Over active thyroid and pregnancy
Thyroid Disease & Pregnancy | NIDDK
On this page:
- What role do thyroid hormones play in pregnancy?
- Hyperthyroidism in Pregnancy
- Hypothyroidism in Pregnancy
- Postpartum Thyroiditis
- Is it safe to breastfeed while I’m taking beta-blockers, thyroid hormone, or antithyroid medicines?
- Thyroid Disease and Eating During Pregnancy
- Clinical Trials
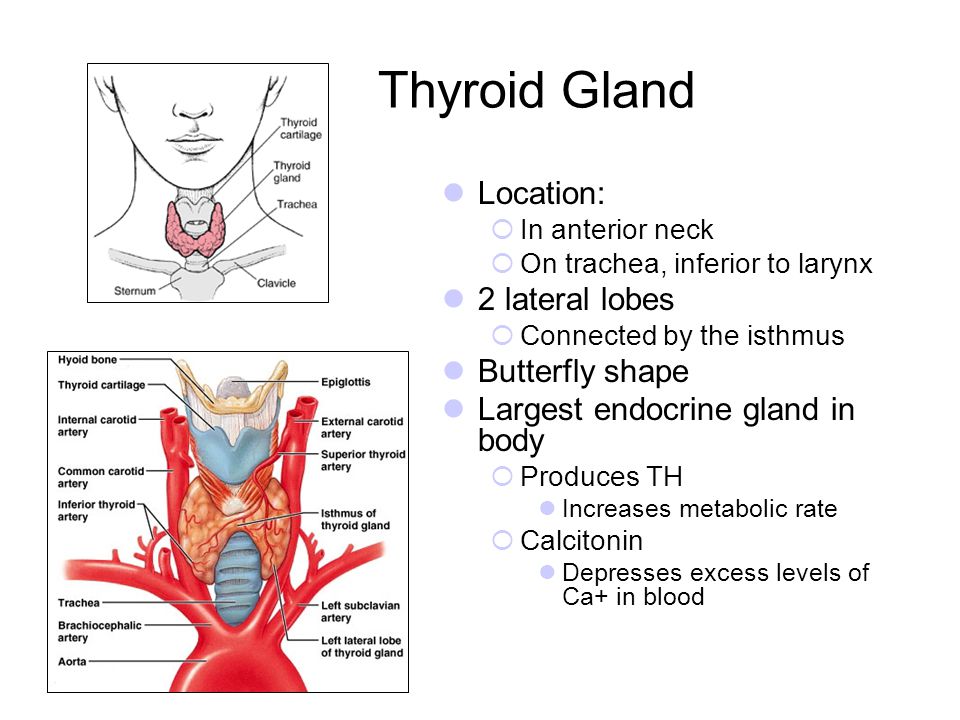
Thyroid disease is a group of disorders that affects the thyroid gland. The thyroid is a small, butterfly-shaped gland in the front of your neck that makes thyroid hormones. Thyroid hormones control how your body uses energy, so they affect the way nearly every organ in your body works—even the way your heart beats.
The thyroid is a small gland in your neck that makes thyroid hormones.Sometimes the thyroid makes too much or too little of these hormones. Too much thyroid hormone is called hyperthyroidism and can cause many of your body’s functions to speed up. “Hyper” means the thyroid is overactive. Learn more about hyperthyroidism in pregnancy. Too little thyroid hormone is called hypothyroidism and can cause many of your body’s functions to slow down. “Hypo” means the thyroid is underactive. Learn more about hypothyroidism in pregnancy.
If you have thyroid problems, you can still have a healthy pregnancy and protect your baby’s health by having regular thyroid function tests and taking any medicines that your doctor prescribes.
What role do thyroid hormones play in pregnancy?
Thyroid hormones are crucial for normal development of your baby’s brain and nervous system. During the first trimester—the first 3 months of pregnancy—your baby depends on your supply of thyroid hormone, which comes through the placenta. At around 12 weeks, your baby’s thyroid starts to work on its own, but it doesn’t make enough thyroid hormone until 18 to 20 weeks of pregnancy.
Two pregnancy-related hormones—human chorionic gonadotropin (hCG) and estrogen—cause higher measured thyroid hormone levels in your blood.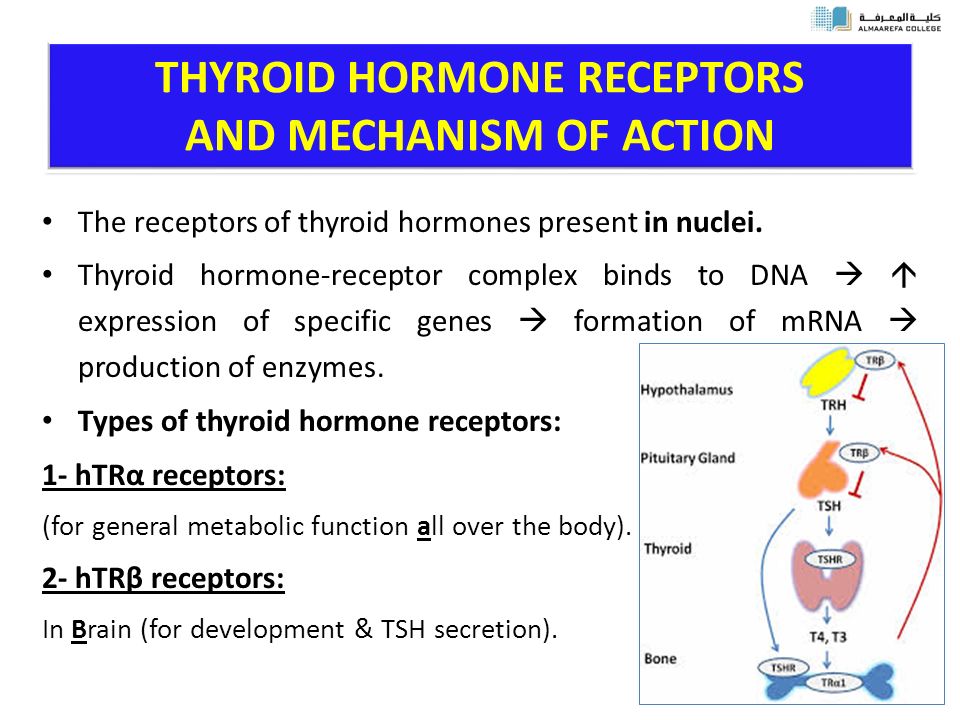 The thyroid enlarges slightly in healthy women during pregnancy, but usually not enough for a health care professional to feel during a physical exam.
The thyroid enlarges slightly in healthy women during pregnancy, but usually not enough for a health care professional to feel during a physical exam.
Thyroid problems can be hard to diagnose in pregnancy due to higher levels of thyroid hormones and other symptoms that occur in both pregnancy and thyroid disorders. Some symptoms of hyperthyroidism or hypothyroidism are easier to spot and may prompt your doctor to test you for these thyroid diseases.
Another type of thyroid disease, postpartum thyroiditis, can occur after your baby is born.
Hyperthyroidism in Pregnancy
What are the symptoms of hyperthyroidism in pregnancy?
Some signs and symptoms of hyperthyroidism often occur in normal pregnancies, including faster heart rate, trouble dealing with heat, and tiredness.
Other signs and symptoms can suggest hyperthyroidism:
- fast and irregular heartbeat
- shaky hands
- unexplained weight loss or failure to have normal pregnancy weight gain
What causes hyperthyroidism in pregnancy?
Hyperthyroidism in pregnancy is usually caused by Graves’ disease and occurs in 1 to 4 of every 1,000 pregnancies in the United States.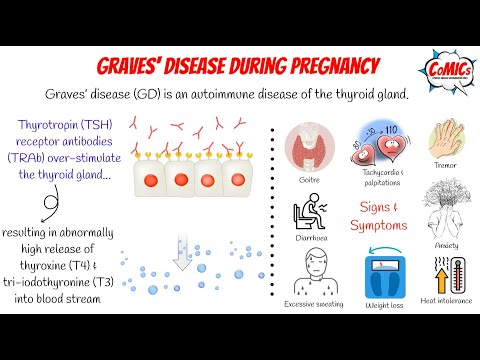 1 Graves’ disease is an autoimmune disorder. With this disease, your immune system makes antibodies that cause the thyroid to make too much thyroid hormone. This antibody is called thyroid stimulating immunoglobulin, or TSI.
1 Graves’ disease is an autoimmune disorder. With this disease, your immune system makes antibodies that cause the thyroid to make too much thyroid hormone. This antibody is called thyroid stimulating immunoglobulin, or TSI.
Graves’ disease may first appear during pregnancy. However, if you already have Graves’ disease, your symptoms could improve in your second and third trimesters. Some parts of your immune system are less active later in pregnancy so your immune system makes less TSI. This may be why symptoms improve. Graves’ disease often gets worse again in the first few months after your baby is born, when TSI levels go up again. If you have Graves’ disease, your doctor will most likely test your thyroid function monthly throughout your pregnancy and may need to treat your hyperthyroidism.1 Thyroid hormone levels that are too high can harm your health and your baby’s.
If you have Graves’ disease, your doctor will most likely test your thyroid function monthly during your pregnancy..jpg)
Rarely, hyperthyroidism in pregnancy is linked to hyperemesis gravidarum—severe nausea and vomiting that can lead to weight loss and dehydration. Experts believe this severe nausea and vomiting is caused by high levels of hCG early in pregnancy. High hCG levels can cause the thyroid to make too much thyroid hormone. This type of hyperthyroidism usually goes away during the second half of pregnancy.
Less often, one or more nodules, or lumps in your thyroid, make too much thyroid hormone.
How can hyperthyroidism affect me and my baby?
Untreated hyperthyroidism during pregnancy can lead to
- miscarriage
- premature birth
- low birthweight
- preeclampsia—a dangerous rise in blood pressure in late pregnancy
- thyroid storm—a sudden, severe worsening of symptoms
- congestive heart failure
Rarely, Graves’ disease may also affect a baby’s thyroid, causing it to make too much thyroid hormone. Even if your hyperthyroidism was cured by radioactive iodine treatment to destroy thyroid cells or surgery to remove your thyroid, your body still makes the TSI antibody. When levels of this antibody are high, TSI may travel to your baby’s bloodstream. Just as TSI caused your own thyroid to make too much thyroid hormone, it can also cause your baby’s thyroid to make too much.
When levels of this antibody are high, TSI may travel to your baby’s bloodstream. Just as TSI caused your own thyroid to make too much thyroid hormone, it can also cause your baby’s thyroid to make too much.
Tell your doctor if you’ve had surgery or radioactive iodine treatment for Graves’ disease so he or she can check your TSI levels. If they are very high, your doctor will monitor your baby for thyroid-related problems later in your pregnancy.
Tell your doctor if you’ve had surgery or radioactive iodine treatment for Graves’ disease.An overactive thyroid in a newborn can lead to
- a fast heart rate, which can lead to heart failure
- early closing of the soft spot in the baby’s skull
- poor weight gain
- irritability
Sometimes an enlarged thyroid can press against your baby’s windpipe and make it hard for your baby to breathe. If you have Graves’ disease, your health care team should closely monitor you and your newborn.
How do doctors diagnose hyperthyroidism in pregnancy?
Your doctor will review your symptoms and do some blood tests to measure your thyroid hormone levels. Your doctor may also look for antibodies in your blood to see if Graves’ disease is causing your hyperthyroidism. Learn more about thyroid tests and what the results mean.
How do doctors treat hyperthyroidism during pregnancy?
If you have mild hyperthyroidism during pregnancy, you probably won’t need treatment. If your hyperthyroidism is linked to hyperemesis gravidarum, you only need treatment for vomiting and dehydration.
If your hyperthyroidism is more severe, your doctor may prescribe antithyroid medicines, which cause your thyroid to make less thyroid hormone. This treatment prevents too much of your thyroid hormone from getting into your baby’s bloodstream. You may want to see a specialist, such as an endocrinologist or expert in maternal-fetal medicine, who can carefully monitor your baby to make sure you’re getting the right dose.
Doctors most often treat pregnant women with the antithyroid medicine propylthiouracil (PTU) during the first 3 months of pregnancy. Another type of antithyroid medicine, methimazole, is easier to take and has fewer side effects, but is slightly more likely to cause serious birth defects than PTU. Birth defects with either type of medicine are rare. Sometimes doctors switch to methimazole after the first trimester of pregnancy. Some women no longer need antithyroid medicine in the third trimester.
Small amounts of antithyroid medicine move into the baby’s bloodstream and lower the amount of thyroid hormone the baby makes. If you take antithyroid medicine, your doctor will prescribe the lowest possible dose to avoid hypothyroidism in your baby but enough to treat the high thyroid hormone levels that can also affect your baby.
Antithyroid medicines can cause side effects in some people, including
- allergic reactions such as rashes and itching
- rarely, a decrease in the number of white blood cells in the body, which can make it harder for your body to fight infection
- liver failure, in rare cases
Stop your antithyroid medicine and call your doctor right away if you develop any of these symptoms while taking antithyroid medicines:
- yellowing of your skin or the whites of your eyes, called jaundice
- dull pain in your abdomen
- constant sore throat
- fever
If you don’t hear back from your doctor the same day, you should go to the nearest emergency room.
You should also contact your doctor if any of these symptoms develop for the first time while you’re taking antithyroid medicines:
- increased tiredness or weakness
- loss of appetite
- skin rash or itching
- easy bruising
If you are allergic to or have severe side effects from antithyroid medicines, your doctor may consider surgery to remove part or most of your thyroid gland. The best time for thyroid surgery during pregnancy is in the second trimester.
Radioactive iodine treatment is not an option for pregnant women because it can damage the baby’s thyroid gland.
Hypothyroidism in Pregnancy
What are the symptoms of hypothyroidism in pregnancy?
Symptoms of an underactive thyroid are often the same for pregnant women as for other people with hypothyroidism. Symptoms include
- extreme tiredness
- trouble dealing with cold
- muscle cramps
- severe constipation
- problems with memory or concentration

Most cases of hypothyroidism in pregnancy are mild and may not have symptoms.
What causes hypothyroidism in pregnancy?
Hypothyroidism in pregnancy is usually caused by Hashimoto’s disease and occurs in 2 to 3 out of every 100 pregnancies.1 Hashimoto’s disease is an autoimmune disorder. In Hashimoto’s disease, the immune system makes antibodies that attack the thyroid, causing inflammation and damage that make it less able to make thyroid hormones.
How can hypothyroidism affect me and my baby?
Untreated hypothyroidism during pregnancy can lead to
- preeclampsia—a dangerous rise in blood pressure in late pregnancy
- anemia
- miscarriage
- low birthweight
- stillbirth
- congestive heart failure, rarely
These problems occur most often with severe hypothyroidism.
Because thyroid hormones are so important to your baby’s brain and nervous system development, untreated hypothyroidism—especially during the first trimester—can cause low IQ and problems with normal development.
How do doctors diagnose hypothyroidism in pregnancy?
Your doctor will review your symptoms and do some blood tests to measure your thyroid hormone levels. Your doctor may also look for certain antibodies in your blood to see if Hashimoto’s disease is causing your hypothyroidism. Learn more about thyroid tests and what the results mean.
How do doctors treat hypothyroidism during pregnancy?
Treatment for hypothyroidism involves replacing the hormone that your own thyroid can no longer make. Your doctor will most likely prescribe levothyroxine, a thyroid hormone medicine that is the same as T4, one of the hormones the thyroid normally makes. Levothyroxine is safe for your baby and especially important until your baby can make his or her own thyroid hormone.
Your thyroid makes a second type of hormone, T3. Early in pregnancy, T3 can’t enter your baby’s brain like T4 can. Instead, any T3 that your baby’s brain needs is made from T4. T3 is included in a lot of thyroid medicines made with animal thyroid, such as Armour Thyroid, but is not useful for your baby’s brain development. These medicines contain too much T3 and not enough T4, and should not be used during pregnancy. Experts recommend only using levothyroxine (T4) while you’re pregnant.
These medicines contain too much T3 and not enough T4, and should not be used during pregnancy. Experts recommend only using levothyroxine (T4) while you’re pregnant.
Some women with subclinical hypothyroidism—a mild form of the disease with no clear symptoms—may not need treatment.
Your doctor may prescribe levothyroxine to treat your hypothyroidism.If you had hypothyroidism before you became pregnant and are taking levothyroxine, you will probably need to increase your dose. Most thyroid specialists recommend taking two extra doses of thyroid medicine per week, starting right away. Contact your doctor as soon as you know you’re pregnant.
Your doctor will most likely test your thyroid hormone levels every 4 to 6 weeks for the first half of your pregnancy, and at least once after 30 weeks.1 You may need to adjust your dose a few times.
Postpartum Thyroiditis
What is postpartum thyroiditis?
Postpartum thyroiditis is an inflammation of the thyroid that affects about 1 in 20 women during the first year after giving birth1 and is more common in women with type 1 diabetes.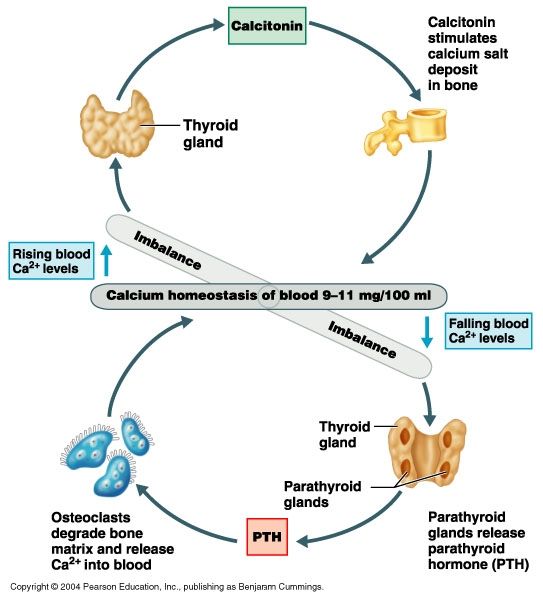 The inflammation causes stored thyroid hormone to leak out of your thyroid gland. At first, the leakage raises the hormone levels in your blood, leading to hyperthyroidism. The hyperthyroidism may last up to 3 months. After that, some damage to your thyroid may cause it to become underactive. Your hypothyroidism may last up to a year after your baby is born. However, in some women, hypothyroidism doesn’t go away.
The inflammation causes stored thyroid hormone to leak out of your thyroid gland. At first, the leakage raises the hormone levels in your blood, leading to hyperthyroidism. The hyperthyroidism may last up to 3 months. After that, some damage to your thyroid may cause it to become underactive. Your hypothyroidism may last up to a year after your baby is born. However, in some women, hypothyroidism doesn’t go away.
Not all women who have postpartum thyroiditis go through both phases. Some only go through the hyperthyroid phase, and some only the hypothyroid phase.
What are the symptoms of postpartum thyroiditis?
The hyperthyroid phase often has no symptoms—or only mild ones. Symptoms may include irritability, trouble dealing with heat, tiredness, trouble sleeping, and fast heartbeat.
Symptoms of the hypothyroid phase may be mistaken for the “baby blues”—the tiredness and moodiness that sometimes occur after the baby is born. Symptoms of hypothyroidism may also include trouble dealing with cold; dry skin; trouble concentrating; and tingling in your hands, arms, feet, or legs.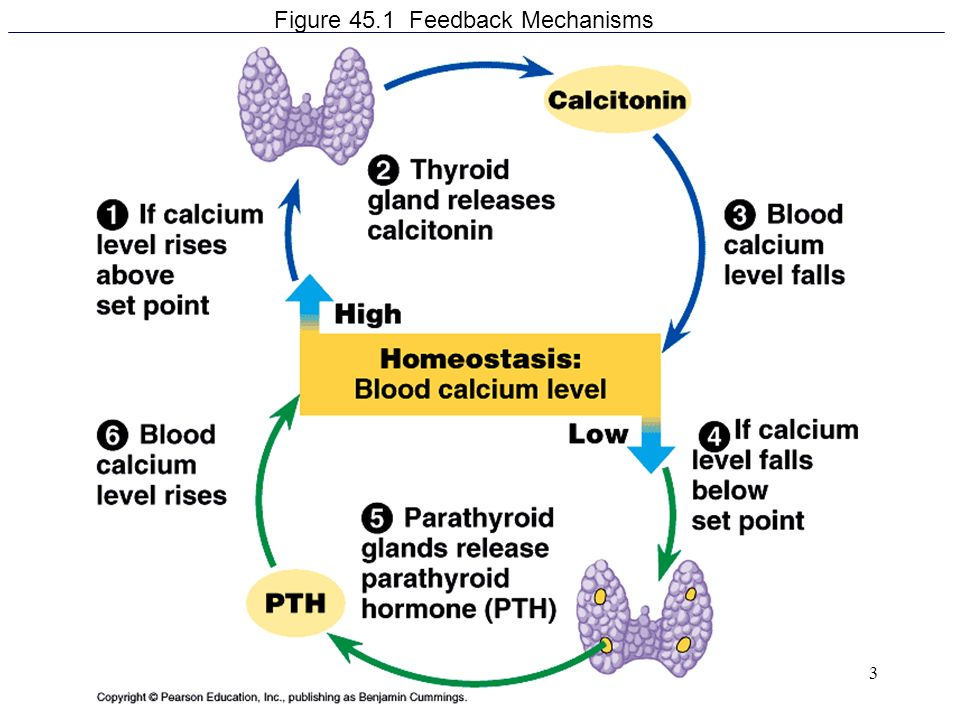 If these symptoms occur in the first few months after your baby is born or you develop postpartum depression, talk with your doctor as soon as possible.
If these symptoms occur in the first few months after your baby is born or you develop postpartum depression, talk with your doctor as soon as possible.
What causes postpartum thyroiditis?
Postpartum thyroiditis is an autoimmune condition similar to Hashimoto’s disease. If you have postpartum thyroiditis, you may have already had a mild form of autoimmune thyroiditis that flares up after you give birth.
Postpartum thyroiditis may last up to a year after your baby is born.How do doctors diagnose postpartum thyroiditis?
If you have symptoms of postpartum thyroiditis, your doctor will order blood tests to check your thyroid hormone levels.
How do doctors treat postpartum thyroiditis?
The hyperthyroid stage of postpartum thyroiditis rarely needs treatment. If your symptoms are bothering you, your doctor may prescribe a beta-blocker, a medicine that slows your heart rate. Antithyroid medicines are not useful in postpartum thyroiditis, but if you have Grave’s disease, it may worsen after your baby is born and you may need antithyroid medicines.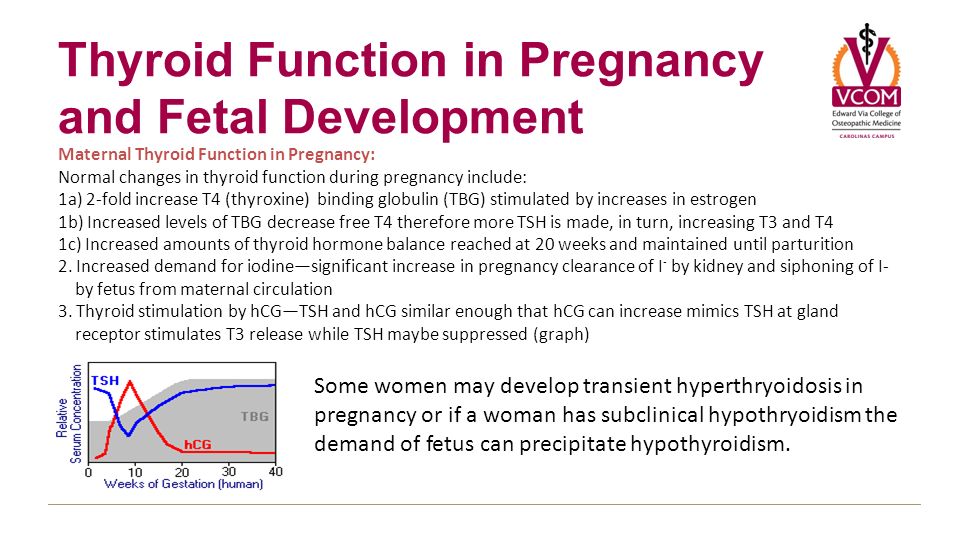
You’re more likely to have symptoms during the hypothyroid stage. Your doctor may prescribe thyroid hormone medicine to help with your symptoms. If your hypothyroidism doesn’t go away, you will need to take thyroid hormone medicine for the rest of your life.
Is it safe to breastfeed while I’m taking beta-blockers, thyroid hormone, or antithyroid medicines?
Certain beta-blockers are safe to use while you’re breastfeeding because only a small amount shows up in breast milk. The lowest possible dose to relieve your symptoms is best. Only a small amount of thyroid hormone medicine reaches your baby through breast milk, so it’s safe to take while you’re breastfeeding. However, in the case of antithyroid drugs, your doctor will most likely limit your dose to no more than 20 milligrams (mg) of methimazole or, less commonly, 400 mg of PTU.
Thyroid Disease and Eating During Pregnancy
What should I eat during pregnancy to help keep my thyroid and my baby’s thyroid working well?
Because the thyroid uses iodine to make thyroid hormone, iodine is an important mineral for you while you’re pregnant. During pregnancy, your baby gets iodine from your diet. You’ll need more iodine when you’re pregnant—about 250 micrograms a day.1 Good sources of iodine are dairy foods, seafood, eggs, meat, poultry, and iodized salt—salt with added iodine. Experts recommend taking a prenatal vitamin with 150 micrograms of iodine to make sure you’re getting enough, especially if you don’t use iodized salt.1 You also need more iodine while you’re breastfeeding since your baby gets iodine from breast milk. However, too much iodine from supplements such as seaweed can cause thyroid problems. Talk with your doctor about an eating plan that’s right for you and what supplements you should take. Learn more about a healthy diet and nutrition during pregnancy.
During pregnancy, your baby gets iodine from your diet. You’ll need more iodine when you’re pregnant—about 250 micrograms a day.1 Good sources of iodine are dairy foods, seafood, eggs, meat, poultry, and iodized salt—salt with added iodine. Experts recommend taking a prenatal vitamin with 150 micrograms of iodine to make sure you’re getting enough, especially if you don’t use iodized salt.1 You also need more iodine while you’re breastfeeding since your baby gets iodine from breast milk. However, too much iodine from supplements such as seaweed can cause thyroid problems. Talk with your doctor about an eating plan that’s right for you and what supplements you should take. Learn more about a healthy diet and nutrition during pregnancy.
Clinical Trials
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and other components of the National Institutes of Health (NIH) conduct and support research into many diseases and conditions.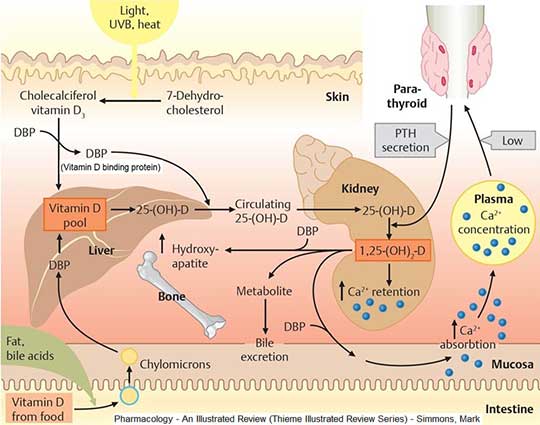
What are clinical trials, and are they right for you?
Clinical trials are part of clinical research and at the heart of all medical advances. Clinical trials look at new ways to prevent, detect, or treat disease. Researchers also use clinical trials to look at other aspects of care, such as improving the quality of life for people with chronic illnesses. Find out if clinical trials are right for you.
What clinical trials are open?
Clinical trials that are currently open and are recruiting can be viewed at www.ClinicalTrials.gov.
References
Hyperthyroidism in Pregnancy | American Thyroid Association
WHAT ARE THE NORMAL CHANGES IN THYROID FUNCTION ASSOCIATED WITH PREGNANCY?
HORMONE CHANGES. A normal pregnancy results in a number of important physiological and hormonal changes that alter thyroid function. These changes mean that laboratory tests of thyroid function must be interpreted with caution during pregnancy. Thyroid function tests change during pregnancy due to the influence of two main hormones: human chorionic gonadotropin (hCG), the hormone that is measured in the pregnancy test and estrogen, the main female hormone. HCG can weakly turn on the thyroid and the high circulating hCG levels in the first trimester may result in a slightly low TSH. When this occurs, the TSH will be slightly decreased in the first trimester and then return to normal throughout the duration of pregnancy. Estrogen increases the amount of thyroid hormone binding proteins in the serum which increases the total thyroid hormone levels in the blood since >99% of the thyroid hormones in the blood are bound to these proteins. However, measurements of “Free” hormone (that are not bound to protein, representing the active form of the hormone) usually remain normal. The thyroid is functioning normally if the TSH and Free T4 remain in the trimester-specific normal ranges throughout pregnancy.
HCG can weakly turn on the thyroid and the high circulating hCG levels in the first trimester may result in a slightly low TSH. When this occurs, the TSH will be slightly decreased in the first trimester and then return to normal throughout the duration of pregnancy. Estrogen increases the amount of thyroid hormone binding proteins in the serum which increases the total thyroid hormone levels in the blood since >99% of the thyroid hormones in the blood are bound to these proteins. However, measurements of “Free” hormone (that are not bound to protein, representing the active form of the hormone) usually remain normal. The thyroid is functioning normally if the TSH and Free T4 remain in the trimester-specific normal ranges throughout pregnancy.
SIZE CHANGES. The thyroid gland can increase in size during pregnancy (enlarged thyroid = goiter). However, pregnancy-associated goiters occur much more frequently in iodine-deficient areas of the world. It is relatively uncommon in the United States.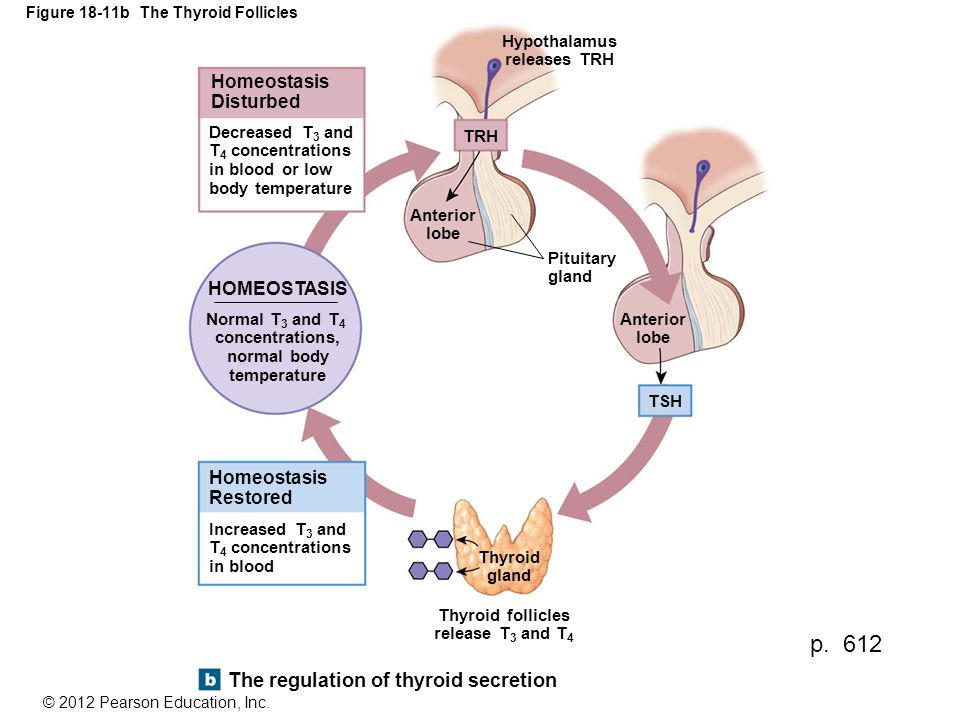 If very sensitive imaging techniques (ultrasound) are used, it is possible to detect an increase in thyroid volume in some women. This is usually only a 10-15% increase in size and is not typically apparent on physical examination by the physician. However, sometimes a significant goiter may develop and prompt the doctor to measure tests of thyroid function.
If very sensitive imaging techniques (ultrasound) are used, it is possible to detect an increase in thyroid volume in some women. This is usually only a 10-15% increase in size and is not typically apparent on physical examination by the physician. However, sometimes a significant goiter may develop and prompt the doctor to measure tests of thyroid function.
WHAT IS THE THYROID GLAND?
The thyroid gland is a butterfly-shaped endocrine gland that is normally located in the lower front of the neck. The thyroid’s job is to make thyroid hormones, which are secreted into the blood and then carried to every tissue in the body. Thyroid hormone helps the body use energy, stay warm and keep the brain, heart, muscles, and other organs working as they should.
WHAT IS THE INTERACTION BETWEEN THE THYROID FUNCTION OF THE MOTHER AND THE BABY?
For the first 18-20 weeks of pregnancy, the baby is completely dependent on the mother for the production of thyroid hormone. By mid-pregnancy, the baby’s thyroid begins to produce thyroid hormone on its own. The baby, however, remains dependent on the mother for ingestion of adequate amounts of iodine, which is essential to make the thyroid hormones. The World Health Organization recommends iodine intake of 250 micrograms/day during pregnancy to maintain adequate thyroid hormone production. Because iodine intakes in pregnancy are currently low in the United States, the ATA recommends that US women who are planning pregnancy, pregnant, or breastfeeding should take a daily supplement containing 150 mcg of iodine.
The baby, however, remains dependent on the mother for ingestion of adequate amounts of iodine, which is essential to make the thyroid hormones. The World Health Organization recommends iodine intake of 250 micrograms/day during pregnancy to maintain adequate thyroid hormone production. Because iodine intakes in pregnancy are currently low in the United States, the ATA recommends that US women who are planning pregnancy, pregnant, or breastfeeding should take a daily supplement containing 150 mcg of iodine.
Hyperthyroidism & Pregnancy
WHAT ARE THE MOST COMMON CAUSES OF HYPERTHYROIDISM DURING PREGNANCY?
Overall, the most common cause of hyperthyroidism in women of childbearing age is Graves’ disease (see Graves’ Disease brochure), which occurs in 0.2% of pregnant patients. In addition to other usual causes of hyperthyroidism (see Hyperthyroidism brochure), very high levels of hCG, seen in severe forms of morning sickness (hyperemesis gravidarum), may cause transient hyperthyroidism in early pregnancy. The correct diagnosis is based on a careful review of history, physical exam and laboratory testing.
The correct diagnosis is based on a careful review of history, physical exam and laboratory testing.
WHAT ARE THE RISKS OF GRAVES' DISEASE/ HYPERTHYROIDISM TO THE MOTHER?
Graves’ disease may present initially during the first trimester or may be exacerbated during this time in a woman known to have the disorder. In addition to the classic symptoms associated with hyperthyroidism, inadequately treated maternal hyperthyroidism can result in early labor and a serious complication known as pre-eclampsia. Additionally, women with active Graves’ disease during pregnancy are at higher risk of developing very severe hyperthyroidism known as thyroid storm. Graves’ disease often improves during the third trimester of pregnancy and may worsen during the post partum period.
WHAT ARE THE RISKS OF GRAVES' DISEASE/ HYPERTHYROIDISM TO THE BABY?
The risks to the baby from Graves’ disease are due to one of three possible mechanisms:
- UNCONTROLLED MATERNAL HYPERTHYROIDISM: Uncontrolled maternal hyperthyroidism has been associated with fetal tachycardia (fast heart rate), small for gestational age babies, prematurity, stillbirths and congenital malformations (birth defects).
 This is another reason why it is important to treat hyperthyroidism in the mother.
This is another reason why it is important to treat hyperthyroidism in the mother. - EXTREMELY HIGH LEVELS OF THYROID STIMULATING IMMUNOGLOBLULINS (TSI): Graves’ disease is an autoimmune disorder caused by the production of antibodies that stimulate the thyroid gland referred to as thyroid stimulating immunoglobulins (TSI). These antibodies do cross the placenta and can interact with the baby’s thyroid. High levels of maternal TSI’s have been known to cause fetal or neonatal hyperthyroidism, but this is uncommon (only 1-5% of women with Graves’ disease during pregnancy). Fortunately, this typically only occurs when the mother’s TSI levels are very high (many times above normal). Measuring TSI in the mother with Graves’ disease is recommended in early pregnancy and, if initially elevated, again around weeks 18-22.When a mother with Graves’ disease requires antithyroid drug therapy during pregnancy, fetal hyperthyroidism is rare because antithyroid drugs also cross the placenta and can prevent the fetal thyroid from becoming overactive.
 Of potentially more concern to the baby is when the mother has been treated for Graves’ disease (for example radioactive iodine or surgery) and no longer requires antithyroid drugs. It is very important to tell your doctor if you have been treated for Graves’ Disease in the past so proper monitoring can be done to ensure the baby remains healthy during the pregnancy.
Of potentially more concern to the baby is when the mother has been treated for Graves’ disease (for example radioactive iodine or surgery) and no longer requires antithyroid drugs. It is very important to tell your doctor if you have been treated for Graves’ Disease in the past so proper monitoring can be done to ensure the baby remains healthy during the pregnancy. - ANTI-THYROID DRUG THERAPY (ATD). Methimazole (Tapazole) or propylthiouracil (PTU) are the ATDs available in the United States for the treatment of hyperthyroidism (see Hyperthyroidism brochure). Both of these drugs cross the placenta and can potentially impair the baby’s thyroid function and cause fetal goiter. Use of either drug in the first trimester of pregnancy has been associated with birth defects, although the defects associated with PTU are less frequent and less severe. Definitive therapy (thyroid surgery or radioactive iodine treatment) may be considered prior to pregnancy in order to avoid the need to use PTU or methimazole in pregnancy.
 When ATDs are required, PTU is preferred until week 16 of pregnancy. It is recommended that the lowest possible dose of ATD be used to control maternal hyperthyroidism in order to minimize the development of hypothyroidism in the baby. Overall, the benefits to the baby of treating a mother with hyperthyroidism during pregnancy outweigh the risks if therapy is carefully monitored.
When ATDs are required, PTU is preferred until week 16 of pregnancy. It is recommended that the lowest possible dose of ATD be used to control maternal hyperthyroidism in order to minimize the development of hypothyroidism in the baby. Overall, the benefits to the baby of treating a mother with hyperthyroidism during pregnancy outweigh the risks if therapy is carefully monitored.
WHAT ARE THE TREATMENT OPTIONS FOR A PREGNANT WOMAN WITH GRAVES' DISEASE/ HYPERTHYROIDISM?
Mild hyperthyroidism (slightly elevated thyroid hormone levels, minimal symptoms) often is monitored closely without therapy as long as both the mother and the baby are doing well. When hyperthyroidism is severe enough to require therapy, anti-thyroid medications are the treatment of choice, with PTU being preferred in the first trimester. The goal of therapy is to keep the mother’s free T4 in the high-normal to mildly elevated range on the lowest dose of antithyroid medication. Addition of levothyroxine to ATDs (“block-and-replace”) is not recommended. Targeting this range of free hormone levels will minimize the risk to the baby of developing hypothyroidism or goiter. Maternal hypothyroidism should be avoided. Therapy should be closely monitored during pregnancy. This is typically done by following thyroid function tests (TSH and thyroid hormone levels) monthly.
Targeting this range of free hormone levels will minimize the risk to the baby of developing hypothyroidism or goiter. Maternal hypothyroidism should be avoided. Therapy should be closely monitored during pregnancy. This is typically done by following thyroid function tests (TSH and thyroid hormone levels) monthly.
In patients who cannot be adequately treated with anti-thyroid medications (i.e. those who develop an allergic reaction to the drugs), surgery is an acceptable alternative. Surgical removal of the thyroid gland is safest in the second trimester.
Radioiodine is contraindicated to treat hyperthyroidism during pregnancy since it readily crosses the placenta and is taken up by the baby’s thyroid gland. This can cause destruction of the gland and result in permanent hypothyroidism.
Beta-blockers can be used during pregnancy to help treat significant palpitations and tremor due to hyperthyroidism. They should be used sparingly due to reports of impaired fetal growth associated with long-term use of these medications.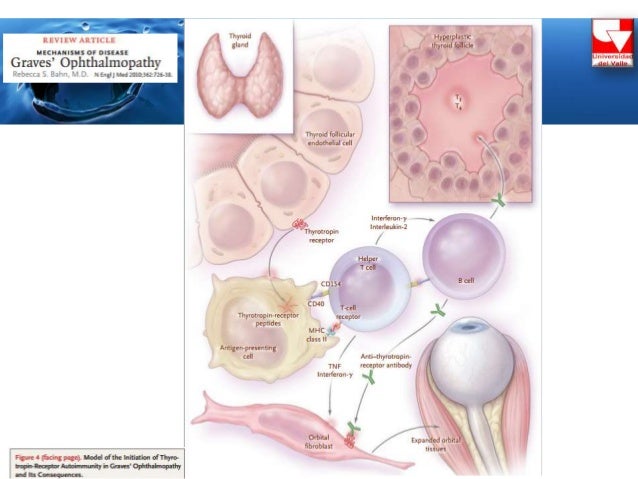 Typically, these drugs are only required until the hyperthyroidism is controlled with anti-thyroid medications.
Typically, these drugs are only required until the hyperthyroidism is controlled with anti-thyroid medications.
WHAT IS THE NATURAL HISTORY OF GRAVES' DISEASE AFTER DELIVERY?
Graves’ disease typically worsens in the postpartum period or may occur then for the first time. When new hyperthyroidism occurs in the first months after delivery, the cause may be either Graves’ disease or postpartum thyroiditis and testing with careful follow-up is needed to distinguish between the two. Higher doses of anti-thyroid medications may be required during this time. As usual, close monitoring of thyroid function tests is necessary.
CAN THE MOTHER WITH GRAVES' DISEASE, WHO IS BEING TREATED WITH ANTI-THYROID DRUGS, BREASTFEED HER INFANT?
Yes. Although very small quantities of both PTU and methimazole are transferred into breast milk, total daily doses of up to 20mg methimazole or 450mg PTU are considered safe and monitoring of the breastfed infants’ thyroid status is not required.
Click ‘+’ button to see all topics
PDF Resources
Hyperthyroidism in Pregnancy Brochure PDF
Hyperthyroidism in Pregnancy FAQ
Articles
Vibhavasu Sharma, MD, FACE Albany Medical College, Albany, NY August 13, 2020 Thyroid disease…
Read More
From Clinical Thyroidology® for the Public: GUEST BLOG FROM THE IODINE GLOBAL NETWORK Timing matters for…
Read More
October 2, 2018—The American Thyroid Association (ATA) will hold its 88th Annual Meeting on October…
Read More
More Articles on Hyperthyroidism in Pregnancy
FURTHER INFORMATION
For information on thyroid patient support organizations, please visit the Patient Support Links section on the ATA website at www.thyroid.org
changes in modern medical and diagnostic paradigms
The problem of the relationship between autoimmune thyroid disease and reproductive disorders has become more and more discussed in recent years. Clinical and experimental studies have shown that impaired thyroid function leads to severe pregnancy complications: spontaneous pathological abortion, stillbirth, miscarriage, fetal abnormalities. This relationship was confirmed not only in women with thyrotoxicosis and hypothyroidism, but also in women with preserved thyroid function, in whose blood serum high titers of antibodies (AT) to thyroperoxidase (AT-TPO), thyroglobulin (AT-TG) and thyroid-stimulating receptors were detected. hormone (AT-rTTH) [1]. nine0003
Clinical and experimental studies have shown that impaired thyroid function leads to severe pregnancy complications: spontaneous pathological abortion, stillbirth, miscarriage, fetal abnormalities. This relationship was confirmed not only in women with thyrotoxicosis and hypothyroidism, but also in women with preserved thyroid function, in whose blood serum high titers of antibodies (AT) to thyroperoxidase (AT-TPO), thyroglobulin (AT-TG) and thyroid-stimulating receptors were detected. hormone (AT-rTTH) [1]. nine0003
The prevalence of primary overt hypothyroidism among pregnant women is 2%, subclinical - up to 15% [2]. Hypothyroidism during pregnancy is most dangerous for the development of the fetus and, first of all, for its central nervous system (CNS) [3]. Moreover, the disease of the mother has a more adverse effect on the formation and functioning of the central structures of the fetal brain than hypothyroidism caused by a violation of the laying of the thyroid gland of the fetus [4, 5]. This is explained by the fact that in the first half of pregnancy (up to 18–20 weeks) the fetal thyroid gland practically does not function, neuronal migration and other important early stages of intrauterine brain development largely depend on the intake of maternal thyroid hormones from the mother. nine0003
This is explained by the fact that in the first half of pregnancy (up to 18–20 weeks) the fetal thyroid gland practically does not function, neuronal migration and other important early stages of intrauterine brain development largely depend on the intake of maternal thyroid hormones from the mother. nine0003
In recent years, special attention has been paid to the relationship between the carriage of antibodies to the thyroid gland and reproductive function in women. The frequency of detection of thyroid antibodies (AB-TPO, AB-TG) in pregnant women according to various sources is 10-20% [6]. It was noted that in women with elevated levels of thyroid antibodies, even in the euthyroid state, the frequency of complications of pregnancy and childbirth is significantly higher. In 16% of pregnant women with antibodies to the thyroid gland and normal levels of thyroid-stimulating hormone (TSH) in the first trimester, there was an increase in TSH by more than 4 mU/l, and 33-50% developed postpartum thyroiditis [7].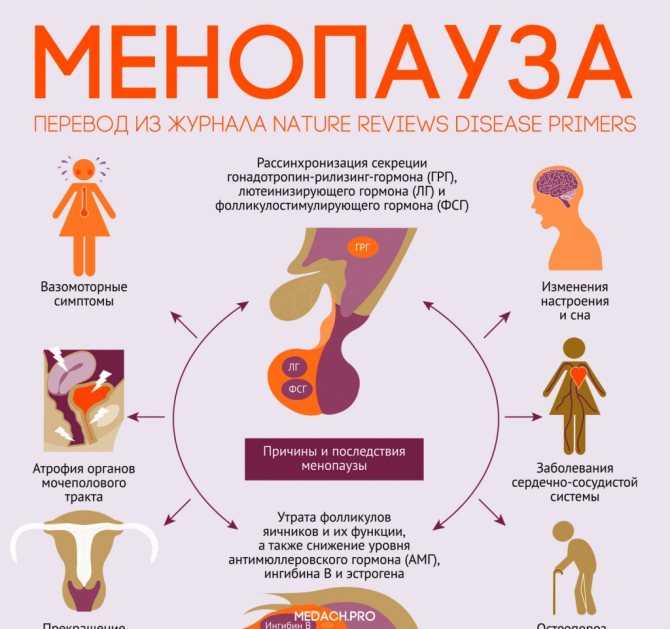 A number of researchers have presented evidence of the negative impact of subclinical changes in the level of TSH in the presence of AB-TPO carriage on the incidence of obstetric complications - this is an increased risk of preterm birth and spontaneous pathological abortions, intrauterine growth retardation, gestational hypertension and other pathologies [8]. The association between the carriage of Ab-TPO and the risk of preterm birth (1.7-fold increase) was identified in two prospective population-based studies that included a total of 7585 pregnant women from two Dutch cohorts [9].
A number of researchers have presented evidence of the negative impact of subclinical changes in the level of TSH in the presence of AB-TPO carriage on the incidence of obstetric complications - this is an increased risk of preterm birth and spontaneous pathological abortions, intrauterine growth retardation, gestational hypertension and other pathologies [8]. The association between the carriage of Ab-TPO and the risk of preterm birth (1.7-fold increase) was identified in two prospective population-based studies that included a total of 7585 pregnant women from two Dutch cohorts [9].
Recently, there have been controversies in the scientific community related to the interpretation of laboratory tests to assess thyroid function during pregnancy. This is primarily due to the fact that during pregnancy there is a change in the metabolism of thyroid hormones and a dynamically changing interaction between the pituitary-thyroid systems of the mother and fetus. Currently available immunometric methods for the determination of thyroid hormones are essentially approximate and evaluative tests, are not direct methods for determining the concentration of the hormone and are very sensitive to changes in the level of binding proteins.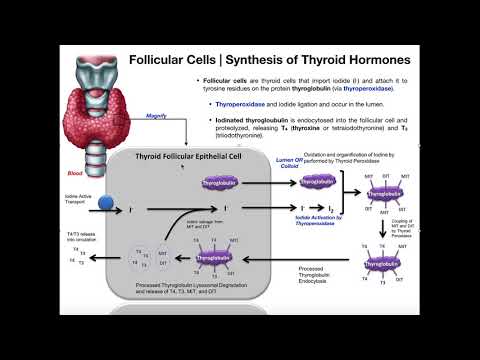 In this regard, it is extremely relevant to use high-performance liquid chromatography in combination with tandem mass spectrometry (HPLC-MS/MS) to develop reliable and accurate trimester-specific intervals for thyroid hormones during pregnancy. nine0003
In this regard, it is extremely relevant to use high-performance liquid chromatography in combination with tandem mass spectrometry (HPLC-MS/MS) to develop reliable and accurate trimester-specific intervals for thyroid hormones during pregnancy. nine0003
In this review, we presented the modern principles of diagnostics and therapeutic approaches to pregnancy management in women with autoimmune thyroid pathology.
Pathogenesis
According to the literature, it is known that autoimmune diseases occur in 3-8% of the world's population [10], develop up to 10 times more often in women than in men, and are characterized by a long course. Great interest is currently being paid to the diagnosis and treatment of autoimmune diseases of the thyroid gland, especially in women, since this pathology has been actively progressing in recent decades [11] and affects the reproductive status [12]. Currently, there is no single point of view on the role of antibodies to thyroid tissue in the genesis of reproductive dysfunction in women.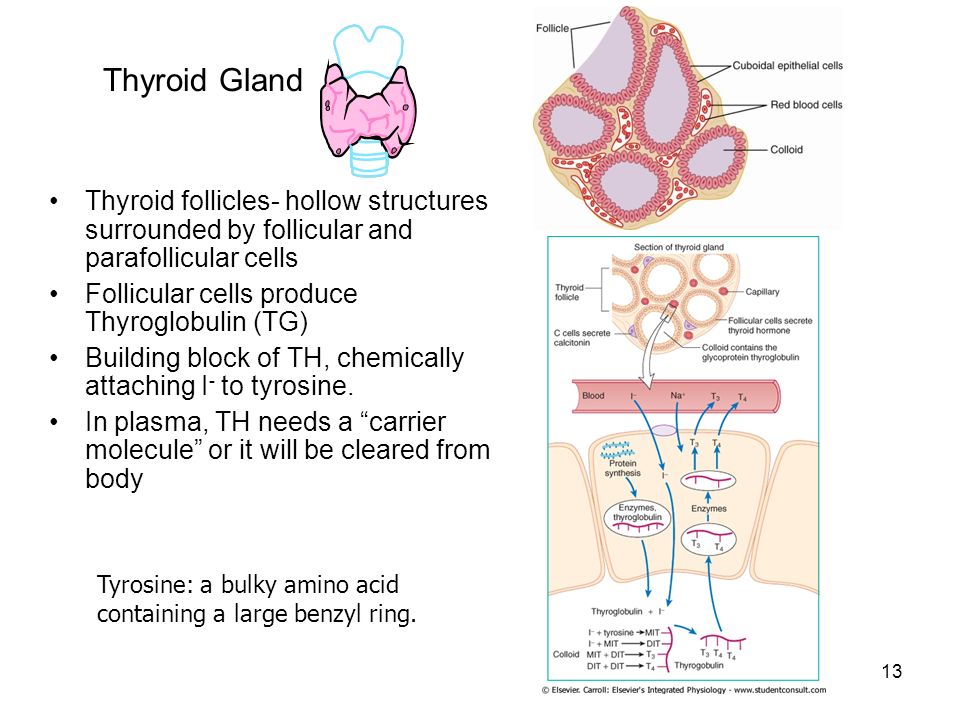 In the general population, an average of 10% of pregnant women are carriers of AT-TPO [13]. nine0003
In the general population, an average of 10% of pregnant women are carriers of AT-TPO [13]. nine0003
AIT is an HLA-associated disease. At the same time, atrophic and hypertrophic forms of AIT are associated with different haplotypes. However, the triggering processes in chronic AIT are not completely clear. In the blood of patients with AIT, as a rule, antibodies to various thyroid antigens are detected, most often AB-TPO, AT-TG, less often - blocking AB-rTTG. In addition, at the onset of the disease, stimulating AT-rTTH can also be detected transiently. Nevertheless, one of the controversial points in the mechanism of development of AIT is the role of antithyroid antibodies. According to researchers, Ab-TPO can lead to the formation of immune complexes that promote the release of biologically active substances that cause destructive changes in the thyroid gland, reducing its function [14]. According to other authors, AT-TPO is an indicator of destructive manifestations of the thyroid gland, and AT-TG is the result of compensatory mechanisms of the body, since the level of these antibodies depends on the number of stimulated receptors for thyroid hormones [15]. nine0003
nine0003
At the moment, it has been proven that the incidence of antithyroid antibodies does not coincide with the prevalence of both overt and subclinical hypothyroidism. This fact indicates that AT can be detected even in individuals who do not have functional or structural changes in the thyroid gland [16]. According to J. Hollowell et al. [17], AB-TPO was found in 12% of the examined patients without thyroid diseases and AB-TG in 10% [17]. At the same time, some researchers consider the detection of Ab-TPO to be a sign of a possible dysfunction of the thyroid gland in the future, and even low titers of these antibodies correlate with lymphoid infiltration of the thyroid tissue. Foreign researchers argue that an elevated level of AT-TPO is a statistically significant sign of AIT, and the presence of thyroid-specific antibodies in the blood serum (AT-TG 1:100 and above, AT-TPO 1:32 and above) is an indicator of autoimmune damage to the thyroid gland [ 17]. According to the research work of G. Karanikas et al. [nineteen], elevated titers of AT-TPO correlate with a high frequency of production of Th/Tc1 cytokines by T cells, which are responsible for the damage to thyroid cells.
Karanikas et al. [nineteen], elevated titers of AT-TPO correlate with a high frequency of production of Th/Tc1 cytokines by T cells, which are responsible for the damage to thyroid cells.
However, despite the significant interest of researchers in this pathology, there is currently no consensus on the etiology of AIT. Some scientists suggest genetic predisposition as the dominant cause in the development of AIT [20], in particular, when studying the genes of the HLA system, a combination with the genes HLA-B8, HLA-DR3, HLA-DR4 was indicated [21].
5 new gene variants TPO , ATXN2 , BACh3 , MAGI3 and KALRN associated with carriage of AT-TPO . The combination of these gene variants was associated with an increased risk of developing hypothyroidism and a reduced risk of developing goiter. Variations in the MAGI3 and BACh3 genes are associated with an increased risk of hyperthyroidism, and the MAGI3 variant is also associated with an increased risk of hypothyroidism [22].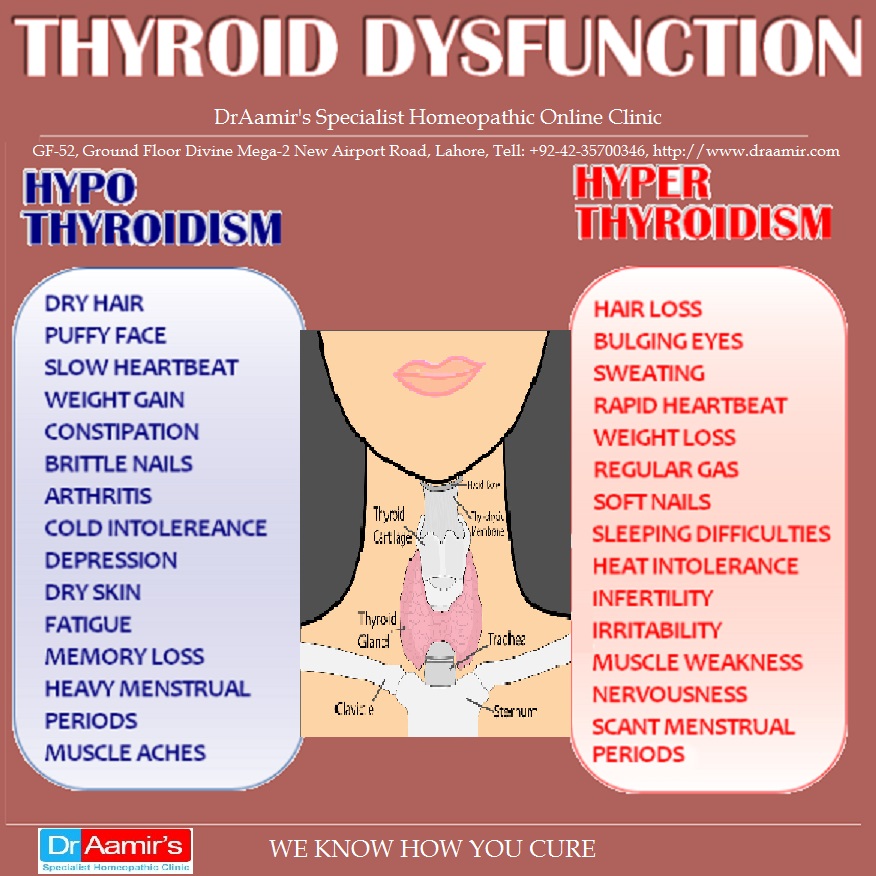 nine0003
nine0003
A separate point for discussion is microchimerism (MC) - the presence in the tissues and / or circulatory system of the “host organism” of a small number of genetically distinct cells capable of long-term persistence. The presence of microchimeric cells in a woman's body is a common occurrence and a consequence of a normal pregnancy. The long-term consequences of this phenomenon have become the subject of close attention relatively recently. Currently, MC is considered as one of the promising theories of the pathogenesis of autoimmune diseases. This phenomenon is associated with the risk of developing thyroid dysfunction as a result of its autoimmune damage, and can also have a direct impact on the course of subsequent pregnancies and the implementation of autoimmune reactions. Fetal and maternal microchimeric cells are able to persist in the body for a long time and can be detected in the blood and tissues decades after the end of pregnancy [23–25]. Mc is more often detected in thyroid tissue and peripheral blood in patients with autoimmune thyroid disorders than in healthy individuals [26]. It is assumed that Mc is able to provoke a local immune reaction against maternal antigens in the gland tissue, and also be a target for the maternal immune system. The prevalence of suspected genetic markers among mother-infant couples with fetal M.Ch. also supports the involvement of MH in the pathogenesis of autoimmune thyroid disorders. This phenomenon is considered as one of the attractive hypotheses of the genesis of autoimmune thyroid disorders, which could explain the prevalence of the incidence among women of reproductive age and frequent manifestations in the postpartum period. nine0003
It is assumed that Mc is able to provoke a local immune reaction against maternal antigens in the gland tissue, and also be a target for the maternal immune system. The prevalence of suspected genetic markers among mother-infant couples with fetal M.Ch. also supports the involvement of MH in the pathogenesis of autoimmune thyroid disorders. This phenomenon is considered as one of the attractive hypotheses of the genesis of autoimmune thyroid disorders, which could explain the prevalence of the incidence among women of reproductive age and frequent manifestations in the postpartum period. nine0003
Autoimmune thyroiditis and pregnancy
When pregnancy occurs in women who are carriers of Ab-TPO without impaired thyroid function, the risk of developing hypothyroidism and relative gestational hypothyroxinemia increases, which can lead to a number of perinatal and obstetric complications [27]. The pathogenesis of these disorders remains unclear to date.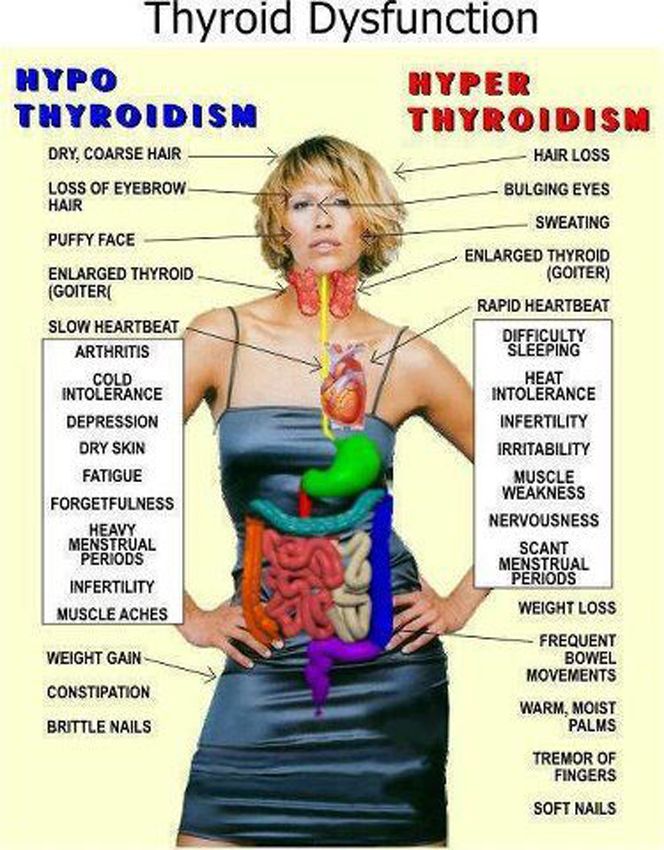 It is possible that antithyroid antibodies are a marker of generalized autoimmune dysfunction, which results in spontaneous pathological abortion. According to the literature, 30-50% of AT carriers develop thyroid dysfunction after childbirth. According to different authors, thyroid dysfunction in the postpartum period can develop in women even in the absence of antibodies to the thyroid gland. Thus, there are no clear prognostic criteria for the development of thyroid dysfunction both during pregnancy, in the postpartum period, and throughout life. nine0003
It is possible that antithyroid antibodies are a marker of generalized autoimmune dysfunction, which results in spontaneous pathological abortion. According to the literature, 30-50% of AT carriers develop thyroid dysfunction after childbirth. According to different authors, thyroid dysfunction in the postpartum period can develop in women even in the absence of antibodies to the thyroid gland. Thus, there are no clear prognostic criteria for the development of thyroid dysfunction both during pregnancy, in the postpartum period, and throughout life. nine0003
Diagnostic difficulties
Thyroid dysfunction can occur at any stage of pregnancy, it should be borne in mind that the principles of diagnosis and treatment of thyroid diseases in pregnant women differ significantly from those generally accepted. There are various methods of screening for thyroid dysfunction: from a simple examination of only high-risk pregnant women, to a total screening of all pregnant women, regardless of the gestational age. On the one hand, given the prevalence and potential danger of thyroid disorders during pregnancy, a number of professional associations and communities recommend an assessment of thyroid function in all pregnant women and women planning pregnancy. On the other hand, researchers from the American College of Obstetrics and Gynecology argued as early as 2002 that proposals for routine screening of pregnant women are premature in the absence of data showing improved outcomes with levothyroxine sodium therapy [28]. Current work in this area shows mixed results. We now have the report of the Cochrane Research Group, which published in 2015 an analysis of two randomized controlled trials involving 26,408 women (one study included 21,839women and others - 4562) [29]. The authors report that universal screening for thyroid dysfunction during pregnancy increased the number of women diagnosed with hypothyroidism and hyperthyroidism and, as a result, increased the number of patients treated for these conditions.
On the one hand, given the prevalence and potential danger of thyroid disorders during pregnancy, a number of professional associations and communities recommend an assessment of thyroid function in all pregnant women and women planning pregnancy. On the other hand, researchers from the American College of Obstetrics and Gynecology argued as early as 2002 that proposals for routine screening of pregnant women are premature in the absence of data showing improved outcomes with levothyroxine sodium therapy [28]. Current work in this area shows mixed results. We now have the report of the Cochrane Research Group, which published in 2015 an analysis of two randomized controlled trials involving 26,408 women (one study included 21,839women and others - 4562) [29]. The authors report that universal screening for thyroid dysfunction during pregnancy increased the number of women diagnosed with hypothyroidism and hyperthyroidism and, as a result, increased the number of patients treated for these conditions. However, routine screening and follow-up treatment failed to identify clear benefits or negative responses for women and/or their children. No change in the proportion of women with preeclampsia and preterm birth (based on a study of 4562 women), the number of children with disabilities (intelligence quotient (IQ) less than 85 at age 3) - a study of 794 children born to mothers with hypothyroidism). Although the studies included large sample sizes, no clear differences in outcomes for mothers and their children were found between routine screening and screening at the time of seeking care (case finding) or no screening at all. Thus, it is clear that more research and evidence is needed to evaluate the potential short-term and long-term advantages or disadvantages of various screening methods. nine0003
However, routine screening and follow-up treatment failed to identify clear benefits or negative responses for women and/or their children. No change in the proportion of women with preeclampsia and preterm birth (based on a study of 4562 women), the number of children with disabilities (intelligence quotient (IQ) less than 85 at age 3) - a study of 794 children born to mothers with hypothyroidism). Although the studies included large sample sizes, no clear differences in outcomes for mothers and their children were found between routine screening and screening at the time of seeking care (case finding) or no screening at all. Thus, it is clear that more research and evidence is needed to evaluate the potential short-term and long-term advantages or disadvantages of various screening methods. nine0003
It is important to clarify that the detection of an increase in the level of TSH is not always synonymous with a decrease in the concentration of free thyroxine 4 (svT 4 ).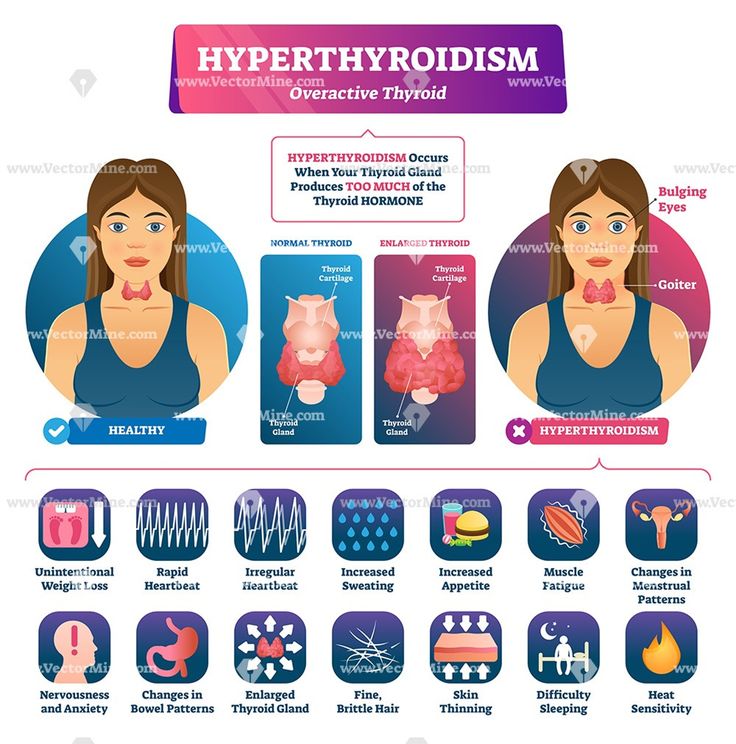 Most often, an elevated TSH level in a pregnant woman is detected when the content of fT 4 is normal - this is regarded as subclinical hypothyroidism. Conversely, a low level of fT 4 can be detected against the background of a normal TSH level - a similar situation is called isolated hypothyroxinemia (IH). IG of pregnant women is rare, if it is established in the first trimester, therapy with levothyroxine sodium may be recommended. The rationale for the appointment of therapy is the proven association of IG at the beginning of pregnancy with disorders of the neuropsychic development of the child. But at the moment, there are no studies demonstrating a clinically significant improvement in neurocognitive functions in children born to mothers with IH due to treatment. Therapy with levothyroxine sodium is not recommended for IG detected in the II and III trimesters of pregnancy. Since the minimum level of svt is 4 often observed and physiologically determined at the end of pregnancy, there is a high risk of developing iatrogenic thyrotoxicosis in the absence of evidence of the potential beneficial effect of levothyroxine sodium therapy.
Most often, an elevated TSH level in a pregnant woman is detected when the content of fT 4 is normal - this is regarded as subclinical hypothyroidism. Conversely, a low level of fT 4 can be detected against the background of a normal TSH level - a similar situation is called isolated hypothyroxinemia (IH). IG of pregnant women is rare, if it is established in the first trimester, therapy with levothyroxine sodium may be recommended. The rationale for the appointment of therapy is the proven association of IG at the beginning of pregnancy with disorders of the neuropsychic development of the child. But at the moment, there are no studies demonstrating a clinically significant improvement in neurocognitive functions in children born to mothers with IH due to treatment. Therapy with levothyroxine sodium is not recommended for IG detected in the II and III trimesters of pregnancy. Since the minimum level of svt is 4 often observed and physiologically determined at the end of pregnancy, there is a high risk of developing iatrogenic thyrotoxicosis in the absence of evidence of the potential beneficial effect of levothyroxine sodium therapy. Increased levels of thyroid hormones during pregnancy are regarded as a physiological process of adaptation, but, according to recent studies, excessively high concentrations of fT 4 have no less negative effect on the child's CNS than its low content: hyperthyroxinemia can contribute to a decrease in the IQ of the child and a decrease in gray volume substances [30]. In this regard, it is necessary to take a very balanced approach to the appointment of levothyroxine sodium and evaluate the justification for any medical interventions during pregnancy, taking into account not only the health of the woman, but also the child. nine0003
Increased levels of thyroid hormones during pregnancy are regarded as a physiological process of adaptation, but, according to recent studies, excessively high concentrations of fT 4 have no less negative effect on the child's CNS than its low content: hyperthyroxinemia can contribute to a decrease in the IQ of the child and a decrease in gray volume substances [30]. In this regard, it is necessary to take a very balanced approach to the appointment of levothyroxine sodium and evaluate the justification for any medical interventions during pregnancy, taking into account not only the health of the woman, but also the child. nine0003
In view of the fact that during pregnancy there is a change in the activity of the thyroid gland and the interaction of the pituitary-thyroid systems of the mother and the fetus changes dynamically, an accurate assessment of the function of the thyroid gland in the mother remains a difficult task at the present time. There is an active discussion in the scientific community and a single agreement has not yet been reached regarding the meaning of the “norm” and, accordingly, the tactics of management.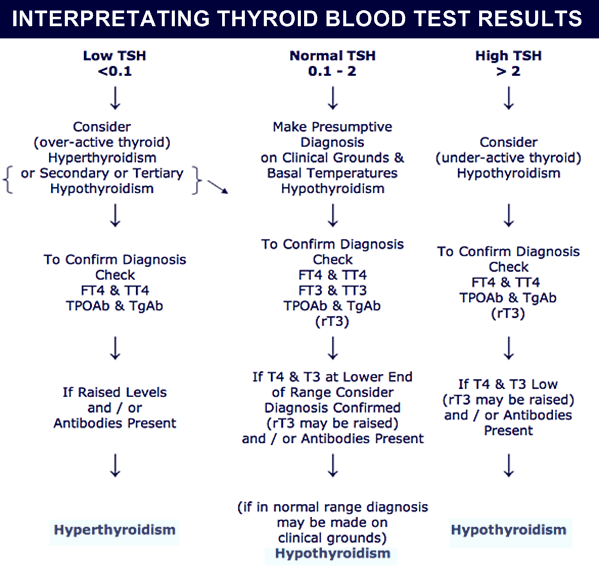 The principal point of discussion is the issue of determining diagnostic reference levels and interpreting the results of laboratory tests to assess hormonal status during pregnancy. nine0003
The principal point of discussion is the issue of determining diagnostic reference levels and interpreting the results of laboratory tests to assess hormonal status during pregnancy. nine0003
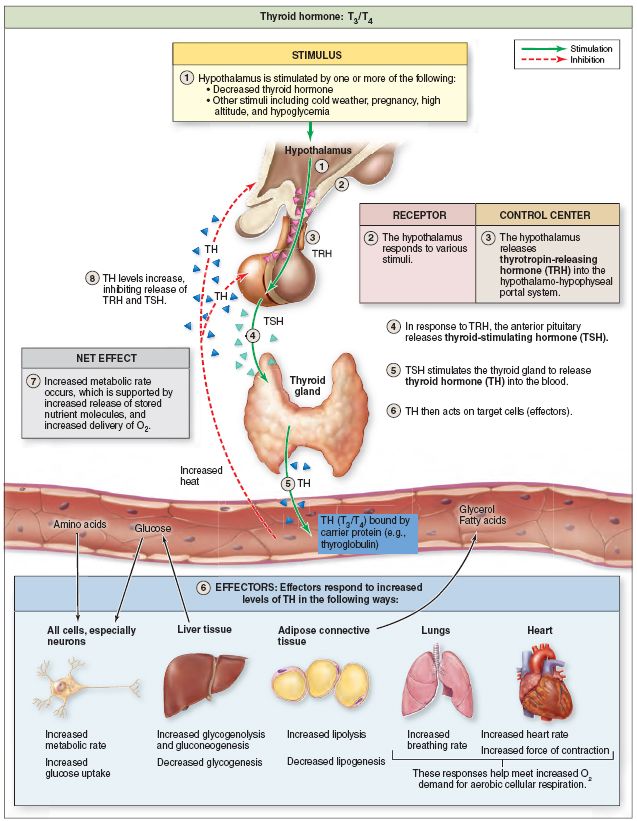
The TSH level is the first to respond to an increase in thyroid activity during pregnancy: there is a downward shift in the TSH level, while both the lower (by about 0.1-0.2 mU / l) and the upper limit of the maternal TSH level (by about 0 .5–1.0 mU/l) relative to the standard limits of TSH [31]. The greatest decrease in serum TSH levels is observed during the first trimester (the peak of TSH secretion by the 8th week of pregnancy) due to direct stimulation of the placental human chorionic gonadotropin (hCG) TSH receptor, thereby directly increasing the production of thyroid hormones. In the future, as pregnancy progresses, the level of TSH gradually increases and reaches a maximum in the third trimester, but generally remains lower than in non-pregnant women [32]. Since the concentration of hCG is higher in a multiple pregnancy than in a single pregnancy, the decrease in the boundaries of the control interval for the TSH level in this case is more significant [31].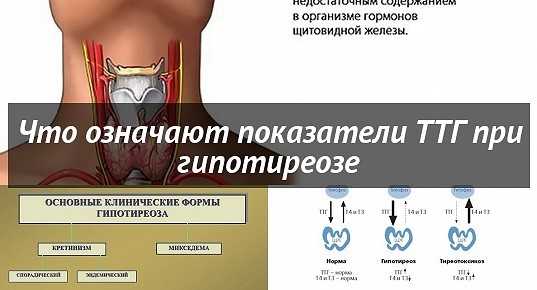 nine0003
nine0003
Over the past two decades, a number of recommendations and guidelines have been published regarding aspects of the diagnosis and treatment of thyroid diseases during pregnancy and the postpartum period. It is accepted by the American Thyroid Association (ATA) that reference intervals for TSH levels during pregnancy should be narrowed by the high value, and since 2011, trimester-specific TSH levels have been used in many countries around the world and in our country. Note that the ATA recommendations were based on the results of 6 cohort studies conducted in the United States and some European countries, and it was also separately stipulated that these standards are offered only for laboratories that, for whatever reason, do not have their own established standards. Recommended trimester-specific reference intervals for TSH were as follows: 1st trimester 0.1–2.5 mU/L; II trimester 0.2-3 mU/l; III trimester 0.3-3 mU/l. nine0003
European guidelines for the diagnosis and treatment of thyroid diseases during pregnancy, published in 2012, demonstrate the agreement of the expert community to reduce the upper limits of trimester-specific reference ranges for TSH levels in pregnant women [33]. It is these ranges that are currently used by domestic clinicians to assess the function of the thyroid gland and as target when performing replacement therapy for pregnant women with hypothyroidism. In 2017, updated ATA clinical guidelines were released, which revised the reference TSH values for pregnant women. This is based on recent screening studies showing that pregnancy in general is characterized by relatively low TSH levels in virtually all populations, but the extent of this decline varies greatly between different racial and ethnic groups. It is also necessary to take into account differences in iodine availability. Studies involving pregnant women in Asia, India, and some European countries have demonstrated significant geographical heterogeneity in the intensity of the fall in TSH levels in the first trimester, revealing the predominance of a slight decrease in upper values [34-37]. Similar data were obtained from studies of pregnant women in Korea — a moderate decrease in TSH in the first trimester by 0.
It is these ranges that are currently used by domestic clinicians to assess the function of the thyroid gland and as target when performing replacement therapy for pregnant women with hypothyroidism. In 2017, updated ATA clinical guidelines were released, which revised the reference TSH values for pregnant women. This is based on recent screening studies showing that pregnancy in general is characterized by relatively low TSH levels in virtually all populations, but the extent of this decline varies greatly between different racial and ethnic groups. It is also necessary to take into account differences in iodine availability. Studies involving pregnant women in Asia, India, and some European countries have demonstrated significant geographical heterogeneity in the intensity of the fall in TSH levels in the first trimester, revealing the predominance of a slight decrease in upper values [34-37]. Similar data were obtained from studies of pregnant women in Korea — a moderate decrease in TSH in the first trimester by 0.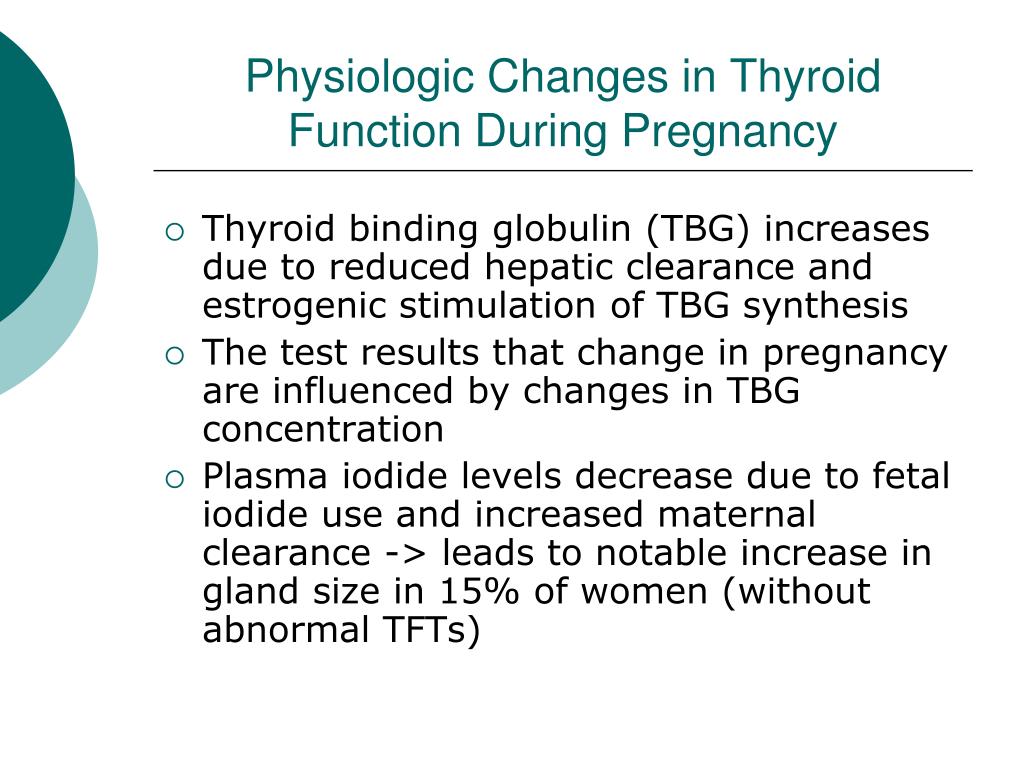 5–1.0 mU/l [38]. A recent study of 4800 pregnant women in China showed that although a downward shift in the TSH reference range occurred at weeks 7-12, the upper reference limit was not significantly lowered from 5.31 to 4.34 mU/L [ 35]. nine0003
5–1.0 mU/l [38]. A recent study of 4800 pregnant women in China showed that although a downward shift in the TSH reference range occurred at weeks 7-12, the upper reference limit was not significantly lowered from 5.31 to 4.34 mU/L [ 35]. nine0003
Most research groups recognize the limited utility of the current trimester-specific ranges for TSH, as they are based on populations with adequate iodine intake, and the sample included women without thyroid A.T. The relevance of conducting national studies in order to establish population reference values of thyroid hormones, taking into account iodine intake, the presence of antibodies to thyroid tissue and, according to some studies, body mass index becomes obvious. nine0003
Thus, taking into account the accumulated data and the latest ATA recommendations, at present, the ideal option is to use TSH “normal” intervals for pregnant women, defined for a specific region (country), taking into account ethnic and geographical characteristics. Unfortunately, in Russia at present there are no data from national population studies of TSH levels and clinical recommendations based on them for the diagnosis and treatment of thyroid diseases during pregnancy. In such a situation, the ATA clinical guidelines suggest that professionals use a TSH level of 4 mU/L as the upper “normal” reference value, which for most laboratory tests represents a decrease in the upper population TSH value of about 0.5 mU/L. nine0003
Unfortunately, in Russia at present there are no data from national population studies of TSH levels and clinical recommendations based on them for the diagnosis and treatment of thyroid diseases during pregnancy. In such a situation, the ATA clinical guidelines suggest that professionals use a TSH level of 4 mU/L as the upper “normal” reference value, which for most laboratory tests represents a decrease in the upper population TSH value of about 0.5 mU/L. nine0003
Determining the level of T 4 during pregnancy
Free T 4 is the biologically active part of the total T 4 , which is only about 0.03%, and the main volume of the hormone is associated with serum proteins, primarily with thyroxin-binding globulin (TSG). Determination of the content of fT 4 in the vast majority of laboratories is carried out by immunometric methods, the accuracy of the results of which depends on many factors: dilution, temperature, buffer composition, AT affinity, etc. [39]. Currently available immunometric methods for the determination of thyroid hormones are essentially approximate and evaluative tests (do not give an accurate indication of the hormone concentration) and are very sensitive to changes in the level of binding proteins [40]. Determining the content of fT 4 during pregnancy is associated with methodological difficulties arising as a result of biochemical changes occurring in the mother's body [41]. Serum of pregnant women is characterized by higher concentrations of TSH, which peaks around the 16th week of pregnancy, non-esterified fatty acids and lower levels of albumin compared to non-pregnant women. High concentrations of TSH tend to lead to higher total T 4 and, accordingly, to a regular, from a methodological point of view, underestimation of the level of svt 4 [42]. As a result, clinicians need to differentiate between true hypothyroxinemia in pregnant women, which requires the appointment of replacement therapy with levothyroxine sodium, and methodically determined underestimation of the level of FTT 4 , which does not require any intervention.
[39]. Currently available immunometric methods for the determination of thyroid hormones are essentially approximate and evaluative tests (do not give an accurate indication of the hormone concentration) and are very sensitive to changes in the level of binding proteins [40]. Determining the content of fT 4 during pregnancy is associated with methodological difficulties arising as a result of biochemical changes occurring in the mother's body [41]. Serum of pregnant women is characterized by higher concentrations of TSH, which peaks around the 16th week of pregnancy, non-esterified fatty acids and lower levels of albumin compared to non-pregnant women. High concentrations of TSH tend to lead to higher total T 4 and, accordingly, to a regular, from a methodological point of view, underestimation of the level of svt 4 [42]. As a result, clinicians need to differentiate between true hypothyroxinemia in pregnant women, which requires the appointment of replacement therapy with levothyroxine sodium, and methodically determined underestimation of the level of FTT 4 , which does not require any intervention. Because serum levels of fT 4 fluctuate significantly during pregnancy and there is wide variability between measurement methods, interpretation of measured values of fT 4 requires both method specific and trimester specific ranges.
Because serum levels of fT 4 fluctuate significantly during pregnancy and there is wide variability between measurement methods, interpretation of measured values of fT 4 requires both method specific and trimester specific ranges.
The possibility of using new HPLC-MS/MS technology to develop reliable and proven trimester-specific intervals for thyroid hormones during pregnancy has been widely discussed. The emergence and development of HPLC-MS/MS technology provides high performance, almost 100% specificity, and the necessary sensitivity compared to immunoassay methods [43, 44]. Currently, MS/MS technology is widely used for routine diagnostics in endocrinological laboratories, and primarily for determining the main spectrum of steroids, as well as their numerous metabolites. nine0003
Subclinical hypothyroidism (SH) during pregnancy
Observational studies covering more than the last three decades show that FH statistically significantly increases obstetric risk, the incidence of pregnancy complications and adverse outcomes for the child, primarily for its CNS [45]. At the same time, there is no evidence that treatment of subclinical hypothyroidism during pregnancy with levothyroxine sodium improves cognitive functions in a child. This was demonstrated in the CATS study, which screened thyroid function in 21,846 pregnant women [46]. More recently, a prospective 5-year study conducted in the United States evaluated the results of treatment of subclinical hypothyroidism, detected for the first time in the first trimester of pregnancy [47]. A total of 9 screeningsOf 7,288 pregnant women, the study included 677 women with SH, defined as an increase in TSH levels of more than 4.0 mU/l with a normal FTS concentration of 4 . An analysis of the "breakpoint" - IQ score in children under 5 years of age - did not reveal the benefits of treating subclinical hypothyroidism during pregnancy: therapy with levothyroxine sodium did not lead to a clinically significant improvement in cognitive functions in children under 5 years of age. As well as no benefits of therapy with levothyroxine sodium in relation to the course of pregnancy and reducing the risk of obstetric complications have been identified.
At the same time, there is no evidence that treatment of subclinical hypothyroidism during pregnancy with levothyroxine sodium improves cognitive functions in a child. This was demonstrated in the CATS study, which screened thyroid function in 21,846 pregnant women [46]. More recently, a prospective 5-year study conducted in the United States evaluated the results of treatment of subclinical hypothyroidism, detected for the first time in the first trimester of pregnancy [47]. A total of 9 screeningsOf 7,288 pregnant women, the study included 677 women with SH, defined as an increase in TSH levels of more than 4.0 mU/l with a normal FTS concentration of 4 . An analysis of the "breakpoint" - IQ score in children under 5 years of age - did not reveal the benefits of treating subclinical hypothyroidism during pregnancy: therapy with levothyroxine sodium did not lead to a clinically significant improvement in cognitive functions in children under 5 years of age. As well as no benefits of therapy with levothyroxine sodium in relation to the course of pregnancy and reducing the risk of obstetric complications have been identified. Some results of the study are of interest, for example, a dynamic assessment of thyroid hormones in the first trimester - a trend towards a decrease in the content of fT 9 was noted0069 4 only when the TSH level is 4.8 mU/l or more.
Some results of the study are of interest, for example, a dynamic assessment of thyroid hormones in the first trimester - a trend towards a decrease in the content of fT 9 was noted0069 4 only when the TSH level is 4.8 mU/l or more.
If FH is detected in a woman trying to conceive naturally in the absence of anti-thyroid antibodies, therapy with low doses of levothyroxine sodium (25–50 mcg) may be recommended to avoid progression of hypothyroidism in the event of pregnancy.
If FH is diagnosed in a woman planning IVF, levothyroxine sodium is recommended to achieve a TSH level of less than 2.5 mU/L. There is insufficient evidence that levothyroxine sodium therapy improves pregnancy success after assisted reproductive technologies in women who carry TPO antibodies without reducing thyroid function. However, the administration of levothyroxine sodium in this situation may be considered in light of its potential benefits compared to its minimal risk. In such cases, 25–50 micrograms of levothyroxine sodium is a typical starting dose. nine0003
nine0003
Treatment
Available evidence supports the benefit of initiating therapy as early as possible. Therefore, with manifest hypothyroidism first detected during pregnancy, it is necessary to promptly prescribe levothyroxine sodium. The calculation of the dose of the drug for starting treatment is defined as 2.3 μg per 1 kg of body weight per day with the first control of the TSH level after 2 weeks. In the future, to correct therapy, control determinations of TSH levels should be performed every 4 weeks throughout the first half of pregnancy (up to 16-20 weeks of pregnancy) and at least once in the period from the 26th to the 32nd week. The goal of hypothyroidism therapy is to maintain TSH levels within trimester-specific reference intervals (if defined), and if this is not available, then it is reasonable to maintain TSH levels below 2.5 mU/L [31]. It has not been confirmed that achieving a lower TSH level (less than 1. 5 mU/L) is associated with the clinical benefit of such therapy [48]. If a woman is already receiving levothyroxine sodium, its dose should be increased by 30-50%. nine0003
5 mU/L) is associated with the clinical benefit of such therapy [48]. If a woman is already receiving levothyroxine sodium, its dose should be increased by 30-50%. nine0003
In the presence of an increased risk of developing hypothyroidism during pregnancy, only dynamic monitoring is recommended, in particular, this applies to patients with euthyroidism and carriage of antibodies to thyroid tissue or after surgical treatment (thyroid resection, hemithyroidectomy) or radioiodine therapy for thyroid disease. Based on the conclusions made on the basis of studies on the treatment of hypothyroidism during pregnancy, it can be recommended to monitor the level of TSH in these women every 4 weeks during the first half of pregnancy, then it is considered sufficient to control the level of TSH in the period of 26-32 weeks of pregnancy [31, 49]. Since it has not been proven that levothyroxine sodium therapy can reduce the risk of preterm birth and spontaneous pathological termination of pregnancy in AT carriers of pregnant women without thyroid dysfunction, prophylactic treatment with levothyroxine sodium preparations is not carried out in this group [31].
Women who received levothyroxine sodium therapy before pregnancy should reduce the dose of the drug to baseline after delivery with TSH control after 6 weeks. If levothyroxine sodium is first started during pregnancy (especially when the dose of the drug is ≤50 µg/day), then after delivery, treatment should be discontinued with TSH control also after 6 weeks [31]. Current clinical practice is mainly focused on preventing the negative consequences of low thyroid hormone levels during pregnancy. At the same time, recent research data have shown that both low and high concentrations of thyroid hormones have a negative impact on the development of the fetal brain and its morphological structure, and are also closely associated with neuropsychiatric disorders in children and adolescents [30, 50, 51 ]. nine0003
In 2016, the results of a prospective cohort population study integrated into the Generation R Study (Rotterdam, the Netherlands) were published, in which the association of maternal thyroid function with the child's IQ (assessed using non-verbal intelligence tests) and brain morphology (assessed using magnetic -resonance tomography, MRI) [30].
IQ data were obtained from 3839 children, and MRI of the brain was performed on 646 children. Concentration of svt 4 in maternal serum showed an inverted U-shaped relationship with IQ and gray matter volume in the child. Equally for both low and high concentrations of fT 4 , this association corresponded to a reduction in the average level of IQ by 1.4-3.8 points. At the same time, the level of maternal TSH was not associated with a decrease in the level of children's IQ or disturbances in brain morphology. All associations remained similar after excluding women with overt hypothyroidism and hyperthyroidism. Established relationship between the level of svt 4 mother and child's cortical volume suggest that levothyroxine sodium therapy during pregnancy, which is often initiated in women with subclinical hypothyroidism, may be associated with a potential risk of adverse neurodevelopmental outcomes in the child when the goal of treatment is to maintain the level of svt 4 at the upper limit of normal.
Micronutrients and autoimmune thyroiditis nine0016
It seems appropriate to consider separately the issue of prescribing micronutrients to patients with AIT. Iodine in a physiological dose (about 200 mcg/day) is not able to induce the development of hypothyroidism and does not adversely affect thyroid function in the already existing hypothyroidism caused by AIT. In the presence of antibodies to the thyroid gland and in the absence of a decrease in its function, it is recommended to prescribe iodine preparations for the entire period of pregnancy and lactation [52]. When prescribing drugs containing iodine in pharmacological doses (more than 1 mg / day) to patients with AIT, one should be aware of the possible risk of manifestation of hypothyroidism (or an increase in the need for thyroid hormones in subclinical and overt hypothyroidism) and monitor thyroid function. Currently, discussions are underway about the positive effect of selenium, however, in accordance with the 2017 ATA recommendations, the administration of selenium preparations to pregnant women with high titers of Ab-TPO is not recommended [31]. Further research is needed on this topic. nine0003
Further research is needed on this topic. nine0003
The urgency of the problem of diagnosing and treating hypothyroidism in women of reproductive age is determined by the high incidence of infertility, obstetric and perinatal complications, since reduced thyroid function plays an important role. A pregnant woman with hypothyroidism has an increased risk of obstetric complications: intrauterine fetal death, hypertension, placental abruption, perinatal complications. Thyroid hormone therapy significantly improves pregnancy outcomes. Hypothyroidism in pregnant women is an absolute indication for the appointment of replacement therapy at a calculated dose of 2.3 μg per 1 kg of body weight per day. If a woman with compensated hypothyroidism plans pregnancy, the dose of levothyroxine sodium should be increased immediately after its onset by 25-30%. In the future, the control of the adequacy of therapy is carried out by the level of thyroid-stimulating hormone, which must be carried out every 6-8 weeks. nine0003
nine0003
The management of subclinical hypothyroidism and the appointment of replacement therapy remain debatable. Based on the opinion of the majority of experts, given the presence of proven potential risks for the mother and fetus, it is recommended to start replacement therapy with levothyroxine sodium with a subclinical increase in the level of thyroid-stimulating hormone against the background of the presence of an increased titer of antibodies to the thyroid gland.
Recent considerations suggest that the upper trimester-specific cut-off values for thyroid-stimulating hormone can be increased and a thyroid-stimulating hormone level of 4 mU/L can be used. nine0003
Since geographic and ethnic differences in the population influence thyroid-stimulating hormone levels in pregnant women, this should be taken into account when developing reference intervals along with iodine intake and anti-thyroid tissue antibody carriage using high performance liquid chromatography in combination with tandem mass spectrometry.
Funding source. Search and analytical work during the preparation of the manuscript was carried out with the financial support of the Russian Science Foundation grant No. 17-75-30035 “Autoimmune endocrinopathies with multiple organ lesions: genomic, postgenomic and metabolomic markers. Genetic risk prediction, monitoring, early predictors, personalized correction and rehabilitation. nine0003
The authors declare no conflict of interest.
The authors declare no conflicts of interest.
Credits
Nadezhda Mikhailovna Platonova — https://orcid.org/0000-0001-6388-1544; eLibrary SPIN: 4053-3033
Makolina Natalia Pavlovna — https://orcid.org/0000-0003-3805-7574; eLibrary SPIN: 7210-9512
Rybakova Anastasia Andreevna — e-mail: [email protected]; https://orcid.org/0000-0002-1248-9099; eLibrary SPIN: 8275-6161
Ekaterina Anatolyevna Troshina — https://orcid. org/0000-0002-8520-8702; eLibrary SPIN: 8821-8990
org/0000-0002-8520-8702; eLibrary SPIN: 8821-8990
Corresponding author: Rybakova Anastasia Andreevna — https://orcid.org/0000-0002-1248-9099;br />eLibrary SPIN: 8275-6161
Features of the management of pregnant women with thyroid pathology. Recommendations for screening, management and monitoring
Authors: A.V. Kaminsky, T.F. Tatarchuk, T.V. Avramenko and others
01/20/2017
The thyroid gland (TG) is one of the most important organs, the functional state of which determines the possibility of conception, bearing and birth of healthy children. Thyroid hormones are needed for the formation of the brain and heart of the unborn child. The trace element iodine is necessary for the synthesis of these hormones, and its deficiency causes the development of iodine deficiency conditions at any age - in the fetus, children and adults.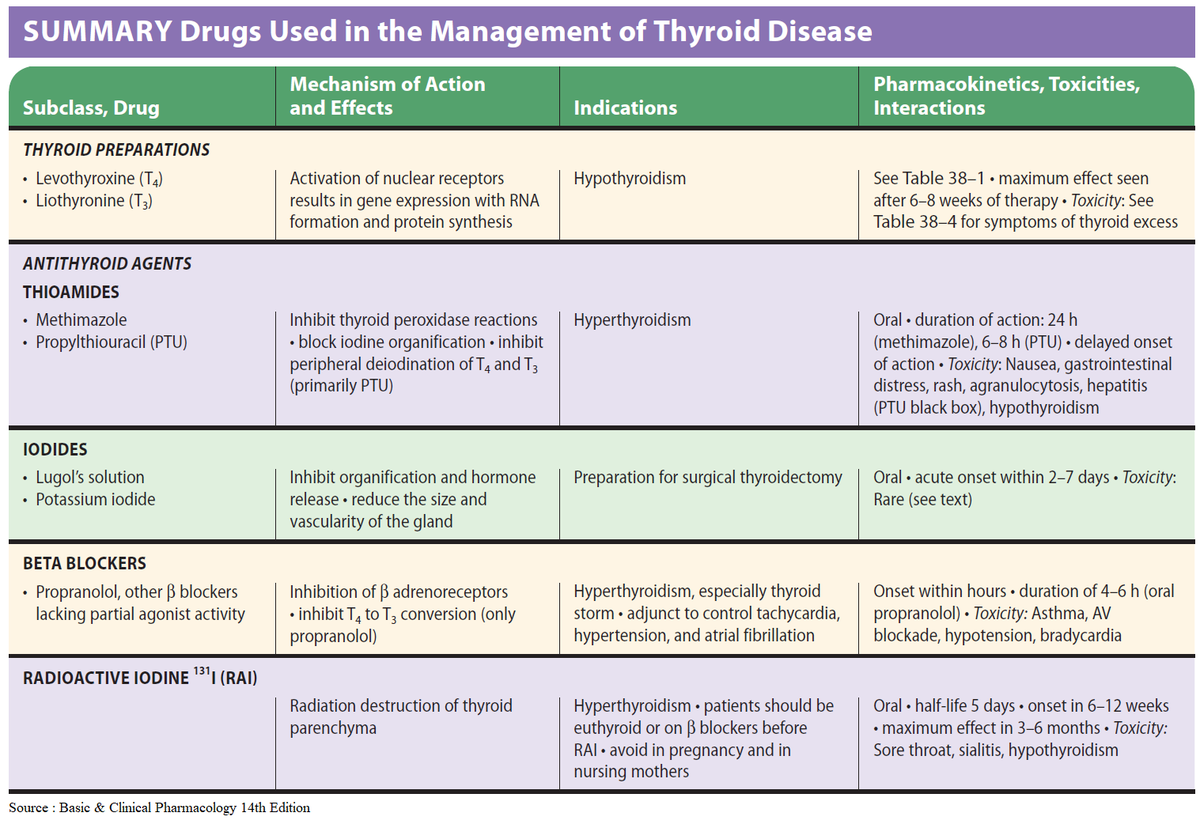 In addition, iodine deficiency often contributes to a decrease in intelligence in individuals and the nation as a whole. nine0216
In addition, iodine deficiency often contributes to a decrease in intelligence in individuals and the nation as a whole. nine0216
In Ukraine, the frequency of thyroid pathology is significantly increased. In general, among the population, it occurs in 20-30% of adults, and among the victims of the Chernobyl accident, about 50%. The most common problems are nodular goiter and diffuse non-toxic goiter, which are caused by the presence of natural iodine deficiency. Another common pathology is autoimmune thyroiditis associated with a lack of the trace element selenium. Thyroid function disorders (hypothyroidism, hyperthyroidism) are diagnosed infrequently - in 2-5% of the population, but with the highest frequency (up to 12%) - among pregnant women or women who cannot become pregnant, and in those who resort to in vitro fertilization - up to 20%. nine0003
In 2001, the World Health Organization (WHO) first introduced the term "iodine deficiency diseases" to refer to all pathological conditions that develop in the population as a result of iodine deficiency, which can be reversible with normalization of iodine intake.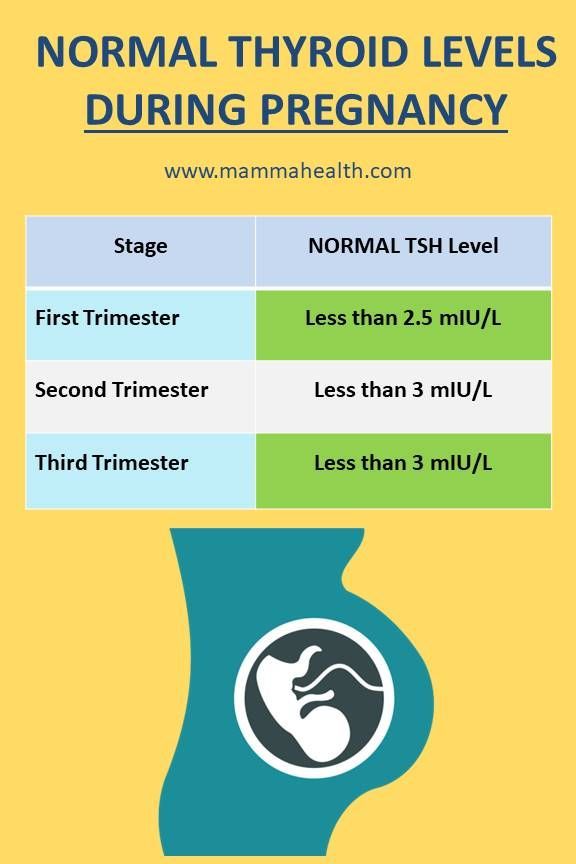 These include not only thyroid diseases (nodular goiter, hyperthyroidism, hypothyroidism), but also others: infertility, decreased intelligence, some disorders and malformations (Table 1).
These include not only thyroid diseases (nodular goiter, hyperthyroidism, hypothyroidism), but also others: infertility, decreased intelligence, some disorders and malformations (Table 1).
The whole territory of Europe, including Ukraine, is iodine deficient. One can only argue about which region lacks iodine more. Natural deficiency of iodine and some other trace elements (selenium, zinc, etc.), vitamins (groups B, D), poor ecology, chemicalization contribute to the occurrence of thyroid pathology and other disorders that prevent normal conception and bearing healthy offspring. nine0003
In some European countries (Switzerland, Germany, Austria, etc.), effective endemic iodine prophylaxis over the past 100 years has made it possible to achieve great success and exclude them from the list of scarce ones. Armenia, Azerbaijan, Turkmenistan, Georgia, Belarus and Kazakhstan managed to almost completely solve the problem of iodine deficiency in the diet of the population through the use of mass iodine prophylaxis in the form of enrichment of edible salt with iodine.
On average, an adult resident of Ukraine receives only 50-80 mcg of iodine per day, which is below the required level of 150 mcg/day (within 100-250 mcg/day). For pregnant and lactating women, the daily requirement for iodine should be above -250 mcg, so they and their children are the most vulnerable groups of the population (Table 2). nine0227 The average daily dose of iodine 150 mcg corresponds to the median urinary iodine concentration of 100 mcg/l [2].
Thyroid and pregnancy
Thyroid dysfunction may interfere with pregnancy or lead to miscarriage already in the presence of subclinical hypothyroidism (thyroid stimulating hormone (TSH) level of 4 mIU/L or higher in non-pregnant; 3 mIU/L or higher in pregnant women ). Fortunately, most thyroid diseases that affect pregnancy are easily diagnosed and corrected. The difficulty lies in the very awareness of the presence of a problem on the part of the thyroid gland. nine0003
Very often, the symptoms that accompany these disorders are minor, are of a general nature: weakness, increased fatigue, drowsiness during the day, insomnia at night, sometimes a violation of the stool or menstrual cycle. In hyperthyroidism, tachycardia, poor heat tolerance are observed, in hypothyroidism, dry skin and / or constipation.
In hyperthyroidism, tachycardia, poor heat tolerance are observed, in hypothyroidism, dry skin and / or constipation.
Timely detection of thyroid disorders in pregnant women, along with diabetes mellitus, is a very important task, therefore thyroid tests (TSH, ATPO, ATTG, thyroglobulin) and determination of glycemia (fasting glucose, standard glucose tolerance test, glycosylated hemoglobin) are mandatory when planning pregnancy, monitoring its development, as well as after childbirth. nine0003
In the early stages of pregnancy (up to 3-4 months), the fetus functions only due to the thyroid hormones of its mother. This period, especially the first 4 weeks after conception, is especially critical. During these periods, the greatest number of miscarriages associated with iodine deficiency or hypothyroidism in the mother occurs.
Today in Ukraine a significant number of women are diagnosed with subclinical hypothyroidism, the main cause of which is iodine deficiency. The human body is characterized by high sensitivity to iodine deficiency and significant resistance to excess iodine for a long time. nine0003
The human body is characterized by high sensitivity to iodine deficiency and significant resistance to excess iodine for a long time. nine0003
Therefore, according to the WHO recommendation, pregnant women, especially those living in iodine-deficient regions, are required to additionally receive iodine-containing tablets for the entire period. The average iodine requirement in adults is, according to WHO, 150 mcg (100-250 mcg) daily, and for pregnant women more than 250 mcg/day. The safe level of iodine for residents of most regions is up to 1000 mcg / day in total. In the conditions of Ukraine, it is almost impossible to achieve it.
Considering that adults in Ukraine actually receive only 50-80 micrograms of iodine per day, the ideal dosage of iodine for pregnant women is 200 micrograms in the form of original potassium iodide tablets taken once daily after meals at a convenient time of day. The drug is recommended for use throughout the entire period of pregnancy, lactation and a year before the planned conception.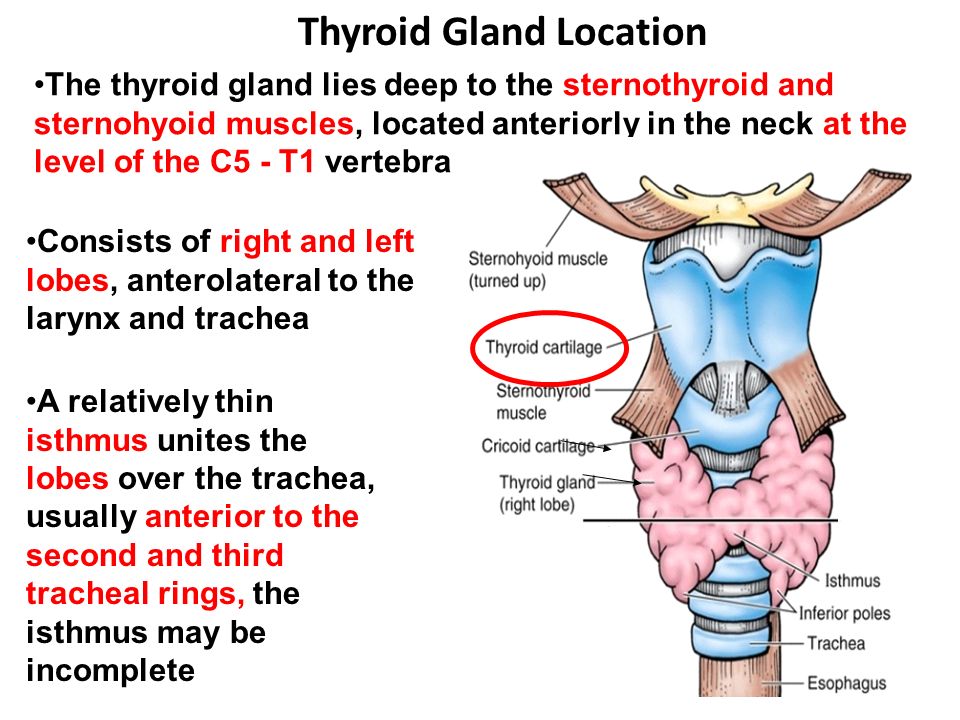 At the same time, periodic (every 4-6 months) monitoring of thyroid parameters (TSH and thyroglobulin, sometimes ATPO) is welcome. nine0003
At the same time, periodic (every 4-6 months) monitoring of thyroid parameters (TSH and thyroglobulin, sometimes ATPO) is welcome. nine0003
After 4 months of intrauterine development, the fetus begins to function its own thyroid gland, which actively captures the iodine absorbed by the mother and synthesizes the amount of thyroid hormones necessary for the body. Therefore, the efficiency of synthesis depends on the daily intake of iodine by the mother.
Diagnosis of thyroid dysfunction
The main function of the thyroid gland is the production of hormones: thyroxine (T4), triiodothyronine (T3), calcitonin. Receptors for them are present in all cells, their effects determine the physiological capabilities of the organism. Any deviation of their concentration in the blood from the norm violates the efficiency of the tissues. The functioning of the thyroid gland is regulated by the hypothalamus and pituitary gland through the release of the latter TSH, which acts as a stimulator of thyrocytes. With a decrease in thyroid function, the pituitary gland increases the secretion of TSH, forcing them to work more intensively, and with excessive production of thyroid hormones, thyroid-stimulating stimulation decreases. Thus, there is an inverse relationship between the concentrations of TSH and thyroid hormones. This feedback mechanism is used in the diagnosis of thyroid dysfunction (Table 3). nine0003
With a decrease in thyroid function, the pituitary gland increases the secretion of TSH, forcing them to work more intensively, and with excessive production of thyroid hormones, thyroid-stimulating stimulation decreases. Thus, there is an inverse relationship between the concentrations of TSH and thyroid hormones. This feedback mechanism is used in the diagnosis of thyroid dysfunction (Table 3). nine0003
Given the dominant role of the pituitary gland in the regulation of thyroid function, which responds to minor changes in the level of thyroid hormones, the determination of TSH concentration is a more sensitive test than the free fractions of hormones (FT3, FT4). This is also due to the fact that they, like all biologically active substances, exist in two molecular optical isoforms - active levorotatory and biologically inactive dextrorotatory. Their sum is FT3 and FT4, and the ratio of isoforms (enantomers) may vary depending on the presence of iodine deficiency, inflammation in the thyroid gland, and other reasons. So, for substitution therapy, a highly purified levorotatory isoform of FT4 is used - the drug L-thyroxine. nine0003
So, for substitution therapy, a highly purified levorotatory isoform of FT4 is used - the drug L-thyroxine. nine0003
Features of thyroid dysfunction in pregnant women
When pregnancy occurs, estrogen synthesis increases, which can lead to a decrease in thyroid function and an increase in TSH concentration in approximately 20% of women during the first trimester. At the same time, other women, on the contrary, may experience a decrease in TSH levels due to an increase in the levels of chorionic gonadotropin (which reaches a peak by 10-12 weeks of pregnancy), which in 2% of cases gives a clinic of transient gestational thyrotoxicosis. This condition is characterized by mild manifestations of excess thyroid hormones and uncontrolled vomiting during the first trimester, the so-called toxicosis of pregnant women. Monitoring of TSH in pregnant women who receive thyroxine replacement therapy or have thyroid pathology should be carried out in a stable situation - every 1-2 months. Due to the special risk for the mother and fetus, physiological characteristics for pregnant women, other norms for TSH levels are recommended (Table 4). nine0003
Due to the special risk for the mother and fetus, physiological characteristics for pregnant women, other norms for TSH levels are recommended (Table 4). nine0003
Diagnosis of iodine deficiency
Iodine is an important trace element that is needed for the synthesis of thyroid hormones, the normal functioning of the mammary glands, stomach, and other tissues (skin, eyes, brain). Lack of iodine leads to disruption of various physiological processes.
From the body, 90% of iodine is excreted in the urine, 10% in the bile. This factor is used in epidemiological (large-scale) scientific studies to study the level of iodine supply in a particular area. With such a one-time study, urine is collected from hundreds to thousands of inhabitants in 1-2 days and the concentration of iodine is analyzed. Despite the rapid change in its content in the body every 3 days, depending on the nature of nutrition, in a large group of observations it is possible to level such a statistical error in the change in ioduria. Therefore, on the recommendation of WHO, the study of ioduria is carried out only in scientific studies in large groups. nine0003
Therefore, on the recommendation of WHO, the study of ioduria is carried out only in scientific studies in large groups. nine0003
For an individual assessment of iodine sufficiency in 1994 and 2007, WHO/UNICEF proposed other indicators of the iodine status of the population - determination of thyroglobulin levels in children, adults and pregnant women, as well as the concentration of TSH in the blood of newborns (neonatal screening on the 4-5th day in full-term; on the 7-14th day in premature babies).
Thyroglobulin is a protein that is synthesized by the thyroid gland and enters the blood in a small amount. However, with the development of goiter or with a lack of iodine, its concentration increases. Studies have shown that the individual level of thyroglobulin reliably coincides with ioduria. Unlike the latter, the amount of thyroglobulin in the blood changes slowly, over months, so it can be used as a marker of iodine deficiency, as well as track its changes over time during treatment with iodine preparations.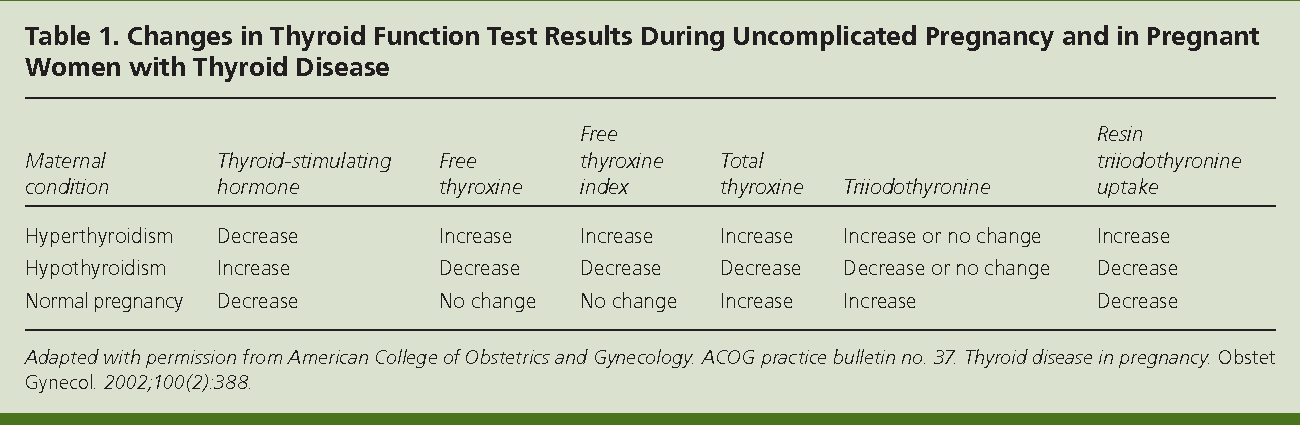 Its blood level of 10 mg / l or more indicates the presence of mild iodine deficiency, 20-40 mg / l - moderate, over 40 mg / l - severe deficiency. Thyroglobulin is also used as a tumor marker when its concentration is 67 mg/l or higher, including in patients with thyroidectomy. It increases in differentiated thyroid cancer (Table 5). nine0003
Its blood level of 10 mg / l or more indicates the presence of mild iodine deficiency, 20-40 mg / l - moderate, over 40 mg / l - severe deficiency. Thyroglobulin is also used as a tumor marker when its concentration is 67 mg/l or higher, including in patients with thyroidectomy. It increases in differentiated thyroid cancer (Table 5). nine0003
Management and monitoring of pregnant women with hypothyroidism
When a woman is pregnant, her body needs enough thyroid hormone to support the development of the fetus and her own needs. Uncontrolled thyroid hormone deficiency can lead to critical pregnancy complications such as preterm birth, preeclampsia, miscarriage, postpartum hemorrhage, anemia, placental abruption, and death of the baby or mother.
 The need for thyroid hormones increases significantly during pregnancy, increasing with each trimester, so women with initially normal levels of these hormones in the presence of thyroid disease may develop hypothyroidism. After childbirth, the need for them decreases sharply, often to the level preceding pregnancy. nine0003
The need for thyroid hormones increases significantly during pregnancy, increasing with each trimester, so women with initially normal levels of these hormones in the presence of thyroid disease may develop hypothyroidism. After childbirth, the need for them decreases sharply, often to the level preceding pregnancy. nine0003 Most women who develop hypothyroidism during pregnancy have little or no symptoms associated with it.
The goal of treatment for hypothyroidism is to maintain a normal TSH level, which will indicate the correct balance of thyroid hormones in the blood. The normal TSH level for pregnant women is different from what is allowed in non-pregnant women. Depending on the trimester, the normal range of TSH during pregnancy should be between 0.1-2.5 mIU/L in the 1st trimester to 0.3-3 mIU/L in the 3rd trimester according to US guidelines and similar to European guidelines. The detection of an increase in TSH more than 3-3.5 mIU / l indicates a decrease in thyroid function in a pregnant woman with hypothyroidism, which requires hormone replacement therapy.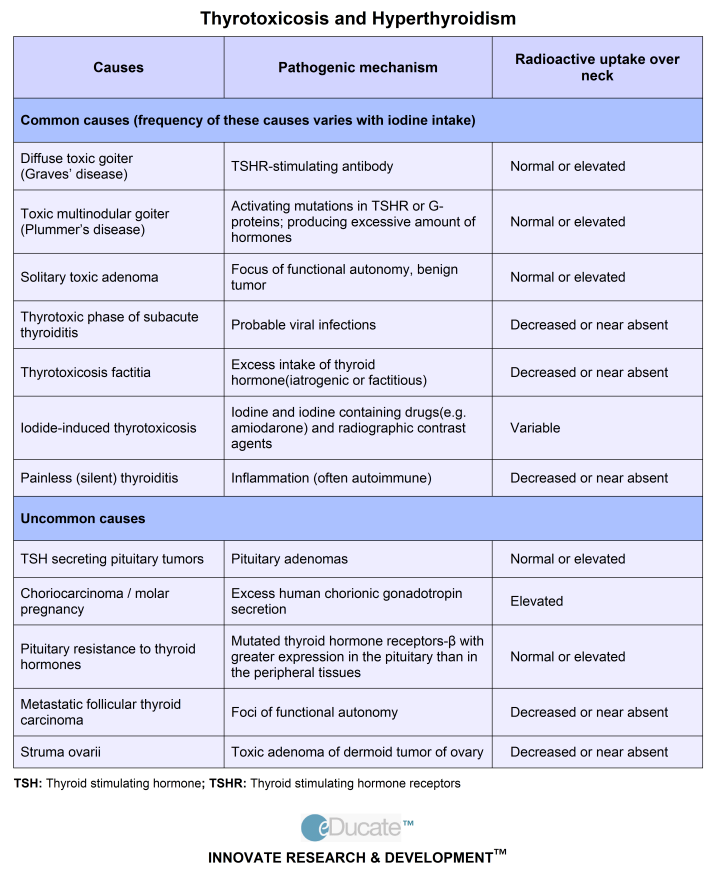 nine0003
nine0003
Adequate treatment and monitoring of hypothyroidism allows you to completely avoid the possible complications associated with it. The treatment of hypothyroidism consists in hormone replacement therapy with thyroid hormones according to the same principles that exist for non-pregnant women. L-thyroxine is initially prescribed at a minimum dose of 25 mcg / day once in the morning, 30 minutes before breakfast, gradually increasing the dose to the required value, which is determined by the level of TSH, which should be within the above-described norm. At the same time, the use of L-thyroxine preparations during pregnancy is absolutely safe if the rules for hormone replacement therapy are taken into account. nine0003
Most patients with hypothyroidism, both pregnant and non-pregnant, need to select a dose of thyroid hormone that will keep the TSH concentration within the ideal range of 0.5-2.5 mIU / l, which will correspond to the level characteristic of 95% of healthy people persons.
Monitoring of established hypothyroidism is carried out, depending on the clinical task, no more than once every 2 weeks and at least 1 time in 1-2 months, optimally monthly throughout the entire period of pregnancy and in the first months after childbirth. nine0003
Adjustment of the dose of L-thyroxine in pregnant women is made every 2 weeks or every month according to the level of TSH. Once the TSH level returns to normal, less frequent checkups are required.
L-thyroxine preparations should be supplemented with iodine preparations (original potassium iodide tablets), usually at a dose of 200 mcg/day, throughout pregnancy until the end of lactation, regardless of the type of thyroid disease. If the problems are chronic, then L-thyroxine and iodine preparations continue to be taken after childbirth (for as long as necessary). nine0003
Isolated (euthyroid) hypothyroxinemia in pregnancy
Isolated hypothyroxinemia (pseudohypothyroidism) is characterized by a low concentration of FT4 with a normal TSH level (ie, euthyroidism).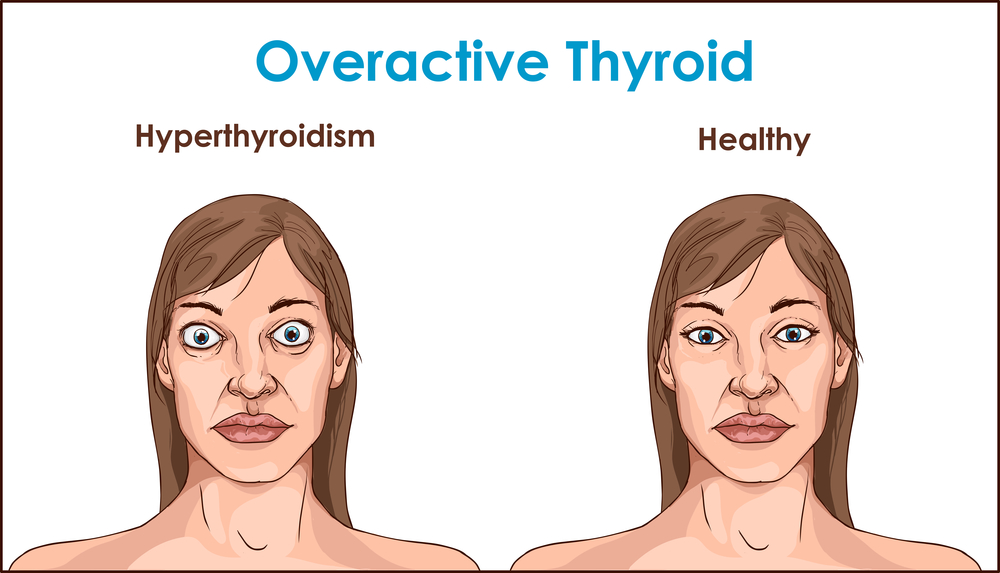 This may be the result of either iodine deficiency or poor laboratory quality (mistake). The use of iodized salt for a long time reduces the likelihood of thyroid diseases and significantly reduces the risk of developing hypothyroxinemia during pregnancy (Fig.). nine0003
This may be the result of either iodine deficiency or poor laboratory quality (mistake). The use of iodized salt for a long time reduces the likelihood of thyroid diseases and significantly reduces the risk of developing hypothyroxinemia during pregnancy (Fig.). nine0003
Approximately 2.5% of healthy women may have an FT4 concentration below the minimum threshold. Nevertheless, they have a high index of pregnancy complications, typical for patients with hypothyroidism.
The presence of isolated hypothyroxinemia leads to spontaneous abortions, premature births, complications in childbirth, perinatal mortality, congenital malformations, fetal macrosomia (body weight over 4000 g), deterioration in neuropsychic development in the offspring (psychomotor deficit associated with gestational diabetes, neonatal intraventricular hemorrhage). nine0003
In such women, it is necessary to investigate the adequacy of iodine supply (thyroglobulin level), if iodine deficiency is detected, replenish with iodine tablets. From a clinical point of view, isolated hypothyroxinemia in pregnant and non-pregnant women does not require L-thyroxine replacement therapy.
From a clinical point of view, isolated hypothyroxinemia in pregnant and non-pregnant women does not require L-thyroxine replacement therapy.
Often, the detection of a low level of FT4 with a normal concentration of TSH indicates a laboratory or methodological error, low quality of diagnostic kits. If such a result is detected, it is necessary to repeat the study of FT4 and TSH, preferably in an alternative laboratory. In many cases, reanalysis does not confirm the original result. nine0003
Management and monitoring of pregnant women with hyperthyroidism
Hyperthyroidism occurs in 0.1-1% of all pregnancies. It is diagnosed when TSH levels are below normal (less than 0.1 mIU/L) and FT4 and/or FT3 levels are above normal (manifest hyperthyroidism). The most common causes of hyperthyroidism are: diffuse toxic goiter (synonyms: thyrotoxicosis; Graves' disease, Basedow's disease) - 80% of cases, transient hyperthyroidism in autoimmune thyroiditis, toxic thyroid adenoma, thyroid cancer, acute (bacterial) or subacute (viral) thyroiditis. Manifest hyperthyroidism in all cases requires treatment, especially in pregnant women. The risks associated with hyperthyroidism are almost the same as with hypothyroidism, the fetus may additionally experience fetal tachycardia. nine0003
Manifest hyperthyroidism in all cases requires treatment, especially in pregnant women. The risks associated with hyperthyroidism are almost the same as with hypothyroidism, the fetus may additionally experience fetal tachycardia. nine0003
In exceptional cases, in women, hyperthyroidism is detected with the development of "ovarian goiter" (struma ovarii), which can develop with ovarian teratoma (2-5% of cases of teratomas), when it contains more than 50% of thyroid tissue cells, or ovarian cystic adenoma ( 1% of all ovarian tumors). These teratomas are usually benign. The symptoms of struma ovarii are similar to those of other ovarian tumors and are nonspecific. Women with struma ovarii may complain of abdominal or pelvic pain and have ascites in 12-17% of cases. Most women have elevated thyroglobulin levels, and a third have an increased concentration of the CA‑125 marker. The final diagnosis is established by cytological or histological examination. An effective method of treating struma ovarii is surgical.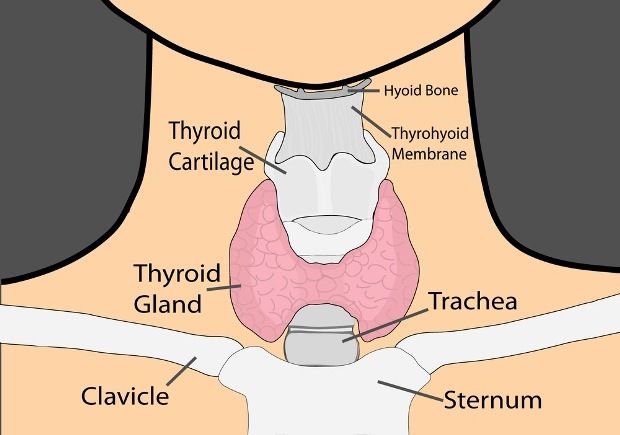 nine0003
nine0003
Subclinical hyperthyroidism is diagnosed when TSH is in the range of 0.1-0.39 mIU/L (non-pregnant) with normal FT4 and FT3 levels. However, in pregnant women, TSH standards differ (Table 4), which does not require treatment. This is also true for pregnant women with transient hyperthyroidism (TSH at the level of 0.1-0.3 mIU / l).
Diffuse toxic goiter (thyrotoxicosis) is an autoimmune disease of the thyroid gland, which is always accompanied by excessive synthesis of thyroid hormones due to the action of thyroid-stimulating antibodies (antibodies to the TSH-AT receptor to r-TSH). Among the most common causes of this disease are tobacco smoking, deficiency of trace elements of iodine and / or selenium, in rare cases, long-term (months to years) use of high doses of iodine (more than 1000-5000 mcg / day). Diagnosis of hyperthyroidism includes the determination of blood TSH, FT4, FT3, antibodies to r-TSH (the main differential criterion), sometimes antibodies to thyroid peroxidase and thyroglobulin. nine0003
nine0003
Treatment begins with the cessation of tobacco smoking, if it has taken place, is based on the suppression of the production of thyroid hormones and their effects through the use of thyreostatics (drugs methimazole, thiamazole, carbimazole and propylthiuracil) for 1.5-2 years on average, titrating the dose to necessary. In case of unsuccessful treatment, the issue of surgical intervention is considered, the condition for which is to achieve compensation for hyperthyroidism.
Monitoring of treatment in pregnant women is carried out every 2-4-6 weeks, determining the levels of TSH, if desired, FT4, FT3, periodically - the concentration of antibodies to r-TSH, glucose in blood plasma. This approach is also used for women who achieve remission of hyperthyroidism before pregnancy with thyreostatics - they have a low risk of relapse of hyperthyroidism during pregnancy, but a high risk of relapse after delivery. In the middle stages of pregnancy, they are monitored for antibodies to r-TSH. nine0227 It is most optimal if a pregnant woman with hyperthyroidism will be jointly supervised by an obstetrician-gynecologist and an endocrinologist.
nine0227 It is most optimal if a pregnant woman with hyperthyroidism will be jointly supervised by an obstetrician-gynecologist and an endocrinologist.
The specificity of the use of thyreostatics in pregnant women is that methimazole, carbimazole and thiamazole penetrate the placental barrier and can cause a teratogenic effect in the first trimester. Their development is associated with the use of high doses of drugs during the first weeks of pregnancy. Therefore, the American Thyroid Association recommends using propylthiuracil preparations in the first trimester of pregnancy, which are associated with a low teratogenic risk, but are characterized by a risk of developing liver dysfunction; and in the 2nd and 3rd trimesters, methimazole preparations. nine0003
Untreated hyperthyroidism is a greater threat to the life and health of the mother and fetus than the risks of using thyreostatics. Antithyroid antibodies can cross the placenta and affect the fetal thyroid. If antibody levels are high enough, the fetus may develop hyperthyroidism or neonatal thyrotoxicosis.
Thyrostatics in pregnant women should be used in a balanced manner, at the lowest effective dose, and hormone preparations (L-thyroxine, corticosteroids) are not prescribed additionally (as adjuvant therapy). Of the beta-blockers, propranolol can be used for a short time. nine0003
In the postpartum period, women with hyperthyroidism who are breastfeeding and receiving antithyroid hormones in small doses can continue taking drugs, which is considered safe and does not affect the thyroid of the child.
Autoimmune thyroiditis
Approximately 11-15% of all women of childbearing age have an increased amount of anti-thyroid antibodies (ATTH, ATPO). In most cases, the so-called carriage of antibodies takes place. Some of them will develop autoimmune thyroiditis with a gradual increase in titer to diagnostically reliable levels (more than 100 IU), while others will not. nine0003
At the onset of pregnancy, approximately 20-40% of these antibody-positive women will develop hypothyroidism before or immediately after delivery.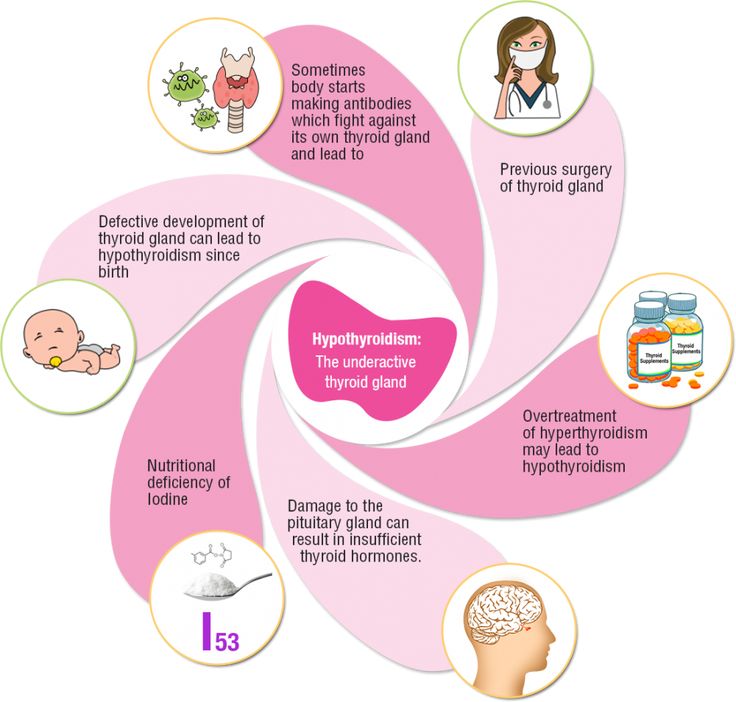 This risk increases with each trimester. It should be noted that ATPO and ATTH titers gradually decrease as pregnancy progresses, which can lead to false negative findings in late pregnancy. An increase in antibody titers to thyroid components is associated with an increased risk of miscarriage, perinatal mortality, preterm birth, neonatal respiratory distress, and aggressive behavior in children. nine0003
This risk increases with each trimester. It should be noted that ATPO and ATTH titers gradually decrease as pregnancy progresses, which can lead to false negative findings in late pregnancy. An increase in antibody titers to thyroid components is associated with an increased risk of miscarriage, perinatal mortality, preterm birth, neonatal respiratory distress, and aggressive behavior in children. nine0003
Some studies have shown beneficial effects of L-thyroxine preparations on pregnancy outcomes in these women. However, confirmed autoimmune thyroiditis does not require thyroid drugs in the absence of hypothyroidism.
Postpartum thyroiditis
Postpartum thyroiditis (postpartum thyroid dysfunction) is an autoimmune thyroid disease that resembles autoimmune thyroiditis in its course. It develops in women in the first 12 months after childbirth, more often after 3-4 months. In a third of women, hyperthyroidism is initially observed, which will be replaced by persistent hypothyroidism. The other third have only a hyperthyroid phase or a hypothyroid phase. According to some members of the American Thyroid Association, this is autoimmune thyroiditis, which was asymptomatic in women with elevated levels of thyroid antibodies (ATPO) even before childbirth, but after childbirth it began to progress rapidly. nine0003
The other third have only a hyperthyroid phase or a hypothyroid phase. According to some members of the American Thyroid Association, this is autoimmune thyroiditis, which was asymptomatic in women with elevated levels of thyroid antibodies (ATPO) even before childbirth, but after childbirth it began to progress rapidly. nine0003
Given the transient nature of this hyperthyroidism, antithyroid drugs are not used because the thyroid gland is not overactive. When diagnosing hypothyroidism, hormone replacement therapy with L-thyroxine preparations and monitoring according to the standard scheme are used. Subsequently, after 12-18 months, in 50-80% of women, thyroid function is restored to normal, the need for hormone replacement therapy with L-thyroxine preparations disappears.
Management and monitoring of pregnant women with nodular goiter
Due to the fact that Ukraine is an iodine-deficient region, there is an increased prevalence of nodular goiter on its territory. Its frequency is approximately 15-20% among adults, up to 34% among victims of the Chernobyl accident. The American Thyroid Association emphasizes that the most obvious manifestations of iodine deficiency are diffuse non-toxic goiter and nodular goiter.
Its frequency is approximately 15-20% among adults, up to 34% among victims of the Chernobyl accident. The American Thyroid Association emphasizes that the most obvious manifestations of iodine deficiency are diffuse non-toxic goiter and nodular goiter.
In most cases, nodular goiter is benign, but in 10% of cases it may be thyroid cancer, which in 90% of patients have a predominantly non-aggressive course.
When pregnancy occurs, the nodes that were diagnosed before it tend to gradually increase in size. This is due to an increased need for iodine, an increasing iodine deficiency in those who do not make up for the increased need for it (with the help of original potassium iodide tablets), excessive thyroid-stimulating stimulation associated with them, and other factors. For all pregnant women, regardless of the presence of any pathology of the thyroid gland, WHO recommends replenishing iodine at a dose of 200 mcg using the original potassium iodide tablets, especially in the area of iodine deficiency.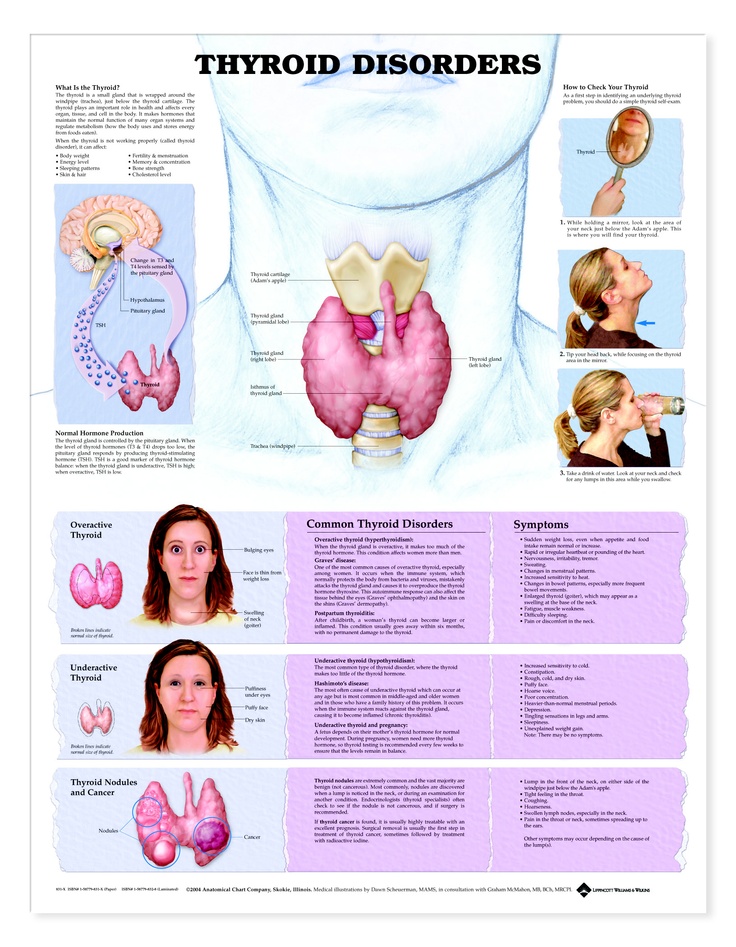 This makes it possible to exclude an increase in the volume of the thyroid gland and nodular goiter in such women. nine0003
This makes it possible to exclude an increase in the volume of the thyroid gland and nodular goiter in such women. nine0003
Monitoring of nodular goiter consists in a periodic (every 3-4 months) study of the concentration of TSH, FT4, thyroglobulin in the blood, and also involves a control ultrasound (ultrasound) of the thyroid gland at the same time. If necessary, pregnant women can undergo a fine-needle aspiration biopsy of the thyroid gland, which, like ultrasound, is a safe procedure.
When thyroid cancer is detected during pregnancy, assessing the possible risks, surgical treatment is postponed until the postpartum period. If the cancer is differentiated, the risks associated with it are low. Hormone therapy with L-thyroxine for such women is carried out with a target decrease in TSH to a level of 0.1-1.5 mIU / l. If surgery is still required due to thyroid cancer, the safest time to perform it is during the second trimester of pregnancy. nine0003
Recommendations for general screening and prevention of endocrine pathology in pregnant women
Starting from the first trimester of pregnancy until the formation of its own functioning thyroid gland, the fetus is provided with maternal hormones that cross the placenta.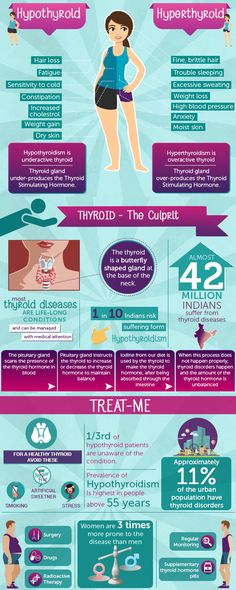 The blood of a newborn may contain up to 20-40% of maternal thyroid hormones [4-5]. Low concentrations of thyroid hormones during embryonic development and early childhood are associated with irreversible brain damage, including mental retardation and neurological damage. A meta-analysis of 18 studies found that iodine deficiency (moderate to severe) was associated with a 13.5-point decrease in mean IQ. nine0003
The blood of a newborn may contain up to 20-40% of maternal thyroid hormones [4-5]. Low concentrations of thyroid hormones during embryonic development and early childhood are associated with irreversible brain damage, including mental retardation and neurological damage. A meta-analysis of 18 studies found that iodine deficiency (moderate to severe) was associated with a 13.5-point decrease in mean IQ. nine0003
The high prevalence among the population of clinically important endocrine pathology in the area of iodine deficiency or environmental risk, which can prevent conception, the normal development of pregnancy and the course of childbirth, affect offspring in the near and long term, forces us to highlight some hormonal markers as screening, that is, those which are effective in most cases, cost-effective ("price-quality"). The analysis of these markers should be carried out in all healthy and with any concomitant pathology. These include fasting plasma glucose and TSH. Desirable additional markers, the study of which will bring an objective benefit, are the concentration of thyroglobulin, as well as ultrasound of the thyroid gland and parathyroid glands.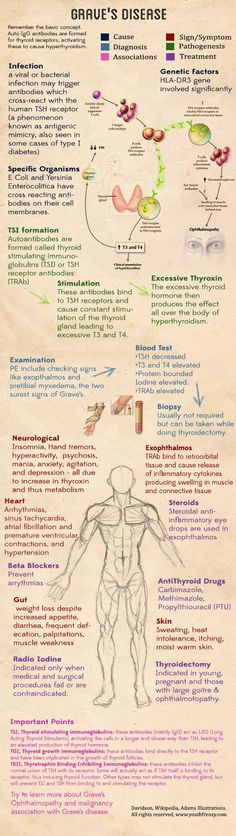 nine0003
nine0003
Every woman, regardless of whether she is planning a pregnancy, registering for pregnancy, diagnosed with infertility, planning in vitro fertilization, or having a miscarriage, should have plasma glucose and TSH levels examined. In 80-90% of women in Ukraine, an increased concentration of thyroglobulin is detected, which indicates the presence of iodine deficiency (Table 5).
The experience of many countries of the world shows that the most effective way to solve the problem of iodine deficiency is to conduct adequate mass, group and individual prophylaxis. According to WHO, all iodine deficiency diseases can be prevented, while the changes caused by iodine deficiency in utero and in early childhood are irreversible and practically untreatable. Therefore, these population groups are primarily at risk of developing the most severe iodine deficiency conditions and require special attention. The highest risk groups are pregnant women and breastfed children. nine0003
Iodine is probably the cheapest and most effective way to prevent the development of iodine deficiency diseases.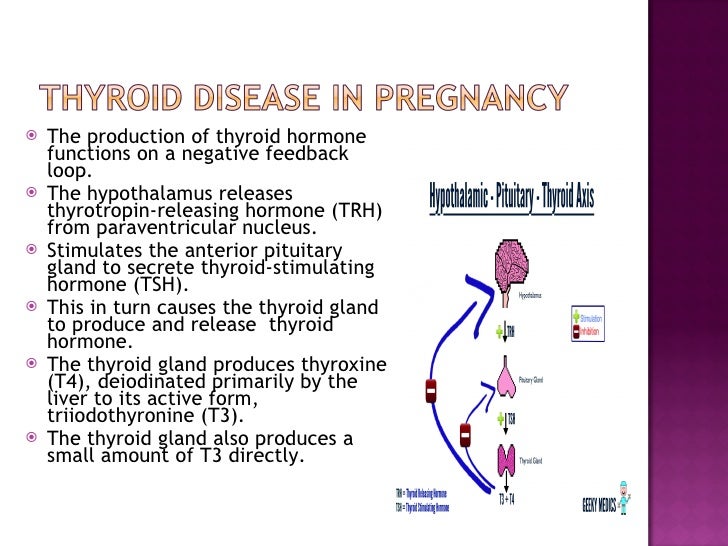 Iodine deficiency cannot be eliminated once and for all. The iodine prophylaxis program should never be terminated because it is carried out in an area where there has always been such a lack of soil and water.
Iodine deficiency cannot be eliminated once and for all. The iodine prophylaxis program should never be terminated because it is carried out in an area where there has always been such a lack of soil and water.
Since iodine is utilized by the body only in a chemically pure state in the form of salts (potassium iodide (KI) and potassium iodate (KIO3) - the main forms of iodine absorbed through the mucous membrane of the gastrointestinal tract), other forms of iodine, including organically bound iodine , like chemically pure iodine, are not absorbed by the human body until they turn into these compounds. nine0003
As a general preventive measure, WHO recommends the use of iodized salt (sodium chloride) in the home. Salt is poison. Since sodium is toxic, the use of household salt is limited to 5-6 g/day. In accordance with the international standard, a person should receive 15-40 mcg of iodine for every 1 g of salt. Sea salt contains a low concentration of iodine - 3 micrograms of iodine per 1 g of sea salt. Therefore, it also needs to be enriched with iodine.
Therefore, it also needs to be enriched with iodine.
Pregnant and lactating mothers, children and adolescents use an active mandatory model of iodine prophylaxis, which consists in prescribing iodine preparations in the form of tablets containing fixed doses of iodide or iodate, rather than dietary supplements produced from plant materials, which are registered under simplified system, without multicenter clinical trials. nine0003
Existing regulatory documents emphasize that iodine prophylaxis should be carried out daily and continuously in case of living in an area where there is a deficiency of micronutrients (Table 6).
Literature
1. Assessment of the Iodine Deficiency Disorders and monitoring their elemination: a guide for program managers, 3rd ed. / WHO. -Geneva, 2007.-P. 1-98.
2. Zimmermann M.B. Iodine deficiency in industrialized countries // Proc Nutr Soc. – 2009. - No. 8. - P. 1-11.
3. WHO/ICCIDD/UNICEF. Indicators for Assessing Iodine Deficiency Disorders and Their Control Through Salt Iodization. Geneva, Switzerland: World Health Organization; 1994.
Geneva, Switzerland: World Health Organization; 1994.
4. Rohner F., Zimmermann M., Jooste P. et al. Biomarkers of Nutrition for Development Iodine. Review // J Nutr. – 2014.–Vol. 144(8). - R. 1322S‑1342S.
5. Bath S.C., Rayman M.P. A review of the iodine status of UK pregnant women and its implications for the offspring // Environ Geochem Health. – 2015.–Vol. 37(4). –P.619-629.
- Number:
- Thematic issue "Gynecology, Obstetrics, Reproductology" No. 4 (24), chest 2016
09.12.2022 Obstetrics/GynecologyDifferentiation Prevention of natural birth defects in the development of the fetus in the female reproductive age with the elimination of methylenetetrahydrofolate reductase gene polymorphism
Looking at the pace of increasing antibiotic resistance, mystic antiseptics may become the first choice in treating vaginal infections.