Injection vs vaccine
Immunisation or vaccination - what's the difference?
Immunisation or vaccination - what's the difference? | Pregnancy Birth and Baby beginning of content8-minute read
Listen
Key facts
- Vaccination is the term used for getting a vaccine — that is, having the injection or taking an oral vaccine dose.
- Immunisation refers to the process of both getting the vaccine and becoming immune to the disease following vaccination.
- Vaccines take time to work, because your immune system needs time to produce an immune response to the vaccine.
- Some vaccines work after one dose, but others require more doses to be effective, and for some you need a ‘booster’ after a certain period, to restore your immunity to disease, which can lessen over time.
- Vaccines available in Australia all undergo strict medical testing and are safe.
What is a vaccine?
Vaccination prepares the immune system to fight against a future infection. Most vaccines contain tiny amounts of dead or weakened viruses or bacteria, called antigens. Your immune system responds to these antigens by training itself to fight disease without you getting sick. This means that your body is better prepared to fight the disease if exposed to it in the future.
Most vaccines are given by injection but some are given orally (by mouth).
What is in vaccines?
Some vaccines contain a very small dose of a live but weakened form of a virus. Some vaccines contain a very small dose of dead bacteria or small parts of bacteria. Other vaccines contain a small dose of a modified toxin produced by bacteria. Newer vaccines, such as most vaccines against COVID-19, contain instructions that ‘teach’ your immune system how to fight a virus.
Vaccines may also contain either a small amount of preservative or a small amount of an antibiotic to preserve the vaccine. Some vaccines also contain a small amount of an aluminium salt, which helps produce a better immune response.
What is the difference between immunisation and vaccination?
The terms ‘vaccination’ and ‘immunisation’ are similar, but don’t exactly mean the same thing. Vaccination is the term used for getting a vaccine — that is, actually having the injection or taking an oral vaccine dose. Immunisation is the process of both getting the vaccine and becoming immune to the disease after vaccination.
How does immunisation work?
All forms of immunisation work in the same way. When someone receives a vaccine, their body produces an immune response in the same way it would after exposure to a disease, but without the person getting the disease. Your immune system’s familiarity with the disease allows your body to make a faster immune response if you are ever exposed to the disease naturally.
Often, your immune system responds fast enough to prevent you from developing any symptoms of the disease. In other cases, it may not occur fast enough to prevent you from developing symptoms, but vaccination will reduce your chance of becoming seriously ill.
What’s the difference between a booster dose and a primary vaccine course?
A primary vaccine course involves the vaccine doses you need for very good protection against a disease.
A booster dose refers to an extra dose of a vaccine that is given after you’ve completed the primary vaccine course. It gives your immune system a 'boost' and helps reach a higher level of protection from the disease.
Booster doses are common with diseases such as COVID-19, tetanus and whooping cough (pertussis).
How long do immunisations take to work?
It takes time for your immune system to respond to a vaccine, usually between 1 and 3 weeks. The time it takes will depend on the vaccine and your age and general health. This means that immunisation will not usually provide protection from infection straight away.
This means that immunisation will not usually provide protection from infection straight away.
How many doses of vaccine do I need to be protected?
Most immunisations need to be given several times to build long-lasting protection. For example, a child who has had only 1 or 2 doses of the DTPa vaccine is only partly protected against diphtheria, tetanus and pertussis (whooping cough). Until they receive all the doses they need, they are still at risk of getting sick if they come into contact with these diseases.
There are some newer vaccines, such as one type of meningococcal ACWY vaccine, that provide long-lasting immunity after only one dose.
How long do immunisations last?
The protective effect of immunisations is not always lifelong. Some, like tetanus vaccine, can last up to 10 years (depending on your age), after which time you will need a booster dose to restore your immunity.
Some immunisations, such as the whooping cough vaccine, give protection for about 5 years after a full course. You need influenza immunisation every year due to the changes to the type of flu virus that are most common in the community from year to year.
You need influenza immunisation every year due to the changes to the type of flu virus that are most common in the community from year to year.
Is everyone protected from disease by immunisation?
Even when all the doses of a vaccine have been given, not everyone is protected against the disease. Most childhood vaccines will give protective immunity to about 9 out of 10 children who have received all the recommended doses.
When vaccination rates in the community are high, the risk of catching vaccine-preventable diseases is low for everyone. This is called herd immunity. This is because immunisation makes these diseases less common in the community, so even someone without immunity is less likely to catch them.
Some vaccines, such as for COVID-19, won’t necessarily prevent you from catching the disease, but can reduce your risk of serious illness.
Other vaccines, such as for whooping cough, prevent disease in most people (about 8 in 10). These vaccines are still important for everyone, as they reduce the chance of you becoming seriously unwell, in the event that you do catch the disease.
How are vaccines approved in Australia?
Before a vaccine becomes available in Australia, it must pass the strict approval processes of the Therapeutic Goods Administration (TGA). This includes assessing every ingredient in the vaccine for safety, quality and effectiveness.
A clinical trial is a scientific study conducted by the makers of a vaccine. Clinical trials of medicines are done in phases.
The TGA carefully assesses the results of clinical trials, as well as the way the trials were run.
The TGA ensures that vaccine manufacturers meet manufacturing quality standards. TGA laboratories check the quality of every batch of a vaccine before it can be used in Australia.
Sometimes a ‘provisional approval pathway’ is needed for the temporary registration of promising new medicines and vaccines. This is only done where the need for early access is greater than any possible risks.
Resources and support
In Australia, vaccines are funded by the National Immunisation Program and protect millions of Australians from vaccine-preventable diseases.
If you have any questions, you can speak to your doctor or call healthdirect on 1800 022 222 (known as NURSE-ON-CALL in Victoria).
FIND A HEALTH SERVICE — The Service Finder can help you find doctors, pharmacies, hospitals and other health services.
Speak to a maternal child health nurse
Call Pregnancy, Birth and Baby to speak to a maternal child health nurse on 1800 882 436 or video call. Available 7am to midnight (AET), 7 days a week.
Sources:
Australian Academy of Science (Who benefits from vaccines?), City of Monash (Immunisation - Frequently Asked Questions), Department of Health and Aged Care (About immunisation), Department of Health and Aged Care (Questions about vaccination), Department of Health and Aged Care (COVID-19 vaccine information), Department of Health and Aged Care (How COVID-19 vaccines work), Department of Health and Aged Care (Vaccine safety), Immunisation coalition (How Vaccines Work), NSW Health (Booster vaccination - frequently asked questions), The Australian Immunisation Handbook (Influenza (flu) vaccine information), The Australian Immunisation Handbook (Pertussis (whooping cough)), The Australian Immunisation Handbook (Meningococcal disease), Therapeutic Goods Administration (COVID-19 vaccine: Information for consumers and health professionals)Learn more here about the development and quality assurance of healthdirect content.
Last reviewed: September 2022
Back To Top
Related pages
- Immunisation and vaccinations for your child
- Vaccinations and pregnancy
Need more information?
Immunisation | Tasmanian Department of Health
Learn about information about immunisation, vaccines and diseases preventable by immunisation in Tasmania.
Read more on Tasmanian Department of Health and Human Services website
Immunisation and vaccinations for your child
Immunisation is a simple, safe and effective way of protecting children against certain diseases. Discover more about childhood vaccinations.
Read more on Pregnancy, Birth & Baby website
Immunisation | SA Health
Information for consumers on vaccines, immunisation programs and immunisation records for you and your children
Read more on SA Health website
Immunisation records | NCIRS
Your immunisation history statement has all your vaccines that have been recorded on the Australian Immunisation Register (AIR)
Read more on National Centre for Immunisation Research and Surveillance (NCIRS) website
What is immunisation? | Australian Government Department of Health and Aged Care
On this page Immunisation or vaccination — what's the difference? Australia's National Immunisation Program Immunisation is a safe and effective way of protecting you and your child against serious diseases.
Read more on Department of Health and Aged Care website
Where can I get immunised? | Australian Government Department of Health and Aged Care
You can get vaccinations from a range of vaccination providers, including your local GP and school-based immunisation programs.
Read more on Department of Health and Aged Care website
Immunisation schedules | NCIRS
The Australian National Immunisation Program (NIP) is funded by the Australian government and implemented by state and territory departments of health
Read more on National Centre for Immunisation Research and Surveillance (NCIRS) website
Kids - Immunisation Coalition
Find out how to protect your kids from infectious diseases. Keep up to date with the Childhood National Immunisation Program schedule.
Keep up to date with the Childhood National Immunisation Program schedule.
Read more on Immunisation Coalition website
Keeping your child’s vaccinations up to date during COVID-19
The COVID-19 pandemic has led to a decrease in children getting their routine childhood vaccinations.
Read more on Pregnancy, Birth & Baby website
Vaccination matters
Ensure your decisions about immunisation are based on fact. When doing your personal reading, make sure your source of information is based on scientific fact.
Read more on Queensland Health website
Disclaimer
Pregnancy, Birth and Baby is not responsible for the content and advertising on the external website you are now entering.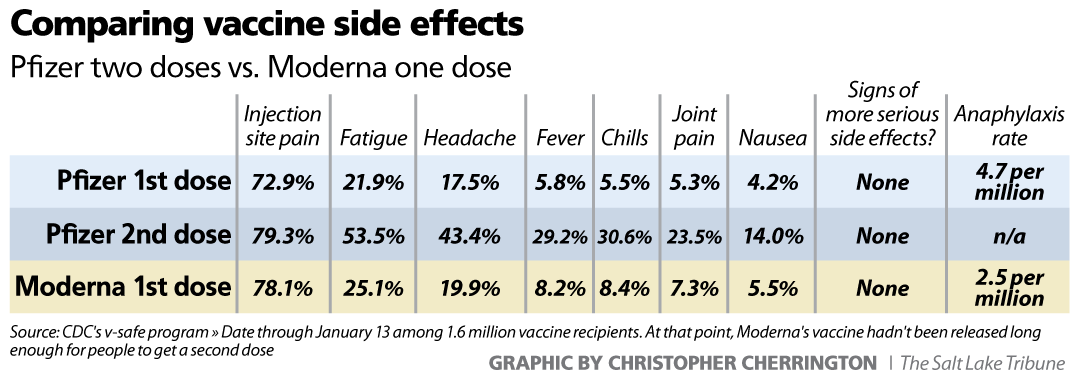
Need further advice or guidance from our maternal child health nurses?
1800 882 436
Video call
- Contact us
- About us
- A-Z topics
- Symptom Checker
- Service Finder
- Linking to us
- Information partners
- Terms of use
- Privacy
Pregnancy, Birth and Baby is funded by the Australian Government and operated by Healthdirect Australia.
Pregnancy, Birth and Baby is provided on behalf of the Department of Health
Pregnancy, Birth and Baby’s information and advice are developed and managed within a rigorous clinical governance framework. This website is certified by the Health On The Net (HON) foundation, the standard for trustworthy health information.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
This information is for your general information and use only and is not intended to be used as medical advice and should not be used to diagnose, treat, cure or prevent any medical condition, nor should it be used for therapeutic purposes.
The information is not a substitute for independent professional advice and should not be used as an alternative to professional health care. If you have a particular medical problem, please consult a healthcare professional.
Except as permitted under the Copyright Act 1968, this publication or any part of it may not be reproduced, altered, adapted, stored and/or distributed in any form or by any means without the prior written permission of Healthdirect Australia.
Support this browser is being discontinued for Pregnancy, Birth and Baby
Support for this browser is being discontinued for this site
- Internet Explorer 11 and lower
We currently support Microsoft Edge, Chrome, Firefox and Safari. For more information, please visit the links below:
For more information, please visit the links below:
- Chrome by Google
- Firefox by Mozilla
- Microsoft Edge
- Safari by Apple
You are welcome to continue browsing this site with this browser. Some features, tools or interaction may not work correctly.
Difference Between Immunization & Vaccination
Immunization means to make someone immune to something. Vaccination, by contrast, according to Dorland’s Medical Dictionary, just means to inject “a suspension of attenuated or killed microorganisms…administered for prevention…or treatment of infectious disease.”
Most people do not realize that when you receive a shot or a vaccine it does not mean you are immunized. Many people believe that once you are vaccinated you are completely protected. That belief is wrong.
The use of the word “immunization” instead of “vaccination” is everywhere. Most importantly, news outlets tell the public that immunization is the same as vaccination. However, there is a large difference between the two.
Most importantly, news outlets tell the public that immunization is the same as vaccination. However, there is a large difference between the two.
Vaccines contain a dead or live weakened germ that can cause a particular disease, like tetanus, or parts of a germ. When we are given a vaccine shot, our body immediately produces antibodies against the germ.
It is at this point that most believe the body’s defense mechanism kicks in and immunity will occur in the event that the said antigen gains entry again into the body but, this is not the case with all vaccines.
Vaccination Doesn’t Guarantee Immunity
Vaccination does not guarantee immunity. Natural immunity happens only after one recovers from the actual disease. During the disease, the microorganism usually has to pass through many of the body’s natural immune defense systems—in the nose, throat, lungs, digestive tract and lymph tissue—before it reaches the bloodstream.
As it does, the microorganism triggers many biological events that are essential in building true natural immunity. When a child gets a new disease, he may feel sick for several days, but, in the vast majority of cases, he will recover.
When a child gets a new disease, he may feel sick for several days, but, in the vast majority of cases, he will recover.
According to the Center for Disease and Prevention Control, CDC, not all that receive a vaccination will have immunity.
Defending what they believe is a misconception that the majority of people who get disease have been vaccinated, they state, “In fact it is true that in an outbreak those who have been vaccinated often outnumber those who have not – even with vaccines such as measles, which we know to be about 98% effective when used as recommended.”
“This is explained by two factors. No vaccine is 100% effective. Most routine childhood vaccines are effective for 85% to 95% of recipients.”
Everyone’s Immune System Reacts Differently
For reasons related to the individual, some will not develop immunity.
The second fact is that in a country such as the United States the people who have been vaccinated vastly outnumber those who have not. Here’s a hypothetical example of how these two factors work together.
Here’s a hypothetical example of how these two factors work together.
The National Network of Immunization Information provides, “Although vaccines have very high effectiveness rates, they are not completely effective for 100% of the people who receive them. For example, a full series of measles vaccine will protect 99 of 100 children from measles and polio vaccine will protect 99 of 100 children from polio. This means when there is a disease outbreak, the very small number of people for whom the vaccine did not work may still be able to catch the disease. Because almost all of our children are immunized and only few are not, it can be the case that during an epidemic the majority of cases occur among children who were immunized. However, the fact remains that those who have not received the vaccine are much more likely to catch the disease.”
Types of Vaccines
There are several different approaches that are used when vaccines are made. Each approach has its own way that it keeps the virus from sickening the recipient of the vaccine. When a company is developing a vaccine, they will have to choose which one of these methods works best to make the vaccine the most effective.
When a company is developing a vaccine, they will have to choose which one of these methods works best to make the vaccine the most effective.
Here are the types of vaccines that are used:
Weakening Vaccines (Live Attenuated)
Some viruses reproduce thousands of times within the body. The bacteria invades the body and then it multiplies exponentially. This type of vaccine involves the introduction of the live virus into the body. While you may think that injecting a virus into the body will have harmful effects, the opposite holds true with these types of vaccines. The theory behind these vaccines is that they are intended to “educate” the body to defend itself from the virus. Once that occurs, the viruses will have difficulty reproducing within the body. Here, much of the work is done before the virus is injected into the body. The virus is grown outside of its normal environment. When that happens, it becomes a weakened form of itself. By the time that it is introduced to the body, it is unable to reproduce itself.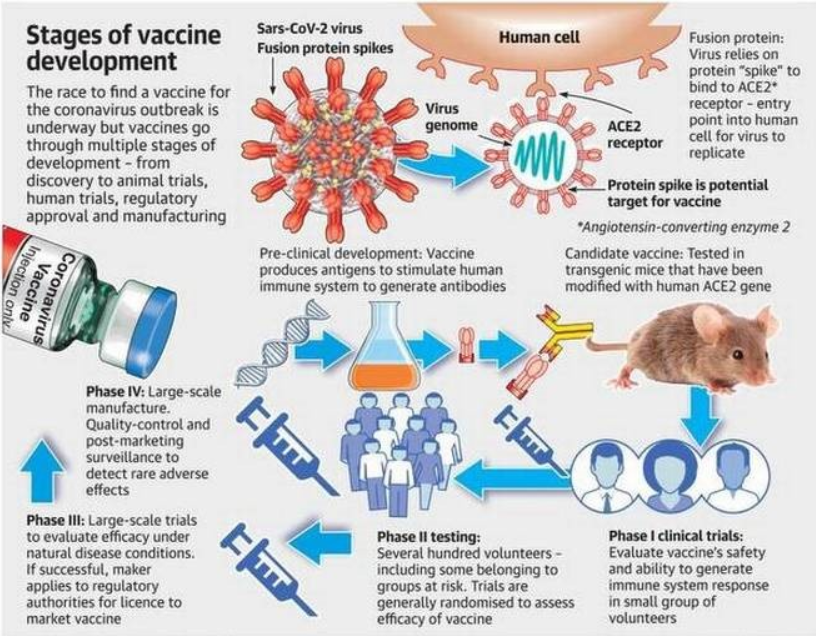
The advantages of live attenuated vaccines include:
- They are relatively easy to create for the viruses that they protect against
- They do not need to be taken more than once or twice
- They help strengthen the immune system since they are very close to the actual virus
However, there are some disadvantages to this type of virus. They include:
- Since they are weakened viruses, they can always re-strengthen and cause the sickness
- They must be well-stored and refrigerated which makes them difficult to ship to certain places
- Attenuated vaccines do not work well with bacterial viruses
Attenuated vaccines are used to immunize against the following diseases:
- Measles, mumps, and rubella
- Chickenpox
- Smallpox
- Yellow Fever
Inactivation Vaccines
Instead of introducing a weakened form of the virus into the body, this type of vaccine aims to completely stop the virus from reproducing. Usually, this type of vaccine will rely on a chemical or a pathogen to achieve that result. The body will continue to recognize the virus so it will keep producing the cells that will protect against the virus. The pathogen will still continue to exist, but it will not be able to replicate within the body.
Usually, this type of vaccine will rely on a chemical or a pathogen to achieve that result. The body will continue to recognize the virus so it will keep producing the cells that will protect against the virus. The pathogen will still continue to exist, but it will not be able to replicate within the body.
The advantages of inactivation vaccines include:
- No form of the disease will be produced by the body whatsoever. This includes even a milder form.
- This is not a type of vaccine that cannot be given to those with weakened immune systems
However, unlike live attenuated vaccines, one or two injections will generally not be enough to provide lifetime protection against these diseases. In addition to the initial vaccination, several long-term booster shots will be necessary to continue to provide immunity against the diseases.
Types of diseases that are protected against by this type of vaccine include:
- Polio
- Rabies
- Hepatitis A
Subunit and Conjugate Vaccines
Subunit vaccines use only a part of the virus in order to create the immunization. They take out only the essential part of the antigen in order to produce the vaccine and leave everything else. This will usually mean that a protein is used that resides on the surface of the virus. This type of vaccine is suitable for an instance where the body needs to protect against one specific part of the virus in order to protect against the entire disease. One of the advantages to this type of vaccine is that the body can give a targeted and focused response to the part of the germ that has been selected.
They take out only the essential part of the antigen in order to produce the vaccine and leave everything else. This will usually mean that a protein is used that resides on the surface of the virus. This type of vaccine is suitable for an instance where the body needs to protect against one specific part of the virus in order to protect against the entire disease. One of the advantages to this type of vaccine is that the body can give a targeted and focused response to the part of the germ that has been selected.
Conjugate vaccines use two different components instead of one. They use parts of the coats of bacteria. These parts are then linked to a protein. The combination of the coats of bacteria and the protein becomes the vaccine. It is the combination of the piece of bacteria and the carrier protein that gives the vaccine its effectiveness.
Some of the positive attributes of these types of vaccines include:
- Those with weakened immune system and other health problems can receive the vaccine without fear of getting the disease
- The immune response is strong and targeted
Those who receive this type of vaccine will likely require booster shots in order to receive continuous protection from the disease. In other words, recipients will not be able to have just one vaccination.
In other words, recipients will not be able to have just one vaccination.
Some of the diseases that this type of vaccine protects against include:
- Shingles
- HPV
- Hepatitis B
- Meningococcal disease
- Whooping cough
- Pneumococcal disease
Toxoid Vaccines
Toxoid vaccines use the harmful thing that is produced by the disease called the toxin, inactivating it with a chemical and reintroducing it into the body. The inactivated toxin becomes the vaccine. As opposed to vaccinating against the entire germ, these vaccinate against the part of the germ that causes the disease. These types of vaccines are considered to be bacterial in nature because they contain the actual bacteria. In other words, when the inactivated toxins are introduced, they stimulate the production of antibodies to the toxin. However, they will not cause the disease itself to take root.
These vaccines can be given to people who have immune systems that are weakened or compromised. However, the amount of antibodies that is produced by the body declines over time with these types of vaccines. Thus, periodic booster shots are necessary to maintain the proper level of protection.
However, the amount of antibodies that is produced by the body declines over time with these types of vaccines. Thus, periodic booster shots are necessary to maintain the proper level of protection.
Some of the diseases treated by this type of vaccine include:
- Tetanus Diphtheria
How Are Vaccines Administered?
There are several different ways that vaccines are administered. Most vaccines are administered through the form of an injection. However, advances in the medical field have diversified the means of delivery of new types of vaccines. Here are some means of administering vaccines through means other than an injection.
- Nasal Spray – flu injections are given to patients through their nose as a mist.
- Inhalant – There is a type of measles vaccine that is inhalable through the mouth.
- Orally – Certain vaccines can given in tablet form.
- Microneedle – Certain flu vaccines can shoot the substance into the body without the use of needles.

What Are the Types of Immunization?
There are two different types of immunization. Each one has different features to it. The two types are active and passive immunizations. Below is some information on each of the two kinds of immunity.
Active Immunization
The term “active immunization” characterizes how the body responds to receiving a vaccine. The vaccine stimulates the production of antibodies. Then, the antibodies will actively fight off the virus or the bacteria. The vaccine recipient will usually need a strong immune system in order to achieve the desired result. Active immunization cannot occur until the recipient has been exposed to the particular pathogen that is contained in the vaccine. The immunity is triggered by the exposure to the pathogen. Vaccines are considered to be a form of active immunization.
Passive Immunization
Passive immunity comes from the transfer of antibodies of an immune person to the body of a person who is not immune. This can happen naturally, such as when a mother passes immunity to a disease to the child through the fetus. It can also occur through a transfer in a substance such as immune globulin, which is given when protection from a certain disease is necessary. This protection occurs immediately, as opposed to active immunization, which takes time for the body to develop the necessary antibodies.
This can happen naturally, such as when a mother passes immunity to a disease to the child through the fetus. It can also occur through a transfer in a substance such as immune globulin, which is given when protection from a certain disease is necessary. This protection occurs immediately, as opposed to active immunization, which takes time for the body to develop the necessary antibodies.
What Are the Differences Between Active and Passive Immunity?
- Active immunity takes some time to develop, usually as a response to receiving the vaccine. Passive immunity comes immediately upon transfer.
- Passive immunity is not permanent and may last for a certain limited period of time. Active immunity is longer-lasting and is either permanent or lasts a long time until a subsequent booster shot is needed.
- In passive immunity, the antibodies comes from outside the body. In active immunity, the antibodies come from within the body in response to the introduction of another substance.

- Passive immunity is usually artificially induced.
Immunization and Vaccine Side Effects
There are three different possible reactions, or lack thereof, that people can experience when they have received immunizations and vaccinations. They are as follows:
- No Side Effects – The majority of people who receive immunizations and vaccinations do not experience any side effects. Sometimes, there is soreness or redness at the site of injection, but nothing further. If there are minor side effects, it is often a sign that the vaccination is working insofar as the body is beginning to build up the resistance necessary to immunize against the disease.
- Common Side Effects – While these side effects may be painful or temporarily debilitating, they are not much about which to be overly concerned. Among other things, these side effects consist of pain and itching at the injection site, dizziness, fever, nausea and a mild rash.
 Most often, these side effects pass after a period of time.
Most often, these side effects pass after a period of time. - Serious Side Effects – In some instances, patients can have severe side effects based on how their bodies react to the vaccination. This could mean seizures, brain damage and in a worst-case scenario, death.
The most important thing is to know what to do when you or someone in your family begins to experience side effects from a vaccination. Here are several important considerations that span both legal and medical issues.
- See a Medical Provider Immediately – If you believe that the side effects are anything more than common, it is essential to be seen by a healthcare provider immediately. From the obvious standpoint, adverse reactions to vaccination can endanger health both in the short and long terms. From a legal perspective, it is essential to document the complications that have been caused by an immunization.
- Report the Side Effect – There is a Vaccine Adverse Event Reporting System that allows people to report any complications.
 Tracking any side effects is essential in order to both know about them and prevent widespread complications if possible.
Tracking any side effects is essential in order to both know about them and prevent widespread complications if possible. - Contact an Attorney – There is a National Vaccine Injury Compensation Program that exists for those who have suffered injury from receiving vaccines. This is a special court that has been set up which has its own rules and procedures. A claim must be filed in order to be eligible for compensation. An attorney who specializes in vaccine injuries will know the most effective way to file a claim for compensation.
Common Side Effects
- Pain, redness, tenderness or swelling at injection site
- Fatigue
- Headache
- Itching at injection site
- Nausea
- Dizziness or fainting (most common in adolescents)
- Fever
- Mild rash
Serious Side Effects
- Severe Allergic Reaction (Anaphylaxis)
- Seizures
- Brain Damage
- Blood Disorders
- Bowel Obstruction
- Nerve Damage
- Death
People Ask:
Vaccine Injury Reporting
When one has sustained an injury as a result of being administered a vaccine, there is a different mechanism whereby one would receive compensation. Vaccines have both their own means of the reporting the injury as well as for filing a claim. This is not the same process that one would follow when they are injured by a medical device or medication.
Vaccines have both their own means of the reporting the injury as well as for filing a claim. This is not the same process that one would follow when they are injured by a medical device or medication.
Why Are Vaccines Different Than Other Medical Claims?
Congress passed the National Childhood Vaccine Injury Act in 1986 as a means of encouraging vaccine makers to produce their products while, at the same time, protecting patients from harm. The intent behind the statute was to keep manufacturers from having to face litigation from the products that they sold. On the other side, Congress also wanted to encourage people to be vaccinated by ensuring that they could be compensated for any injuries that they suffered.
How Are Vaccine Injuries Reported?
While the FDA has its own adverse event reporting database, the Centers for Disease Control, in tandem with the FDA, has its own Vaccine Adverse Event Reporting System for vaccine injuries. VAERS exists to serve as a repository for information about vaccine related injuries that can be reported to the database by anyone. There are several steps that one should take in order to report a vaccine injury to VAERS.
There are several steps that one should take in order to report a vaccine injury to VAERS.
- Contact healthcare provider – The first person to inform of an injury is the physician
- Report the injury – Patients can submit an online report to VAERS
- Update VAERS – If there are medical records pertaining to treatment, they should be uploaded into VAERS.
VAERS is helpful to track various metrics that relate to vaccines. Among the uses of VAERS are:
- It helps to detect any trends in injuries
- Allows people to track the safety of new vaccines
- Gives transparency to adverse events for new vaccinations
- Can identify any patient risk factors for certain vaccine injuries
The National Vaccine Injury Compensation Program
The VICP is the fund that allows those who have suffered vaccine-related injuries to receive compensation for their injuries. The main difference between vaccines and drugs is that, with regard to the VICP, the manufacturers are not paying out the settlement. That is because the VICP is intended to shield them from liability in order to encourage them to enter and stay in what is a critical field. The VICP was initially established because there was a scare related to a certain vaccine in the early 1980s that resulted in a threat to the vaccine supply when vaccine makers lost several large lawsuits.
That is because the VICP is intended to shield them from liability in order to encourage them to enter and stay in what is a critical field. The VICP was initially established because there was a scare related to a certain vaccine in the early 1980s that resulted in a threat to the vaccine supply when vaccine makers lost several large lawsuits.
The VICP is funded by a fee that is assessed by the federal government on the sale of every single childhood vaccination. The fees are then placed in the fund which is used to pay out claims as they are filed. This means that patients end up funding the settlements through the vaccines that are purchased.
There is a special mechanism for the VICP that must be followed in order to receive compensation. The process is started by filing a petition with the United States Court of Federal Claims (COFC), which has exclusive jurisdiction over vaccination cases. COFC has Special Masters whose job it is to decide these claims. Before the Special Master issues a decision, the Department of Health and Human Services will make their own recommendation. Then, the Department of Justice develops a report that includes a medical recommendation and legal analysis. After that, the Special Master may hold a hearing and decide the issue of compensation. The petitioner has the right to appeal the Special Master’s decision.
Then, the Department of Justice develops a report that includes a medical recommendation and legal analysis. After that, the Special Master may hold a hearing and decide the issue of compensation. The petitioner has the right to appeal the Special Master’s decision.
It is best to hire an attorney to help with drafting the petition since the case will likely be decided initially on the basis of the petition and any hearing that the Special Master may hold. Given the scientific complexity of vaccines and their injuries, an attorney may best be able to present a case.
What makes COVID-19 vaccines based on messenger RNA (mRNA) different?
Infectious disease specialist Tobias Hohl explains that the messenger RNA vaccine induces the body's own cells to produce a protein fragment found in the COVID-19 pathogen, and this triggers a protective immune response.
Pfizer-BioNTech (Comirnaty®) and Moderna (Spikevax™) COVID-19 vaccines use messenger ribonucleic acid (mRNA) to elicit an immune response that may protect against future infection. Memorial Sloan Kettering Infectious Disease Specialist Tobias Hohl talks about mRNA-based vaccine technology. nine0003
Memorial Sloan Kettering Infectious Disease Specialist Tobias Hohl talks about mRNA-based vaccine technology. nine0003
Conventional vaccines involve the introduction of a weakened or inactivated infectious agent into the body. Messenger RNA (mRNA)-based vaccines, such as the Pfizer and Moderna COVID-19 vaccines, train cells to produce a protein that triggers an immune response in the event of infection. After the vaccine is injected into the shoulder, the mRNA enters the cells near the injection site and induces them to start producing the same protein found in the COVID-19 pathogen. The immune system recognizes this protein and begins to produce antibodies that can fight the virus if a vaccinated person is subsequently infected. nine0003
Truth: None of the vaccines interact with or alter DNA in any way and therefore cannot cause cancer. Messenger RNA is not the same as DNA, so it cannot combine with DNA and change the genetic code. Messenger RNA is fragile, and it tells your cells how to make antibodies against SARS-CoV-2. mRNA does not enter the nucleus of the cell - the part where DNA is contained. Therefore, the myth that an mRNA-based vaccine can somehow block the activity of cancer-suppressing genes is not true. nine0003
mRNA does not enter the nucleus of the cell - the part where DNA is contained. Therefore, the myth that an mRNA-based vaccine can somehow block the activity of cancer-suppressing genes is not true. nine0003
The COVID-19 vaccine does not expose you to the virus that causes COVID-19.
back to top of pageThese are the first mRNA-based vaccines to be made and tested in large-scale phase 3 human clinical trials. The advantage of mRNA technology over traditional approaches is that it allows faster development and production scale-up. Vaccine development has traditionally taken decades. What we have today is an incredible scientific achievement. nine0003 back to top of page
If mRNA is not stored at a low temperature, it is easily and rapidly degraded. Once introduced into the body, this is not a problem, since the mRNA does not take long to complete its function. However, it may be necessary to keep vaccines stable for several days or even weeks before they are administered. With traditional vaccines, it didn't really matter. Weakened or inactivated versions of the virus can remain stable for longer periods without cold temperatures. nine0003
With traditional vaccines, it didn't really matter. Weakened or inactivated versions of the virus can remain stable for longer periods without cold temperatures. nine0003
In clinical studies, Pfizer vaccine was stored at -70 degrees Celsius (-94 degrees Fahrenheit), which involves the use of dry ice. The Moderna vaccine was stored at -20 degrees Celsius (-4 degrees Fahrenheit), which is closer to the temperature in a conventional refrigerator. Both vaccines should still be stored frozen - vaccine approval is based on data from clinical trials, so conditions need to remain the same. nine0003
The idea is that the first shot prepares the immune system to recognize the virus, while the second boosts the immune response. The second Pfizer vaccine is given three weeks after the first; for Moderna, this interval is four weeks. It is important that both vaccinations are given with the same vaccine and that the same procedures are followed that have ensured such successful results in clinical trials.
We have data on millions of people and know that these vaccines are very effective in preventing severe COVID-19.
COVID-19 Information for Patients and Caregivers
We know it may be difficult to navigate challenges related to COVID-19. We’ve put together information and answers to frequently asked questions about COVID-19 and your cancer care.
To learn moreSome people experience mild or moderate side effects, but they do not last long - about one to three days. The most common side effects include pain at the injection site, weakness (feeling tired), headache, aches and fever. They are more common after the second shot and you may need to rest more. Serious side effects are rare and treatable. nine0003
It's important for people to understand that these data are reviewed by independent teams of experts not affiliated with the drug companies. These are specialists who have no interest in the development or commercialization of vaccines. Personally, I would not hesitate to vaccinate with any of these vaccines and would advise my relatives and colleagues to do the same.
Personally, I would not hesitate to vaccinate with any of these vaccines and would advise my relatives and colleagues to do the same.
Vaccines are an important part of the response to the pandemic. But we know that wearing masks and washing our hands can give us extra protection, especially as new strains emerge. nine0003
December 6, 2021
Additional resources
- Information leaflet for vaccinees and caregivers on emergency use of COVID-19 vaccines
- Pfizer-BioNTech (Comirnaty®) Vaccine Instructions for Use
- Instructions for use of the Moderna vaccine (Spikevax™)
- Johnson & Johnson Emergency Vaccine Authorization Information Sheet nine0042
What you need to know about Novavax COVID-19 Vaccine
The Emergency Medical Devices Technical Advisory Group has included Nuvaxovid (NVX-CoV2373) and Covovax (NVX-CoV2373) COVID-19 vaccines in list of funds to use in emergencies on December 20, 2021 and December 17, 2021, respectively.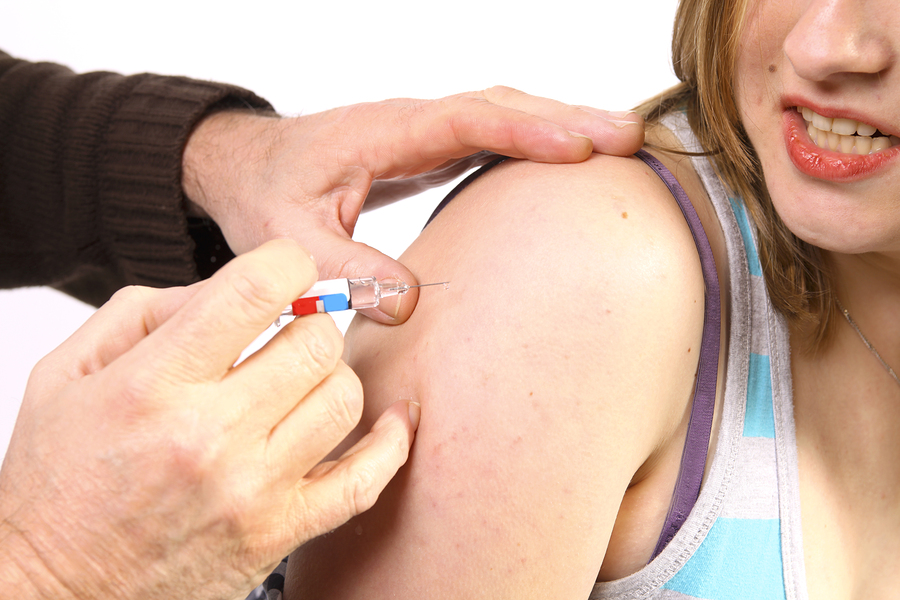
Novavax vaccine will be manufactured at two different facilities. In Europe, the vaccine is approved by the European Medicines Agency and will be produced under the trade name Nuvaxovid, while in India it is approved by the Comptroller General medicines of India and will be manufactured by the Serum Institute of India under the trade name Covovax. nine0003
The WHO Strategic Advisory Group of Experts (SAGE) on Immunization has issued interim recommendations for the use of Novavax (NVX-CoV2373). This article provides a summary of them.
In this material, the vaccine is referred to as Novavax (NVX-CoV2373).
Vaccine reference documentation is available here (in English).
Who is this vaccine suitable for?
The vaccine can be safely and effectively administered to all individuals 12 years of age and older. In accordance with the WHO Roadmap for Vaccine Priority and the WHO Values Framework, the elderly, workers healthcare and immunocompromised patients.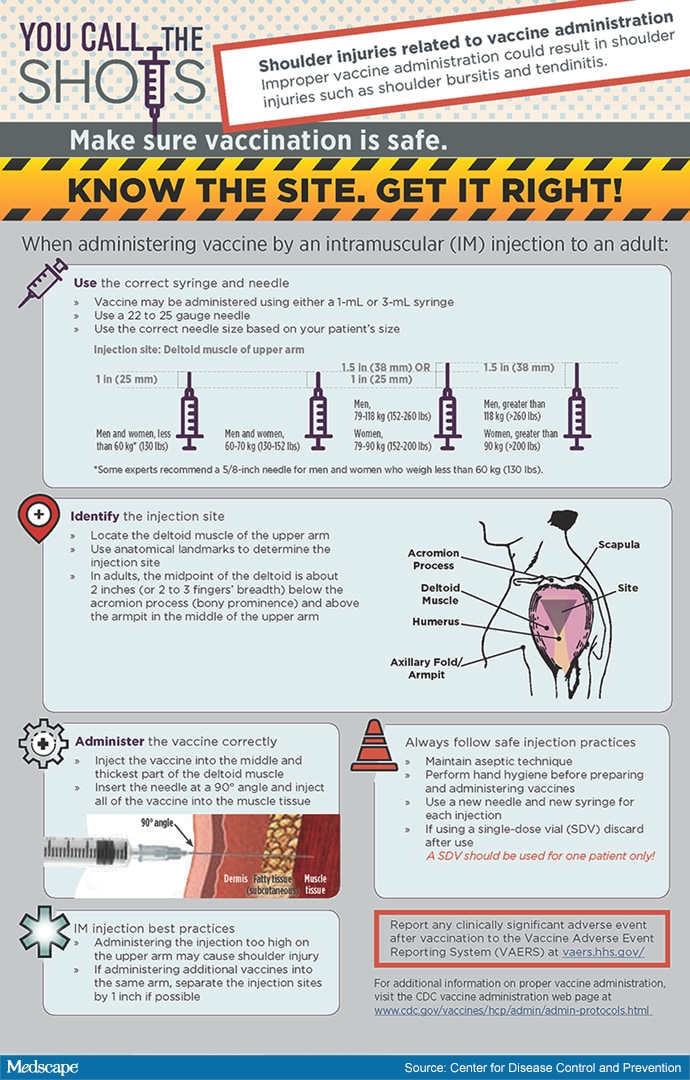 The highest priority group includes adolescents with moderate and severe immunodeficiencies. nine0003
The highest priority group includes adolescents with moderate and severe immunodeficiencies. nine0003
Novavax may be offered to people who have had COVID-19 in the past. They can optionally delay vaccination for a period of 3 months from the time of infection.
Should pregnant and breastfeeding women be vaccinated?
During the accumulation of pharmacovigilance data on the use of NVX-CoV2373 for immunization of pregnant women at the stage after the introduction of the vaccine, no problems were identified with the safety of vaccination during pregnancy, and based on data obtained earlier with the use other protein-based vaccines during pregnancy, its effectiveness is expected to be comparable to that for non-pregnant women of a similar age. nine0003
Given the increased risk of severe outcomes, WHO has included pregnant women as a high priority for COVID-19 vaccination. WHO recommends the use of Novavax (NVX-CoV2373) during pregnancy if the benefits of vaccination for the pregnant woman are outweigh potential risks.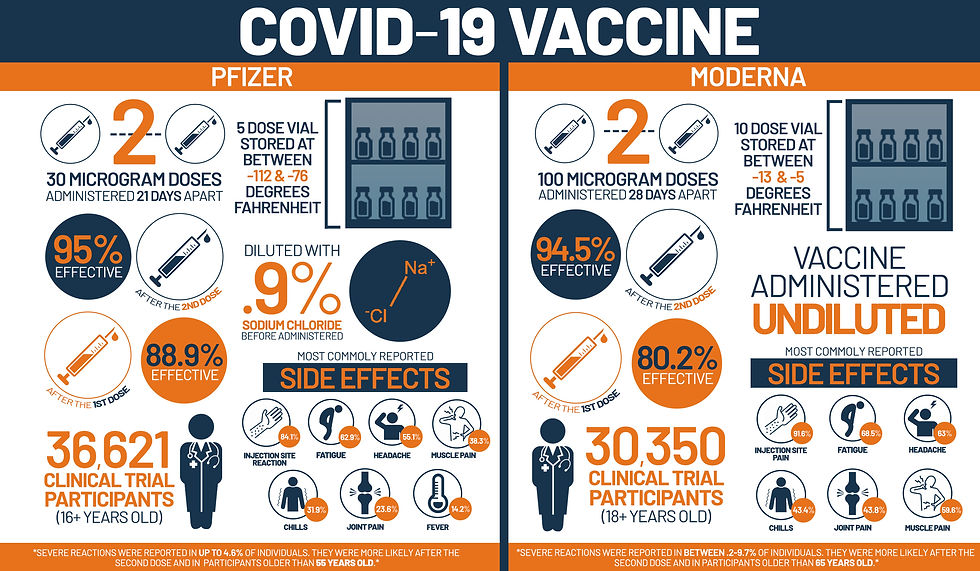 To help the pregnant woman make this decision, she should be provided with information about the risks of COVID-19 during pregnancy, the expected benefits of vaccination based on the local epidemiological situation, and current the lack of data on the safety of vaccines in pregnant women. WHO does not recommend pregnancy testing before vaccination. WHO does not recommend postponing or terminating pregnancy due to vaccination. nine0003
To help the pregnant woman make this decision, she should be provided with information about the risks of COVID-19 during pregnancy, the expected benefits of vaccination based on the local epidemiological situation, and current the lack of data on the safety of vaccines in pregnant women. WHO does not recommend pregnancy testing before vaccination. WHO does not recommend postponing or terminating pregnancy due to vaccination. nine0003
WHO recommends the use of Novavax (NVX-CoV2373) during breastfeeding under the same conditions as if not. The effectiveness of the vaccine in breastfeeding women is expected to be similar to its effectiveness in other categories of adults. Because Novavax (NVX‑CoV2373) is not a live virus vaccine, it is unlikely that clinical or biological hazards will occur with its use in infants. WHO does not recommend interrupting breastfeeding due to vaccination. nine0003
For whom is the vaccine contraindicated?
This vaccine is not recommended for use in persons under 12 years of age.
Vaccination is contraindicated in persons with a history of anaphylactic reactions to any of the components of the vaccine.
Individuals with PCR-confirmed acute COVID-19 should not be vaccinated until the acute course of the disease has ended and the isolation conditions have been met.
Persons with a body temperature above 38.5°C should postpone vaccination until the fever has passed. nine0003
Is the vaccine safe?
SAGE reviewed the safety and efficacy data for the vaccine in detail and recommended it for use in people aged 12 years and older. When evaluating a drug for emergency use, WHO considers not only its safety and effectiveness, but also the quality of production.
Extremely rare cases of myocarditis and pericarditis have been reported, but these reactions usually lasted for several days after vaccination and were usually mild. nine0003
How effective is this vaccine?
The efficacy of Novavax (NVX-CoV2373) was evaluated in Phase II and Phase III clinical trials.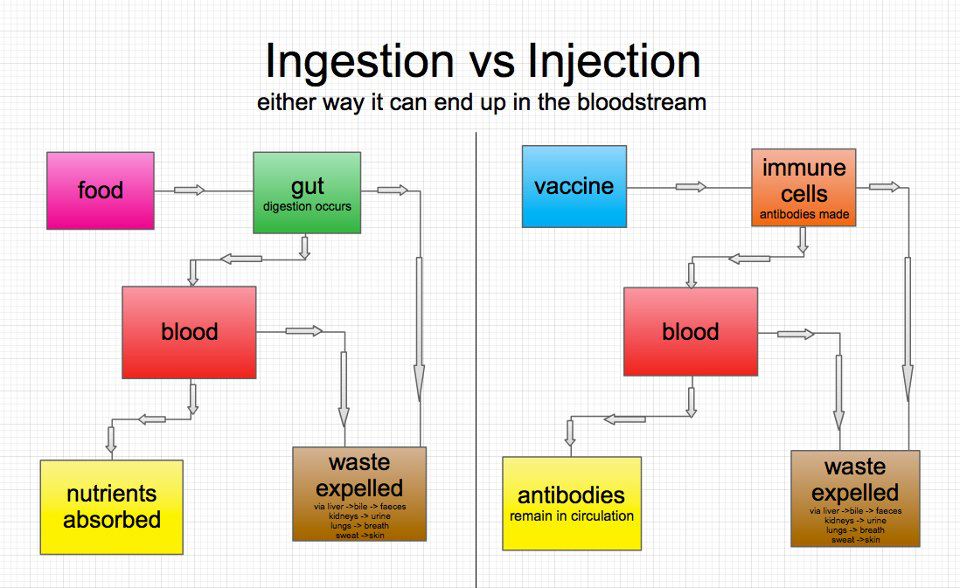 Two Phase III trials determined vaccine efficacy against mild, moderate and severe disease to be 90%.
Two Phase III trials determined vaccine efficacy against mild, moderate and severe disease to be 90%.
The efficacy of Novavax (NVX-CoV2373) in vaccinating adolescents aged 12–17 years was assessed in an interim analysis of data from pediatric patients additionally enrolled in an ongoing Phase 3 trial in the United States. States. For this population group, the effectiveness of the vaccine reached 80%. nine0003
What is the recommended dose of the vaccine?
The SAGE recommended regimen is for two doses of Novavax (NVX-CoV2373) (0.5 ml) intramuscularly. Between the introduction of primary doses of NVX-CoV2373, it is recommended to maintain an interval of eight weeks.
The first booster dose is recommended 4-6 months after the completion of the primary vaccination series. In line with the WHO vaccine prioritization roadmap, it should be proposed first to the most priority populations (i.e. the elderly, those with moderate and severe immunodeficiencies, and health care workers). nine0003
nine0003
To further reduce the risk of severe illness, death and disruption of health services, WHO recommends that countries consider introducing all older people, all those with moderate and severe immunodeficiencies conditions regardless of age, adults with underlying medical conditions, pregnant women and healthcare workers with a second booster dose 4-6 months later.
There are currently no data available to recommend a booster dose in adolescents aged 12-18 years, except in immunocompromised adolescents. nine0003
Can this vaccine be combined with other vaccines?
SAGE recognizes as a complete primary series two heterologous doses of COVID-19 vaccines approved by WHO for emergency use. However, there are only limited data on the use of Novavax (NVX-CoV2373) in heterologous mode.
Does the vaccine prevent infection and transmission of the virus?
As there is currently insufficient data to assess the impact of the vaccine on virus transmission, it is necessary to continue to observe epidemic control measures, including the use of masks, keeping a distance from other people, wash hands, ventilate rooms, and apply other protective measures that may be necessary in certain conditions depending on the epidemiology of COVID-19and the potential risk of emergence of new variants of the virus. Both vaccinated and unvaccinated persons should continue to comply with the recommendations of state authorities regarding anti-epidemic measures and restrictions. SAGE will update these recommendations as information is analyzed on the impact of vaccination on virus transmission and the formation indirect protection.
Both vaccinated and unvaccinated persons should continue to comply with the recommendations of state authorities regarding anti-epidemic measures and restrictions. SAGE will update these recommendations as information is analyzed on the impact of vaccination on virus transmission and the formation indirect protection.
Does the vaccine protect against new variants of the SARS-CoV-2 virus?
In a multi-strain multi-strain (alpha, beta, and delta) phase III trial conducted in the US and Mexico, vaccine efficacy against mild, moderate, or severe COVID-19amounted to 90%.
In light of these findings, WHO recommends using Novavax
(NVX-CoV2373) according to the WHO Vaccine Priority Roadmap even when known variants of concern are circulating in the country (BVO). If new VZVs become available that reduce the effectiveness of the vaccine, these recommendations will be updated accordingly. Sufficient data are not yet available for the omicron variant.
How does this vaccine compare to other vaccines used against COVID-19?
Due to different study design approaches, it is not possible to directly compare vaccines, but in general, all vaccines approved by WHO for emergency use are extremely effective in preventing severe course of COVID-19 and the associated need for hospitalization.