in the first trimester of pregnancy should be treated for hypothyroidism. Conversely, women with a TSH of 2.5 or less, do not need levothyroxine treatment. For women with TSH measured between these (2.5-10), ATA recommendations for treatment vary and may depend on whether or not the mother has TPO antibodies. When TPO antibodies are positive, treatment is recommended when the TSH is above 4 and should be considered when the TSH is between 2.5-4.0. However, when there are no TPO antibodies (i.e. negative), current ATA recommendations are less strong and suggest that treatment ‘may be considered’ when TSH is between 2.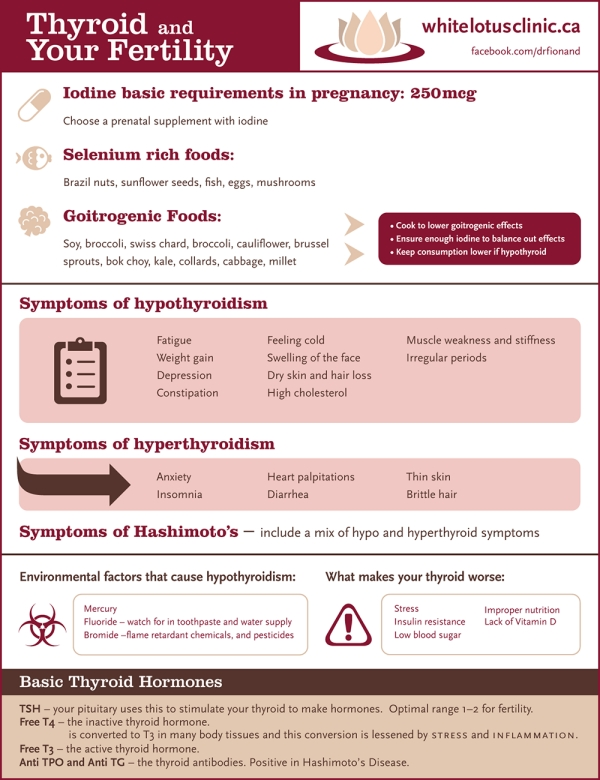 5-10.0 mIU/L. These recommendations are based on the degree of evidence that exists that treatment with levothyroxine would be beneficial.
5-10.0 mIU/L. These recommendations are based on the degree of evidence that exists that treatment with levothyroxine would be beneficial.
HOW SHOULD A WOMAN WITH HYPOTHYROIDISM BE TREATED DURING PREGNANCY?
The goal of treating hypothyroidism in a pregnant woman is adequate replacement of thyroid hormone. Ideally, hypothyroid women should have their levothyroxine dose optimized prior to becoming pregnant. Levothyroxine requirements frequently increase during pregnancy, usually by 25 to 50 percent. Hypothyroid women taking levothyroxine should independently increase their dose by 20%–30% as soon as pregnancy is diagnosed and should notify their doctor for prompt testing and further evaluation. One means of accomplishing the dose increase is to take two additional tablets weekly of their usual daily levothyroxine dosage. Thyroid function tests should be checked approximately every 4 weeks during the first half of pregnancy to ensure that the woman has normal thyroid function throughout pregnancy. As soon as delivery of the child occurs, the woman may go back to her usual prepregnancy dose of levothyroxine. It is also important to recognize that prenatal vitamins contain iron and calcium that can impair the absorption of thyroid hormone from the gastrointestinal tract. Consequently, levothyroxine and prenatal vitamins should not be taken at the same time and should be separated by at least 4 hours.
As soon as delivery of the child occurs, the woman may go back to her usual prepregnancy dose of levothyroxine. It is also important to recognize that prenatal vitamins contain iron and calcium that can impair the absorption of thyroid hormone from the gastrointestinal tract. Consequently, levothyroxine and prenatal vitamins should not be taken at the same time and should be separated by at least 4 hours.
SPECIAL CONSIDERATIONS FOR WOMEN WITH A HISTORY OF GRAVES' DISEASE
In addition to the dosing and testing considerations explained in this brochure, women with a history of Graves’ disease who were treated with radioiodine (RAI) or surgical thyroidectomy should also have Graves’ antibodies (TRAb) tested early in pregnancy to assess the risk of passing antibodies on to the fetus. If antibodies are elevated, follow-up testing is recommended at weeks 18- 22, and if antibodies are still elevated, additional follow-up is recommended at weeks 30-34 to evaluate the need for fetal and neonatal monitoring.
Printable Brochures
Hypothyroidism in Pregnancy Brochure PDF
Hypothyroidism in Pregnancy FAQ PDF
Hipotiroidismo Durante el Embarazo
Articles
Vibhavasu Sharma, MD, FACE Albany Medical College, Albany, NY August 13, 2020 Thyroid disease…
Read More
From Clinical Thyroidology® for the Public: GUEST BLOG FROM THE IODINE GLOBAL NETWORK Timing matters for…
Read More
October 2, 2018—The American Thyroid Association (ATA) will hold its 88th Annual Meeting on October…
Read More
More Articles on Hypothyroidism in Pregnancy
FURTHER INFORMATION
For information on thyroid patient support organizations, please visit the Patient Support Links section on the ATA website at www.thyroid.org
Hypothyroidism and Pregnancy | Johns Hopkins Medicine
Facts about hypothyroidism and pregnancy
Hypothyroidism is a condition marked by an underactive thyroid gland and may be present during pregnancy. Many symptoms of hypothyroidism are similar to pregnancy symptoms. For example, fatigue, weight gain, and abnormal menstruation are common to both. Having low thyroid hormone levels may even interfere with becoming pregnant or be a cause of miscarriage.
Many symptoms of hypothyroidism are similar to pregnancy symptoms. For example, fatigue, weight gain, and abnormal menstruation are common to both. Having low thyroid hormone levels may even interfere with becoming pregnant or be a cause of miscarriage.
What are the symptoms of hypothyroidism?
Hypothyroidism is a common condition. It can go undetected if symptoms are mild. Hypothyroidism means the thyroid is underactive and making insufficient amounts of thyroid hormones. Symptoms of hypothyroidism may be mild and may start slowly. The following are the most common symptoms of hypothyroidism:
-
Feeling tired
-
Unable to stand cold temperatures
-
Hoarse voice
-
Swelling of the face
-
Weight gain
-
Constipation
-
Skin and hair changes, including dry skin and loss of eyebrows
-
Carpal tunnel syndrome (hand tingling or pain)
-
Slow heart rate
-
Muscle cramps
-
Trouble concentrating
-
Irregular menstrual periods
The symptoms of hypothyroidism may resemble other conditions or medical problems.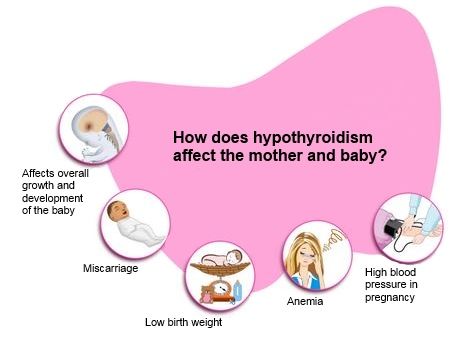 Always talk with your healthcare provider for a diagnosis.
Always talk with your healthcare provider for a diagnosis.
How does hypothyroidism affect the fetus?
During the first few months of pregnancy, the fetus relies on the mother for thyroid hormones. Thyroid hormones are important in normal brain development and growth of the fetus. Hypothyroidism in the mother can have long-lasting effects on the fetus.
How is thyroid function tested?
You will have blood test that measures thyroid hormone (thyroxine, or T4) and serum TSH (thyroid-stimulating hormone) levels to check for hypothyroidism. Hypothyroidism is often suspected when TSH levels are above normal and T4 levels are below normal.
Who should undergo thyroid function screening?
Routine screening for hypothyroidism during pregnancy is not recommended. A pregnant woman with symptoms of hypothyroidism, a history of hypothyroidism, or with other endocrine system conditions should be screened.
How is hypothyroidism treated during pregnancy?
Thyroid hormone replacement is used to treat the mother. Dosage of thyroid hormone replacement therapy is based on the individual's levels of thyroid hormones. Thyroid hormone levels may change during pregnancy. And, the thyroid replacement dosing may also change. Thyroid hormone levels need to be checked every 4 weeks during the first half of pregnancy. The treatment is safe and essential to both mother and fetus. Routine screening for all newborns includes a test of thyroid hormone levels.
Dosage of thyroid hormone replacement therapy is based on the individual's levels of thyroid hormones. Thyroid hormone levels may change during pregnancy. And, the thyroid replacement dosing may also change. Thyroid hormone levels need to be checked every 4 weeks during the first half of pregnancy. The treatment is safe and essential to both mother and fetus. Routine screening for all newborns includes a test of thyroid hormone levels.
Seminar Webinar: Thyroid Disease, an Often Surprising Diagnosis
Join endocrinologist Paul Ladenson, M.D., as he outlines the signs and symptoms of the various thyroid disorders and discusses the interplay among other diseases and the thyroid. The webinar recording is presented as part of A Woman’s Journey Conversations That Matter webinar series.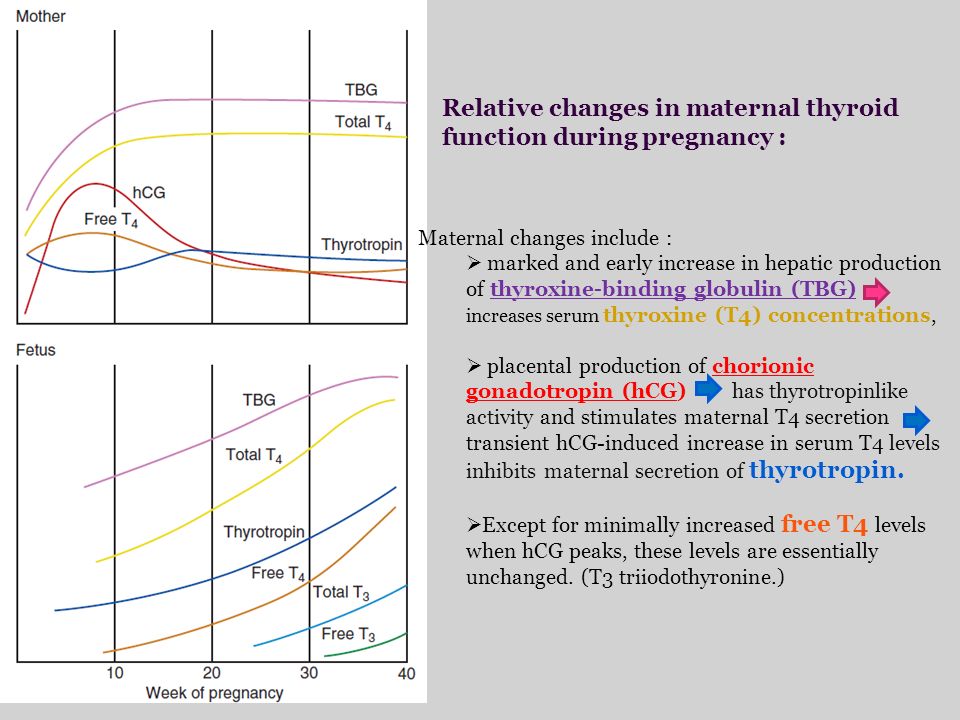
Watch Now
Thyroid gland - keep under control
Important hormones
The main task of the thyroid gland - is the production of hormones: thyroxine - T4 (tetraiodothyronine) and T3 (triiodothyronine). It is these hormones that throughout life support the work of the brain, heart, muscles, regulate the metabolism in the body. So our mental abilities, body weight, physical activity, sexual development, skeletal bone strength, skin and hair condition, sleep and appetite depend on the thyroid gland. The thyroid gland works correctly - a person is alert, active, calm, feels good and looks good. There are violations in its activity - the symptoms can be very different: for example, let's take body weight. With increased production of thyroid hormones, a person can eat as much as he wants, but will lose weight. And with a reduced function, you can eat nothing at all, but gain weight, because the so-called mucous edema develops in the body.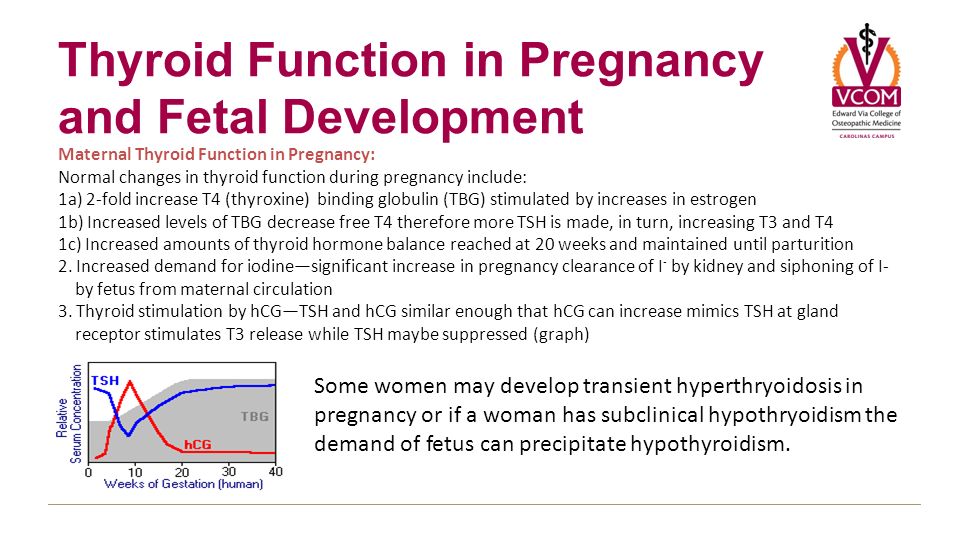 And the matter will not be at all in nutrition, but in the thyroid gland.
And the matter will not be at all in nutrition, but in the thyroid gland.
Essential iodine
Various factors affect how the thyroid gland functions: even stress and insomnia can disrupt the production of its hormones. But still, most of all, for normal functioning of the thyroid gland, iodine is needed, it is from it that thyroid hormones consist of 65%. Our body cannot produce this microelement on its own, we get it only from the outside - from food, water or medicines. And if there is little iodine in the diet, then, therefore, the thyroid gland will not be able to produce the required amount of hormones. In ordinary life, of course, this is also bad, but not yet so critical, but during pregnancy, iodine deficiency can cause real problems. After all, now this trace element is needed not only by a woman, but also by her child. Both the very bearing of the baby and his health will be under threat: after all, as mentioned above, the thyroid gland affects all organs and systems.
Development of the baby
Let's start with the fact that the baby is completely dependent on the mother's thyroid gland. In the unborn child, the thyroid gland, although it begins to form already at the 4–5th week of pregnancy, but it begins to function, that is, it begins to produce hormones only at 12 weeks, and it is capable of finally working at full strength by the 16–17th week. pregnancy. Until that time, the development of the child and the laying of all his organs and systems are "under the protection" of the mother's thyroid gland. And if a woman has little iodine, then this means that some system or organ of the baby may suffer. And even when the child’s own thyroid gland is formed and starts working, she can still take iodine only from the mother’s body.
Iodine deficiency most strongly affects intellectual development, even if a child is born physically healthy, his mental abilities may be lower than those of his peers.
In slow motion
Lack of iodine leads to the development of hypothyroidism – decrease in the production of thyroid hormones , which means that energy is produced less intensively and all processes in the body slow down. At first, the woman feels weak, she constantly wants to sleep. Hair fades, splits and falls out, nails exfoliate and break. There is excess weight, constipation, a feeling of chilliness. With developed hypothyroidism, the face becomes puffy, the eyelids swell, dry skin flakes and sometimes even turns slightly yellow. Due to swelling of the vocal cords, the voice may become lower. Many of these symptoms of hypothyroidism are similar to the usual “troubles” of pregnancy (especially weakness, drowsiness, excess weight, constipation), but if they are significantly pronounced, there is a reason for examining the thyroid gland. So if the expectant mother is tired, looks bad, she does not care what is happening around, do not blame everything on pregnancy or beriberi. Perhaps there are problems with the thyroid gland.
At first, the woman feels weak, she constantly wants to sleep. Hair fades, splits and falls out, nails exfoliate and break. There is excess weight, constipation, a feeling of chilliness. With developed hypothyroidism, the face becomes puffy, the eyelids swell, dry skin flakes and sometimes even turns slightly yellow. Due to swelling of the vocal cords, the voice may become lower. Many of these symptoms of hypothyroidism are similar to the usual “troubles” of pregnancy (especially weakness, drowsiness, excess weight, constipation), but if they are significantly pronounced, there is a reason for examining the thyroid gland. So if the expectant mother is tired, looks bad, she does not care what is happening around, do not blame everything on pregnancy or beriberi. Perhaps there are problems with the thyroid gland.
Change of mood
When pregnancy begins, when the thyroid gland starts working for two, it enlarges a little and produces a little more hormones. This is a common occurrence, and after pregnancy everything will return to normal.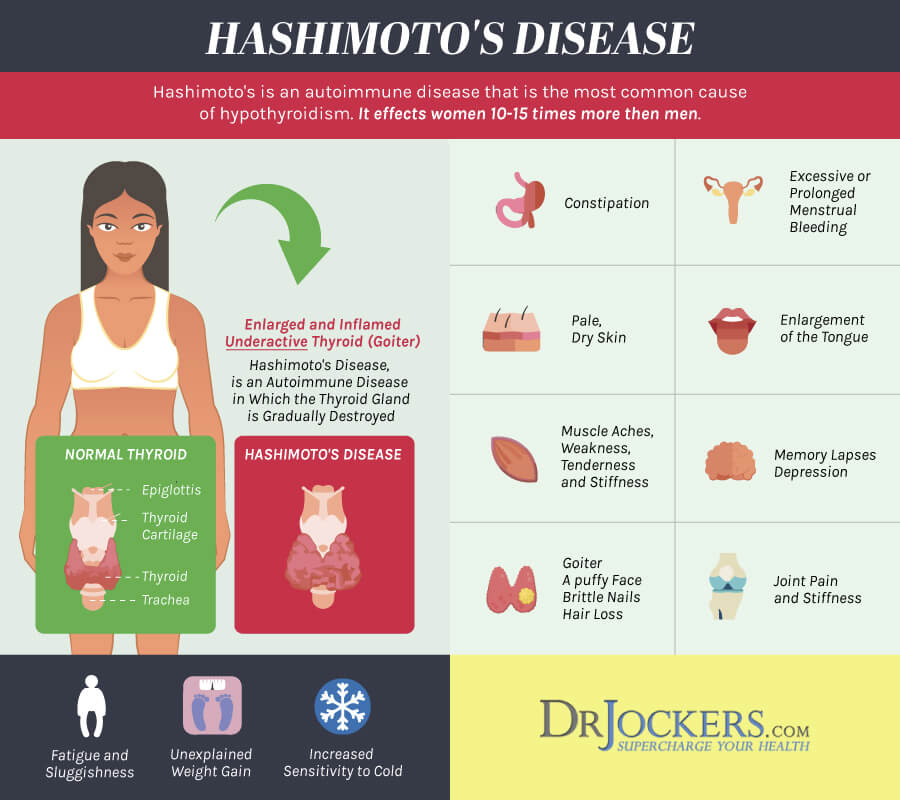 But there are women in whom, for some reason, the thyroid gland begins to produce too many hormones and without the influence of pregnancy, hyperthyroidism (or thyrotoxicosis) occurs. Then other symptoms appear - imbalance, tearfulness, agitation, increased appetite, slight trembling in the hands, sleep disturbances. The body temperature can rise to small values for no reason, the head often hurts, the heart beats faster, and blood pressure rises. If such symptoms appear, especially if there is still vomiting that seems to be normal for pregnant women with significant weight loss, an examination of the thyroid gland is necessary.
But there are women in whom, for some reason, the thyroid gland begins to produce too many hormones and without the influence of pregnancy, hyperthyroidism (or thyrotoxicosis) occurs. Then other symptoms appear - imbalance, tearfulness, agitation, increased appetite, slight trembling in the hands, sleep disturbances. The body temperature can rise to small values for no reason, the head often hurts, the heart beats faster, and blood pressure rises. If such symptoms appear, especially if there is still vomiting that seems to be normal for pregnant women with significant weight loss, an examination of the thyroid gland is necessary.
Going to the doctor
If the future mother has not been to an endocrinologist before, now is the time to visit this specialist and, possibly, do a blood test to determine the level of thyroid hormones.
Even if everything is in order with the thyroid gland, the doctor will still recommend taking either a separate medicine with iodine or a vitamin complex.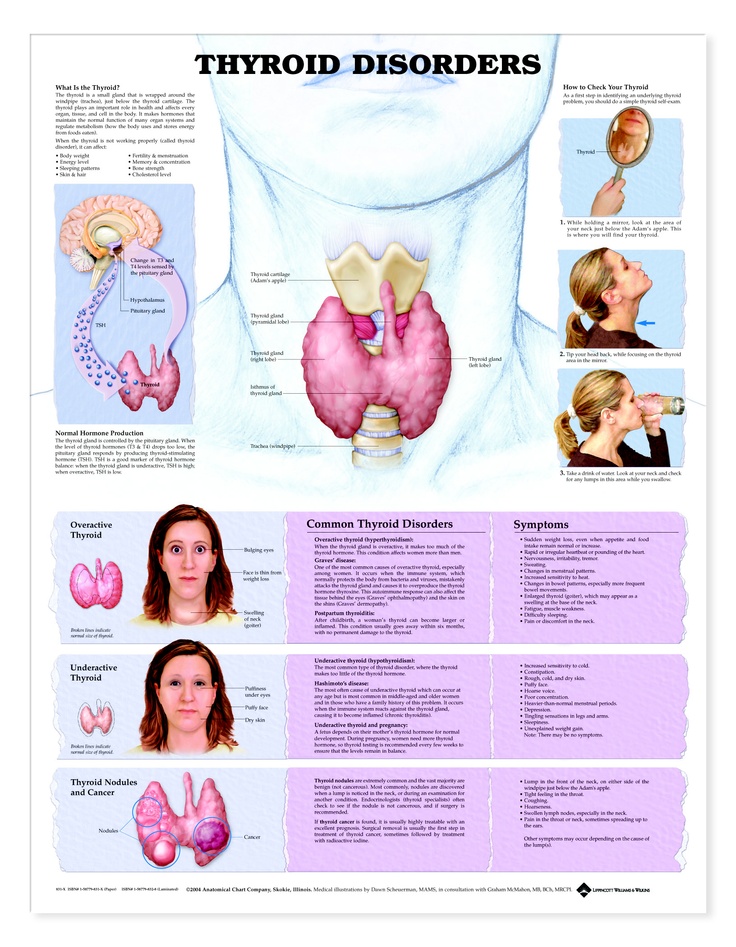 The fact is that in Russia, in most regions, both water and food contain little iodine, so that it does not come with enough food. Moreover, if before pregnancy the daily dose of iodine was 150 mcg, now the expectant mother should take 200 mcg already. But still, before taking even harmless vitamins with iodine, it is better to check the level of thyroid hormones.
The fact is that in Russia, in most regions, both water and food contain little iodine, so that it does not come with enough food. Moreover, if before pregnancy the daily dose of iodine was 150 mcg, now the expectant mother should take 200 mcg already. But still, before taking even harmless vitamins with iodine, it is better to check the level of thyroid hormones.
Also, in order to prevent iodine deficiency, you can salt your food with iodized salt (although salt itself is not healthy). A lot of iodine is found in sea fish, seaweed, squid, persimmon, feijoa, dates, dried figs, dairy products and meat.
Pay attention to the thyroid gland, and it will definitely help you look and feel good!
Attention! Prices for services in different clinics may vary. To clarify the current cost, select clinic
The administration of the clinic takes all measures to update the prices for programs in a timely manner, however, in order to avoid possible misunderstandings, we recommend that you clarify the cost of services by phone / with the managers of the clinic
Clinical Hospital MD GROUPClinical Hospital Lapino-1 "Mother and Child"Clinic KG "Lapino" in Odintsovo (branch)Clinic "Mother and Child" Khodynskoye PoleClinic "Mother and Child" KuntsevoClinic "Mother and Child" Savelovskaya Clinic "Mother and Child" » South-WestClinic "Mother and Child" NovogireevoClinic "Mother and Child" Lefortovo
All areasSpecialist consultations (adults)Specialist consultations (children)Molecular genetics laboratoryGeneral clinical studiesTreatment roomTelemedicine for adultsTherapeutic studiesUltrasound examinations for adults
01.
Specialist consultations (adults)
02.
Specialist consultations (children)
03.
Laboratory of molecular genetics
04.
General clinical studies
05.
Procedure cabinet
06.
Telemedicine for adults
07.
Therapeutic studies
08.
Ultrasound studies of adults. Nothing found
The administration of the clinic takes all measures to timely update the price list posted on the website, however, in order to avoid possible misunderstandings, we advise you to clarify the cost of services and the timing of the tests by calling
Thyroid disorders and pregnancy | Burumkulova
In their practice, both endocrinologists and obstetrician-gynecologists often encounter various diseases of the thyroid gland (thyroid gland) in pregnant women, which is of significant clinical and scientific interest both for studying the pathology of these disorders and in terms of their treatment.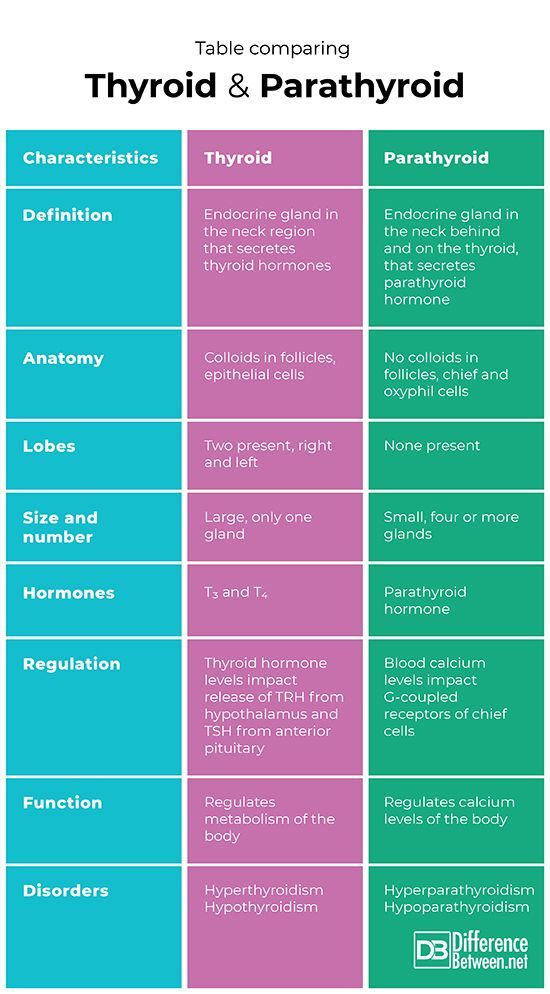
As you know, pregnancy often leads to goiter. An increase in the size and volume of the thyroid gland during pregnancy is observed due to both a more intensive blood supply to the thyroid tissue and an increase in the mass of the thyroid tissue. 3 factors can stimulate thyroid function during pregnancy: an increase in the degree of binding of thyroid hormones (TG) to blood proteins, an increase in the level of chorionic gonadotropin (CG) in the blood of pregnant women, and an insufficient supply of iodine to the thyroid gland due to increased excretion of iodine in the urine during pregnancy (see . drawing).
Increased binding of TG to blood proteins. More than 99% of TG circulating in the blood is bound to plasma proteins: thyroxine-binding globulin (TSG), thyroxine-binding prealbumin and albumin. The relative distribution of the amount of TG binding to various binding proteins directly depends on the degree of their affinity and concentration. 80% of TG is associated with TSH. The bound and inactive TG fractions are in equilibrium with the "free" unbound fraction, which represents only a small fraction of all circulating TG: 0.03-0.04% for free thyroxine (swT 4 ) and 0.3-0.5% for free triiodothyronine (swt 3 ). However, it is this fraction that provides all the metabolic and biological activity of TH.
The bound and inactive TG fractions are in equilibrium with the "free" unbound fraction, which represents only a small fraction of all circulating TG: 0.03-0.04% for free thyroxine (swT 4 ) and 0.3-0.5% for free triiodothyronine (swt 3 ). However, it is this fraction that provides all the metabolic and biological activity of TH.
During pregnancy, already a few weeks after conception, the serum level of TSH progressively increases as a result of stimulation by a significant amount of estrogens produced by the placenta. Then the level of TSH reaches a plateau, which is maintained until the moment of delivery. Conversely, the level of 2 other circulating binding proteins tends to decrease, mainly as a result of passive
Scheme of thyroid stimulation during pregnancy
dilution due to increased vascular pool (blood depot).
Increased TSH production during pregnancy results in an increase in total TG levels. The levels of total T 4 (vb 4 ) and T 3 (vb 3 ) increase significantly during the first half of pregnancy and reach a plateau by the 20th week, remaining at the same level thereafter. Transient decrease in the amount of svt 4 and svTz on the feedback principle stimulates the release of thyroid-stimulating hormone (TSH) and restoration of homeostasis of the level of free forms of TG.
Transient decrease in the amount of svt 4 and svTz on the feedback principle stimulates the release of thyroid-stimulating hormone (TSH) and restoration of homeostasis of the level of free forms of TG.
Adequate maintenance of thyroid homeostasis is disturbed in about 1/3 of pregnant women, which leads to the development of a state of relative hypothyroxinemia.
Stimulation of thyroid function during pregnancy hCG. CG is secreted by the placenta only in primates. It is produced in large quantities by placental syncytiotrophoblasts, especially in the first quarter of pregnancy. The most important function of hCG is the stimulation of steroidogenesis, first in the corpus luteum, then in the placenta.
The value of hCG for thyroid stimulation in women during pregnancy is not fully understood. It is known that there is a correlation between the suppression of TSH secretion and an increase in the concentration of hCG, as well as between the level of hCG and the level of fT 4 . CG is able to have a direct stimulating effect on the mother's thyroid gland (and this effect is most pronounced at the end of the first trimester of pregnancy) due to the molecular similarity of CG to TSH. Acting in early pregnancy as a weak 'analogue' of TSH, hCG is responsible for the slight increase in serum FTT levels 4 and svt 3 and, as a result, for a decrease in serum TSH levels. In the vast majority of healthy pregnant women, the stimulatory effect of CG on the thyroid gland is short and insignificant. However, in 1-2% of all pregnant women during the first trimester of pregnancy, there is a decrease in the concentration of TSH and an increase in the level of sT 3 , which is accompanied by a clinic of thyrotoxicosis. This syndrome is called ''gestational transient thyrotoxicosis'' (GTT).
CG is able to have a direct stimulating effect on the mother's thyroid gland (and this effect is most pronounced at the end of the first trimester of pregnancy) due to the molecular similarity of CG to TSH. Acting in early pregnancy as a weak 'analogue' of TSH, hCG is responsible for the slight increase in serum FTT levels 4 and svt 3 and, as a result, for a decrease in serum TSH levels. In the vast majority of healthy pregnant women, the stimulatory effect of CG on the thyroid gland is short and insignificant. However, in 1-2% of all pregnant women during the first trimester of pregnancy, there is a decrease in the concentration of TSH and an increase in the level of sT 3 , which is accompanied by a clinic of thyrotoxicosis. This syndrome is called ''gestational transient thyrotoxicosis'' (GTT).
Possible reasons for the increase in the level of CG and the development of GTT may be the following: 1) unbalanced production of CG due to transient overexpression of the gene encoding the P-subunit of CG; 2) changes in the degree of glycosylation of the CG molecule, which in turn leads to a prolongation of its half-life; 3) an increase in the mass of placental trophoblast syncytial cells in some women (for example, in multiple pregnancies).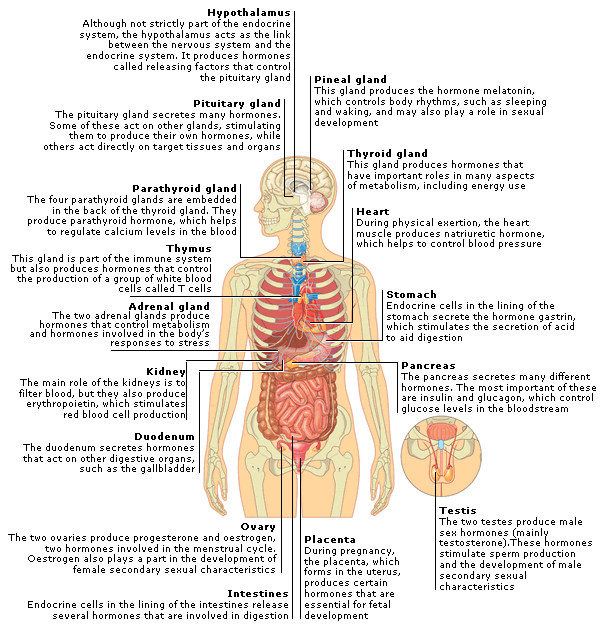 In multiple pregnancy, the concentration of hCG increases in proportion to the number of placentas.
In multiple pregnancy, the concentration of hCG increases in proportion to the number of placentas.
GTT is often accompanied by uncontrollable vomiting of pregnant women (hyperemesis gravidatum), which makes its diagnosis difficult due to the fact that nausea and vomiting are in principle characteristic of early pregnancy. This condition is usually transient and resolves by the second trimester of pregnancy. The diagnosis of HTT is made on the basis of an elevated level of hCG, a slightly suppressed concentration of TSH, an increase in serum levels of fT 4 and FT 3 to the levels characteristic of hyperthyroidism. Treatment with thyreostatics GTT is not indicated; with severe clinical symptoms, only a short course of β-blockers is sufficient.
Thus, it is important for clinicians to know that the symptoms of thyrotoxicosis during pregnancy have specific differences and may result not only from an autoimmune process in the thyroid gland, but also from hormonal changes inherent in pregnancy itself.
Reduced availability of iodine while increasing the need for it during pregnancy. The increased need for iodine during pregnancy is due to two factors. On the one hand, during pregnancy, there is an additional loss of iodine from the mother's body due to increased renal clearance of iodide, on the other hand, the loss of iodide in the second half of pregnancy increases due to the fact that part of the maternal pool of inorganic iodide is consumed by the fetoplacental complex and is used for synthesis TG thyroid of the fetus.
For women living in countries with sufficient iodine intake (such as Japan, the United States or Scandinavia), iodine loss during pregnancy is not significant because daily iodine intake is more than 150-200 mcg/day and remains satisfactory in throughout the entire pregnancy.
At the same time, in regions with moderate and severe iodine deficiency in the biosphere, which include the vast majority of Russia, reduced iodine intake (less than 100 mcg/day) is a rather severe factor in thyroid stimulation during pregnancy.
The risk of developing thyroid disease during pregnancy is higher in women with a history of goiter (diffuse or nodular), and the number and size of the nodules may increase during pregnancy. Repeated pregnancy leads to a further increase in the size of the thyroid gland and increased nodulation.
In 1989, D. Glinoer et al. proposed a hypothesis according to which increased thyroid stimulation during pregnancy can lead to the formation of diffuse non-toxic goiter (DNG), and pregnancy is one of the factors causing pathological changes in the thyroid gland.
In clinical practice, the following biochemical parameters have been proposed to detect increased thyroid stimulation during pregnancy.
— Presence of relative hypothyroxinemia observed in about 1/3 of all pregnant women. For its diagnosis, certain ratios T 4 /TSG are recommended.
- Increased secretion of T 3 , manifested in an increase in the ratio of T 3 / T 4 more than 0..jpg) 025 and reflecting the stimulation of the thyroid gland in conditions of iodine deficiency.
025 and reflecting the stimulation of the thyroid gland in conditions of iodine deficiency.
- Change in the concentration of TSH in the blood. After the initial phase of suppression of the TSH level due to the high secretion of CG at the end of the first trimester of pregnancy, the TSH level progressively increases and its concentration by the time of delivery doubles in relation to the initial one. The increase in TSH levels usually remains within the normal range (<4 mU/l).
- Change in the concentration of thyroglobulin (Tg) in the blood serum. The serum level of Tg is a sensitive indicator of thyroid stimulation, which often increases during pregnancy: its increase is already observed in the first trimester, but is most pronounced in the third trimester and by the time of delivery. By the time of delivery, 60% of pregnant women have an elevated level of Tg in the blood.
An increase in Tg concentration correlates with other indicators of thyroid stimulation, such as a slight increase in TSH levels and an increase in the ratio of T 3 /T 4 more than 0.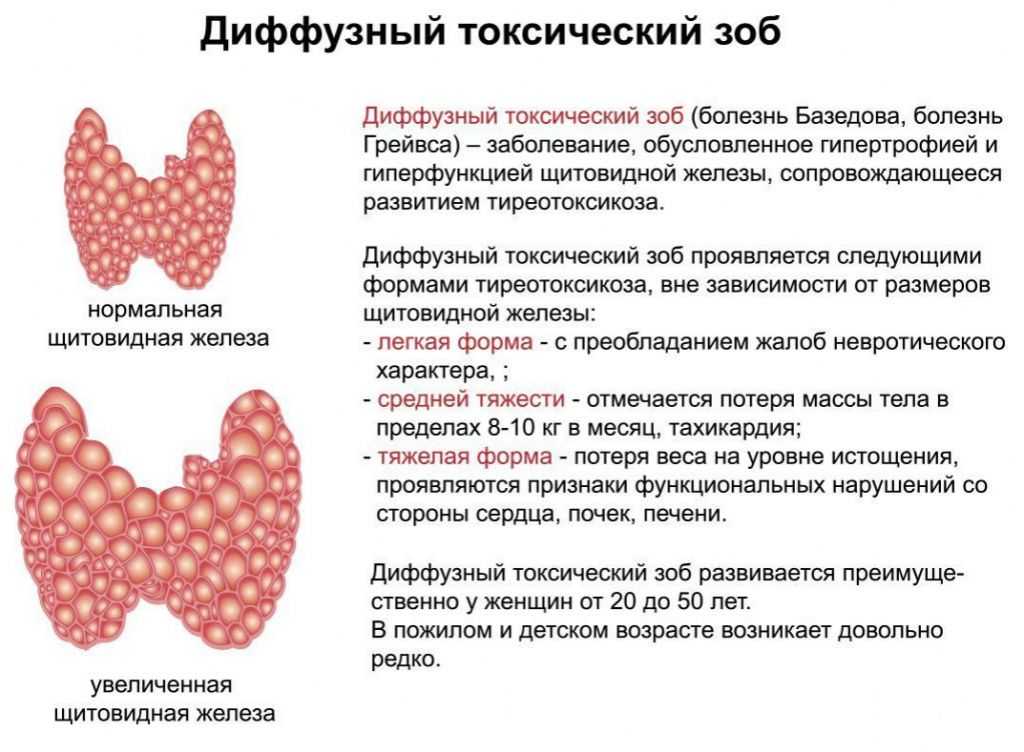 025. The presence of a correlation between the level of Tg and the volume of the thyroid gland (according to ultrasound - ultrasound confirms that the level of Tg in the blood is a fairly reliable biochemical marker of the goiterogenic effect of pregnancy.
025. The presence of a correlation between the level of Tg and the volume of the thyroid gland (according to ultrasound - ultrasound confirms that the level of Tg in the blood is a fairly reliable biochemical marker of the goiterogenic effect of pregnancy.
intellectual and physical development of the child.As is known, the thyroid gland of the fetus acquires the ability to concentrate iodine and synthesize iodothyronines at 10-12 weeks of intrauterine development.0113 4 , vT 4 and TSH reach adult levels around the 36th week of pregnancy.
The issue of placental permeability for triglycerides has been debatable for a long time. It is currently assumed that maternal and fetal thyroid glands are regulated autonomously, but not independently of each other. Apparently, the transplacental transfer of TG from the mother's body to the fetus is observed only at an early stage of intrauterine development.
In addition, the activity of the thyroid gland of the fetus is completely dependent on the intake of iodine from the mother's body. As a result of both insufficient intake of iodine in the mother’s body and a low intrathyroid iodine reserve, fetal thyroid stimulation occurs, which is reflected in a significant increase (compared with those of the mother) in the levels of neonatal TSH and Tg, as well as the development of goiter in the fetus. The development of hypothyroidism in the prenatal and neonatal periods can lead to an irreversible decrease in the child's mental development up to endemic cretinism.
As a result of both insufficient intake of iodine in the mother’s body and a low intrathyroid iodine reserve, fetal thyroid stimulation occurs, which is reflected in a significant increase (compared with those of the mother) in the levels of neonatal TSH and Tg, as well as the development of goiter in the fetus. The development of hypothyroidism in the prenatal and neonatal periods can lead to an irreversible decrease in the child's mental development up to endemic cretinism.
For the treatment of DND during pregnancy in regions with insufficient iodine intake, it is advisable to recommend iodine intake at the rate of 150-250 mcg/day. To do this, you can use the Antistrumine drug available in the pharmacy network (1000 μg of potassium iodide in 1 tablet), 1 tablet 1-2 times a week.
Another iodine preparation is "Potassium iodide-200" tablets, manufactured by Berlin-Chemie. They must be taken daily. An alternative may be imported multivitamins containing a daily dose of iodine (150 micrograms).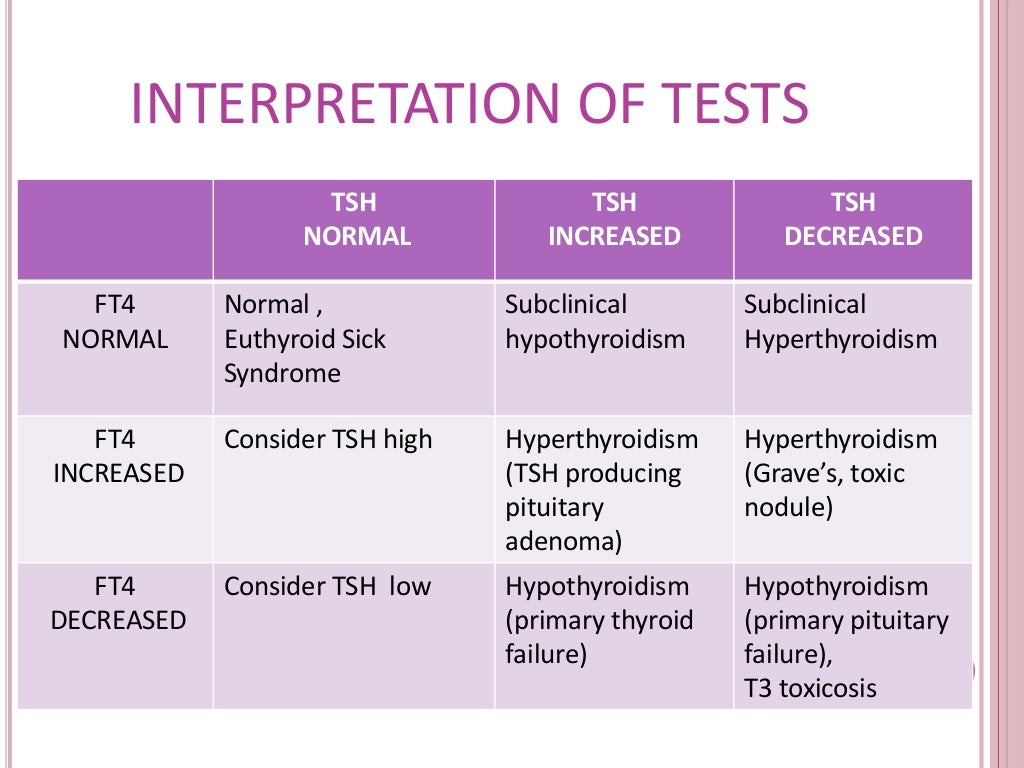 As a rule, these prescriptions will be enough to prevent further growth of the goiter and even achieve a reduction in its volume.0005
As a rule, these prescriptions will be enough to prevent further growth of the goiter and even achieve a reduction in its volume.0005
In the presence of a large goiter before pregnancy or with its rapid growth at the beginning of pregnancy, a combination of iodine and thyroid hormones is justified: either Thyreocomb containing 70 µg T 4 , 10 µg T 3 and 150 µg iodine, or 50-100 mcg T 4 daily and additionally 1 tablet of antistrumine 2-3 times a week. This allows you to quickly and effectively restore the normal function of the mother's thyroid gland and prevent the goiter effect of pregnancy.
The development of hyperthyroidism during pregnancy is relatively rare and occurs in 0.05-3% of pregnant women. In most cases, its cause is diffuse toxic goiter (DTG), while toxic adenoma or multinodular toxic goiter are much less common.
The main difficulty in diagnosing thyrotoxicosis during pregnancy is that many clinical symptoms and signs of thyrotoxicosis can be masked by manifestations of a normal pregnancy (tachycardia, weakness, irritability, vegetative disorders, etc. ).
).
Diagnosis of DTG must be confirmed by history, ultrasound of the thyroid gland, as well as the study of the levels of TSH, sT 3 and especially sT 4 in the blood.
A typical error in the interpretation of the results of the study of hormonal function in pregnant women can be considered the determination of levels of vT 9vT 4 and vT 3 , which does not reflect the true functional state of the thyroid gland.
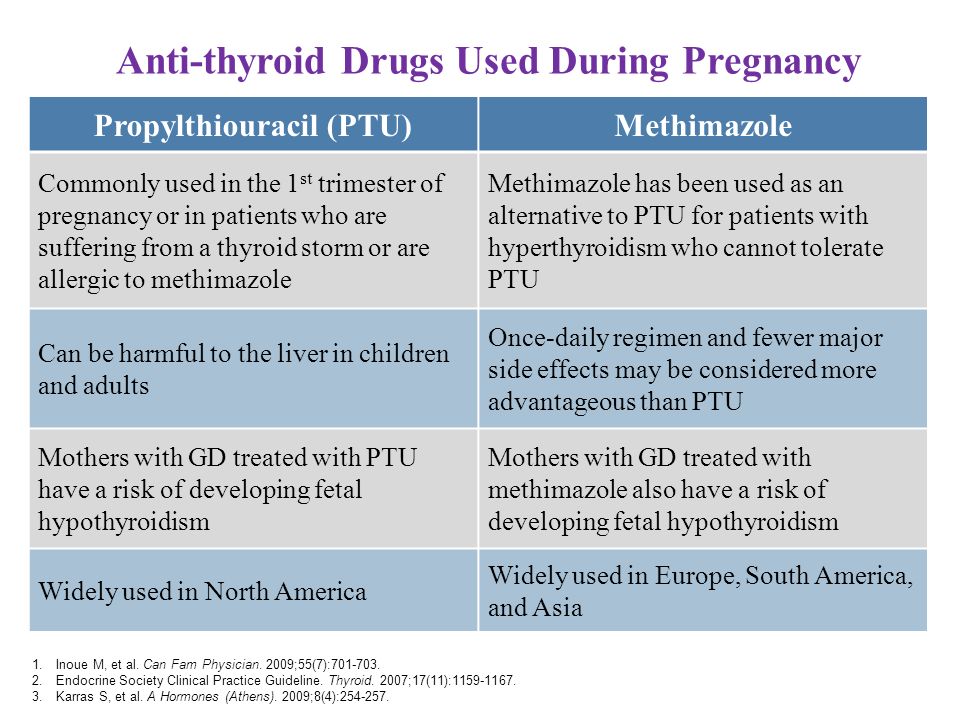
Thyrostatic drugs (mercasolil, methimazole, propylthiouracil) are preferred in all countries for the treatment of DTG in pregnant women. Surgical treatment is recommended only in exceptional cases, such as severe side effects, very large goiter, suspected malignancy, or the need to use high doses of thyreostatics to maintain maternal euthyroidism. The optimal time for subtotal resection of the thyroid gland is the second trimester of pregnancy. The appointment of iodides during pregnancy is contraindicated because of the risk of developing hypothyroidism in the fetus and goiter due to the Wolf-Chaikov effect.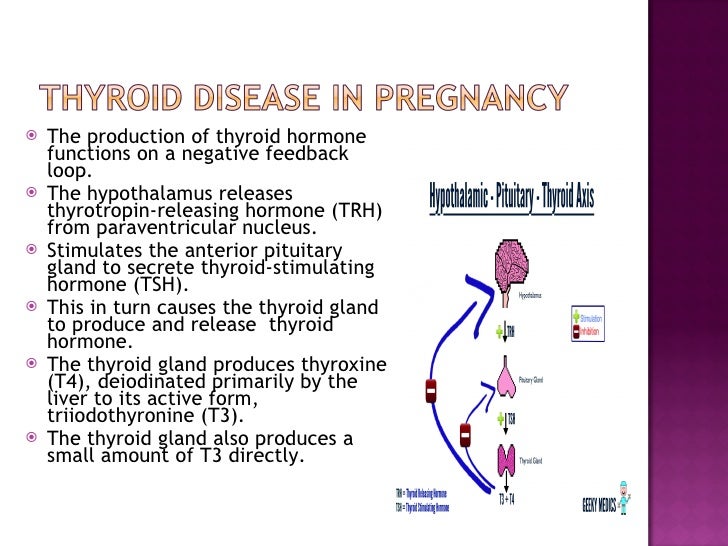
What principles should be followed when treating a pregnant woman with DTG?
- The choice of a specific thyreostatic is determined both by the doctor's personal experience and the availability of a particular drug. In our country, mercasolil (1-methyl-2-mercaptoimidazole) or its analogues (methimazole, thiamazole) are more often used to treat DTG during pregnancy. Abroad, in a similar situation, preference is given to propylthiouracil (6-propyl-2-thiouracil). At present, a preparation of this group under the name Propicil (Kali-Khemi) has been registered and made available in Russia.
The frequency of side effects of therapy is the same for propylthiouracil and mercazolil. Both drugs cross the placenta, and excessive doses of them can cause the development of hypothyroidism and goiter in utero and neonatal periods.
Prescribing propylthiouracil during pregnancy, however, has a number of advantages. Firstly, the kinetics of propylthiouracil does not change during pregnancy, secondly, the half-life of propylthiouracil from the blood does not depend on the presence of hepatic or renal insufficiency, thirdly, propylthiouracil binds to proteins to a greater extent compared to mercazolil and has limited lipophilicity, which hinders its penetration through biological membranes such as the placenta and mammary gland epithelium.
- Clinical improvement in treatment with thionamides appears already by the end of the 1st week of therapy, and euthyroidism is achieved after 4-6 weeks. As a result of the well-known immunosuppressive effect of pregnancy, manifested by an increase in the number of T-suppressors and a decrease in the number of T-helpers, DTG during pregnancy tends to spontaneous remission. Knowledge of this feature of the course of thyrotoxicosis during pregnancy makes it possible to control the function of the thyroid gland of the mother with the help of relatively low initial and maintenance doses of thyreostatics. The drugs should be administered at the lowest possible initial dosage (no higher than 10-15 mg of mercazolil or 100 mg of propylthiouracil per day) with the transition to a maintenance dose (2.5 mg/day for mercazolil and 50 mg/day for propylthiouracil).
- Treatment according to the "block and replace" method with high doses of thionamides in combination with replacement therapy T 4 during pregnancy is contraindicated.
 With this regimen T 4 maintains euthyroidism only in the mother, at the same time it can cause hypothyroidism in fetus, since high doses of thyreostatics, in contrast to T 4 , easily pass through the placenta.
With this regimen T 4 maintains euthyroidism only in the mother, at the same time it can cause hypothyroidism in fetus, since high doses of thyreostatics, in contrast to T 4 , easily pass through the placenta. - The use of p-adrenergic antagonists during pregnancy complicated by the development of thyrotoxicosis is undesirable, since they can cause a decrease in placental weight, intrauterine growth retardation, postnatal bradycardia and hypoglycemia, and also weaken the response to hypoxia, p-blockers can only be used for a short the period for preparation for surgical treatment or with the development of a thyrotoxic crisis.
- The optimal method for monitoring the effectiveness of the treatment of thyrotoxicosis during pregnancy is to determine the concentration of SvTz and SvT 4 in the blood. The levels of fT 4 and fT 3 in the mother's blood serum during treatment with thyreostatics should be maintained at the upper limit of the norm in order to avoid hypothyroidism in the fetus.
Due to the physiological changes in TSH secretion during the various phases of pregnancy, the TSH blood level is not a reliable criterion for judging the adequacy of treatment. At the same time, a very high level of TSH indicates the development of drug-induced hypothyroidism and requires immediate withdrawal or reduction of the dose of thionamides. Recommended by a number of authors, ultrasound determination of the size of the thyroid gland of the fetus and the study of the level of TSH, T 3 T 4 in the blood of the fetus, unfortunately, is available only to a small circle of highly specialized medical institutions and cannot yet be widely used.
- If there is stable compensation in the last months of pregnancy, thyreostatic drugs can be canceled. At the same time, one should be aware of the frequent recurrence of thyrotoxicosis in the postpartum period.
- During lactation, thionamides may pass into breast milk, with mercazolil to a greater extent than propylthiouracil.
 However, there is evidence that low doses of thionamides (up to 15 mg mercazolil and 150 mg propylthiouracil) taken by a woman while breastfeeding do not appear to affect the infant's thyroid function.
However, there is evidence that low doses of thionamides (up to 15 mg mercazolil and 150 mg propylthiouracil) taken by a woman while breastfeeding do not appear to affect the infant's thyroid function.
Why is it so important to treat thyrotoxicosis during pregnancy?
Pregnancy thyrotoxicosis increases the risk of stillbirth, preterm labor or preeclampsia. There is also an increase in the incidence of neonatal mortality and the likelihood of a child being born with a lack of body weight. Decompensated thyrotoxicosis can cause and aggravate cardiovascular insufficiency in the mother, as well as contribute to the development of a thyrotoxic crisis during labor pains and attempts.
It should be noted that the above complications are more often observed in the development of thyrotoxicosis during pregnancy than in the case of pregnancy in women with previously treated DTG. There is no doubt that adequate control and treatment of thyrotoxicosis in the mother are the main factor in improving the prognosis of pregnancy and childbirth.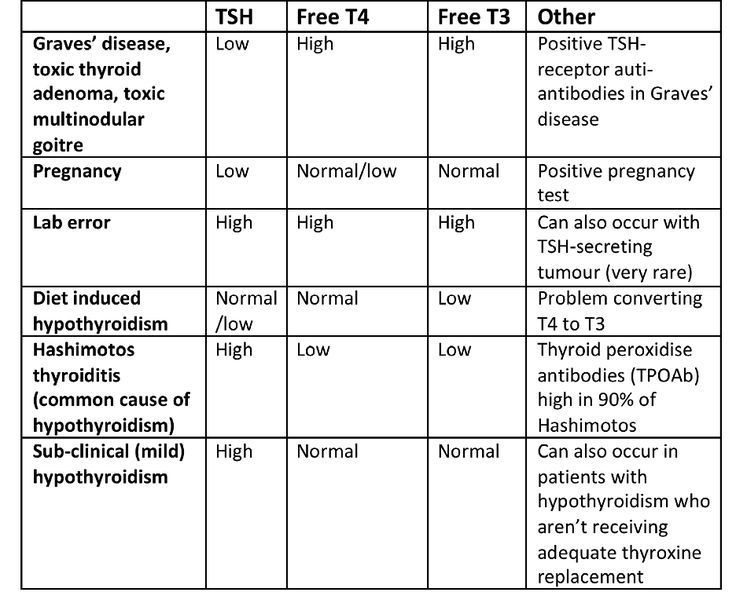
Children born to mothers with decompensated DTG have an increased risk of congenital malformations and other fetal disorders (6%). At the same time, in children whose mothers during pregnancy were in a state of drug euthyroidism during treatment with methimazole, the frequency of fetal disorders is similar to that among children of healthy euthyroid mothers (< 1%).
There is no information in the literature about the teratogenic effects of propylthiouracil, while: methimazole is extremely rarely accompanied by a congenital malformation of the skin (aplasi cutis). Studies of the intellectual development of children exposed to thyreostatics during fetal development also did not reveal deviations from normal indicators.
All of these data suggest that untreated maternal thyrotoxicosis can cause fetal malformations and other complications of pregnancy, and that the benefits of thyreostatic treatment outweigh any possible teratogenic effects associated with these drugs.
Infants whose mothers suffered from autoimmune thyrotoxicosis during pregnancy may develop fetal or neonatal hyperthyroidism:
Intrauterine thyrotoxicosis develops when the function of the mature thyroid gland of the fetus is stimulated by a large amount of immunoglobulins and maternal blood. This condition may develop only after about the 25th week of pregnancy. Fetal thyrotoxicosis can be established by measuring the heart rate (above 160 per minute), determining the level of TSH or integral TG level obtained by amniocentesis or cordocentesis, as well as ultrasound, which allows establish the presence of goiter in the fetus. The basis of the treatment of fetal thyrotoxicosis is its temporary administration of thyrostatic therapy to the mother, and the heart rate is reduced!!! fetus during treatment should be within 140 beats per minute. 9lasts 2-3 months, may be a placenta! passage of thyroid-stimulating immunoglobulins. The clinical symptoms of neonatal thyrotoxicosis are tachycardia, hypersensitivity, growth retardation, increased bone age, goiter (not always), premature, craniostenosis, increased mortality and morbidity.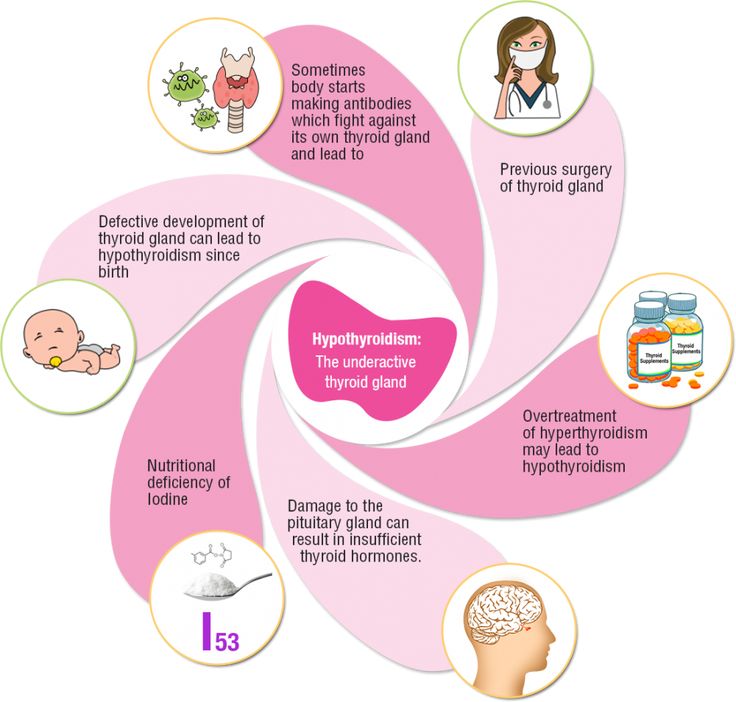
Neonatal hyperthyroidism requires the earliest and most active treatment with thionamides. Newborns are prescribed methimazole (0.5-1 mg / kg body weight per day) or propylthiouracil (5-10 mg / kg body weight per day) in 3 divided doses. It is possible to prescribe propranolol to slow down the heart rate and reduce catecholamine activity. In severe disease, a saturated solution of iodide (1 drop of solution per day for no more than 3 weeks) can be given to inhibit the release of previously synthesized triglycerides.
In severe cases, the addition of glucocorticoids is necessary, which, in addition to the general effect, also have the ability to block the conversion of T 4 vT 3 .
The most common causes of primary hypothyroidism in pregnant women are chronic autoimmune thyroiditis (AIT) and the condition after thyroid resection for DTG and various forms of goiter. Hypothyroidism due to AIT in most cases is detected and compensated before pregnancy, but sometimes its debut coincides with pregnancy.
In order to detect AIT during pregnancy, it is necessary to examine pregnant women with suspected thyroid dysfunction for the presence of antibodies to thyroglobulin and thyroid peroxidase in the blood serum.
As previously described, due to the immunosuppressive effects of pregnancy, previously diagnosed AIT may tend to remit during pregnancy with relapse in the postpartum period.
The most typical symptoms of hypothyroidism during pregnancy are weakness, increased dryness of the skin, fatigue and constipation, however, it should be remembered that these symptoms can also be manifestations of pregnancy itself in the absence of a decrease in thyroid function. The diagnosis of hypothyroidism during pregnancy is made on the basis of a decrease in the level of fT 4 and increased serum TSH levels.
The selection of an adequate dose of T 4 is carried out under the control of the level of TSH and sT 4 in the blood serum (100-150 mcg of T 4 per day).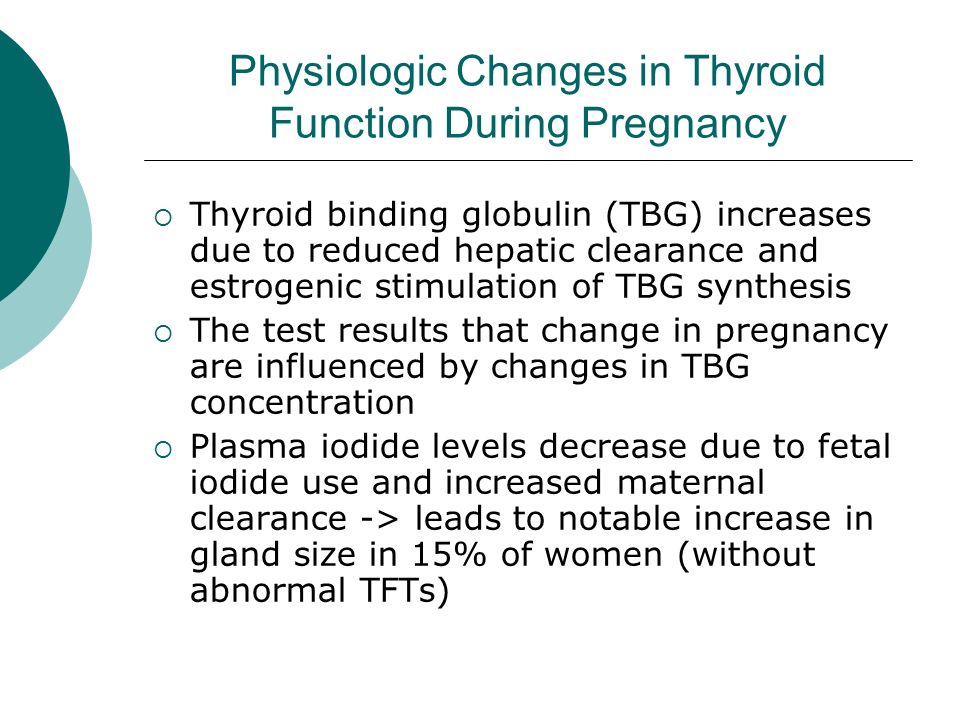 Until recently, it was believed that pregnant women with previously treated hypothyroidism do not need to increase the dose of T 4 on the basis that the increased need for thyroid hormones is compensated by an increase in their concentration in blood serum and a decrease in metabolic conversion of T 4 . However, it has now become clear that women suffering from hypothyroidism and receiving T 4 replacement therapy often need to increase the dose of T 4 during pregnancy.
Until recently, it was believed that pregnant women with previously treated hypothyroidism do not need to increase the dose of T 4 on the basis that the increased need for thyroid hormones is compensated by an increase in their concentration in blood serum and a decrease in metabolic conversion of T 4 . However, it has now become clear that women suffering from hypothyroidism and receiving T 4 replacement therapy often need to increase the dose of T 4 during pregnancy.
Probable reasons for the increased need for triglycerides during pregnancy can be both an increase in body weight with increasing gestational age, and adaptive regulation of the hypothalamus-pituitary-thyroid axis, as well as possible changes in peripheral metabolism T 4 due to the presence of feto-placental complex.
Inadequate treatment of maternal hypothyroidism can lead to pregnancy complications such as anemia, preeclampsia, placental abruption, postpartum hemorrhage, and cardiovascular dysfunction.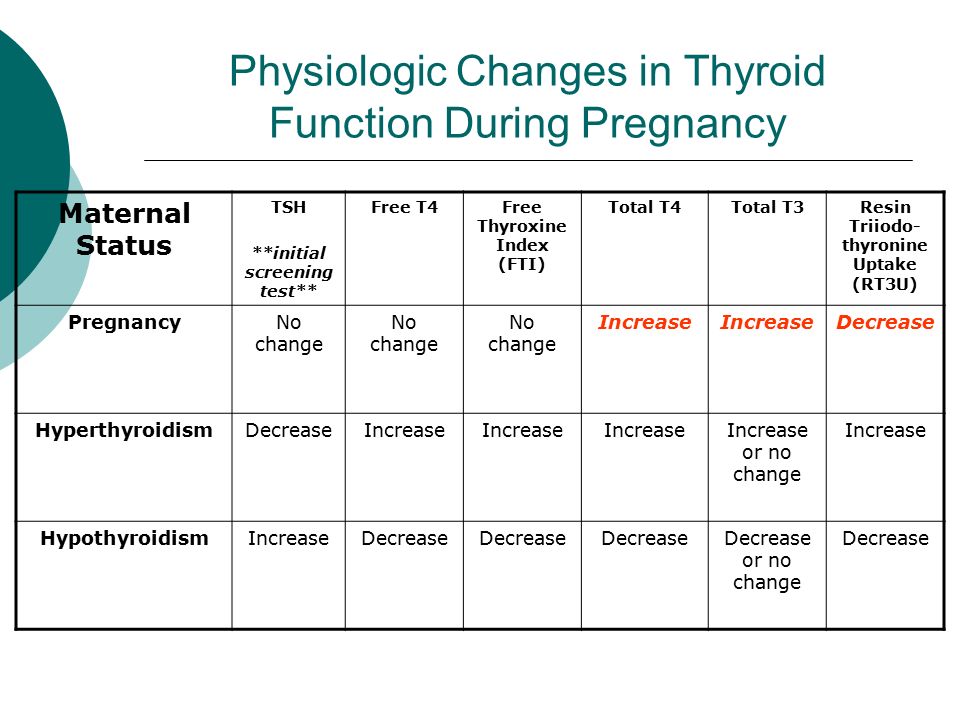 In addition, in the fetus and neonate with congenital hypothyroidism, the transplacental passage of maternal T 4 during early pregnancy may play a critical role in normal brain development.
In addition, in the fetus and neonate with congenital hypothyroidism, the transplacental passage of maternal T 4 during early pregnancy may play a critical role in normal brain development.
Blocking antibodies to TSH receptors that cross the placenta to the fetus can cause fetal and neonatal hypothyroidism (similar to fetal and neonatal hyperthyroidism). It is important to note that children of mothers suffering from hypothyroidism with the presence of antibodies that block the TSH receptor have an increased risk of developing intrauterine or postpartum hypothyroidism, even if the mother reaches the euthyroid state after T replacement therapy 4 .
Fetal hypothyroidism is accompanied by intrauterine growth retardation, bradycardia, delayed development of ossification nuclei, as well as impaired development of the fetal central nervous system.
Neonatal hypothyroidism usually lasts for 1-4 months (half-life of maternal antibodies from the baby's blood averages 3 weeks). The most typical signs of hypothyroidism in the early postnatal period are postterm pregnancy (gestational age > 42 weeks), high birth weight (> 4 kg), macroglossia, peripheral cyanosis and edema, difficulty breathing, low rough voice when crying and screaming. The diameter of the occipital fontanel exceeds 5 mm, the period of jaundice lengthens (> 3 days). In the future, drowsiness, decreased appetite, decreased activity, hypothermia, dryness and pallor of the skin may occur.
The most typical signs of hypothyroidism in the early postnatal period are postterm pregnancy (gestational age > 42 weeks), high birth weight (> 4 kg), macroglossia, peripheral cyanosis and edema, difficulty breathing, low rough voice when crying and screaming. The diameter of the occipital fontanel exceeds 5 mm, the period of jaundice lengthens (> 3 days). In the future, drowsiness, decreased appetite, decreased activity, hypothermia, dryness and pallor of the skin may occur.
Other causes of transient hypothyroidism may be functional immaturity of the hypothalamic-pituitary system in premature infants, as well as severe iodine deficiency in the mother during pregnancy and the appointment of high doses of thyreostatics for DTG. The proven role of TSH in fetal development, as well as the effect of TG on growth and development in the neonatal period (especially during the 1st year of life) necessitates screening for congenital (including transient) hypothyroidism.
Transient hypothyroxinemia in most cases resolves on its own with the disappearance of the cause that caused it. In some cases, the appointment of a newborn T 4 at a dose of 10-15 mcg / kg body weight per day in a short course (3-4 weeks) is indicated.
In some cases, the appointment of a newborn T 4 at a dose of 10-15 mcg / kg body weight per day in a short course (3-4 weeks) is indicated.
In the postpartum period, 4-16.7% of women with no history of thyroid disease may develop postpartum thyroiditis (PT). The etiology of this disease is still not fully understood. The revealed relationship between PT and the detection of autoantibodies to thyroid tissue (to thyroid peroxidase and microsomal antigens), the presence of certain HLA markers and lymphocytic infiltration of thyroid tissue allows us to consider PT as a type of AIT.
During the PT there is a certain phase. After an optional phase of destructive hyperthyroidism, which occurs in the form of painless asymptomatic thyroiditis (1-4th month of the postpartum period), in about 23% of cases, a phase of persistent hypothyroidism occurs (5-7th month of the postpartum period).
Clinical manifestations of hypothyroidism in PT are usually typical (weakness, dry skin, tendency to constipation, etc. ). When scanning the thyroid gland, a reduced absorption of the radioactive isotope of iodine is noted. The ultrasound picture of PT is characterized by a diffuse or multifocal decrease in thyroid echogenicity and structural changes characteristic of lymphocytic infiltration of the thyroid gland and disorders of thyroid morphology.
). When scanning the thyroid gland, a reduced absorption of the radioactive isotope of iodine is noted. The ultrasound picture of PT is characterized by a diffuse or multifocal decrease in thyroid echogenicity and structural changes characteristic of lymphocytic infiltration of the thyroid gland and disorders of thyroid morphology.
With the development of persistent hypothyroidism, replacement therapy T 4 is prescribed according to the usual scheme.
Currently, the relationship between the presence in the postpartum period of an increased titer of antibodies to thyroid tissue and postpartum depression is being investigated. It is assumed that these antibodies can modulate the function of neurotransmitters, as well as affect cytokine receptors in the brain.
In conclusion, I would like to note that timely and adequate treatment of thyroid diseases in pregnant women contributes both to the normal course of pregnancy and to the correct physical and intellectual development of the child.