Placenta maternal side
‘Fetal side’ of the placenta: anatomical mis-annotation of carbon particle ‘transfer’ across the human placenta
arising from H. Bové et al. Nature Communications https://doi.org/10.1038/s41467-019-11654-3 (2019)
In utero exposure to environmental agents is a critical driver of diseases manifesting in childhood and adulthood1,2, and direct fetal contact with potentially harmful substances is largely determined by the ability of the material to cross the placenta, which forms a selective barrier between maternal and fetal circulations. In a recent Nature Communications article, Bové et al. present data which they report as demonstrating that ‘ambient black carbon particles reach the fetal side of human placenta’3, which centres on their demonstration that carbon particles are detectable in placental villous tissue, as visualised by two-photon microscopy. Most people will interpret the term ‘fetal side’ to mean that particles have moved from the maternal circulation into cells adjacent to the fetal circulation, yet, the data presented shows that carbon particles are contained in the placental villous tissue, and does not demonstrate transfer to the fetal side. The data presented are interesting, and the techniques used a valid way of studying entry of carbon particles into tissues, yet the conclusion as stated in the title is open to misinterpretation, which was strongly evident in media coverage of this publication.
Whether carbon particles, or other pollutants, can cross the placenta to the fetus is an important question to investigate. The images presented in Bové et al., show the presence of carbon particles primarily in trophoblast, the outermost cellular layer that contacts maternal blood, indicating that particles are likely trapped in the maternally-facing side of the placenta. Villous tissue was sampled at different depths within the placenta. However, increased distance from the maternal attachment site does not correspond to greater risk of fetal exposure, as even in deeper locations the particles are still within the trophoblast layer in contact with maternal blood and are protected from the fetal circulation. It is also very important that samples of maternal decidua are not confused as being placental tissue.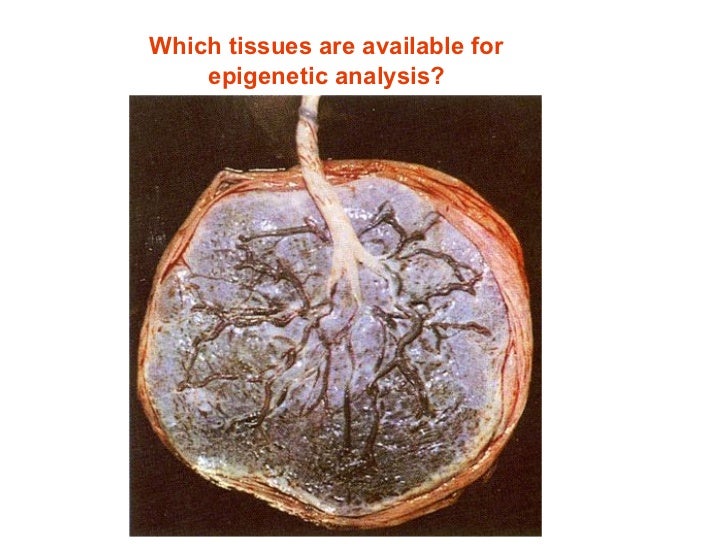 Here, we argue the importance of employing accurate terminology in studies of the maternal-fetal interface to avoid misinterpretation of research findings. Indeed, it can be argued that there is no ‘fetal side’ of the placenta, as the human placenta is, in its entirety, a fetal organ.
Here, we argue the importance of employing accurate terminology in studies of the maternal-fetal interface to avoid misinterpretation of research findings. Indeed, it can be argued that there is no ‘fetal side’ of the placenta, as the human placenta is, in its entirety, a fetal organ.
To understand transfer of substances from mother to fetus, it is vital to appreciate the complex structure of the placenta, which enables maternal and fetal blood to come into close proximity, whilst maintaining physical separation. Figure 1a illustrates a cross-section of the human placenta at term. The functional area for exchange of gases and nutrients is the chorionic villous tissue, containing placental blood vessels that connect directly into the fetal circulation via the umbilical cord. These villi are bathed in maternal blood within the intervillous space. The maternal endometrium at the site of placental attachment undergoes remodelling, forming the decidua basalis. At birth, the placenta is delivered with an attached layer of maternal decidua, enabling the study of both placental and decidual tissues ex vivo. The different cellular make-up and structure of these two tissue types can be seen in Fig. 1b.
The different cellular make-up and structure of these two tissue types can be seen in Fig. 1b.
a The umbilical vein and arteries containing fetal blood reach the placenta at the cord insertion site and spread across the chorionic plate, branching extensively to provide the tree-like shape of the placenta. The branched vessels are embedded in a stromal core covered by trophoblast cells which are completely bathed in maternal blood within the intervillous space. Anchoring villi attach the placenta to the maternal endometrium (decidua). The three dashed-line boxes indicate the potential biopsy locations utilised by Bove et al. (1) and (2) are standard placental biopsies, comprising chorionic villous tissue that is fetal in origin, whilst excluding contamination with maternal decidual tissue. Regardless of whether a placental biopsy is taken near to the chorionic plate (1) or close to the decidua (2), the tissue is totally fetal. (3) represents an alternative possible location for the ‘maternal side’ biopsy employed by Bové et al. which is a mixed tissue biopsy comprising fetal chorionic villous tissue and anchoring villi, and a layer of maternal decidual tissue. Created with Biorender.com. b Micrograph of term maternal-fetal interface demonstrating the different structure of the decidua layer (maternal) that is delivered with the placenta (fetal). Decidua contains a mix of stromal cells, leucocytes and invaded extravillous trophoblast. Tissue stained with antibodies to Cytokeratin 7, which marks placental trophoblast. Nuclei counterstained with haematoxylin.
(3) represents an alternative possible location for the ‘maternal side’ biopsy employed by Bové et al. which is a mixed tissue biopsy comprising fetal chorionic villous tissue and anchoring villi, and a layer of maternal decidual tissue. Created with Biorender.com. b Micrograph of term maternal-fetal interface demonstrating the different structure of the decidua layer (maternal) that is delivered with the placenta (fetal). Decidua contains a mix of stromal cells, leucocytes and invaded extravillous trophoblast. Tissue stained with antibodies to Cytokeratin 7, which marks placental trophoblast. Nuclei counterstained with haematoxylin.
Full size image
Sampling location is central to the study by Bové et al., as it is important to know whether the tissue contains fetal and/or maternal components, but it is not well described, and unorthodox definitions have been utilised to describe the sites of biopsies. Bové et al. take what they term a ‘fetal’ biopsy by excising chunks of villous tissue underneath the chorionic plate.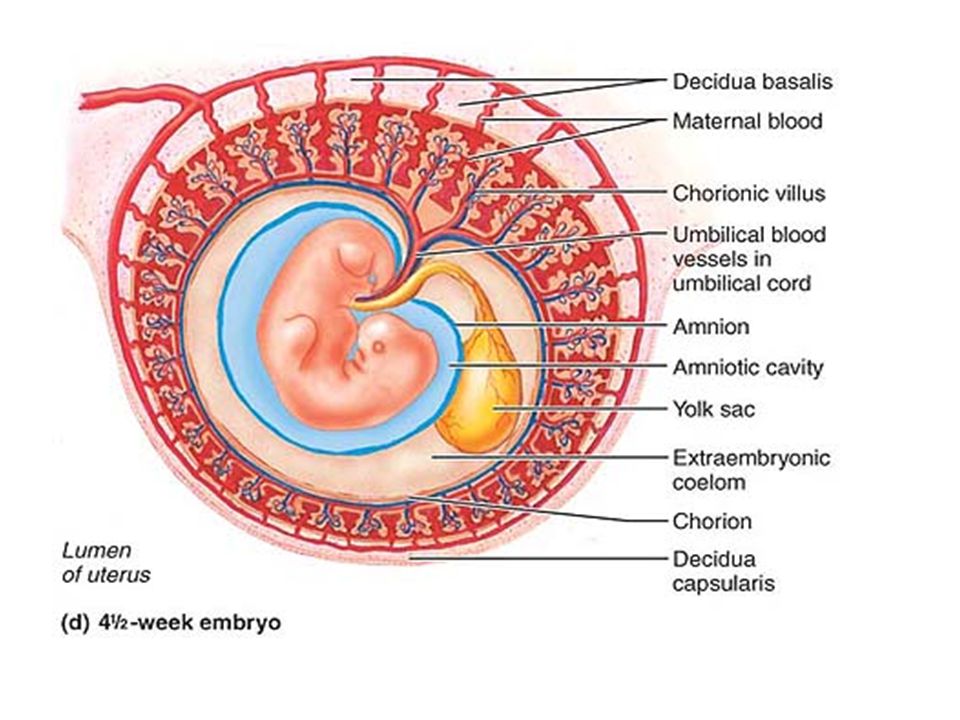 Box 1 in Fig. 1a shows our interpretation of the location of this biopsy, which is a standard placental biopsy of chorionic villous tissue. The nature of the ‘maternal’ biopsy is more ambiguous, being described as taken at the ‘side facing towards…the mother’. This could describe the location indicated by box 2 in Fig. 1a; which would be the same in composition as the ‘fetal’ biopsy indicated by box 1. Alternatively, it could describe the location shown in box 3 of Fig. 1a, which would yield a mixed biopsy of fetal placental tissue and maternal decidua basalis. Either approach is fine, if combined with the correct terminology, rather than the terminology used in this paper. A chorionic villous sample, taken nearer the decidua (box 2) is not the ‘maternal side of the placenta’; it is a placental sample, the same as a sample taken a bit nearer the chorion (box 1). A mixed biopsy of placenta and decidua (box 3) is also not the ‘maternal side of the placenta’, in the way it is being interpreted in this study.
Box 1 in Fig. 1a shows our interpretation of the location of this biopsy, which is a standard placental biopsy of chorionic villous tissue. The nature of the ‘maternal’ biopsy is more ambiguous, being described as taken at the ‘side facing towards…the mother’. This could describe the location indicated by box 2 in Fig. 1a; which would be the same in composition as the ‘fetal’ biopsy indicated by box 1. Alternatively, it could describe the location shown in box 3 of Fig. 1a, which would yield a mixed biopsy of fetal placental tissue and maternal decidua basalis. Either approach is fine, if combined with the correct terminology, rather than the terminology used in this paper. A chorionic villous sample, taken nearer the decidua (box 2) is not the ‘maternal side of the placenta’; it is a placental sample, the same as a sample taken a bit nearer the chorion (box 1). A mixed biopsy of placenta and decidua (box 3) is also not the ‘maternal side of the placenta’, in the way it is being interpreted in this study. The importance of consistent sampling of placentas, and methods for achieving this, is reviewed by Burton et al.4.
The importance of consistent sampling of placentas, and methods for achieving this, is reviewed by Burton et al.4.
The word decidua does not appear in this paper, although we think that this is probably what is being examined in the ‘maternal biopsies’. Unfortunately, although summary data of particles within the biopsies are provided in bar graphs (Supplementary Fig. 4), Bové et al. do not show any images of the ‘maternal biopsies’, so it is not possible to determine what tissue/cells are examined, and whether particles are in placental tissue or in decidua (as shown by us in Fig. 1b). This is central to the interpretation of this study, so it is concerning that these details are not reported. The lower particle count reported on the ‘maternal side’ of the placenta could be due to microscopy being performed on a completely different tissue; the decidua basalis.
Once an understanding of the gross anatomy of the human placenta is recognised, transfer studies must also consider the complex cellular organisation of the placental villus, the major site of exchange between maternal and fetal circulations (Fig. 2). The floating villi are covered in a multinucleated syncytium in direct contact with maternal blood. Underneath this lie the cytotrophoblast cells and the inner stromal core, containing placental macrophages, fibroblasts, and a capillary endothelium containing fetal blood5. Thus, for particles to move from maternal to fetal circulations, they must cross several cell layers and basement membranes. All these cell types arise from the blastocyst, and are therefore fetal in origin. Although fundamental to the interpretation of particle localisation, none of these cell types are mentioned in this study.
2). The floating villi are covered in a multinucleated syncytium in direct contact with maternal blood. Underneath this lie the cytotrophoblast cells and the inner stromal core, containing placental macrophages, fibroblasts, and a capillary endothelium containing fetal blood5. Thus, for particles to move from maternal to fetal circulations, they must cross several cell layers and basement membranes. All these cell types arise from the blastocyst, and are therefore fetal in origin. Although fundamental to the interpretation of particle localisation, none of these cell types are mentioned in this study.
The surface of the placenta facing the maternal blood is covered by a multinucleate syncytiotrophoblast with a microvillous surface to facilitate exchange. The syncytiotrophoblast layer is formed by the fusion of the underlying cytotrophoblast cells.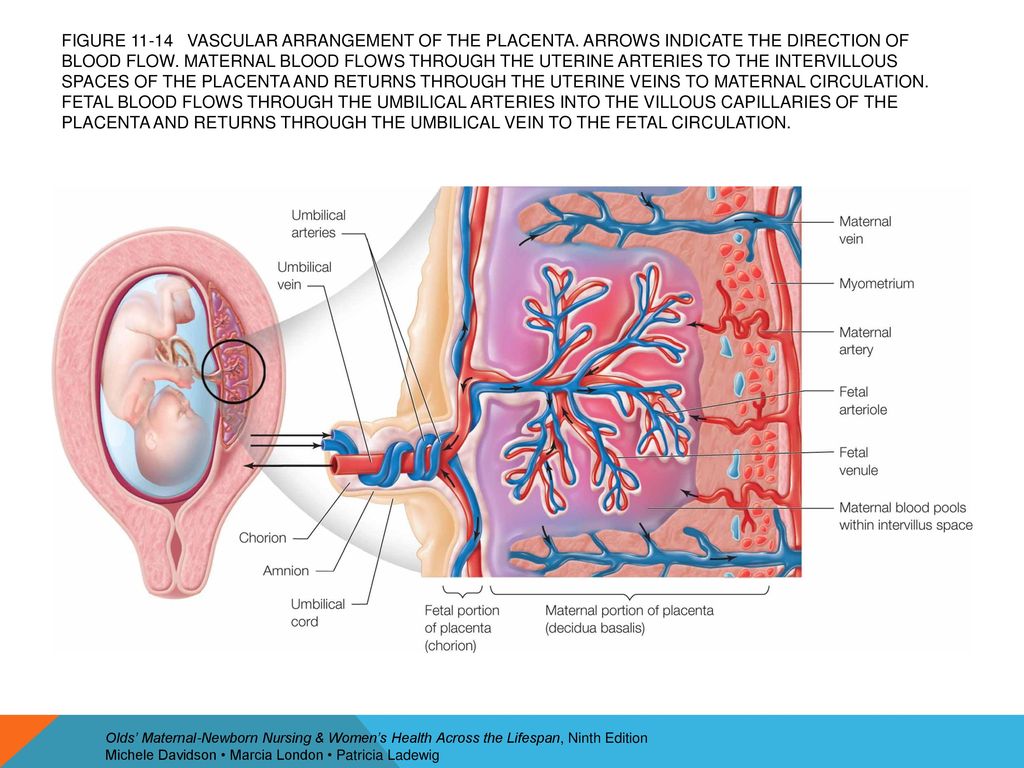 Beneath this lies the stroma, which contains fibroblasts and placental macrophages, and placental blood vessels lined by endothelial cells. a Diagram of a cross-section through a placental villus, showing the fetal syncytiotrophoblast in contact with maternal blood and fetal blood in the capillary endothelium. b Semi-thin section of term placenta stained with Toluidine blue. Fetal BV; fetal blood vessel. c Whole-mount term placenta stained with lectins illustrating the syncytiotrophoblast in contact with maternal blood in green, stroma in blue and the fetal capillaries in magenta. d Electron micrograph illustrating the three main cell layers between the maternal and fetal circulations.
Beneath this lies the stroma, which contains fibroblasts and placental macrophages, and placental blood vessels lined by endothelial cells. a Diagram of a cross-section through a placental villus, showing the fetal syncytiotrophoblast in contact with maternal blood and fetal blood in the capillary endothelium. b Semi-thin section of term placenta stained with Toluidine blue. Fetal BV; fetal blood vessel. c Whole-mount term placenta stained with lectins illustrating the syncytiotrophoblast in contact with maternal blood in green, stroma in blue and the fetal capillaries in magenta. d Electron micrograph illustrating the three main cell layers between the maternal and fetal circulations.
Full size image
A micrograph presented by Bové et al. shows a cross-section of term placental villous tissue familiar to placental biologists. Carbon particles are visible within this tissue (reported as the ‘fetal side’). Unfortunately, the magnification and resolution are too low to precisely identify the particle location. Nevertheless, it appears most particles are lodged in the syncytiotrophoblast, the first barrier layer of the placenta. The syncytiotrophoblast is highly endocytic and constantly samples its environment through fluid phase endocytosis6. It is, therefore, not surprising that carbon particles could be taken up into this cell layer, if they are present in the maternal circulation. Critically, the syncytiotrophoblast is an important barrier, trapping harmful components to prevent transfer to the fetus. Hence, the presence of particles in the syncytium does not demonstrate transfer per se. This is exemplified by a recent study7 where macrophage-enriched placental cells were examined for ingested carbon particles; the bulk of these, when visualised by transmission electron microscopy, were of trophoblast origin as can be attested by one of us (CJ) who analysed the cell samples. This is not to say that such ingestion does not have deleterious effects, as there is evidence of reduced cellular growth and altered protein expression of first trimester trophoblast cultured with urban pollution particles8.
Nevertheless, it appears most particles are lodged in the syncytiotrophoblast, the first barrier layer of the placenta. The syncytiotrophoblast is highly endocytic and constantly samples its environment through fluid phase endocytosis6. It is, therefore, not surprising that carbon particles could be taken up into this cell layer, if they are present in the maternal circulation. Critically, the syncytiotrophoblast is an important barrier, trapping harmful components to prevent transfer to the fetus. Hence, the presence of particles in the syncytium does not demonstrate transfer per se. This is exemplified by a recent study7 where macrophage-enriched placental cells were examined for ingested carbon particles; the bulk of these, when visualised by transmission electron microscopy, were of trophoblast origin as can be attested by one of us (CJ) who analysed the cell samples. This is not to say that such ingestion does not have deleterious effects, as there is evidence of reduced cellular growth and altered protein expression of first trimester trophoblast cultured with urban pollution particles8. The trophoblast and syncytiotrophoblast are not mentioned in the interpretation of the results.
The trophoblast and syncytiotrophoblast are not mentioned in the interpretation of the results.
To definitively demonstrate penetration of the placental barrier, certain criteria must be met. Specifically, higher magnification images are required to enable proper visualisation of the location of particles within cell layers other than the syncytiotrophoblast such as the stroma or capillary endothelium; regions that are not mentioned in the entirety of the paper. The interpretation of potential transfer across the placental barrier towards the fetus would be justified if particles were clearly demonstrated to have crossed the syncytiotrophoblast and to be present within the villous stroma and/or the endothelium of fetal blood vessels (or in cord blood), substantiated by co-localisation of cell lineage-specific markers.
It could alternatively be argued that Bové et al. have shown the presence of carbon particles in the villous stroma; potentially there are two particles located either on the basal side of cytotrophoblast cells or within the stroma in Fig.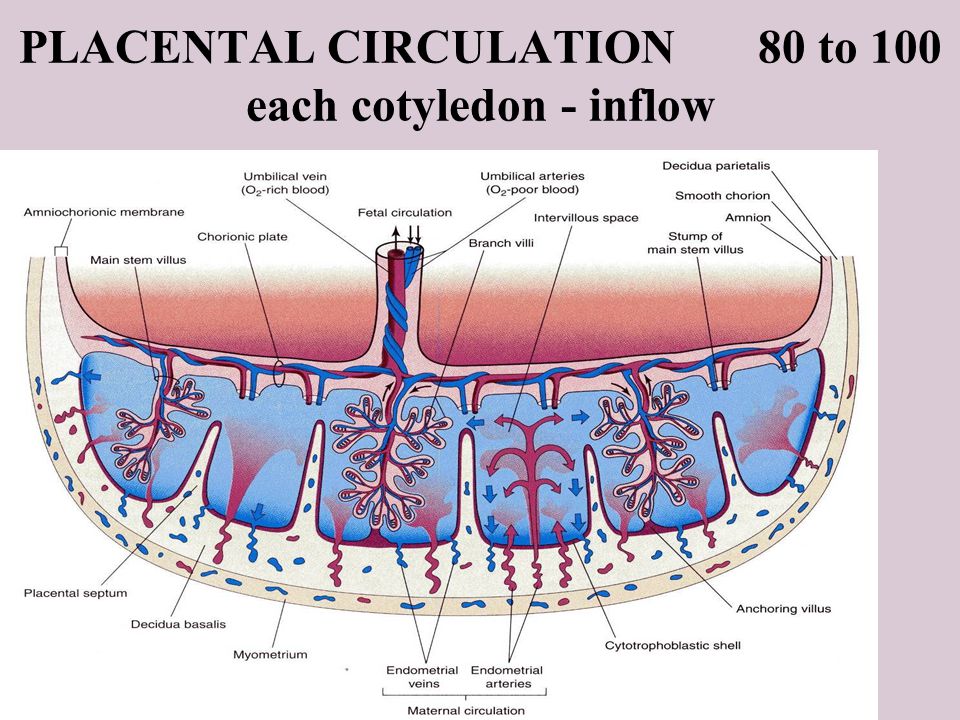 2 (red and blue inset boxes). It is very hard to judge this, as the stroma is not mentioned anywhere in the paper, and the images presented are not of sufficient magnification nor resolution for the reader to determine this localisation themselves. Supplementary Fig. 2a shows carbon particles inside a cell that does not appear to be trophoblast, which is intriguing. The authors describe this location as ‘inside the placental tissue’. Unfortunately, it is not stated what cell type it is. It is certainly not trophoblast, nor is it endothelial, as it has no basal lamina. It does not resemble a fibroblast nor a macrophage. Without more details of its location, it is hard to interpret this data. Likewise, Supplementary Fig. 2b shows aggregates inside some type of cell, but it is not possible to conclude what cell type this is and where the particles are located within the placenta without the provision of some orientating information.
2 (red and blue inset boxes). It is very hard to judge this, as the stroma is not mentioned anywhere in the paper, and the images presented are not of sufficient magnification nor resolution for the reader to determine this localisation themselves. Supplementary Fig. 2a shows carbon particles inside a cell that does not appear to be trophoblast, which is intriguing. The authors describe this location as ‘inside the placental tissue’. Unfortunately, it is not stated what cell type it is. It is certainly not trophoblast, nor is it endothelial, as it has no basal lamina. It does not resemble a fibroblast nor a macrophage. Without more details of its location, it is hard to interpret this data. Likewise, Supplementary Fig. 2b shows aggregates inside some type of cell, but it is not possible to conclude what cell type this is and where the particles are located within the placenta without the provision of some orientating information.
Growing evidence points to a link between maternal exposure to environmental pollutants during pregnancy and adverse effects on fetal and childhood health9,10,11,12,13.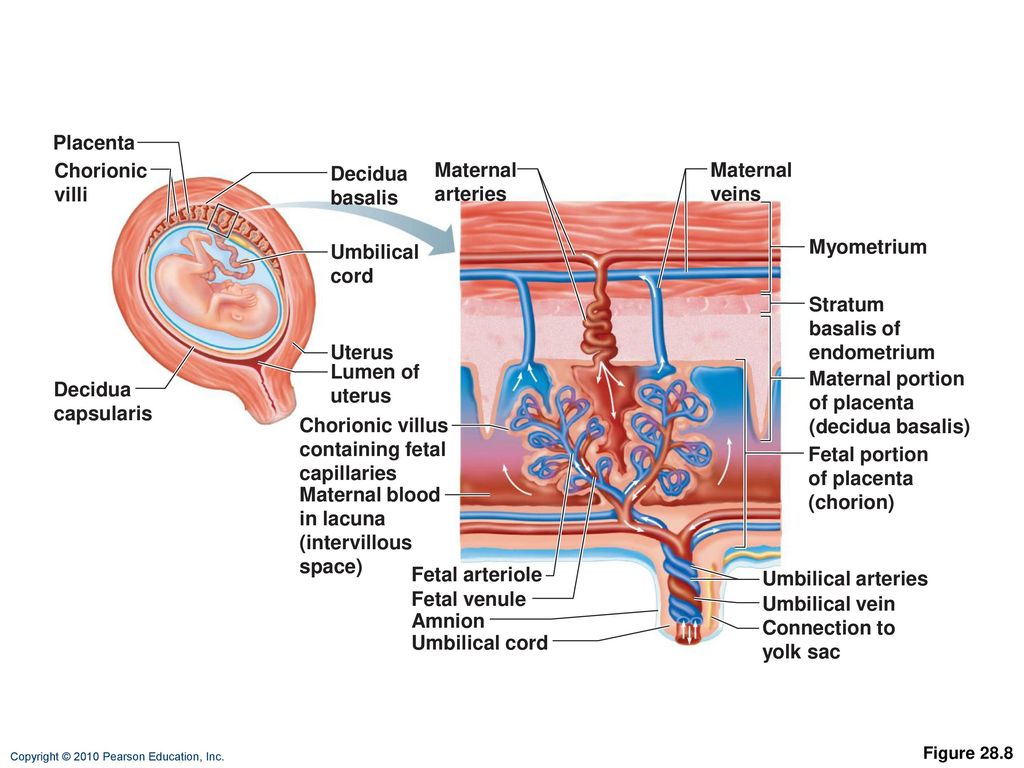 However, the negative effects observed in offspring after perinatal/in utero exposure to environmental contaminants do not necessarily indicate that the pollutants reached the fetus, as indirect effects via the mother and the placenta could be responsible. The study by Bové et al. nicely supports previous suggestions that carbon particles can be taken up from maternal blood into the human placenta. However, the information presented to the reader does not demonstrate maternal to ‘fetal side’ transfer, although we don’t rule out that this is possible. The terminology employed in this article is not in common usage in the academic community, and misrepresents the structure and function of the human placenta and nature of the feto-maternal interface.
However, the negative effects observed in offspring after perinatal/in utero exposure to environmental contaminants do not necessarily indicate that the pollutants reached the fetus, as indirect effects via the mother and the placenta could be responsible. The study by Bové et al. nicely supports previous suggestions that carbon particles can be taken up from maternal blood into the human placenta. However, the information presented to the reader does not demonstrate maternal to ‘fetal side’ transfer, although we don’t rule out that this is possible. The terminology employed in this article is not in common usage in the academic community, and misrepresents the structure and function of the human placenta and nature of the feto-maternal interface.
Similar inaccurate ‘fetal side’ terminology has been utilised in other recent papers on the topics of placental uptake/transfer of SARS-COV-214 and microplastics15. Worryingly, these misinterpretations are further amplified in the media, as there is strong public interest in studies with implications for fetal and infant health. More definitive reporting of the tissue composition of the biopsies analysed, as well as higher resolution/magnification images that enable accurate localisation of the carbon particles within precise anatomical sites, are necessary to support a convincing case for carbon particle transfer across the human placenta. Only with both a sufficiently rigorous technical approach, clearly presented data and accurate interpretation can we reliably advance understanding of the environmental determinants of pregnancy outcome and offspring health.
More definitive reporting of the tissue composition of the biopsies analysed, as well as higher resolution/magnification images that enable accurate localisation of the carbon particles within precise anatomical sites, are necessary to support a convincing case for carbon particle transfer across the human placenta. Only with both a sufficiently rigorous technical approach, clearly presented data and accurate interpretation can we reliably advance understanding of the environmental determinants of pregnancy outcome and offspring health.
Methods
Human subjects
Where required, we have complied with all relevant ethical regulations, and informed consent was obtained from participants. Ethical approval was provided by the Northern X Regional Ethics Committee (New Zealand; NTX/12/06/057/AM09) or Southampton and Southwest Hampshire Local Research Ethics Committee (UK; 11/SC/0529).
Immunohistochemistry
Paraformaldehyde-fixed term human placenta-decidua biopsies were snap frozen in OCT compound, cut and subjected to heat induced antigen retrieval. Sections were blocked in 10% Normal Goat Serum and stained with 2 μg/mL cytokeratin 7 antibody (clone OV-TL 12/30, Dako Laboratories #M7018). Secondary and tertiary steps were undertaken using a Histostain-plus kit (Thermofisher Scientific). Colour was developed with AEC, and sections counterstained in Gills II Haematoxylin, prior to mounting with Aquamount.
Sections were blocked in 10% Normal Goat Serum and stained with 2 μg/mL cytokeratin 7 antibody (clone OV-TL 12/30, Dako Laboratories #M7018). Secondary and tertiary steps were undertaken using a Histostain-plus kit (Thermofisher Scientific). Colour was developed with AEC, and sections counterstained in Gills II Haematoxylin, prior to mounting with Aquamount.
Immunofluorescence
Paraformaldehyde-fixed term human placental villi were stained with FITC-Aleuria Aurantia Lectin to visualise blood vessels, Biotin Datura Stramonium Lectin with Streptavidin 680 to visualise the trophoblast and Rhodamine-labelled Pisum sativum Agglutinin which binds the stroma (all Vector Laboratories), and imaged by confocal microscopy.
Electron microscopy
Villous tissue from term placenta was fixed in 2.5% glutaraldehyde in 0 1 M cacodylate buffer, post-fixed in 1% osmium tetroxide and embedded in epoxy resin. Semi-thin sections were stained with 1% toluidine blue in 1% borax for examination at the light microscopic level. Ultrathin sections from suitable areas were stained with uranyl acetate and lead citrate before examining in an AEI EM6B electron microscope.
Ultrathin sections from suitable areas were stained with uranyl acetate and lead citrate before examining in an AEI EM6B electron microscope.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All relevant data are available from the authors upon request.
References
Luyten, L. J. et al. Children’s microvascular traits and ambient air pollution exposure during pregnancy and early childhood: Prospective evidence to elucidate the developmental origin of particle-induced disease. BMC Med. https://doi.org/10.1186/s12916-020-01586-x (2020).
Pedersen, M. et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir. Med. https://doi.org/10.1016/S2213-2600(13)70192-9 (2013).
Bové, H.
 et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 10, 1–7 (2019).
et al. Ambient black carbon particles reach the fetal side of human placenta. Nat. Commun. 10, 1–7 (2019).Article ADS Google Scholar
Burton, G. J. et al. Optimising sample collection for placental research. Placenta 35, 9–22 (2014).
Article CAS Google Scholar
Jones, C. J. P. & Fox, H. Ultrastructure of the normal human placenta. Electron Microsc. Rev. 4, 129–178 (1991).
Article CAS Google Scholar
Fuchs, R. & Ellinger, I. Endocytic and transcytotic processes in villous syncytiotrophoblast: role in nutrient transport to the human fetus. Traffic 5, 725–738 (2004).
Article CAS Google Scholar
Liu, N. M. et al. Evidence for the presence of air pollution nanoparticles in placental tissue cells. Sci. Total Environ. https://doi.org/10.1016/j.scitotenv.2020.142235 (2021).
Familari, M. et al. Exposure of trophoblast cells to fine particulate matter air pollution leads to growth inhibition, inflammation and ER stress. PLoS ONE https://doi.org/10.1371/journal.pone.0218799 (2019).
Grippo, A. et al. Air pollution exposure during pregnancy and spontaneous abortion and stillbirth. Rev. Environ. Health. https://doi.org/10.1515/reveh-2017-0033 (2018).
Liu, Y. & Peterson, K. E. Maternal exposure to synthetic chemicals and obesity in the offspring: recent findings. Curr. Environ. Health Rep. https://doi.org/10.1007/s40572-015-0068-6 (2015).
Kishi, R. et al. Ten years of progress in the Hokkaido birth cohort study on environment and children’s health: cohort profile —updated 2013.
 Environ. Health Prev. Med. https://doi.org/10.1007/s12199-013-0357-3 (2013).
Environ. Health Prev. Med. https://doi.org/10.1007/s12199-013-0357-3 (2013).Baldacci, S. et al. Environmental and individual exposure and the risk of congenital anomalies: a review of recent epidemiological evidence. Epidemiol. Prev. https://doi.org/10.19191/EP18.3-4.S1.P001.057 (2018).
Bilbo, S. D., Block, C. L., Bolton, J. L., Hanamsagar, R. & Tran, P. K. Beyond infection—maternal immune activation by environmental factors, microglial development, and relevance for autism spectrum disorders. Exp. Neurol. https://doi.org/10.1016/j.expneurol.2017.07.002 (2018).
Patanè, L. et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am. J. Obstet. Gynecol. MFM 2, 100145 (2020).
Article Google Scholar
Ragusa, A. et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 146, 106274 (2021).
Article CAS Google Scholar
Download references
Acknowledgements
B. Holder is supported by the National Institutes of Health (Grant 5R01HD093801). N.G.-L. is supported by the Wayne State University Perinatal Research Initiative and Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). J. James is supported by a Health Research Council Sir Charles Hercus Research Fellowship (16/043). H. Jones is supported by the National Institutes of Health (R01HD090657 & R01HD091527).
Author information
Author notes
These authors contributed equally: Beth Holder, Carolyn J.
 P. Jones.
P. Jones.
Authors and Affiliations
Institute of Reproductive and Developmental Biology, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, UK
Beth Holder
Maternal and Fetal Health Research Centre, School of Medical Sciences, University of Manchester, Manchester Academic Health Sciences Centre, St Mary’s Hospital, Manchester, UK
John D. Aplin, Alexander E. P. Heazell & Carolyn J. P. Jones
Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services Detroit, MI, USA
Nardhy Gomez-Lopez
Department of Obstetrics and Gynaecology, School of Medicine, FMHS, University of Auckland, Auckland, New Zealand
Joanna L. James
Departments of Physiology and Functional Genomics & Obstetrics and Gynecology, College of Medicine, University of Florida, Gainesville, FL, USA
Helen Jones
The University of Southampton, Southampton, UK
Rohan M.
 Lewis
LewisWayne State University School of Medicine, Detroit, MI, USA
Gil Mor
Flinders Health and Medical Research Institute, Flinders University, Bedford Park, SA, Australia
Claire T. Roberts
The University of Adelaide, Adelaide, SA, Australia
Sarah A. Robertson
Department of Environmental Immunology, Helmholtz Centre for Environmental Research, Leipzig, Germany
Ana C. Zenclussen
Authors
- Beth Holder
View author publications
You can also search for this author in PubMed Google Scholar
- John D. Aplin
View author publications
You can also search for this author in PubMed Google Scholar
- Nardhy Gomez-Lopez
View author publications
You can also search for this author in PubMed Google Scholar
- Alexander E. P. Heazell
View author publications
You can also search for this author in PubMed Google Scholar
- Joanna L.
James
View author publications
You can also search for this author in PubMed Google Scholar
- Carolyn J. P. Jones
View author publications
You can also search for this author in PubMed Google Scholar
- Helen Jones
View author publications
You can also search for this author in PubMed Google Scholar
- Rohan M. Lewis
View author publications
You can also search for this author in PubMed Google Scholar
- Gil Mor
View author publications
You can also search for this author in PubMed Google Scholar
- Claire T. Roberts
View author publications
You can also search for this author in PubMed Google Scholar
- Sarah A. Robertson
View author publications
You can also search for this author in PubMed Google Scholar
- Ana C.
Zenclussen
View author publications
You can also search for this author in PubMed Google Scholar
Contributions
B.H. conceived the manuscript. All authors contributed to writing and critical review of the manuscript, with equal contribution by B.H. and C.J.P.J. Figs. 1a and 2a were created by B.H., Fig. 1b was provided by J.J., Fig. 2b, d were provided by C.J.P.J., and Fig. 2c was provided by R.M.L.
Corresponding author
Correspondence to Beth Holder.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Margherita Turco and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Reporting summary
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
|
The placenta is a fetomaternal organ. DeciduaThe endometrium (lining of the uterus) of the mother is known as the decidua (cast off), consisting of three regions named by location. Table 7 - Regions of the Decidua
As the embryo enlarges, the decidua capsularis
becomes stretched and smooth. FunctionThe placental membrane separates maternal blood from fetal blood. The fetal part of the placenta is known as the chorion. The maternal component of the placenta is known as the decidua basalis. Oxygen and nutrients in the maternal blood in the intervillous spaces diffuse through the walls of the villi and enter the fetal capillaries. Carbon dioxide and waste products diffuse from blood in the fetal capillaries through the walls of the villi to the maternal blood in the intervillous spaces. The PlacentaAlthough the placental membrane is often referred to as the placental barrier, many substances, both helpful and harmful, can cross it to affect the developing embryo. Structure
Primary chorionic villi are
solid outgrowths of cytotrophoblast that protrude into the
syncytiotrophoblast. Secondary chorionic villi have a core of loose connective tissue, which grows into the primary villi about the third week of development. Tertiary chorionic villi contain embryonic blood vessels that develop from mesenchymal cells in the loose connective tissue core. These blood vessels connect up with vessels that develop in the chorion and connecting stalk and begin to circulate embryonic blood about the third week of development.
Figure 4 - Structure of placenta and chorionic villi Table 8 - Substances that Cross the Placental Membrane
Amniotic FluidAmniotic fluid has three main functions: it protects the
fetus physically, it provides room for fetal movements, and helps to regulate
fetal body temperature. The umbilical cord is a composite structure formed by contributions from: Fetal connecting (body) stalk Yolk sac Amnion The umbilical cord contains the right and left umbilical arteries, the left umbilical vein, and mucous connective tissue. Presence of only one umbilical artery may suggest the presence of cardiovascular anomalies. Fetal circulation involves three circulatory shunts: the ductus
venosus, which allows blood from the placenta to bypass
the liver, and the ductus arteriosus
and foramen ovale, which together
allow blood to bypass the developing
lungs. Multiple PregnancyDizygotic twins are derived from two zygotes that were fertilized independently (i.e., two oocytes and two spermatozoa). Consequently, they are associated with two amnions, two chorions, and two placentas, which may (65%) or may not (35%) be fused. Dizygotic twins are only as closely genetically related as any two siblings. Monozygotic twins
(30%) are derived from one zygote that splits into two parts.
This type of twins commonly has two amnions, one chorion, and one
placenta. If the embryo splits
early in the second week after the amniotic cavity has formed, the twins will
have one amnion, one chorion, and one placenta.
Monozygotic twins are genetically identical, but may have physical
differences due to differing developmental environments (e.g., unequal division
of placental circulation). Placenta PreviaThe fetus implants in such a way that the placenta or fetal blood vessels grow to block the internal os of the uterus. See implantation. Erythroblastosis FetalisSome erythrocytes produced in the fetus routinely escape into the mothers systemic circulation. When fetal erythrocytes are Rh-positive but the mother is Rh-negative, the mothers body can form antibodies to the Rh antigen, which cross the placental barrier and destroy the fetus. The immunological memory of the mothers immune system means this problem is much greater with second and subsequent pregnancies. OligohydramniosDeficiency of amniotic fluid (less than 400 ml in late pregnancy). It can result from renal agenesis because the fetus is unable to contribute urine to the amniotic fluid volume. |
Maternity hospital №7, Novosibirsk - What is the placenta?
Anna Valentinovna Yakimova, obstetrician-gynecologist, MD talks about the placenta and placental insufficiency.
Picture from the site http://s7ya7.zdorovo-zivi.ru
Placenta. The name of the organ comes from lat. placenta - cake, cake, pancake. This is an extra-embryonic organ, consisting of villi, thanks to which the fetus is nourished, respired, and waste products are removed from its blood. There are free and fixing (anchor) villi. The placenta is formed in the place where the embryo was implanted, arises as a result of the connection of the chorion - extraembryonic tissue with a thickened uterine mucosa (decidual tissue). Despite the fact that the blood of the mother and fetus does not mix, as they are separated by the placental barrier, the fetus receives all the necessary nutrients and oxygen from the mother's blood. In addition, the placenta produces hormones that ensure the preservation of pregnancy. The placenta has two surfaces. The surface that faces the fetus is called the fetal. It is covered with a smooth shell - the amnion, through which large vessels shine through. The one that is attached to the wall of the uterus is called maternal. nine0003
The main structural unit of the placenta is cotelidone. Cotyledon of the placenta is conditionally comparable to a tree. Each cotyledon is formed by a stem villus, from which, like branches of a tree, villi of the second and third order extend, containing vessels. The central part of the cotyledon forms a cavity, which is surrounded by many villi. Between the cotyledons there is a space - mejvirus, which on the maternal side is limited by partitions (septa) extending from the mucous membrane of the uterus. Most of the placental villi are freely immersed in the intervillous space (free villi) and are bathed in maternal blood. The spiral arteries, which are small branches of the arteries that supply the uterus, open into the intervillous space and supply oxygen-rich blood to the intervillous space. Due to the pressure difference, which is higher in the mother's arterial bed compared to the intervillous space, oxygenated blood from the mouths of the spiral arteries is directed through the center of the cotyledon to the villi, washes them, reaches the chorionic plate and returns to the maternal circulation through the venous orifices through the separating septa. . In this case, the blood flow of the mother and fetus are separated from each other. Those. maternal and fetal blood does not mix. Thus, the concept of the placental barrier appears: the blood of the mother of the fetus does not mix, because they are separated by the wall of the villus, loose connective tissue inside the villus and the wall of the vessel that is inside the villus, and in which the blood of the fetus circulates. nine0003
At the end of pregnancy, the placenta is a soft disk 15-18 cm in diameter, 2-4 cm thick in the central part, weighing about 500-600 g. their capillaries - 12 m2. The placenta, membranes, and umbilical cord together form the afterbirth, which is expelled from the uterus after the baby is born.
Normally, the placenta is attached to the uterine cavity on its anterior or posterior surface, sometimes in the fundus. If the placenta is attached in the lower part of the uterine cavity, close to the internal opening of the cervix - the internal pharynx, then its blood supply is often insufficient and the fetus may suffer from lack of oxygen and nutrients - a phenomenon called placental insufficiency occurs. nine0003
Placental insufficiency is a violation of all or some of the functions of the placenta, ultimately leading to oxygen starvation (hypoxia), fetal growth retardation or death and / or early termination of pregnancy.
Placental insufficiency may occur early in pregnancy due to impaired placental formation, for example, if the spiral arteries that supply blood to the villi do not lose the ability to narrow their lumen in response to exposure to vasoconstrictor substances. It is possible that there is a violation of the development of blood vessels inside the villi, the vessels can form in the central part of the villus, and not close to its wall, then the transfer of nutrients from the mother's blood to the fetal blood and the flow of metabolic products back will be difficult. The process of development of the placenta (in particular, vascular formation) occurs to a greater extent in the first and second trimesters of pregnancy, ending at about 30-32 weeks. After this period, involutive processes predominate ("aging", immuring the villi with fibrinoid). Along with the processes of involution in the placenta during pregnancy, young villi develop, more often avascular, which, however, only partially compensate for the function of mature villi containing vessels that have “fallen out” of the circulation. nine0003
In another scenario, placental insufficiency occurs at a later date, as a result of damage to the placenta in inflammatory processes, diabetes in a pregnant woman, or high blood pressure, when blood flow to the uterus is disturbed, which can also occur with increased maternal blood clotting . It is believed that it is the violation of the uteroplacental circulation that plays the main role in the formation of the intrauterine growth retardation syndrome. Before the advent of Doppler ultrasound research methods in obstetric practice, there were no non-invasive methods for studying blood flow in the mother-placenta-fetus system. To date, Doppler is the most preferred instrumental method that provides useful information in relation to the detection of blood flow disorders and the determination of pregnancy management tactics for placental insufficiency. If there are symptoms of placental insufficiency - a mismatch in the height of the uterine fundus, signs of a threat of early termination of pregnancy, rapid or slow fetal heart rate (normal: 120-160 beats per minute), an altered amount of amniotic fluid - it is necessary to conduct an ultrasound examination, Doppler blood flow in the vessels of the uterus, umbilical cord and fetus, and in the third trimester - cardiotocography. The earliest sign of placental dysfunction is a decrease in its production of hormones and proteins, therefore, in pregnant women with a known risk of placental dysfunction (for example, if she has a chronic inflammatory process or often / constantly increased blood pressure, etc.), you can examine the level placental lactogen, progesterone, unconjugated estriol in the blood. And on the basis of the data obtained, predict the further development of pregnancy and take preventive measures. nine0003
Can placental insufficiency be treated? The answer to this question is ambiguous. It is possible to influence the development of the placenta when it is incomplete, improving the conditions for development - by eliminating, for example, the inflammatory process, normalizing blood pressure, lowering the tone of the uterus, and normalizing blood clotting. You can influence the metabolism in the cells of the placenta - by doing this, for example, by introducing drugs into the body of a pregnant woman that help improve the utilization of glucose by cells. It is possible to influence the tone of the vessels, reduce the permeability of the wall and, in this way, the swelling of the tissues decreases and the penetration of oxygen into them improves. But we must understand that we do not cure placental insufficiency, we are only trying to influence the compensatory mechanisms that exist in the placenta, if this succeeds, the manifestations of placental insufficiency disappear. But more often, treatment is aimed at prolonging the pregnancy to the term of a viable fetus. nine0003
But if the signs of placental insufficiency increase, and the gestation period is more than 32 weeks, when even in a normal placenta it undergoes involution, the placenta initially developed incorrectly, and now it performs its functions worse, the fetus suffers, then it is useless to treat placental insufficiency, it is better to carry out early delivery and to nurse the newborn without the risk of intrauterine death, which is possible if the placenta depletes its reserves. Sometimes it is necessary to make a decision on early delivery and at an earlier date, when the treatment of placental insufficiency is impossible and the delay threatens the life of the fetus. nine0003
Makarov I.O. • The placenta and its role in the development of pregnancy
Ultrasound scanner WS80
An ideal tool for prenatal examinations. Unique image quality and a full range of diagnostic programs for an expert assessment of a woman's health.
From the very beginning of pregnancy and up to its end, the mother-placenta-fetus system is formed and functions . The most important component of this system is the placenta , which is a complex organ in the formation of which derivatives 9 take part0009 trophoblast and embryoblast , as well as decidual tissue . The function of the placenta, first of all, is aimed at providing sufficient conditions for the physiological course of pregnancy and the normal development of the fetus. These functions include: respiratory, nutritional, excretory, protective, endocrine. All metabolic, hormonal, immune processes during pregnancy are provided through the vascular system of the mother and fetus . Despite the fact that the blood of the mother and fetus does not mix, since there are 9 of them0009 shares the placental barrier , the fetus receives all the necessary nutrients and oxygen from the mother's blood. The main structural component of the placenta is villous tree .
In the normal development of pregnancy, there is a relationship between the growth of the fetus, its body weight and the size, thickness, weight of the placenta. Up to 16 weeks of pregnancy, the development of the placenta outstrips the growth rate of the fetus. In case of death of the embryo (fetus) growth and development inhibition occurs chorionic villi and progression of involution-dystrophic processes in the placenta. Having reached the required maturity at 38-40 weeks of pregnancy, the processes of formation of new vessels and villi in the placenta stop.
Scheme of the structure of the placenta and uteroplacental circulation
1 - umbilical cord arteries
2 - stem villus
3 - decidual septum
4 - decidua
5 - myometrium
6 - veins
7 - spiral arteries
8 - chorion
9 - amnion
10 - intervillous space
11 - vein of the umbilical cord
12 - cotyledon
The mature placenta is a disc-shaped structure 15-20 cm in diameter and 2.5-3.5 cm thick. Its mass reaches 500-600 g. The maternal surface of the placenta, which faces the uterine wall, has a rough surface formed by the structures of the basal part of the decidua. The fetal surface of the placenta , which faces the fetus, is covered with an amniotic membrane . Under it are visible vessels that go from the place of attachment of the umbilical cord to the edge of the placenta. The structure of the fruiting part of the placenta is represented by numerous chorionic villi , which are combined into structural formations - cotyledons. Each cotyledon is formed by a stem villus with branches containing fetal vessels. The central part of the cotyledon forms a cavity, which is surrounded by many villi. In a mature placenta, there are 30 to 50 cotyledons. Cotyledon of the placenta is conditionally comparable to a tree, in which the supporting villi of the 1st order are its trunk, the villi of the 2nd and 3rd orders are large and small branches, the intermediate villi are small branches, and the terminal villi are leaves. Cotyledons are separated from each other by partitions (septa) emanating from the basal plate. nine0003
Intervillous space on the fetal side is formed by the chorionic plate and villi attached to it, and on the maternal side it is limited by the basal plate, decidua and septa extending from it. Most of the placental villi are freely immersed in the intervillous space and are bathed in maternal blood . There are also anchor villi, which are fixed to the basal decidua and provide attachment of the placenta to the wall of the uterus. nine0003
Diagram of blood circulation in the fetus
1 - superior vena cava
2 - oval hole
3 - inferior vena cava
4 - venous duct
5 - portal sinus
6 - portal vein
7 - vein of the umbilical cord
8 - umbilical cord arteries
9 - placenta
10 - epigastric arteries
11 - ductus arteriosus
Spiral arteries , which are the terminal branches of the uterine and ovarian arteries, supplying the pregnant uterus , open into the intervillous space with 120-150 orifices, providing a constant flow of maternal oxygen-rich blood into the intervillous space. Due to pressure difference , which is higher in the maternal arterial bed compared to the intervillous space, blood saturated with oxygen from the mouths of the spiral arteries is directed through the center of the cotyledon to the villi, washes them, reaches the chorionic plate and returns to the dividing septa maternal blood flow through venous orifices. In this case, the blood flow of the mother and fetus are separated from each other. Those. maternal and fetal blood does not mix among themselves.
The transfer of blood gases, nutrients , metabolic products and other substances from maternal blood to fetal and back is carried out at the moment of contact of the villi with the mother's blood through the placental barrier . It is formed by the outer epithelial layer of the villus, the stroma of the villus, and the wall of the blood capillary located inside each villus. Fetal blood flows through this capillary. Saturated in this way with oxygen, the blood of the fetus from the capillaries of the villi is collected into larger vessels, which eventually combine into vein of the umbilical cord , through which oxygenated blood flows to the fetus . Having given up oxygen and nutrients in the body of the fetus, the blood, depleted in oxygen and rich in carbon dioxide, flows from the fetus through two umbilical arteries to the placenta , where these vessels divide radially in accordance with the number of cotyledons. As a result of further branching of the vessels inside the cotyledons, the fetal blood again enters the capillaries of the villi and is again saturated with oxygen, and the cycle repeats. Due to the passage through the placental barrier of blood gases and nutrients, the respiratory, nutritional and excretory functions of the placenta are realized. At the same time, oxygen enters the bloodstream of the fetus and carbon dioxide and other metabolic products of the fetus are excreted .
At the same time, proteins, lipids, carbohydrates, microelements, vitamins, enzymes and much more are transported towards the fetus.
Scheme of the placental barrier
1 - capillary endothelium of terminal villi
2 - villus capillary
3 - villus stroma
4 - epithelial cover of villi nine0003
The placenta performs an important protective (barrier) function through the placental barrier, which is selectively permeable in both directions. In the normal course of pregnancy, the permeability of the placental barrier increases up to 32-34 weeks of pregnancy, after which it decreases in a certain way. However, unfortunately, a fairly large number of drugs, nicotine, alcohol, narcotic substances, pesticides, other toxic chemicals, as well as a number of pathogens of infectious diseases penetrate the placental barrier relatively easily into the fetal circulation, which has an adverse effect on the fetus. In addition, under the influence of pathogenic factors, the barrier function of the placenta is even more disturbed. nine0003
The placenta is anatomically and functionally related to the amnion (aqueous membrane) that surrounds the fetus. The amnion is a thin membrane that lines the surface of the placenta facing the fetus, passes to the umbilical cord and merges with the skin of the fetus at the umbilical ring. Amnion is actively involved in the exchange of amniotic fluid , in a number of metabolic processes, and also performs a protective function.
Connects placenta and fetus umbilical cord , which is a cord-like formation. The umbilical cord contains two arteries and one vein . Two arteries in the umbilical cord carry oxygen-depleted blood from the fetus to the placenta. The vein of the umbilical cord carries oxygenated blood to the fetus. The vessels of the umbilical cord are surrounded by a gelatinous substance, which was called "warton jelly" .