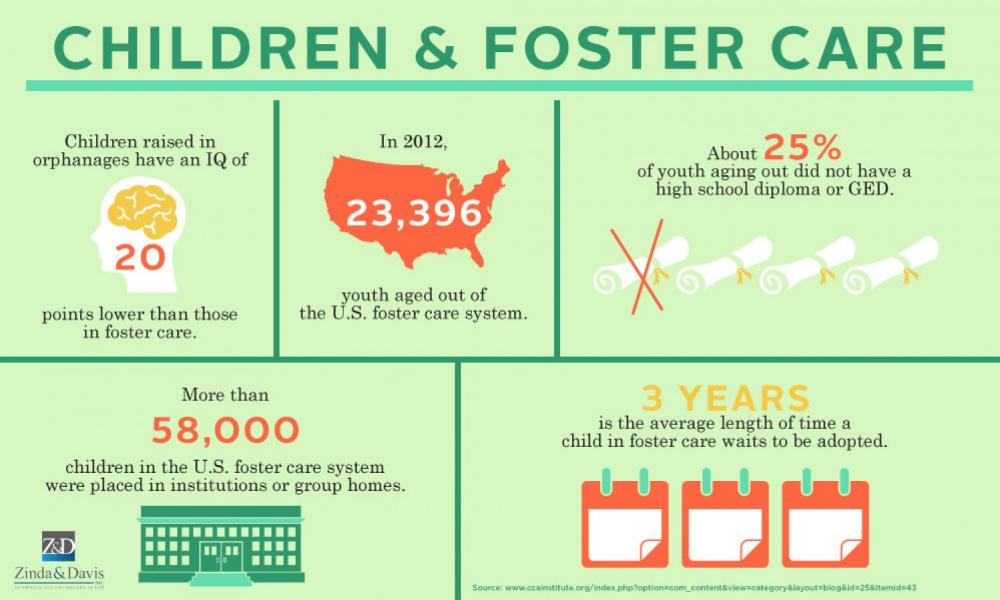
How much tamiflu should a child take
Tamiflu® (oseltamivir phosphate) | Dosing for Flu (Influenza) Treatment
Taking Tamiflu at thefirst sign of flu symptoms
may reduce the amount of
time you’re sick.
Tamiflu for children
Taking Tamiflu at thefirst sign of flu symptoms
may reduce the amount of
time you’re sick.
Tamiflu for children
How to Take Tamiflu
Take Tamiflu within 48 hours of flu symptom onset. You have a choice of capsules or liquid. It’s best to take Tamiflu with food—there is less chance of stomach upset if you take it with a light snack or a meal.
If you have already been exposed to the flu, but you don't have any symptoms, you can take Tamiflu for up to 6 weeks to help prevent you from getting sick.
Flu Treatment
For adults and teens aged 13 years and older
- Take 75 mg, twice daily, for 5 days.
For children 2 weeks of age to 12 years old
- Take 1 dose, based on your child's weight, twice daily, for 5 days.
- Tamiflu is available in capsule and liquid form.
- See full prescribing information for dosing based on your child's weight.
Flu Prevention
For adults and teens aged 13 years and older
- Take 1 dose, once daily, for 10 days or as long as prescribed.
For children from 1 to 12 years old
- Take 1 dose, based on your child's weight, once daily, for 10 days or as long as prescribed.
- See full prescribing information for dosing based on your child's weight.
- Tamiflu is not approved for flu prevention in children less than 1 year of age.
FAQs
Below are several commonly asked questions and their answers. If you have additional questions not covered here, be sure to ask your doctor.
What should I do if I miss a dose of Tamiflu?
If you forget to take your medicine, take the missed dose as soon as you remember, unless it is 2 hours or less before your next scheduled dose. Then continue to take Tamiflu at the usual times. Do not take 2 doses at a time to make up for a missed dose. If you miss several doses, tell your doctor and follow the advice given to you.
Then continue to take Tamiflu at the usual times. Do not take 2 doses at a time to make up for a missed dose. If you miss several doses, tell your doctor and follow the advice given to you.
Can I take Tamiflu with other medications?
Your doctor or healthcare provider may recommend taking over-the-counter medications to help lessen your symptoms.
As with any medication, be sure to discuss with your doctor any over-the-counter or prescription medicines you are currently taking before beginning Tamiflu therapy.
How do I store Tamiflu?
Follow these guidelines:
- Store Tamiflu capsules at room temperature between 68°F to 77°F (20°C to 25°C).
- Store liquid Tamiflu in the refrigerator for up to 17 days between 36°F to 46°F (2°C to 8°C).
- Store liquid Tamiflu for up to 10 days at room temperature between 68°F to 77°F (20°C to 25°C).
- Safely throw away any unused Tamiflu that is out of date or no longer needed.
How do I administer liquid Tamiflu?
If you are taking liquid Tamiflu or giving it to a loved one, your pharmacist should give you a small measuring cup or dosing syringe, since there is not a dosing device in the carton. Follow your doctor’s instructions on how to take Tamiflu and which dose to take.
Follow your doctor’s instructions on how to take Tamiflu and which dose to take.
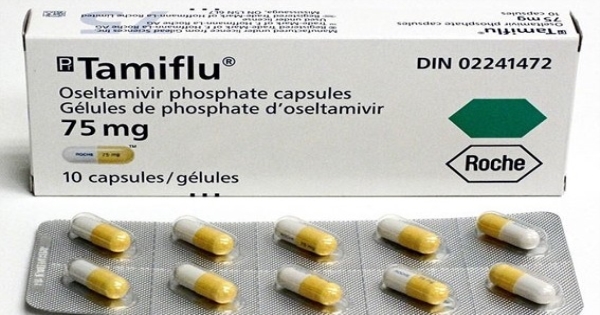
Should Children Take Tamiflu? - Children's Health
Share:
When kids are sick, all you want is to help them feel better. With bacterial infections, you might give them antibiotics, but when it comes to viruses like the flu, you may not have many options.
Besides rest and fluids, one safe way to treat the flu in children over 2 weeks old is with an antiviral medicine called Tamiflu.
What does Tamiflu do?
Tamiflu is a medication that shortens the duration of the flu and the severity of its symptoms by a day or a day and a half. It is not going to cure the flu the way an antibiotic cures a bacterial infection.
Though Tamiflu doesn't cure the flu, it still might help your child. Tamiflu is most often used for the following purposes in children.
Tamiflu for treatment in high-risk children
Tamiflu can be an important medicine for kids who are at a high risk for life-threatening flu complications. Your child may be at a high risk if they are younger than 5 or if they have:
Your child may be at a high risk if they are younger than 5 or if they have:
- Asthma
- Diabetes
- Heart disease
- Metabolic conditions
- Neurologic conditions
- Neuromuscular disorders
Tamiflu may help reduce your child's risk for severe illness or hospitalization if they have any of these conditions.
Tamiflu for flu prevention in high-risk children
Tamiflu may also help prevent high-risk children from getting the flu if they are around another person with the flu. For instance, if your child has asthma and their sibling gets the flu, your child with asthma may benefit from taking Tamiflu before they show symptoms.
Tamiflu may prevent a child from getting the flu or may make the condition less severe if they do contract the flu. Your child can take Tamiflu for up to six weeks.
Your child should also receive a flu shot every year and practice good hand hygiene to prevent the flu.
Tamiflu for low-risk children who just started flu symptoms
If your child is over the age of 5 and generally healthy, they may or may not benefit from using Tamiflu. If your child does take Tamiflu, it's important that it’s administered to children as soon as symptoms begin.
Is Tamiflu effective after 48 hours?
Tamiflu is most effective if it is administered within 48 hours of flu symptoms starting. If your child is at day four or five of the flu and is generally healthy, it may not be worth it to start taking Tamiflu at that point.
What are symptoms of flu in kids?
Flu symptoms in children can include:
- Cough
- Fatigue
- Headache
- Sore throat
- Muscle aches
- Runny or stuffy nose
- Fever or feverish chills
Tamiflu may help your child have these symptoms for about one day less than normal or may make these symptoms less severe.
What are the side effects of Tamiflu in children?
Tamiflu rarely has side effects. However, side effects may be more common in kids who are fructose intolerant (fruit sugar). The liquid form of Tamiflu contains sorbitol (a type of fructose) that can rarely cause nausea and vomiting in some kids. Non-liquid forms of Tamiflu do not have this side effect.
If children are generally healthy, it may not be worth risking this side effect. Instead, you may want to focus on helping them rest, drinking plenty of fluids and healing while their bodies fight off the flu.
While everyone wants medicine to help make their child feel better, it depends on the situation to know if Tamiflu will help your child. If it's a high-risk situation, your child may benefit from Tamiflu. If it's low risk, discuss the medication with your child's pediatrician and let them decide if Tamiflu is right.
Tamiflu is most effective if prescribed within 48 hours of flu symptoms occurring.
See other facts about Tamiflu and kids via @Childrens.
Click to Tweet
Get care now
We know that getting sick is never convenient. But now you can videoconference with a health care provider 24 hours a day, 7 days a week with Virtual Visit by Children's Health Virtual Care. Get treated right from your smartphone, tablet or computer for allergies, common colds and flu, cuts and more. Download the Virtual Visit app today.
Children’s Health Family Newsletter
Get health tips and parenting advice from Children’s Health experts sent straight to your inbox twice a month. Sign up now.
capsules, 75 mg - RLS Drug Encyclopedia
By mouth , with or without food. Tolerability of the drug can be improved if taken with food.
Adults, adolescents or children who cannot swallow a capsule may also be treated with Tamiflu ® powder for oral suspension.
In cases where Tamiflu ® powder for oral suspension is not available, or if there are signs of aging of the capsules (for example, increased fragility or other physical disorders), it is necessary to open the capsule and empty its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food item (normal sugar or no sugar chocolate syrup, honey, light brown sugar or table sugar dissolved in water, sweet dessert, sweetened condensed milk, applesauce or yogurt) to to hide the bitter taste. The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation. Detailed recommendations are given in Section Extemporaneous preparation of a suspension of Tamiflu ® .
The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation. Detailed recommendations are given in Section Extemporaneous preparation of a suspension of Tamiflu ® .
Standard dosing regimen
Treatment. The drug should be started no later than 2 days after the onset of symptoms of the disease.
Adults and adolescents ≥12 years of age. 75 mg twice a day for 5 days. Increasing the dose of more than 150 mg / day does not increase the effect.
Children weighing >40 kg or 8 to 12 years of age. Children who can swallow capsules can also be treated with 1 caps. 75 mg 2 times a day for 5 days.
Children from 1 to 8 years of age. Recommended Tamiflu ® powder for oral suspension 12 mg/ml or capsules 30 and 45 mg (for children over 2 years of age). To determine the recommended dosing regimen, see the instructions for medical use of Tamiflu ® powder for oral suspension 12 mg/ml or capsules 30 and 45 mg.
Prevention. The drug should be started no later than 2 days after contact with patients.
Adults and adolescents ≥12 years of age. 75 mg once a day by mouth for at least 10 days after contact with the patient. During a seasonal influenza epidemic - 75 mg 1 time per day for 6 weeks. The prophylactic effect lasts as long as the drug is taken.
Children weighing >40 kg or 8 to 12 years of age. Children who can swallow the capsules can also receive prophylactic therapy by taking 1 caps. 75 mg 1 time per day.
Children from 1 to 8 years of age. Recommended Tamiflu ® powder for oral suspension 12 mg/ml or capsules 30 and 45 mg. To determine the recommended dosing regimen, see the instructions for medical use of Tamiflu ® Powder for Oral Suspension 12 mg/ml or caps. 30 and 45 mg. Possible extemporaneous suspension using 75 mg capsules (see Extemporaneous suspension of Tamiflu ® ).
Dosing in special cases
Patients with kidney damage, treatment. Patients with Cl creatinine> 60 ml / min dose adjustment is not required. In patients with Cl creatinine from 30 to 60 ml / min, the dose of Tamiflu ® should be reduced to 30 mg 1 time per day for 5 days.
For patients on chronic hemodialysis, Tamiflu® at the initial dose of 30 mg may be taken prior to dialysis if influenza symptoms occur within 48 hours between dialysis sessions. To maintain plasma concentrations at therapeutic levels Tamiflu ® should be taken at 30 mg after each dialysis session. For patients on peritoneal dialysis, Tamiflu ® should be taken at an initial dose of 30 mg prior to dialysis, then 30 mg every 5 days (see also Dosage in Special Cases and 'Special Instructions').
The pharmacokinetics of oseltamivir in patients with end-stage renal disease (Cl creatinine ?10 ml/min) not on dialysis has not been studied. In this regard, there are no recommendations for dosing in this group of patients.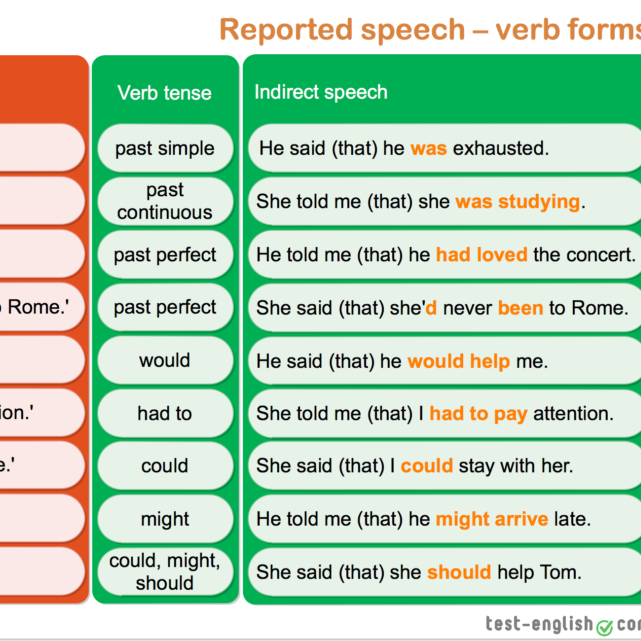
Patients with kidney damage, prophylaxis. Patients with Cl creatinine> 60 ml / min dose adjustment is not required. In patients with Cl creatinine from 30 to 60 ml / min, the dose of Tamiflu ® should be reduced to 30 mg 1 time per day. For patients on permanent hemodialysis, Tamiflu ® at the initial dose of 30 mg can be taken prior to dialysis (1st session). To maintain plasma concentrations at therapeutic levels, Tamiflu ® should be taken at 30 mg after each successive odd dialysis session. Patients on peritoneal dialysis, Tamiflu ® should be taken at an initial dose of 30 mg prior to dialysis, then 30 mg every 7 days (see also Dosing in Special Cases and 'Special Instructions'). The pharmacokinetics of oseltamivir in patients with end-stage renal disease (with Cl creatinine? 10 ml / min) who are not on dialysis have not been studied. In this regard, there are no recommendations for dosing in this group of patients.
Patients with liver damage. Dose adjustment in the treatment and prevention of influenza in patients with mild to moderate hepatic impairment is not required. Safety and pharmacokinetics of Tamiflu ® has not been studied in patients with severe hepatic impairment.
Elderly and senile patients. Dose adjustment is not required for the prevention or treatment of influenza.
Immunocompromised patients (after transplantation). For seasonal influenza prophylaxis in immunocompromised patients ≥1 year of age for 12 weeks, dose adjustment is not required (see Dosage and Administration).
Children. Tamiflu ® in this formulation should not be given to children under 1 year of age.
Extemporaneous formulation of suspension Tamiflu ®
In cases where adults, adolescents and children have a problem swallowing capsules, and Tamiflu ® in powder form for oral suspension is absent or there are signs of aging capsules (e. g. increased fragility or other physical disorders), it is necessary to open the capsule and pour its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food (see above) in order to mask the bitter taste. The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation.
g. increased fragility or other physical disorders), it is necessary to open the capsule and pour its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food (see above) in order to mask the bitter taste. The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation.
If patients require a dose of 75 mg, the following instructions should be followed:
1. Holding 1 caps. 75 mg Tamiflu ® over a small container, carefully open the capsule and pour the powder into the container.
2. Add a small amount (no more than 1 teaspoon) of a suitable sweetened food (to cover the bitter taste) and mix well.
3. Stir the mixture thoroughly and drink immediately after preparation. If a small amount of the mixture remains in the container, then rinse the container with a small amount of water and drink the remaining mixture.
If patients require doses of 30–60 mg, the following instructions should be followed for correct dosing:
1. Holding 1 caps. 75 mg Tamiflu ® over a small container, carefully open the capsule and pour the powder into the container.
Holding 1 caps. 75 mg Tamiflu ® over a small container, carefully open the capsule and pour the powder into the container.
2. Add 5 ml of water to the powder using a syringe labeled to indicate the amount of liquid drawn. Mix thoroughly for 2 minutes.
3. Draw up the required amount of mixture from the container according to the table below into the syringe.
| Body weight, kg | Recommended dose, mg | Amount of Tamiflu ® mixture per 1 reception, ml |
| ?15 | 30 | 2 |
| >15–23 | 45 | 3 |
| >23–40 | 60 | 4 |
There is no need to collect undissolved white powder as it is an inactive excipient. By pressing the plunger of the syringe, inject all its contents into the second container. The remaining unused mixture must be discarded.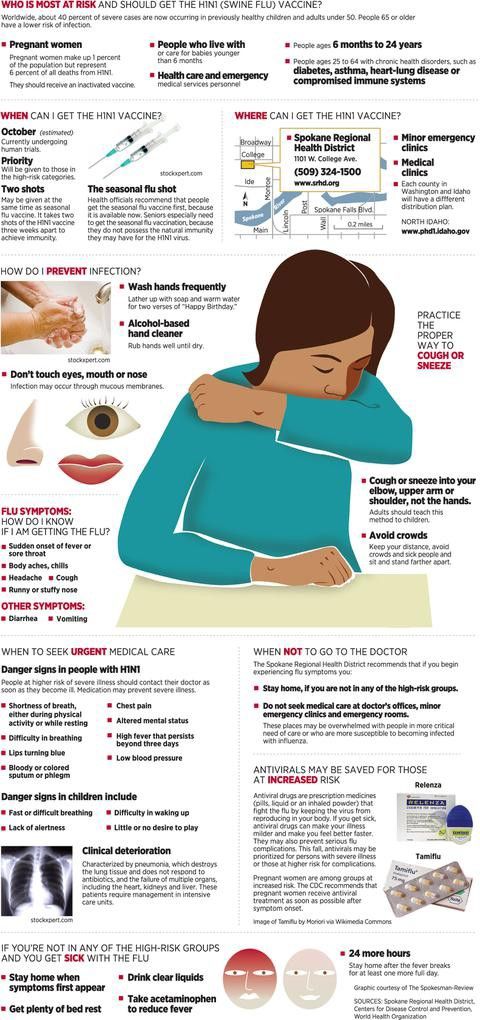
4. In the second container, add a small amount (no more than 1 teaspoon) of a suitable sweetened food to cover the bitter taste and mix well.
5. Stir the mixture thoroughly and drink immediately after preparation. If a small amount of the mixture remains in the container, then rinse the container with a small amount of water and drink the remaining mixture.
Repeat this procedure before each dose.
Capsules, 75 mg. 10 caps. in a blisters (blister) made of triplex (PVC/PE/PVDC) and aluminum foil. 1 bl. placed in a cardboard box.
F. Hoffmann-La Roche Ltd., Grensacherstrasse 124, 4070 Basel, Switzerland.
Senexy CAC, 52 rue Marseille and Jacques Gaucher, 94120 Fontenay-sue-Boa, France.
Catalent Germany Schorndorf GmbH, Steinbeisstraße 2, 73614 Schorndorf, Germany.
Marketing authorization holder. F. Hoffmann-La Roche Ltd., Grensacherstrasse 124, 4070 Basel, Switzerland.
Claims of consumers should be sent to the address of the representative office of F. Hoffmann-La Roche Ltd. 107031, Russia, Moscow, Trubnaya Square, 2.
Hoffmann-La Roche Ltd. 107031, Russia, Moscow, Trubnaya Square, 2.
Tel.: (495) 229-29-99; fax: (495) 229-79-99.
In the case of packaging at OJSC Pharmstandard-Leksredstva, 305022, Russia, Kursk, st. 2nd Aggregate, 1a/18.
Tel./Fax: (4712) 34-03-13.
www.pharmstd.ru
capsules, 75 mg Tolerability of the drug can be improved if taken with food.
Adults, adolescents or children who cannot swallow a capsule may also be treated with Tamiflu ® powder for oral suspension.
In cases where Tamiflu ® powder for oral suspension is not available, or if there are signs of aging of the capsules (for example, increased fragility or other physical disorders), it is necessary to open the capsule and empty its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food item (normal sugar or no sugar chocolate syrup, honey, light brown sugar or table sugar dissolved in water, sweet dessert, sweetened condensed milk, applesauce or yogurt) to to hide the bitter taste. The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation. Detailed recommendations are given in Section Extemporaneous preparation of a suspension of Tamiflu ® .
The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation. Detailed recommendations are given in Section Extemporaneous preparation of a suspension of Tamiflu ® .
Standard dosing regimen
Treatment. The drug should be started no later than 2 days after the onset of symptoms of the disease.
Adults and adolescents ≥12 years of age. 75 mg twice a day for 5 days. Increasing the dose of more than 150 mg / day does not increase the effect.
Children weighing >40 kg or 8 to 12 years of age. Children who can swallow capsules can also be treated with 1 caps. 75 mg 2 times a day for 5 days.
Children from 1 to 8 years of age. Recommended Tamiflu ® powder for oral suspension 12 mg/ml or capsules 30 and 45 mg (for children over 2 years of age). To determine the recommended dosing regimen, see the instructions for medical use of Tamiflu ® powder for oral suspension 12 mg/ml or capsules 30 and 45 mg.
Prevention. The drug should be started no later than 2 days after contact with patients.
Adults and adolescents ≥12 years of age. 75 mg once a day by mouth for at least 10 days after contact with the patient. During a seasonal influenza epidemic - 75 mg 1 time per day for 6 weeks. The prophylactic effect lasts as long as the drug is taken.
Children weighing >40 kg or 8 to 12 years of age. Children who can swallow the capsules can also receive prophylactic therapy by taking 1 caps. 75 mg 1 time per day.
Children from 1 to 8 years of age. Recommended Tamiflu ® powder for oral suspension 12 mg/ml or capsules 30 and 45 mg. To determine the recommended dosing regimen, see the instructions for medical use of Tamiflu ® Powder for Oral Suspension 12 mg/ml or caps. 30 and 45 mg. Possible extemporaneous suspension using 75 mg capsules (see Extemporaneous suspension of Tamiflu ® ).
Dosing in special cases
Patients with kidney damage, treatment. Patients with Cl creatinine> 60 ml / min dose adjustment is not required. In patients with Cl creatinine from 30 to 60 ml / min, the dose of Tamiflu ® should be reduced to 30 mg 1 time per day for 5 days.
For patients on chronic hemodialysis, Tamiflu® at the initial dose of 30 mg may be taken prior to dialysis if influenza symptoms occur within 48 hours between dialysis sessions. To maintain plasma concentrations at therapeutic levels Tamiflu ® should be taken at 30 mg after each dialysis session. For patients on peritoneal dialysis, Tamiflu ® should be taken at an initial dose of 30 mg prior to dialysis, then 30 mg every 5 days (see also Dosage in Special Cases and 'Special Instructions').
The pharmacokinetics of oseltamivir in patients with end-stage renal disease (Cl creatinine ?10 ml/min) not on dialysis has not been studied. In this regard, there are no recommendations for dosing in this group of patients.
Patients with kidney damage, prophylaxis. Patients with Cl creatinine> 60 ml / min dose adjustment is not required. In patients with Cl creatinine from 30 to 60 ml / min, the dose of Tamiflu ® should be reduced to 30 mg 1 time per day. For patients on permanent hemodialysis, Tamiflu ® at the initial dose of 30 mg can be taken prior to dialysis (1st session). To maintain plasma concentrations at therapeutic levels, Tamiflu ® should be taken at 30 mg after each successive odd dialysis session. Patients on peritoneal dialysis, Tamiflu ® should be taken at an initial dose of 30 mg prior to dialysis, then 30 mg every 7 days (see also Dosing in Special Cases and 'Special Instructions'). The pharmacokinetics of oseltamivir in patients with end-stage renal disease (with Cl creatinine? 10 ml / min) who are not on dialysis have not been studied. In this regard, there are no recommendations for dosing in this group of patients.
Patients with liver damage. Dose adjustment in the treatment and prevention of influenza in patients with mild to moderate hepatic impairment is not required. Safety and pharmacokinetics of Tamiflu ® has not been studied in patients with severe hepatic impairment.
Elderly and senile patients. Dose adjustment is not required for the prevention or treatment of influenza.
Immunocompromised patients (after transplantation). For seasonal influenza prophylaxis in immunocompromised patients ≥1 year of age for 12 weeks, dose adjustment is not required (see Dosage and Administration).
Children. Tamiflu ® in this formulation should not be given to children under 1 year of age.
Extemporaneous formulation of suspension Tamiflu ®
In cases where adults, adolescents and children have a problem swallowing capsules, and Tamiflu ® in powder form for oral suspension is absent or there are signs of aging capsules (e. g. increased fragility or other physical disorders), it is necessary to open the capsule and pour its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food (see above) in order to mask the bitter taste. The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation.
g. increased fragility or other physical disorders), it is necessary to open the capsule and pour its contents into a small amount (maximum 1 teaspoon) of a suitable sweetened food (see above) in order to mask the bitter taste. The mixture must be thoroughly mixed and given to the patient as a whole. The mixture should be swallowed immediately after preparation.
If patients require a dose of 75 mg, the following instructions should be followed:
1. Holding 1 caps. 75 mg Tamiflu ® over a small container, carefully open the capsule and pour the powder into the container.
2. Add a small amount (no more than 1 teaspoon) of a suitable sweetened food (to cover the bitter taste) and mix well.
3. Stir the mixture thoroughly and drink immediately after preparation. If a small amount of the mixture remains in the container, then rinse the container with a small amount of water and drink the remaining mixture.
If patients require doses of 30–60 mg, the following instructions should be followed for correct dosing:
1. Holding 1 caps. 75 mg Tamiflu ® over a small container, carefully open the capsule and pour the powder into the container.
Holding 1 caps. 75 mg Tamiflu ® over a small container, carefully open the capsule and pour the powder into the container.
2. Add 5 ml of water to the powder using a syringe labeled to indicate the amount of liquid drawn. Mix thoroughly for 2 minutes.
3. Draw up the required amount of mixture from the container according to the table below into the syringe.
| Body weight, kg | Recommended dose, mg | Amount of Tamiflu ® mixture per 1 reception, ml |
| ?15 | 30 | 2 |
| >15–23 | 45 | 3 |
| >23–40 | 60 | 4 |
There is no need to collect undissolved white powder as it is an inactive excipient. By pressing the plunger of the syringe, inject all its contents into the second container. The remaining unused mixture must be discarded.