Postpartum bleeding treatment
Postpartum Hemorrhage: Prevention and Treatment
ANN EVENSEN, MD, JANICE M. ANDERSON, MD, AND PATRICIA FONTAINE, MD, MS
This is an updated version of the article that appeared in print.
Am Fam Physician. 2017;95(7):442-449
Author disclosure: No relevant financial affiliations.
Postpartum hemorrhage is common and can occur in patients without risk factors for hemorrhage. Active management of the third stage of labor should be used routinely to reduce its incidence. Use of oxytocin after delivery of the anterior shoulder is the most important and effective component of this practice. Oxytocin is more effective than misoprostol for prevention and treatment of uterine atony and has fewer adverse effects. Routine episiotomy should be avoided to decrease blood loss and the risk of anal laceration. Appropriate management of postpartum hemorrhage requires prompt diagnosis and treatment. The Four T's mnemonic can be used to identify and address the four most common causes of postpartum hemorrhage (uterine atony [Tone]; laceration, hematoma, inversion, rupture [Trauma]; retained tissue or invasive placenta [Tissue]; and coagulopathy [Thrombin]). Rapid team-based care minimizes morbidity and mortality associated with postpartum hemorrhage, regardless of cause. Massive transfusion protocols allow for rapid and appropriate response to hemorrhages exceeding 1,500 mL of blood loss. The National Partnership for Maternal Safety has developed an obstetric hemorrhage consensus bundle of 13 patient- and systems-level recommendations to reduce morbidity and mortality from postpartum hemorrhage.
Approximately 3% to 5% of obstetric patients will experience postpartum hemorrhage.1 Annually, these preventable events are the cause of one-fourth of maternal deaths worldwide and 12% of maternal deaths in the United States.2,3 The American College of Obstetricians and Gynecologists defines early postpartum hemorrhage as at least 1,000 mL total blood loss or loss of blood coinciding with signs and symptoms of hypovolemia within 24 hours after delivery of the fetus or intrapartum loss.4,5 Primary postpartum hemorrhage may occur before delivery of the placenta and up to 24 hours after delivery of the fetus. Complications of postpartum hemorrhage are listed in Table 13,6,7; these range from worsening of common postpartum symptoms such as fatigue and depressed mood, to death from cardiovascular collapse.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Routinely use active management of the third stage of labor, preferably with oxytocin (Pitocin). This practice will decrease the risks of postpartum hemorrhage and a postpartum maternal hemoglobin level lower than 9 g per dL (90 g per L), and reduce the need for manual removal of the placenta. | A | 11, 12, 16, 18 |
| Oxytocin is the most effective treatment for postpartum hemorrhage, even if already used for labor induction or augmentation or as part of active management of the third stage of labor. | A | 8, 23, 24 |
In women with postpartum hemorrhage, tranexamic acid (Cyklokapron) given within the first three hours after birth reduces mortality due to bleeding, but not overall mortality. | B | 25 |
| Avoid routine episiotomy, which increases the risk of blood loss and anal sphincter tears, unless urgent delivery is necessary and the perineum is thought to be a limiting factor. | A | 26 |
| When needed, use massive transfusion protocols to decrease the risk of dilutional coagulopathy and other postpartum hemorrhage complications. | C | 7, 39 |
| Interdisciplinary team training with realistic simulation should be used to improve perinatal safety. | C | 47, 48 |
| Anemia |
Anterior pituitary ischemia with delay or failure of lactation (i.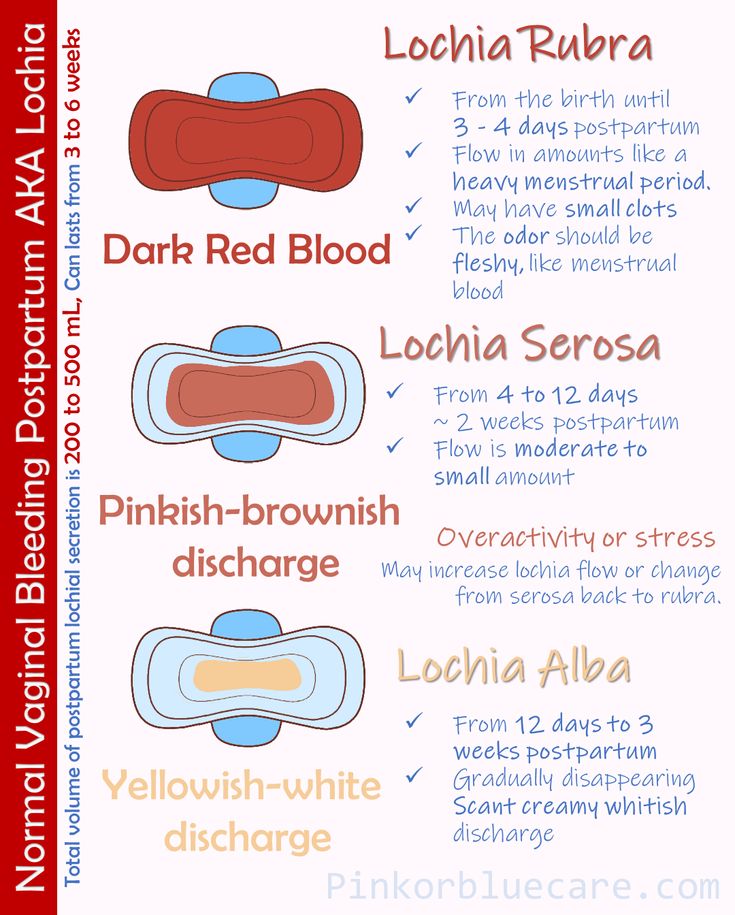 e., Sheehan syndrome or postpartum pituitary necrosis) e., Sheehan syndrome or postpartum pituitary necrosis) |
| Blood transfusion |
| Death |
| Dilutional coagulopathy |
| Fatigue |
| Myocardial ischemia |
| Orthostatic hypotension |
| Postpartum depression |
This review presents evidence-based recommendations for the prevention of and appropriate response to postpartum hemorrhage and is intended for physicians who provide antenatal, intrapartum, and postpartum care.
Prevention
Risk factors for postpartum hemorrhage are listed in Table 2.8 However, 20% of postpartum hemorrhage occurs in women with no risk factors, so physicians must be prepared to manage this condition at every delivery.9 Strategies for decreasing the morbidity and mortality associated with postpartum hemorrhage are listed in Table 3,6,10–14 including the choice to deliver infants in women at high risk of hemorrhage at facilities with immediately available surgical, intensive care, and blood bank services.
| Antepartum hemorrhage |
| Augmented labor |
| Chorioamnionitis |
| Fetal macrosomia |
| Maternal anemia |
| Maternal obesity |
| Multifetal gestation |
| Preeclampsia |
| Primiparity |
| Prolonged labor |
| Readiness by every unit | |
| Have a hemorrhage cart with medications, supplies, checklist, and instruction cards immediately available | |
| Establish a response team and know who to call when help is needed | |
| Establish massive and emergency release transfusion protocols | |
| Institute unit education on protocols and run unit-based drills | |
| Recognition and prevention efforts for every patient | |
| Antenatal assessment | |
| Screen for and treat anemia antenatally | |
| Screen for sickle cell disease and thalassemia in women of African, Southeast Asian, or Mediterranean descent | |
| Obtain sonograms for women at high risk of invasive placenta | |
| Perform delivery in facility with blood bank and in-house surgical services if the patient has a high risk of hemorrhage | |
| Identify Jehovah's Witnesses and other patients who decline blood products | |
| Intrapartum management | |
| Use active management of the third stage of labor in every delivery | |
| Avoid routine episiotomy | |
| Avoid instrumented deliveries, especially forceps | |
| Use perineal warm compresses | |
| Measure cumulative blood loss and track postpartum vital signs | |
| Response for every hemorrhage | |
| Use an emergency management plan with checklists | |
| Provide support program for patients, families, and staff | |
| Reporting and systems learning for every unit | |
| Establish a culture of huddles and postevent debriefs | |
| Complete a multidisciplinary review for systems issues | |
| Establish a perinatal quality improvement committee | |
The most effective strategy to prevent postpartum hemorrhage is active management of the third stage of labor (AMTSL). AMTSL also reduces the risk of a postpartum maternal hemoglobin level lower than 9 g per dL (90 g per L) and the need for manual removal of the placenta.11 Components of this practice include: (1) administering oxytocin (Pitocin) with or soon after the delivery of the anterior shoulder; (2) controlled cord traction (Brandt-Andrews maneuver) to deliver the placenta; and (3) uterine massage after delivery of the placenta.11 Placental delivery can be achieved using the Brandt-Andrews maneuver, in which firm traction on the umbilical cord is applied with one hand while the other applies suprapubic counterpressure15(eFigure A).
AMTSL also reduces the risk of a postpartum maternal hemoglobin level lower than 9 g per dL (90 g per L) and the need for manual removal of the placenta.11 Components of this practice include: (1) administering oxytocin (Pitocin) with or soon after the delivery of the anterior shoulder; (2) controlled cord traction (Brandt-Andrews maneuver) to deliver the placenta; and (3) uterine massage after delivery of the placenta.11 Placental delivery can be achieved using the Brandt-Andrews maneuver, in which firm traction on the umbilical cord is applied with one hand while the other applies suprapubic counterpressure15(eFigure A).
The individual components of AMTSL have been evaluated and compared. Based on existing evidence, the most important component is administration of a uterotonic drug, preferably oxytocin.12,16 The number needed to treat to prevent one case of hemorrhage 500 mL or greater is 7 for oxytocin administered after delivery of the fetal anterior shoulder or after delivery of the neonate compared with placebo.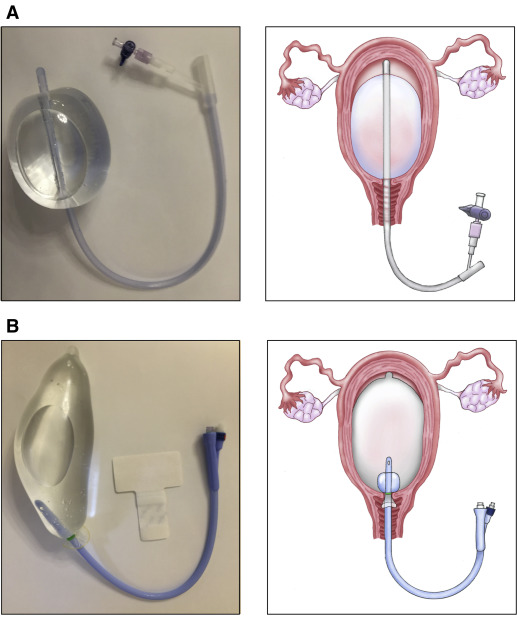 16 The risk of postpartum hemorrhage is also reduced if oxytocin is administered after placental delivery instead of at the time of delivery of the anterior shoulder.17 Dosing instructions are provided in Table 4.6
16 The risk of postpartum hemorrhage is also reduced if oxytocin is administered after placental delivery instead of at the time of delivery of the anterior shoulder.17 Dosing instructions are provided in Table 4.6
| Medication | Dosage | Prevention | Treatment | Contraindications and cautions | Mechanism of action | Adverse effects | Cost* |
|---|---|---|---|---|---|---|---|
| First-line agent | |||||||
| Oxytocin (Pitocin) | Prevention: 10 IU IM or 5 to 10 IU IV bolus | + | + | Overdose or prolonged use can cause water intoxication | Stimulates the upper segment of the myometrium to contract rhythmically, constricting spiral arteries and decreasing blood flow through the uterus | Rare | $1 ($13) for 10 units of injectable solution |
| Treatment: 20 to 40 IU in 1 L normal saline, infuse 500 mL over 10 minutes then 250 mL per hour | Possible hypotension with IV use following cesarean delivery | ||||||
| Second-line agents | |||||||
| Carboprost (Hemabate), a prostaglandin F2-alpha analogue | 250 mcg IM or into myometrium, repeated every 15 to 90 minutes for a total dose of 2 mg | – | + | Avoid in patients with asthma or significant renal, hepatic, or cardiac disease | Improves uterine contractility by increasing the number of oxytocin receptors and causes vasoconstriction | Nausea, vomiting, and diarrhea | NA ($270) for 250 mcg of injectable solution |
| Methylergonovine (Methergine) | 0. 2 mg IM, repeat every two to four hours 2 mg IM, repeat every two to four hours | – | + | Avoid in hypertensive disorders of pregnancy, including chronic hypertension | Causes vasoconstriction and contracts smooth muscles and upper and lower Segments of the uterus tetanically | Nausea, vomiting, and increased blood pressure | $9 (NA) for 0.2 mg of injectable solution |
| Use with caution in patients with human immunodeficiency virus infection who are receiving protease inhibitors | |||||||
| Misoprostol (Cytotec),† a prostaglandin E1 analogue | Prevention: 600 mcg orally Treatment: 800 to 1,000 mcg rectally or 600 to 800 mcg sublingually or orally | Use only when oxytocin is not available | + | Use with caution in patients with cardiovascular disease | Causes generalized smooth muscle contraction | Nausea, vomiting, diarrhea, pyrexia, and shivering | $1 ($5) per 200-mcg tablet |
| Tranexamic acid (Cyklokapron)† | 1 g intravenously over 10 minutes, may be repeated after 30 minutes | – | + | Use within three hours of onset of bleeding | Inhibits breakdown of fibrin and fibrinogen by plasmin | May increase risk of thrombosis and cause visual defects | $24 ($50) |
| Use with caution in patients with renal impairment and with other clotting factors such as prothrombin complex concentrate | |||||||
An alternative to oxytocin is misoprostol (Cytotec), an inexpensive medication that does not require injection and is more effective than placebo in preventing postpartum hemorrhage.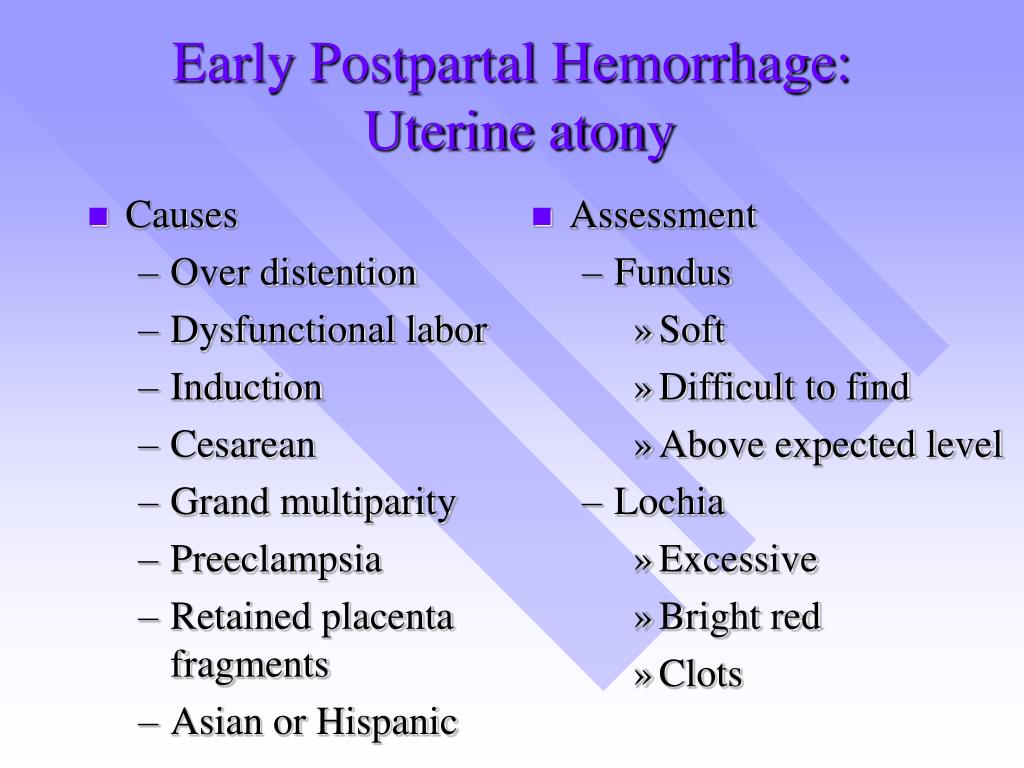 12 However, most studies have shown that oxytocin is superior to misoprostol.12,18 Misoprostol also causes more adverse effects than oxytocin—commonly nausea, diarrhea, and fever within three hours of birth.12,18
12 However, most studies have shown that oxytocin is superior to misoprostol.12,18 Misoprostol also causes more adverse effects than oxytocin—commonly nausea, diarrhea, and fever within three hours of birth.12,18
The benefits of controlled cord traction and uterine massage in preventing postpartum hemorrhage are less clear, but these strategies may be helpful.15,19,20 Controlled cord traction does not prevent severe postpartum hemorrhage, but reduces the incidence of less severe blood loss (500 to 1,000 mL) and reduces the need for manual extraction of the placenta.21
Diagnosis and Management
Diagnosis of postpartum hemorrhage begins with recognition of excessive bleeding and targeted examination to determine its cause (Figure 16). Cumulative blood loss should be monitored throughout labor and delivery and postpartum with quantitative measurement, if possible.22 Although some important sources of blood loss may occur intrapartum (e. g., episiotomy, uterine rupture), most of the fluid expelled during delivery of the infant is urine or amniotic fluid. Quantitative measurement of postpartum bleeding begins immediately after the birth of the infant and entails measuring cumulative blood loss with a calibrated underbuttocks drape, or by weighing blood-soaked pads, sponges, and clots; combined use of these methods is also appropriate for obtaining an accurate measurement.22 Healthy pregnant women can typically tolerate 500 to 1,000 mL of blood loss without having signs or symptoms.9 Tachycardia may be the earliest sign of postpartum hemorrhage. Orthostasis, hypotension, nausea, dyspnea, oliguria, and chest pain may indicate hypovolemia from significant hemorrhage. If excess bleeding is diagnosed, the Four T's mnemonic (uterine atony [Tone]; laceration, hematoma, inversion, rupture [Trauma]; retained tissue or invasive placenta [Tissue]; and coagulopathy [Thrombin]) can be used to identify specific causes (Table 56).
g., episiotomy, uterine rupture), most of the fluid expelled during delivery of the infant is urine or amniotic fluid. Quantitative measurement of postpartum bleeding begins immediately after the birth of the infant and entails measuring cumulative blood loss with a calibrated underbuttocks drape, or by weighing blood-soaked pads, sponges, and clots; combined use of these methods is also appropriate for obtaining an accurate measurement.22 Healthy pregnant women can typically tolerate 500 to 1,000 mL of blood loss without having signs or symptoms.9 Tachycardia may be the earliest sign of postpartum hemorrhage. Orthostasis, hypotension, nausea, dyspnea, oliguria, and chest pain may indicate hypovolemia from significant hemorrhage. If excess bleeding is diagnosed, the Four T's mnemonic (uterine atony [Tone]; laceration, hematoma, inversion, rupture [Trauma]; retained tissue or invasive placenta [Tissue]; and coagulopathy [Thrombin]) can be used to identify specific causes (Table 56). Regardless of the cause of bleeding, physicians should immediately summon additional personnel and begin appropriate emergency hemorrhage protocols.
Regardless of the cause of bleeding, physicians should immediately summon additional personnel and begin appropriate emergency hemorrhage protocols.
| Pathology | Specific cause | Approximate incidence (%) |
|---|---|---|
| Tone | Atonic uterus | 70 |
| Trauma | Lacerations, hematomas, inversion, rupture | 20 |
| Tissue | Retained tissue, invasive placenta | 10 |
| Thrombin | Coagulopathies | 1 |
TONE (UTERINE ATONY)
Uterine atony is the most common cause of postpartum hemorrhage. 9 Brisk blood flow after delivery of the placenta unresponsive to transabdominal massage should prompt immediate action including bimanual compression of the uterus and use of uterotonic medications (Table 46). Massage is performed by placing one hand in the vagina and pushing against the body of the uterus while the other hand compresses the fundus from above through the abdominal wall (eFigure B).
9 Brisk blood flow after delivery of the placenta unresponsive to transabdominal massage should prompt immediate action including bimanual compression of the uterus and use of uterotonic medications (Table 46). Massage is performed by placing one hand in the vagina and pushing against the body of the uterus while the other hand compresses the fundus from above through the abdominal wall (eFigure B).
Uterotonic agents include oxytocin, ergot alkaloids, and prostaglandins. Oxytocin is the most effective treatment for postpartum hemorrhage, even if already used for labor induction or augmentation or as part of AMTSL.8,23,24 The choice of a second-line uterotonic should be based on patient-specific factors such as hypertension, asthma, or use of protease inhibitors. Although it is not a uterotonic, tranexamic acid (Cyklokapron) may reduce mortality due to bleeding from postpartum hemorrhage (but not overall mortality) when given within the first three hours and may be considered as an adjuvant therapy. 25[updated] Table 4 outlines dosages, cautions, contraindications, and common adverse effects of uterotonic medications and tranexamic acid.6
25[updated] Table 4 outlines dosages, cautions, contraindications, and common adverse effects of uterotonic medications and tranexamic acid.6
TRAUMA
Lacerations and hematomas due to birth trauma can cause significant blood loss that can be lessened by hemostasis and timely repair. Episiotomy increases the risk of blood loss and anal sphincter tears; this procedure should be avoided unless urgent delivery is necessary and the perineum is thought to be a limiting factor.26
Vaginal and vulvar hematomas can present as pain or as a change in vital signs disproportionate to the amount of blood loss. Small hematomas can be managed with ice packs, analgesia, and observation. Patients with persistent signs of volume loss despite fluid replacement, as well as those with large (greater than 3 to 4 cm) or enlarging hematomas, require incision and evacuation of the clot.27 The involved area should be irrigated and hemostasis achieved by ligating bleeding vessels, placing figure-of-eight sutures, and creating a layered closure, or by using any of these methods alone.
Uterine inversion is rare, occurring in only 0.04% of deliveries, and is a potential cause of postpartum hemorrhage.27 AMTSL does not appear to increase the incidence of uterine inversion, but invasive placenta does.27,28 The contributions of other conditions such as fundal implantation of the placenta, fundal pressure, and undue cord traction are unclear.27 The inverted uterus usually appears as a bluish-gray mass protruding from the vagina. Patients with uterine inversion may have signs of shock without excess blood loss. If the placenta is attached, it should be left in place until after reduction to limit hemorrhage.27 Every attempt should be made to quickly replace the uterus. The Johnson method of reduction begins with grasping the protruding fundus with the palm of the hand, directing the fingers toward the posterior fornix.27 The uterus is returned to position by lifting it up through the pelvis and into the abdomen (eFigure C). Once the uterus is reverted, uterotonic agents can promote uterine tone and prevent recurrence. If initial attempts to replace the uterus fail or contraction of the lower uterine segment (contraction ring) develops, the use of magnesium sulfate, terbutaline, nitroglycerin, or general anesthesia may allow sufficient uterine relaxation for manipulation.28
Once the uterus is reverted, uterotonic agents can promote uterine tone and prevent recurrence. If initial attempts to replace the uterus fail or contraction of the lower uterine segment (contraction ring) develops, the use of magnesium sulfate, terbutaline, nitroglycerin, or general anesthesia may allow sufficient uterine relaxation for manipulation.28
Uterine rupture can cause intrapartum and postpartum hemorrhage.29 Although rare in an unscarred uterus, clinically significant uterine rupture occurs in 0.8% of vaginal births after cesarean delivery via low transverse uterine incision.30 Induction and augmentation increase the risk of uterine rupture, especially for patients with prior cesarean delivery.31 Before delivery, the primary sign of uterine rupture is fetal bradycardia.31,32 Other signs and symptoms of uterine rupture are listed in eTable A.
| Abdominal tenderness |
| Circulatory collapse |
| Elevation of presenting fetal part |
| Fetal bradycardia* |
| Increasing abdominal girth |
| Loss of uterine contractions |
| Maternal tachycardia |
| Vaginal bleeding |
TISSUE
Retained tissue (i. e., placenta, placental fragments, or blood clots) prevents the uterus from contracting enough to achieve optimal tone. Classic signs of placental separation include a small gush of blood, lengthening of the umbilical cord, and a slight rise of the uterus. The mean time from delivery to placental expulsion is eight to nine minutes.33 Longer intervals are associated with an increased risk of postpartum hemorrhage, with rates doubling after 10 minutes.33 Retained placenta (i.e., failure of the placenta to deliver within 30 minutes) occurs in less than 3% of vaginal deliveries.34,35 If the placenta is retained, consider manual removal using appropriate analgesia.35 Injecting the umbilical vein with saline and oxytocin does not clearly reduce the need for manual removal.35–37 If blunt dissection with the edge of the gloved hand does not reveal the tissue plane between the uterine wall and placenta, invasive placenta should be considered.
e., placenta, placental fragments, or blood clots) prevents the uterus from contracting enough to achieve optimal tone. Classic signs of placental separation include a small gush of blood, lengthening of the umbilical cord, and a slight rise of the uterus. The mean time from delivery to placental expulsion is eight to nine minutes.33 Longer intervals are associated with an increased risk of postpartum hemorrhage, with rates doubling after 10 minutes.33 Retained placenta (i.e., failure of the placenta to deliver within 30 minutes) occurs in less than 3% of vaginal deliveries.34,35 If the placenta is retained, consider manual removal using appropriate analgesia.35 Injecting the umbilical vein with saline and oxytocin does not clearly reduce the need for manual removal.35–37 If blunt dissection with the edge of the gloved hand does not reveal the tissue plane between the uterine wall and placenta, invasive placenta should be considered.
Invasive placenta (placenta accreta, increta, or percreta) can cause life-threatening postpartum hemorrhage.13,34,35 The incidence has increased with time, mirroring the increase in cesarean deliveries.13,34 In addition to prior cesarean delivery, other risk factors for invasive placenta include placenta previa, advanced maternal age, high parity, and previous invasive placenta.13,34 Treatment of invasive placenta can require hysterectomy or, in select cases, conservative management (i.e., leaving the placenta in place or giving weekly oral methotrexate).13
THROMBIN (COAGULATION DEFECTS)
Coagulation defects can cause a hemorrhage or be the result of one. These defects should be suspected in patients who have not responded to the usual measures to treat postpartum hemorrhage or who are oozing from puncture sites. A coagulation defect should also be suspected if blood does not clot in bedside receptacles or red-top (no additives) laboratory collection tubes within five to 10 minutes. Coagulation defects may be congenital or acquired (eTable B). Evaluation should include a platelet count and measurement of prothrombin time, partial thromboplastin time, fibrinogen level, fibrin split products, and quantitative d-dimer assay. Physicians should treat the underlying disease process, if known, and support intravascular volume, serially evaluate coagulation status, and replace appropriate blood components using an emergency release protocol to improve response time and decrease risk of dilutional coagulopathy.7,38,39 [updated]
Coagulation defects may be congenital or acquired (eTable B). Evaluation should include a platelet count and measurement of prothrombin time, partial thromboplastin time, fibrinogen level, fibrin split products, and quantitative d-dimer assay. Physicians should treat the underlying disease process, if known, and support intravascular volume, serially evaluate coagulation status, and replace appropriate blood components using an emergency release protocol to improve response time and decrease risk of dilutional coagulopathy.7,38,39 [updated]
| Acquired |
| Amniotic fluid embolism |
| Consumptive coagulation secondary to excessive bleeding of any origin |
| Disseminated intravascular coagulation secondary to abruption |
| Fetal demise |
| HELLP (hemolysis, elevated liver enzyme levels, and low platelet levels) syndrome |
| Placental abruption |
| Preeclampsia with severe features |
| Sepsis |
| Use of anticoagulants such as aspirin or heparin |
| Chronic or congenital |
| Hemophilia |
| Idiopathic thrombocytopenic purpura |
| Thrombotic thrombocytopenic purpura |
| Von Willebrand disease |
Ongoing or Severe Hemorrhage
Significant blood loss from any cause requires immediate resuscitation measures using an interdisciplinary, stage-based team approach.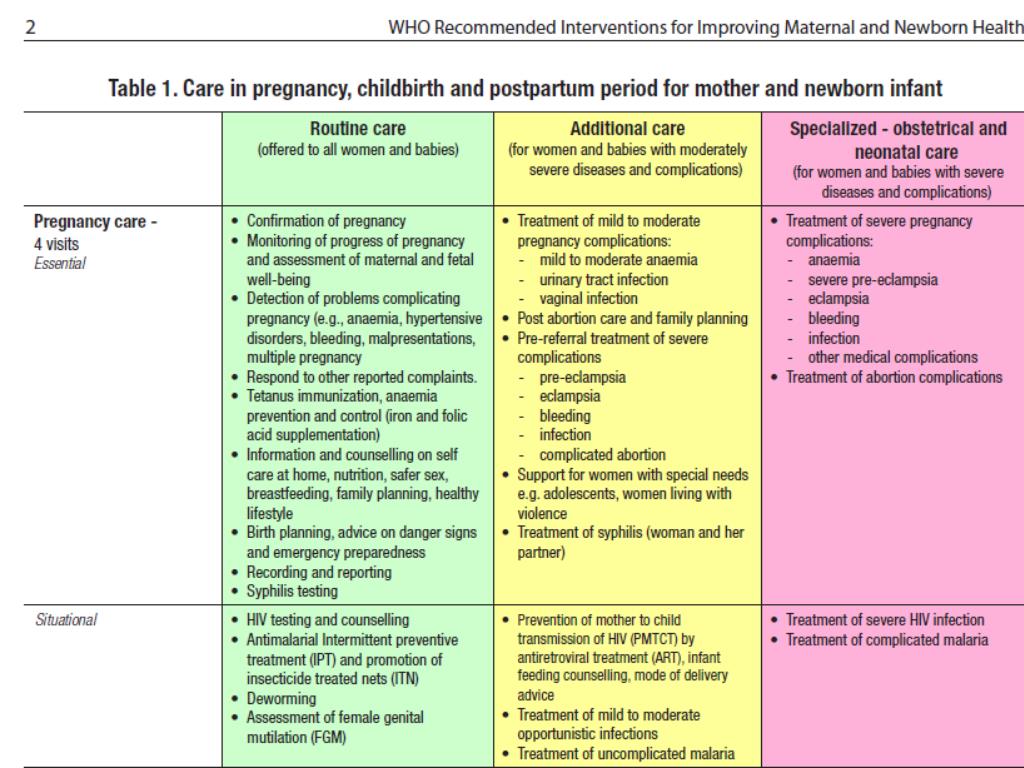 Physicians should perform a primary maternal survey and institute care based on American Heart Association standards and an assessment of blood loss.14,40 Patients should be given oxygen, ventilated as needed, and provided intravenous fluid and blood replacement with normal saline or other crystalloid fluids administered through two large-bore intravenous needles. Fluid replacement volume should initially be given as a bolus infusion and subsequently adjusted based on frequent reevaluation of the patient's vital signs and symptoms. The use of O negative blood may be needed while waiting for type-specific blood.
Physicians should perform a primary maternal survey and institute care based on American Heart Association standards and an assessment of blood loss.14,40 Patients should be given oxygen, ventilated as needed, and provided intravenous fluid and blood replacement with normal saline or other crystalloid fluids administered through two large-bore intravenous needles. Fluid replacement volume should initially be given as a bolus infusion and subsequently adjusted based on frequent reevaluation of the patient's vital signs and symptoms. The use of O negative blood may be needed while waiting for type-specific blood.
Elevating the patient's legs will improve venous return. Draining the bladder with a Foley catheter may improve uterine atony and will allow monitoring of urine output. Massive transfusion protocols to decrease the risk of dilutional coagulopathy and other postpartum hemorrhage complications have been established. These protocols typically recommend the use of four units of fresh frozen plasma and one unit of platelets for every four to six units of packed red blood cells used.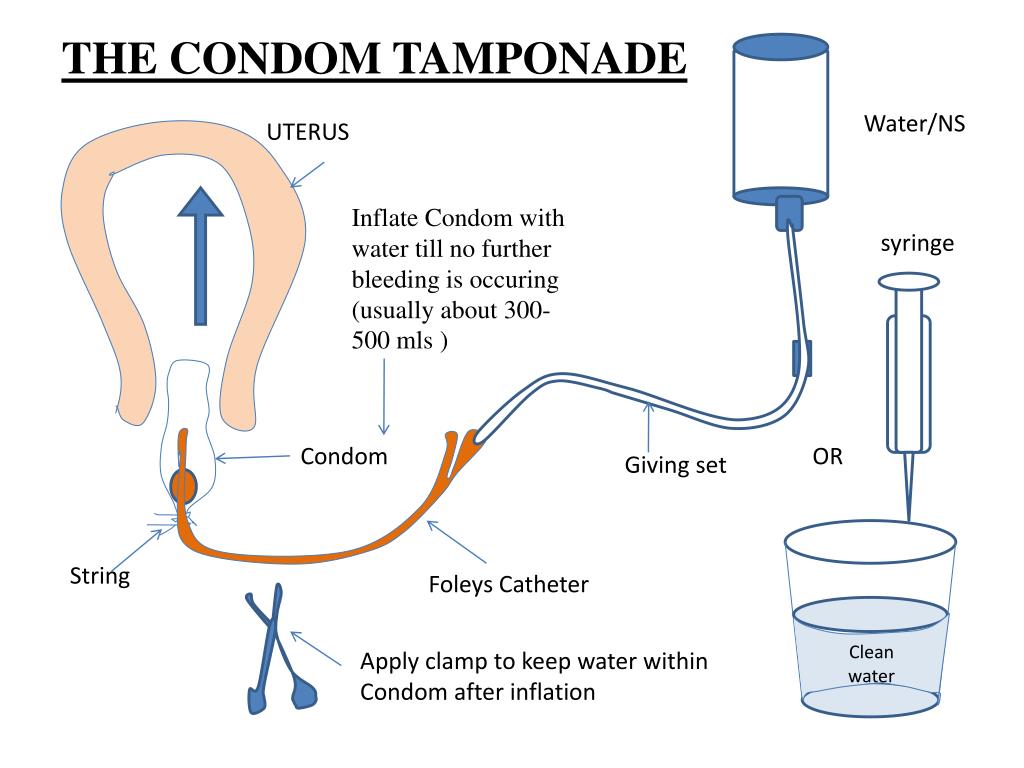 7,39
7,39
Uterus-conserving treatments include uterine packing (plain gauze or gauze soaked with vasopressin, chitosan, or carboprost [Hemabate]), artery ligation, uterine artery embolization, B-lynch compression sutures, and balloon tamponade.7,41–43 Balloon tamponade (in which direct pressure is applied to potential bleeding sites via a balloon that is inserted through the vagina and cervix and inflated with sterile water or saline), uterine packing, aortic compression, and nonpneumatic antishock garments may be used to limit bleeding while definitive treatment or transport is arranged.7,41,44 Hysterectomy is the definitive treatment in women with severe, intractable hemorrhage.
Follow-up of postpartum hemorrhage includes monitoring for ongoing blood loss and vital signs, assessing for signs of anemia (fatigue, shortness of breath, chest pain, or lactation problems), and debriefing with patients and staff. Many patients experience acute and posttraumatic stress disorders after a traumatic delivery.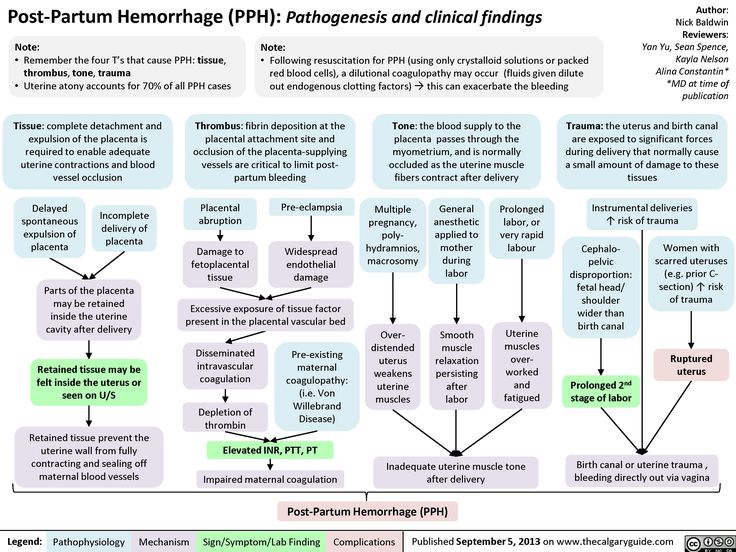 Individual, trauma-focused cognitive behavior therapy can be offered to reduce acute traumatic stress symptoms.45 Debriefing with staff may identify necessary systems-level changes (Table 3).6,10–14
Individual, trauma-focused cognitive behavior therapy can be offered to reduce acute traumatic stress symptoms.45 Debriefing with staff may identify necessary systems-level changes (Table 3).6,10–14
Systems Approach to Prevention and Treatment
Complications of postpartum hemorrhage are common, even in high-resource countries and well-staffed delivery suites. Based on an analysis of systems errors identified in The Joint Commission's 2010 Sentinel Event Alert, the commission recommended that hospitals establish protocols to enable an optimal response to changes in maternal vital signs and clinical condition. These protocols should be tested in drills, and systems problems that interfere with care should be fixed through their continual refinement.46 In response, The Council on Patient Safety in Women's Health Care outlined essential steps that delivery units should take to decrease the incidence and severity of postpartum hemorrhage14(Table 36,10–14). The creation of a hemorrhage cart with supplies, and the use of huddles, rapid response teams, and massive transfusion protocols are among the recommendations. Advanced Life Support in Obstetrics (ALSO) training can be part of a systems approach to improving patient care. The use of interdisciplinary team training with in situ simulation, available through the ALSO program and from TeamSTEPPS (Team Strategies and Tools to Enhance Performance and Patient Safety), has been shown to improve perinatal safety.47,48
The creation of a hemorrhage cart with supplies, and the use of huddles, rapid response teams, and massive transfusion protocols are among the recommendations. Advanced Life Support in Obstetrics (ALSO) training can be part of a systems approach to improving patient care. The use of interdisciplinary team training with in situ simulation, available through the ALSO program and from TeamSTEPPS (Team Strategies and Tools to Enhance Performance and Patient Safety), has been shown to improve perinatal safety.47,48
Data Sources: A PubMed search was completed in Clinical Queries using the key term postpartum hemorrhage. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Cochrane Database of Systematic Reviews, Essential Evidence Plus, National Institute for Health and Care Excellence guidelines, Agency for Healthcare Research and Quality evidence reports, the Institute for Clinical Systems Improvement, and the National Guideline Clearinghouse. Search dates: October 12, 2015, and January 19, 2016.
Search dates: October 12, 2015, and January 19, 2016.
This article is one in a series on “Advanced Life Support in Obstetrics (ALSO),” initially established by Mark Deutchman, MD, Denver, Colo. The coordinator of this series is Larry Leeman, MD, MPH, ALSO Managing Editor, Albuquerque, N.M.
Prevention and Management of Postpartum Hemorrhage
JANICE M. ANDERSON, M.D., AND DUNCAN ETCHES, M.D., M.CL.SC.
Postpartum hemorrhage, the loss of more than 500 mL of blood after delivery, occurs in up to 18 percent of births and is the most common maternal morbidity in developed countries. Although risk factors and preventive strategies are clearly documented, not all cases are expected or avoidable. Uterine atony is responsible for most cases and can be managed with uterine massage in conjunction with oxytocin, prostaglandins, and ergot alkaloids. Retained placenta is a less common cause and requires examination of the placenta, exploration of the uterine cavity, and manual removal of retained tissue. Rarely, an invasive placenta causes postpartum hemorrhage and may require surgical management. Traumatic causes include lacerations, uterine rupture, and uterine inversion. Coagulopathies require clotting factor replacement for the identified deficiency. Early recognition, systematic evaluation and treatment, and prompt fluid resuscitation minimize the potentially serious outcomes associated with postpartum hemorrhage.
Although risk factors and preventive strategies are clearly documented, not all cases are expected or avoidable. Uterine atony is responsible for most cases and can be managed with uterine massage in conjunction with oxytocin, prostaglandins, and ergot alkaloids. Retained placenta is a less common cause and requires examination of the placenta, exploration of the uterine cavity, and manual removal of retained tissue. Rarely, an invasive placenta causes postpartum hemorrhage and may require surgical management. Traumatic causes include lacerations, uterine rupture, and uterine inversion. Coagulopathies require clotting factor replacement for the identified deficiency. Early recognition, systematic evaluation and treatment, and prompt fluid resuscitation minimize the potentially serious outcomes associated with postpartum hemorrhage.
Postpartum hemorrhage, defined as the loss of more than 500 mL of blood after delivery, occurs in up to 18 percent of births. 1,2 Blood loss exceeding 1,000 mL is considered physiologically significant and can result in hemodynamic instability.3 Even with appropriate management, approximately 3 percent of vaginal deliveries will result in severe post-partum hemorrhage.4 It is the most common maternal morbidity in developed countries and a major cause of death worldwide.1,3
1,2 Blood loss exceeding 1,000 mL is considered physiologically significant and can result in hemodynamic instability.3 Even with appropriate management, approximately 3 percent of vaginal deliveries will result in severe post-partum hemorrhage.4 It is the most common maternal morbidity in developed countries and a major cause of death worldwide.1,3
Complications from postpartum hemorrhage include orthostatic hypotension, anemia, and fatigue, which may make maternal care of the newborn more difficult. Post-partum anemia increases the risk of post-partum depression.5 Blood transfusion may be necessary and carries associated risks.6 In the most severe cases, hemorrhagic shock may lead to anterior pituitary ischemia with delay or failure of lactation (i.e., postpartum pituitary necrosis).7,8 Occult myocardial ischemia, dilutional coagulopathy, and death also may occur.9 Delayed postpartum hemorrhage, bleeding after 24 hours as a result of sloughing of the placental eschar or retained placental fragments, also can occur. 10
10
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Active management of the third stage of labor decreases postpartum blood loss and the risk of postpartum hemorrhage (number needed to treat=12). | A | 2,17 |
| Active management of the third stage of labor does not increase the risk of retained placenta. | A | 2,17,18 |
| Oxytocin (Pitocin) is the first choice for prevention of postpartum hemorrhage because it is as effective or more effective than ergot alkaloids or prostaglandins and has fewer side effects. | A | 2,25,26 |
Misoprostol (Cytotec) may be used when other oxytocic agents are not available for prevention of postpartum hemorrhage (number needed to treat=18). | A | 27 |
| Misoprostol may be used for treatment of postpartum hemorrhage, but this agent is associated with more side effects than conventional uterotonic drugs. | A | 37 |
| Routine episiotomy increases anal sphincter tears and blood loss. | A | 11,40 |
Prevention
Risk factors for postpartum hemorrhage include a prolonged third stage of labor, multiple delivery, episiotomy, fetal macrosomia, and history of postpartum hemorrhage.3,4,11,12 However, postpartum hemorrhage also occurs in women with no risk factors, so physicians must be prepared to manage this condition at every delivery.13 Strategies for minimizing the effects of postpartum hemorrhage include identifying and correcting anemia before delivery, being aware of the mother's beliefs about blood transfusions, and eliminating routine episiotomy. 14–16 Reexamination of the patient's vital signs and vaginal flow before leaving the delivery area may help detect slow, steady bleeding.
14–16 Reexamination of the patient's vital signs and vaginal flow before leaving the delivery area may help detect slow, steady bleeding.
The best preventive strategy is active management of the third stage of labor (number needed to treat [NNT] to prevent one case of postpartum hemorrhage = 12).17,18 Hospital guidelines encouraging this practice have resulted in significant reductions in the incidence of massive hemorrhage.19 Active management, which involves administering a uterotonic drug with or soon after the delivery of the anterior shoulder, controlled cord traction, and, usually, early cord clamping and cutting, decreases the risk of postpartum hemorrhage and shortens the third stage of labor with no significant increase in the risk of retained placenta.17,18 Compared with expectant management, in which the placenta is allowed to separate spontaneously aided only by gravity or nipple stimulation, active management decreases the incidence of postpartum hemorrhage by 68 percent. 17
17
Early cord clamping is no longer included in the International Federation of Gynecology and Obstetrics (FIGO) definition of active management of the third stage of labor, and uterine massage after delivery of the placenta has been added.20 Delaying cord clamping for about 60 seconds has the benefit of increasing iron stores and decreasing anemia, which is especially important in preterm infants and in low-resource settings.16,21–23 The delay has not been shown to increase neonatal morbidity or maternal blood loss.16,21,23
Prophylactic administration of oxytocin (Pitocin) reduces rates of postpartum hemorrhage by 40 percent24; this reduction also occurs if oxytocin is given after placental delivery.2,18 Oxytocin is the drug of choice for preventing postpartum hemorrhage because it is at least as effective as ergot alkaloids or prostaglandins and has fewer side effects.2,25,26 Misoprostol (Cytotec) has a role in the prevention of postpartum hemorrhage (NNT = 18)16; this agent has more side effects but is inexpensive, heat- and light-stable, and requires no syringes. 27
27
Diagnosis and Management
The diagnosis of postpartum hemorrhage begins with recognition of excessive bleeding and methodic examination to determine its cause (Figure 1). The “Four Ts” mnemonic (Tone, Trauma, Tissue, and Thrombin) can be used to detect specific causes (Table 1).
| Four Ts | Cause | Approximate incidence (%) |
|---|---|---|
| Tone | Atonic uterus | 70 |
| Trauma | Lacerations, hematomas, inversion, rupture | 20 |
| Tissue | Retained tissue, invasive placenta | 10 |
| Thrombin | Coagulopathies | 1 |
Uterine atony is the most common cause of postpartum hemorrhage. 28 Because hemostasis associated with placental separation depends on myometrial contraction, atony is treated initially by bimanual uterine compression and massage, followed by drugs that promote uterine contraction.
28 Because hemostasis associated with placental separation depends on myometrial contraction, atony is treated initially by bimanual uterine compression and massage, followed by drugs that promote uterine contraction.
Uterine Massage
Brisk blood flow after delivery of the placenta should alert the physician to perform a bimanual examination of the uterus. If the uterus is soft, massage is performed by placing one hand in the vagina and pushing against the body of the uterus while the other hand compresses the fundus from above through the abdominal wall (Figure 2).29 The posterior aspect of the uterus is massaged with the abdominal hand and the anterior aspect with the vaginal hand.
Uterotonic Agents
Uterotonic agents include oxytocin, ergot alkaloids, and prostaglandins. Oxytocin stimulates the upper segment of the myometrium to contract rhythmically, which constricts spiral arteries and decreases blood flow through the uterus.30 Oxytocin is an effective first-line treatment for postpartum hemorrhage31; 10 international units (IU) should be injected intramuscularly, or 20 IU in 1 L of saline may be infused at a rate of 250 mL per hour.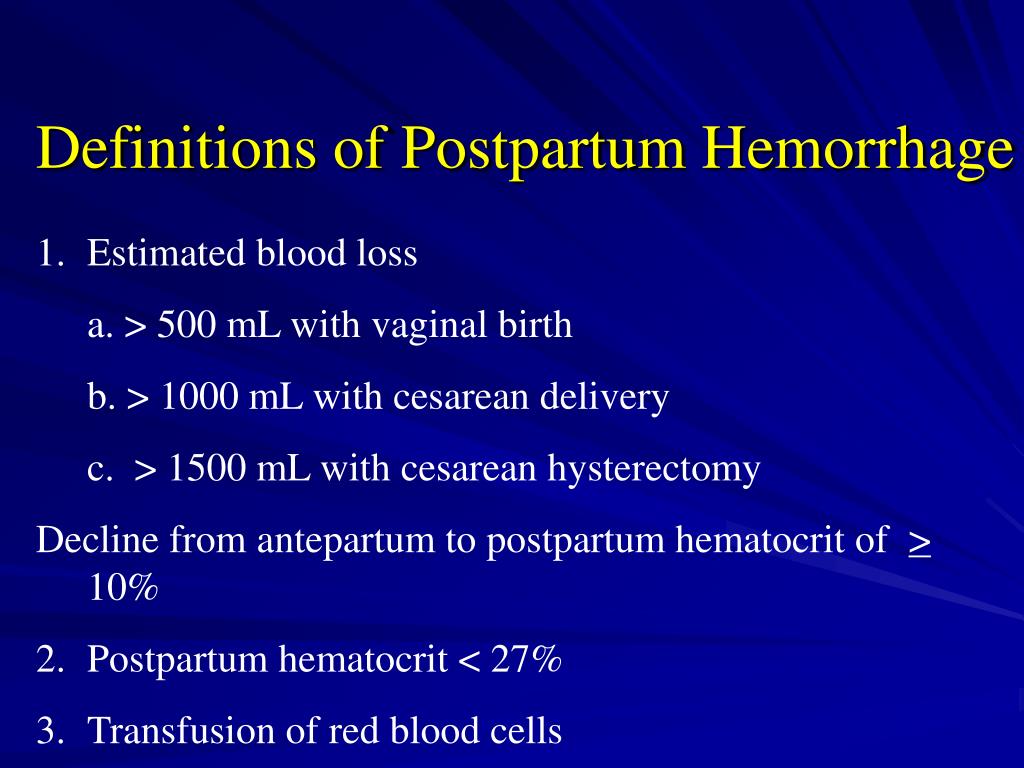 As much as 500 mL can be infused over 10 minutes without complications.10
As much as 500 mL can be infused over 10 minutes without complications.10
Methylergonovine (Methergine) and ergometrine (not available in the United States) are ergot alkaloids that cause generalized smooth muscle contraction in which the upper and lower segments of the uterus contract tetanically.32 A typical dose of methylergonovine, 0.2 mg administered intramuscularly, may be repeated as required at intervals of two to four hours. Because ergot alkaloid agents raise blood pressure, they are contraindicated in women with preclampsia or hypertension.33 Other adverse effects include nausea and vomiting.33
Prostaglandins enhance uterine contractility and cause vasoconstriction.34 The prostaglandin most commonly used is 15-methyl prostaglandin F2a, or carboprost (Hemabate). Carboprost can be administered intramyometrially or intramuscularly in a dose of 0.25 mg; this dose can be repeated every 15 minutes for a total dose of 2 mg. Carboprost has been proven to control hemorrhage in up to 87 percent of patients.35 In cases where it is not effective, chorioamnionitis or other risk factors for hemorrhage often are present.35 Hypersensitivity is the only absolute contraindication, but carboprost should be used with caution in patients with asthma or hypertension. Side effects include nausea, vomiting, diarrhea, hypertension, headache, flushing, and pyrexia.34
Carboprost has been proven to control hemorrhage in up to 87 percent of patients.35 In cases where it is not effective, chorioamnionitis or other risk factors for hemorrhage often are present.35 Hypersensitivity is the only absolute contraindication, but carboprost should be used with caution in patients with asthma or hypertension. Side effects include nausea, vomiting, diarrhea, hypertension, headache, flushing, and pyrexia.34
Misoprostol is another prostaglandin that increases uterine tone and decreases postpartum bleeding.36 Misoprostol is effective in the treatment of postpartum hemorrhage, but side effects may limit its use.28,37 It can be administered sublingually, orally, vaginally, and rectally. Doses range from 200 to 1,000 mcg; the dose recommended by FIGO is 1,000 mcg administered rectally.28,37,38 Higher peak levels and larger doses are associated with more side effects, including shivering, pyrexia, and diarrhea. 28,39 Although misoprostol is widely used in the treatment of postpartum hemorrhage, it is not approved by the U.S. Food and Drug Administration for this indication.
28,39 Although misoprostol is widely used in the treatment of postpartum hemorrhage, it is not approved by the U.S. Food and Drug Administration for this indication.
TRAUMA
Lacerations and hematomas resulting from birth trauma can cause significant blood loss that can be lessened by hemostasis and timely repair. Sutures should be placed if direct pressure does not stop the bleeding. Episiotomy increases blood loss and the risk of anal sphincter tears,11,12,40 and this procedure should be avoided unless urgent delivery is necessary and the perineum is thought to be a limiting factor.14
Hematomas can present as pain or as a change in vital signs disproportionate to the amount of blood loss. Small hematomas can be managed with close observation.41 Patients with persistent signs of volume loss despite fluid replacement, as well as those with large or enlarging hematomas, require incision and evacuation of the clot.41 The involved area should be irrigated and the bleeding vessels ligated. In patients with diffuse oozing, a layered closure will help to secure hemostasis and eliminate dead space.
In patients with diffuse oozing, a layered closure will help to secure hemostasis and eliminate dead space.
Uterine Inversion
Uterine inversion is rare, occurring in 0.05 percent of deliveries.10 Active management of the third stage of labor may reduce the incidence of uterine inversion.42 Fundal implantation of the placenta may lead to inversion; the roles of fundal pressure and undue cord traction are uncertain.10 The inverted uterus usually appears as a bluish-gray mass protruding from the vagina. Vasovagal effects producing vital sign changes disproportionate to the amount of bleeding may be an additional clue. The placenta often is still attached, and it should be left in place until after reduction.42 Every attempt should be made to replace the uterus quickly. The Johnson method of reduction begins with grasping the protruding fundus Figure 3A29) with the palm of the hand and fingers directed toward the posterior fornix (Figure 3B29). The uterus is returned to position by lifting it up through the pelvis and into the abdomen (Figure 3C29).43 Once the uterus is reverted, uterotonic agents should be given to promote uterine tone and to prevent recurrence. If initial attempts to replace the uterus fail or a cervical contraction ring develops, administration of magnesium sulfate, terbutaline (Brethine), nitroglycerin, or general anesthesia may allow sufficient uterine relaxation for manipulation. If these methods fail, the uterus will need to be replaced surgically.42
The uterus is returned to position by lifting it up through the pelvis and into the abdomen (Figure 3C29).43 Once the uterus is reverted, uterotonic agents should be given to promote uterine tone and to prevent recurrence. If initial attempts to replace the uterus fail or a cervical contraction ring develops, administration of magnesium sulfate, terbutaline (Brethine), nitroglycerin, or general anesthesia may allow sufficient uterine relaxation for manipulation. If these methods fail, the uterus will need to be replaced surgically.42
Uterine Rupture
Although rare in an unscarred uterus, clinically significant uterine rupture occurs in 0.6 to 0.7 percent of vaginal births after cesarean delivery in women with a low transverse or unknown uterine scar.44–46 The risk increases significantly with previous classical incisions or uterine surgeries, and to a lesser extent with shorter intervals between pregnancies or a history of multiple cesarean deliveries, particularly in women with no previous vaginal deliveries. 44–48 Compared with spontaneous labor, induction or augmentation increases the rate of uterine rupture, more so if prostaglandins and oxytocin are used sequentially. However, the incidence of rupture is still low (i.e., 1 to 2.4 percent).46,48 Misoprostol should not be used for cervical ripening or induction when attempting vaginal birth after previous cesarean delivery.48
44–48 Compared with spontaneous labor, induction or augmentation increases the rate of uterine rupture, more so if prostaglandins and oxytocin are used sequentially. However, the incidence of rupture is still low (i.e., 1 to 2.4 percent).46,48 Misoprostol should not be used for cervical ripening or induction when attempting vaginal birth after previous cesarean delivery.48
Before delivery, the primary sign of uterine rupture is fetal bradycardia.45 Tachycardia or late decelerations can also herald a uterine rupture, as can vaginal bleeding, abdominal tenderness, maternal tachycardia, circulatory collapse, or increasing abdominal girth.47 Symptomatic uterine rupture requires surgical repair of the defect or hysterectomy. When detected in the postpartum period, a small asymptomatic lower uterine segment defect or bloodless dehiscence can be followed expectantly.47
TISSUE
Classic signs of placental separation include a small gush of blood with lengthening of the umbilical cord and a slight rise of the uterus in the pelvis. Placental delivery can be achieved by use of the Brandt-Andrews maneuver, which involves applying firm traction on the umbilical cord with one hand while the other applies suprapubic counterpressure (Figure 429).49 The mean time from delivery until placental expulsion is eight to nine minutes.4 Longer intervals are associated with an increased risk of postpartum hemorrhage, with rates doubling after 10 minutes.4 Retained placenta (i.e., failure of the placenta to deliver within 30 minutes after birth) occurs in less than 3 percent of vaginal deliveries.50 One management option is to inject the umbilical vein with 20 mL of a solution of 0.9 percent saline and 20 units of oxytocin. This significantly reduces the need for manual removal of the placenta compared with injecting saline alone.51 Alternatively, physicians may proceed directly to manual removal of the placenta, using appropriate analgesia. If the tissue plane between the uterine wall and placenta cannot be developed through blunt dissection with the edge of the gloved hand, invasive placenta should be considered.
Placental delivery can be achieved by use of the Brandt-Andrews maneuver, which involves applying firm traction on the umbilical cord with one hand while the other applies suprapubic counterpressure (Figure 429).49 The mean time from delivery until placental expulsion is eight to nine minutes.4 Longer intervals are associated with an increased risk of postpartum hemorrhage, with rates doubling after 10 minutes.4 Retained placenta (i.e., failure of the placenta to deliver within 30 minutes after birth) occurs in less than 3 percent of vaginal deliveries.50 One management option is to inject the umbilical vein with 20 mL of a solution of 0.9 percent saline and 20 units of oxytocin. This significantly reduces the need for manual removal of the placenta compared with injecting saline alone.51 Alternatively, physicians may proceed directly to manual removal of the placenta, using appropriate analgesia. If the tissue plane between the uterine wall and placenta cannot be developed through blunt dissection with the edge of the gloved hand, invasive placenta should be considered.
Invasive placenta can be life threatening.50 The incidence has increased from 0.003 percent to 0.04 percent of deliveries since 1950s; this increase is likely a result of the increase in cesarean section rates.49 Classification is based on the depth of invasion and can be easily remembered through alliteration: placenta accreta adheres to the myometrium, placenta increta invades the myometrium, and placenta percreta penetrates the myometrium to or beyond the serosa.10 Risk factors include advanced maternal age, high parity, previous invasive placenta or cesarean delivery, and placenta previa (especially in combination with previous cesarean delivery, increasing to 67 percent with four or more).49 The most common treatment for invasive placenta is hysterectomy.49 However, conservative management (i.e., leaving the placenta in place or giving weekly oral methotrexate52 until ⊠ human chorionic gonadotropin levels are 0) is sometimes successful. 53 Women treated for a retained placenta must be observed for late sequelae, including infection and late postpartum bleeding.52,53
53 Women treated for a retained placenta must be observed for late sequelae, including infection and late postpartum bleeding.52,53
THROMBIN
Coagulation disorders, a rare cause of post-partum hemorrhage, are unlikely to respond to the measures described above.10 Most coagulopathies are identified before delivery, allowing for advance planning to prevent postpartum hemorrhage. These disorders include idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, von Willebrand's disease, and hemophilia. Patients also can develop HELLP (hemolysis, elevated liver enzyme levels, and low platelet levels) syndrome or disseminated intravascular coagulation. Risk factors for disseminated intravascular coagulation include severe pre-eclampsia, amniotic fluid embolism, sepsis, placental abruption, and prolonged retention of fetal demise.54,55 Abruption is associated with cocaine use and hypertensive disorders.54 Excessive bleeding can deplete coagulation factors and lead to consumptive coagulation, which promotes further bleeding. Coagulation defects should be suspected in patients who have not responded to the usual measures to treat post-partum hemorrhage, and in those who are not forming blood clots or are oozing from puncture sites.
Coagulation defects should be suspected in patients who have not responded to the usual measures to treat post-partum hemorrhage, and in those who are not forming blood clots or are oozing from puncture sites.
Evaluation should include a platelet count and measurement of prothrombin time, partial thromboplastin time, fibrinogen level, and fibrin split products (i.e., d-dimer). Management consists of treating the underlying disease process, supporting intravascular volume, serially evaluating coagulation status, and replacing appropriate blood components. Administration of recombinant factor VIIa or clot-promoting medications (e.g., tranexamic acid [Cyklokapron]) may be considered.33,54,56
Clinical Approach
Significant blood loss from any cause requires standard maternal resuscitation measures (Figure 1). Blood loss of more than 1,000 mL requires quick action and an interdisciplinary team approach.54 Hysterectomy is the definitive treatment in women with severe, intractable hemorrhage. In patients who desire future fertility, uterus-conserving treatments include uterine packing or tamponade procedures, B-lynch uterine compression sutures, artery ligation, and uterine artery embolization.54,57
In patients who desire future fertility, uterus-conserving treatments include uterine packing or tamponade procedures, B-lynch uterine compression sutures, artery ligation, and uterine artery embolization.54,57
Sitemap
|
|
What is the best drug to reduce excessive blood loss after childbirth? nine0001
What is the problem?
The aim of this Cochrane review was to find out which drug is most effective in preventing excessive blood loss in labor and has the fewest side effects. We collected and analyzed all relevant studies to answer this question (search date: May 24, 2018).
We collected and analyzed all relevant studies to answer this question (search date: May 24, 2018).
Why is this important?
Excessive blood loss after childbirth (postpartum haemorrhage) is a common cause of maternal death during childbirth worldwide. Although most women experience moderate blood loss during childbirth, some women may experience excessive blood loss and this can pose a serious risk to their health and life. To reduce excessive bleeding in childbirth, the administration of a uterine contraction agent (uterotonic) has become standard practice throughout the world. nine0075
Various medications are routinely used in childbirth to reduce excessive bleeding. These are oxytocin, misoprostol, ergometrine, carbetocin, injectable prostaglandins, and combinations of these drugs, each with varying efficacy and side effects. Some reported side effects include vomiting, high blood pressure, and fever. Oxytocin is currently recommended as the standard treatment for reducing excess bleeding. We reviewed all available evidence to compare the efficacy and side effect profile of each drug. nine0075
We reviewed all available evidence to compare the efficacy and side effect profile of each drug. nine0075
What evidence did we find?
We found 196 studies involving 135,559 women. We compared seven uterotonics with each other and with no uterotonic treatment. The studies were carried out in 53 countries. In most studies, women gave birth naturally in a hospital setting.
This analysis suggests that all drugs are effective in preventing blood loss equal to or greater than 500 ml compared to no standard uterotonic treatment. Compared with oxytocin (the standard recommended treatment), the top three treatments for this outcome were ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin. We found that other drugs such as misoprostol, injectable prostaglandins, ergometrine may not (or only slightly) affect this outcome compared to oxytocin. nine0075
All drugs except ergometrine and injectable prostaglandins are effective in preventing blood loss equal to or greater than 1000 ml compared to no treatment. The combination of ergometrine with oxytocin and the combination of misoprostol with oxytocin do not (or only slightly) affect this outcome compared to oxytocin. Uncertainty remains as to whether carbetocin and ergometrine alone affect this outcome. However, misoprostol is less effective in preventing blood loss equal to or greater than 1000 ml compared to oxytocin. nine0075
The combination of ergometrine with oxytocin and the combination of misoprostol with oxytocin do not (or only slightly) affect this outcome compared to oxytocin. Uncertainty remains as to whether carbetocin and ergometrine alone affect this outcome. However, misoprostol is less effective in preventing blood loss equal to or greater than 1000 ml compared to oxytocin. nine0075
Misoprostol in combination with oxytocin reduces the use of additional uterotonics and probably reduces the risk of blood transfusion compared to oxytocin. Carbetocin, injectable prostaglandins, and the combination of ergometrine with oxytocin may also reduce the use of additional uterotonics, but the evidence is low-certainty. No significant differences were found between all agents in terms of maternal mortality or severe complications in childbirth, as these were rare in these studies. nine0075
Two drug combinations were associated with significant side effects. Compared with the oxytocin group, women who received misoprostol plus oxytocin were more likely to experience vomiting and fever.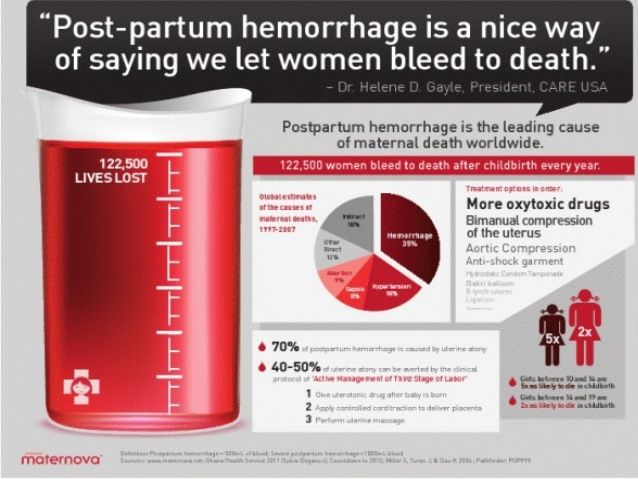 Women who received the combination of ergometrine and oxytocin were also more likely to vomit, and the combination may not (or only slightly) affect the risk of hypertension, but the evidence for this outcome was low-certainty.
Women who received the combination of ergometrine and oxytocin were also more likely to vomit, and the combination may not (or only slightly) affect the risk of hypertension, but the evidence for this outcome was low-certainty.
The analysis performed was similar whether women delivered vaginally or by caesarean section, in hospital or at home, at high or low risk of PPH, were receiving high or low dose misoprostol, and whether they received bolus or infusion of oxytocin, or both. nine0075
What does this mean?
All agents were generally effective in preventing excess bleeding compared with no treatment with uterotonic agents. The combination of ergometrine with oxytocin, carbetocin, and the combination of misoprostol with oxytocin may have additional benefits compared to standard administration of oxytocin. However, these two drug combinations are associated with significant side effects that may be worrisome for women when compared to oxytocin. Carbetocin may have some additional benefit over oxytocin, and does not appear to increase side effects.
 Practice
Practice