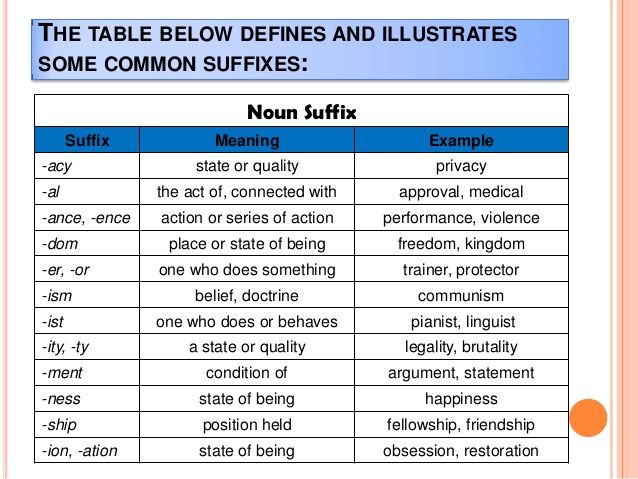
Gbs positive meaning
Group B Strep and Pregnancy (for Parents)
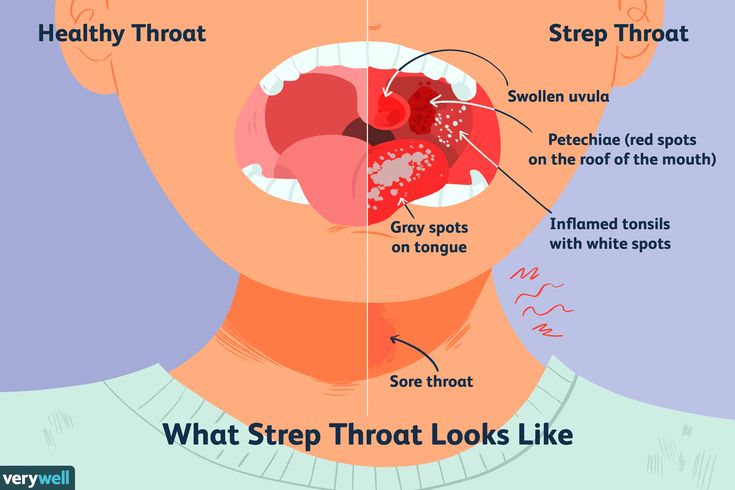
What Is Group B Strep?
Group B Streptococcus (group B strep, GBS) is a type of
bacteriaoften found in the urinary tract, digestive system, and reproductive tracts. The bacteria come and go from our bodies, so most people who have it don't know that they do. GBS usually doesn't cause health problems.
What Problems Can Group B Strep Cause?
Health problems from GBS are not common. But it can cause illness in some people, such as the elderly and those with some medical conditions. GBS can cause infections in such areas of the body as the blood, lungs, skin, or bones.
About 1 out of every 4 women have GBS. In pregnant women, GBS can cause infection of the urinary tract, placenta, womb, and amniotic fluid.
Even if they haven't had any symptoms of infection, pregnant women can pass the infection to their babies during labor and delivery.
How Does Group B Strep Affect Babies?
When women with GBS are treated with antibiotics during labor, most of their babies do not have any problems. But some babies can become very sick from GBS. Premature babies are more likely to be infected with GBS than full-term babies because their bodies and immune systems are less developed.
The two types of GBS disease in babies are:
- Early-onset infections, which happen during the first week of life. Babies often have symptoms within 24 hours of birth.
- Late-onset infections, which develop weeks to months after birth. This type of GBS disease is not well understood.
What Are the Signs & Symptoms of GBS Disease?
Newborns and infants with GBS disease might show these signs:
- a fever
- feeding problems
- breathing problems
- irritability or fussiness
- inactivity or limpness
- trouble keeping a healthy body temperature
Babies with GBS disease can develop serious problems, such as:
- pneumonia
- sepsis
- meningitis (infection of the fluid and lining around the brain).
 Meningitis is more common with late-onset GBS disease and, in some cases, can lead to hearing loss, vision loss, learning disabilities, seizures, and even death.
Meningitis is more common with late-onset GBS disease and, in some cases, can lead to hearing loss, vision loss, learning disabilities, seizures, and even death.
How Is Group B Strep Diagnosed?
Pregnant women are routinely tested for GBS late in the pregnancy, usually between weeks 35 and 37. The test is simple, inexpensive, and painless. Called a culture, it involves using a large cotton swab to collect samples from the vagina and rectum. These samples are tested in a lab to check for GBS. The results are usually available in 1 to 3 days.
If a test finds GBS, the woman is said to be "GBS-positive." This means only that she has the bacteria in her body — not that she or her baby will become sick from it.
GBS infection in babies is diagnosed by testing a sample of blood or spinal fluid. But not all babies born to GBS-positive mothers need testing. Most healthy babies are simply watched to see if they have signs of infection.
How Is Group B Strep Treated?
Doctors will test a pregnant woman to see if she has GBS. If she does, she will get intravenous (IV) antibiotics during labor to kill the bacteria. Doctors usually use penicillin, but can give other medicines if a woman is allergic to it.
If she does, she will get intravenous (IV) antibiotics during labor to kill the bacteria. Doctors usually use penicillin, but can give other medicines if a woman is allergic to it.
It's best for a woman to get antibiotics for at least 4 hours before delivery. This simple step greatly helps to prevent the spread of GBS to the baby.
Doctors also might give antibiotics during labor to a pregnant woman if she:
- goes into labor prematurely, before being tested for GBS
- hasn't been tested for GBS and her water breaks 18 or more hours before delivery
- hasn't been tested for GBS and has a fever during labor
- had a GBS bladder infection during the pregnancy
- had a baby before with GBS disease
Giving antibiotics during labor helps to prevent early-onset GBS disease only. The cause of late-onset disease isn't known, so no method has yet been found to prevent it. Researchers are working to develop a vaccine to prevent GBS infection.
Babies who get GBS disease are treated with antibiotics. These are started as soon as possible to help prevent problems. These babies also may need other treatments, like breathing help and IV fluids.
How Can I Help Prevent Group B Strep Infection?
Because GBS comes and goes from the body, a woman should be tested for it during each pregnancy. Women who are GBS-positive and get antibiotics at the right time during labor do well, and most don't pass the infection to their babies.
If you are GBS-positive and begin to go into labor, go to the hospital rather than laboring at home. By getting IV antibiotics for at least 4 hours before delivery, you can help protect your baby against early-onset GBS disease.
Reviewed by: Thinh Phu Nguyen, MD
Date reviewed: July 2022
Group B Strep In Pregnancy: Test, Risks & Treatment
Overview
What is group B strep?
Group B strep infection (also GBS or group B Streptococcus) is caused by bacteria typically found in a person's vagina or rectal area.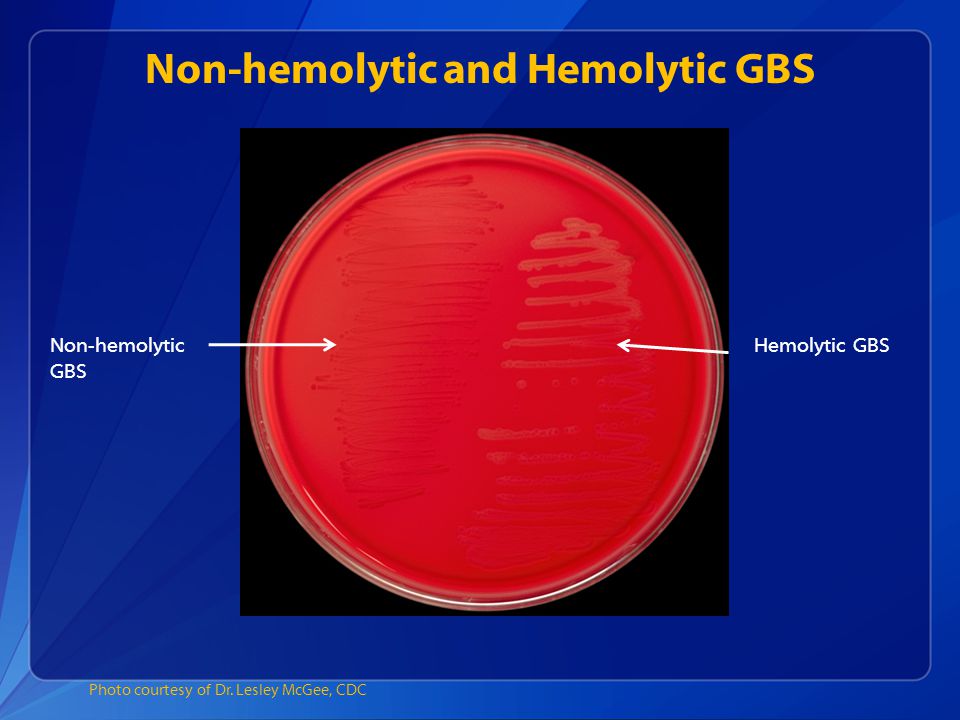 About 25% of pregnant people have GBS, but don't know it because it doesn't cause symptoms. A pregnant person with GBS can pass the bacteria to their baby during vaginal delivery. Infants, older adults or people with a weakened or underdeveloped immune system are more likely to develop complications from group B strep.
About 25% of pregnant people have GBS, but don't know it because it doesn't cause symptoms. A pregnant person with GBS can pass the bacteria to their baby during vaginal delivery. Infants, older adults or people with a weakened or underdeveloped immune system are more likely to develop complications from group B strep.
Most newborns who get GBS don't become sick. However, the bacteria can cause severe and even life-threatening infections in a small percentage of newborns. Healthcare providers screen for group B strep as part of your routine prenatal care. If you test positive, your provider will treat you with antibiotics.
How do you get group B strep?
GBS bacteria naturally occur in areas of your body like your intestines and genital and urinary tracts. Adults can't get it from person-to-person contact or from sharing food or drinks with an infected person. Experts aren't entirely sure why the bacteria spreads, but they know that it’s potentially harmful in babies and people with weakened immune systems.
When do you get tested for group B strep?
The Centers for Disease Control and Prevention (CDC) recommends routine screening for group B strep in all pregnancies. You're screened for GBS between 36 and 37 weeks of pregnancy. Group B strep testing involves your provider taking a swab of your vagina and rectum and then sending it to a lab for analysis.
Can Group B strep affect a developing fetus?
GBS doesn't affect the fetus baby while it's still inside your uterus. However, your baby can get GBS from you during labor and delivery. Taking antibiotics for GBS reduces your chances of passing it to your baby.
How does a baby get GBS?
There are two main types of Group B strep in babies:
- Early-onset infection: Most (75%) babies with GBS become infected in the first week of life. GBS infection is usually apparent within a few hours after birth. Premature babies face greater risk if they become infected, but most babies who get GBS are full-term.
- Late-onset infection: GBS infection can also occur in infants one week to three months after birth. Late-onset infection is less common and is less likely to result in a baby's death than early-onset infection.
How common is GBS?
Group B strep screening during pregnancy has decreased the number of cases. According to the CDC, about 930 babies get early-onset GBS, and 1,050 get late-onset GBS. About 4% of babies who develop GBS will die from it.
Symptoms and Causes
What are the symptoms of group B strep?
Most adults don't experience symptoms of group B strep. It can cause symptoms in older people or people with certain medical conditions, but this is rare. These symptoms include:
- Fever, chills and fatigue.
- Difficulty breathing.
- Chest pains.
- Muscle stiffness.
Newborns with GBS have symptoms like:
- Fever.
- Difficulty feeding.
- Irritability.
- Breathing difficulties.
- Lack of energy.
These symptoms can become serious quickly because newborns lack immunity. Group B strep infection can lead to severe problems like sepsis, pneumonia and meningitis in infants.
Can I pass group B strep to my partner?
Other people that live with you, including other children, aren't at risk of getting sick.
Diagnosis and Tests
Will I be tested for group B strep?
Yes, your healthcare provider will test you for GBS late in your pregnancy, around weeks 36 to 37.
Your obstetrician uses a cotton swab to obtain samples of cells from your vagina and rectum. This test doesn't hurt and takes less than a minute. Then, the sample is sent to a lab where it's analyzed for group B strep. Most people receive their results within 48 hours. A positive culture result means you're a GBS carrier, but it doesn't mean that you or your baby will become sick.
If you’re using a midwife, you might be given instructions on how to test yourself at home and submit the swab to a lab.
Is Group B strep an STI?
No, group B strep isn't an STI (sexually transmitted infection). The type of bacteria that causes GBS naturally lives in your vagina or rectum. It doesn't cause symptoms for most people.
Management and Treatment
What happens if you test positive for group B strep during pregnancy?
Healthcare providers prevent GBS infection in your baby by treating you with intravenous (IV) antibiotics during labor and delivery. The most common antibiotic to treat group B strep is penicillin or ampicillin. Giving you an antibiotic at this time helps prevent the spread of GBS from you to your newborn. It's not effective to treat GBS earlier than at delivery. The antibiotics work best when given at least four hours before delivery. About 90% of infections are prevented with this type of treatment.
One exception to the timing of treatment is when GBS is detected in urine. When this is the case, oral antibiotic treatment begins when GBS is identified (regardless of the stage of pregnancy). Antibiotics should still be given through an IV during labor.
Antibiotics should still be given through an IV during labor.
Any pregnant person who has previously given birth to a baby who developed a GBS infection or who has had a urinary tract infection in this pregnancy caused by GBS will also be treated during labor.
How is group B strep treated in newborns?
Some babies still get GBS infections despite testing and antibiotic treatment during labor. Healthcare providers might take a sample of the baby's blood or spinal fluid to see if the baby has GBS infection. If your baby has GBS, they're treated with antibiotics through an IV.
Prevention
How can I reduce my risk of group B strep?
Anyone can get GBS. Some people are at higher risk due to certain medical conditions or age. The following factors increase your risk for having a baby born with group B strep:
- You tested positive for GBS.
- You develop a fever during labor.
- More than 18 hours pass between your water breaking and your baby being born.

- You have medical conditions like diabetes, heart disease or cancer.
Getting screened for GBS and taking antibiotics (if you're positive) is the best way to protect your baby from infection.
Outlook / Prognosis
What are long term problems of Group B strep in newborns?
Infants with GBS can develop meningitis, pneumonia or sepsis. These illnesses can be life-threatening. Most infants don't develop any long-term issues; however, about 25% of babies with meningitis caused by GBS develop cerebral palsy, hearing problems, learning disabilities or seizures.
Can I get tested for group B strep again?
No, once you test positive for GBS, you're considered positive for the rest of your pregnancy. You will not get re-tested.
Do I need treated for group B strep if I am having a c-section?
No, you don't need antibiotics if you're having a c-section delivery. However, you'll still be tested for GBS because labor could start before your scheduled c-section. If your water breaks and you're GBS positive, your baby is at risk of contracting the disease.
If your water breaks and you're GBS positive, your baby is at risk of contracting the disease.
Living With
When should I see my healthcare provider if I’m positive for group B strep?
In some cases, GBS causes infections during pregnancy. Symptoms of infection include fever, pain and increased heart rate. Let your provider know if you have any of those symptoms as it could lead to preterm labor.
GBS can also cause urinary tract infection (UTI), which requires oral antibiotics.
Talk to your provider about what you can expect during labor and delivery if you have group B strep.
A note from Cleveland Clinic
Try not to panic if your healthcare provider tells you you're positive for group B strep during pregnancy. It's caused by bacteria that occur naturally in your body, not by anything you did wrong. The chances of you passing group B strep to your baby are quite low, especially if you take antibiotics during labor. Talk to your provider about group B strep and share any concerns you have.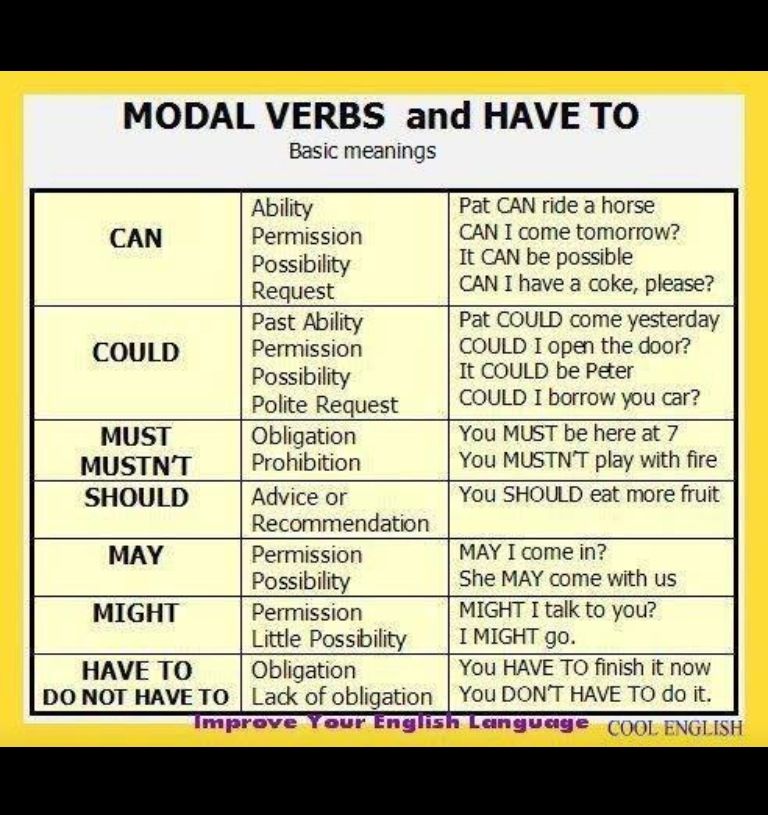 In most cases, testing positive for GBS causes no problems, and your baby is healthy.
In most cases, testing positive for GBS causes no problems, and your baby is healthy.
Inventory card | Knowledge Base | GBS.Market
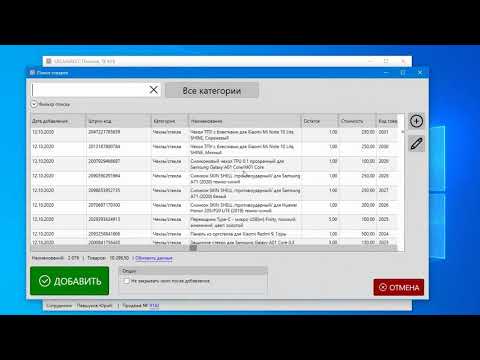
Inventory is the process of recalculating inventory balances. In the process of recounting, surpluses, shortages or re-sorting of goods that are in the warehouse of the outlet are revealed.
In GBS.Market, inventory is carried out in the inventory card. You can open it:
- From the main form menu: Documents - New Inventory
- From the inventory log: “Add” button
Inventory card appearance
Inventory card consists of three sections
- Inventory parameters page
- Inventory process page
Page "Inventory parameters"
The page with inventory parameters looks as shown in the screenshot below. nine0005 Inventory parameters
The following parameters are available for setting:
- Product categories
- Warehouse
- “Load products with inventory less than or equal to zero” option
Categories
for which the balance will be recalculated.
Selection of categories for inventory
In the selection list of categories, mark the necessary ones. nine0005
Inventory can only be carried out for the balance of goods in the same warehouse. You must select the warehouse for which the revision will take place.
If you didn't create additional warehouses, there will be only one.
Load goods with stocks less than or equal to zero
that have a zero or negative remainder.
If the option is disabled, only products with a positive balance will be loaded into the list. nine0005
Click the "Next" button to go to the list of items to be inventoried.
Take Inventory Page
The Take Inventory page displays a list of items, control buttons, a search box, and other controls. The page looks like the screenshot below.
Take Inventory PageForm Menu
The following options are available on the Form Menu:
- File
- Save as .
 .. - allows you to save the contents of the list in Excel/CSV Format
.. - allows you to save the contents of the list in Excel/CSV Format
- Save as .
In the case of the integration with Barcode Harvester, points will be available:
- 9000
- 9000 9000 9000 9000 9000
"Search/Barcode" block
In the "Search/Barcode" panel there is a field for searching and parameters of program behavior. Click on the “settings” button to show more options. nine0005
"Search/barcode" field
The "search/barcode" field allows you to change the quantity of goods on the balance using a barcode or filter the list by the name/part of the barcode.
If the barcode of the item is scanned in the field, the value of “balance after the fact” will be increased by 1 pc.
If a weight barcode is scanned, the value of the field “remainder after the fact” will be increased by the amount indicated in the barcode.
If a part of a product name or a part of a barcode is entered in the field, then the list of products will be filtered, the values of the “remaining after the fact” fields will not be changed for any product.
 nine0005
nine0005 When scanning the barcode, request the quantity
If this option is enabled, then when scanning a barcode, instead of increasing the value of “residual balance”, a window will be displayed for entering the quantity of goods found by matching the barcode.
Entering the value "remaining after the fact"In the window that appears, you must set the quantity of goods that is in stock.
For identical barcodes, fix the number for the first match
If the option is enabled, and more than one match is found by the barcode, the program will increase (or request) the actual balance for the first entry in the list. nine0005
If the option is disabled, then the field “remaining after the fact” will not be changed, and the list of goods will show those items that were found.
Do not show notifications
If this option is enabled, then notifications about balance changes will not be shown.
If the option is disabled, the program will notify you about the successful change in the value of “actual balance”.
Notification appearance
List of goods
An example of a list of goods for which inventory is performed is shown in the screenshot below. nine0005 List of goods in the inventory window
in the list are a standard set of fields:
- barcode
- Category of goods
- Name of goods
- 9000 quantity of goods entered during the inventory
- Balance in the database is the number of goods that should be in stock at the current time according to information from the database
- The difference is the difference between the actual balance and the base balance. If the value is negative, there is a shortage of goods. If positive - excess.
The value of the field “Balance after the fact” can be set in inline editing mode directly in the table.
Edit
Select one or more items to change their actual balance value.
Enter balance for multiple items
You can also double-click on an item in the list to display the quantity change form. nine0005
Delete
Select one or more items in the list to delete them.
It is important to understand that the selected items will only be removed from the inventory list and not from the item catalog. Those. at the end of the inventory, the balances of these products will NOT be adjusted, while the products will remain in the catalog.
Total row
The total row displays the total values for the positions in the list:
- total retail value of goods in fact and in the database
- number of items
- balance in fact and in the database
When you click on the “Update balance from database” button, the value of the “base balance” field will be updated. This can be useful when the balance value for items has changed during the inventory process.
Completion of the inventory
When you click on the “Next” button on the inventory page, the inventory completion window will open.
 nine0005 Inventory completion window
nine0005 Inventory completion window Comment
In the comment field, you can enter some note about the ongoing inventory.
Finish
When you click on the “Finish” button, the inventory document will be saved to the database. For goods that were in the list for recalculation of balances, the balances will be adjusted according to the value of the field “balance after the fact”.
Important If for some goods you have not changed the value of the field "balance after the fact", then their balances in the database will be set to zero. Before saving the inventory, make sure that you have correctly entered the value of the "remaining after the fact" field. nine0005
Inventory deletion will be required to restore zero balances.
You can view the balance of goods in the catalog of goods, and the fact of the inventory will be displayed in the journal in the product card.
Suspend
If you select Suspend, the physical inventory document will be saved with the status Draft.
The balance of goods for which recalculation is taking place will not be changed. An inventory with this status can be continued from the inventory log. nine0005
Cancel
The inventory completion form will be closed so that you can continue working with the current list of items. The inventory itself will not be canceled when this button is pressed.
Try GBS.Market for free
GBS.Market is a convenient and affordable cash register program. Suitable for retail store and cafe. 30 days free!
Download
Group B streptococcal infections in the neonatal period
_Title Group B streptococcal infections in the neonatal period
_Author
_KeywordsStreptococcus agalactae (Group B-streptococci, GBS), even in industrialized countries, continues to be the most common cause of neonatal sepsis. The frequency of perinatal GBS infections increased dramatically by the early 1970s.
 From three independent papers in the April 1973 issue of the Journal of Pediatrics, GBS was the most common cause of neonatal sepsis in the United States. During this time, almost half of the newborns affected by GBS infection died. Even today, neonatal sepsis - despite all the progress made in perinatal medicine - is associated with a very significant morbidity, and even mortality. nine0005
From three independent papers in the April 1973 issue of the Journal of Pediatrics, GBS was the most common cause of neonatal sepsis in the United States. During this time, almost half of the newborns affected by GBS infection died. Even today, neonatal sepsis - despite all the progress made in perinatal medicine - is associated with a very significant morbidity, and even mortality. nine0005 Not only the sudden “emergence” of pathogens in the 1970s, but some other phenomena that accompany neonatal sepsis are still unclear or have become slightly better understood in recent years:
GBS accounts for almost half of the blood culture isolates isolated during the first days of life in newborns with sepsis.
The causative agent quite obviously comes from the birth canal of the mother. Why exactly GBS passes to newborns with such frequency is still unclear. The maternal genital tract is colonized by a variety of other similar or more pathogenic bacteria without being able to play such an epidemiological or similar role. Interestingly, the observation that invasive infection develops only in the proportion of newborns that are postnatally colonized by GBS. nine0005
Interestingly, the observation that invasive infection develops only in the proportion of newborns that are postnatally colonized by GBS. nine0005 At least 10% of pregnant women in Germany are colonized with GBS. Almost half of the children of these mothers will also be colonized, of whom only a small proportion will actually become ill. With a maternal GBS colonization rate of almost 10%, and a transmission rate of almost 50%, the risk of disease in the newborn is about 0.2%. At the same time, such risk factors as prematurity, premature rupture of membranes, population density of microorganisms, etc. play their role; In addition, the magnitude of specific maternal antibodies should also be of particular importance. nine0005
Another phenomenon that has only been somewhat better studied in recent years is the extremely strong activation of the immune system in the context of GBS infection. Clinical effusion (lightning) of GBS sepsis correlates with excessively elevated concentrations of pro-inflammatory cytokine in the blood of the umbilical cord.
 The immunological basis for this has been intensively studied in recent years. Below we present some of this new knowledge, as well as current aspects regarding the clinic, epidemiology, therapy and prevention. nine0005
The immunological basis for this has been intensively studied in recent years. Below we present some of this new knowledge, as well as current aspects regarding the clinic, epidemiology, therapy and prevention. nine0005 Clinical picture
The clinical spectrum of systemic GBS infections in the perinatal period is quite wide and ranges from septic abortion to transient bacteremia with a mild clinical course. It is not always possible to identify any features in the anamnesis of pregnancy and childbirth. Even full-term newborns, without any indication of maternal fever or amnion infection syndrome, may develop a severe septic presentation within a few hours of life. nine0005
Traditionally two forms of neonatal sepsis are distinguished :
- Early sepsis (early onset) and
- Late sepsis (late onset)
The late form includes septicemia, which began from the 2nd week of life until the end of the 3rd month of life.
 More than 80% of all cases of early sepsis caused by GBS appear in the first 24 hours of life.
More than 80% of all cases of early sepsis caused by GBS appear in the first 24 hours of life. As with other pathogens, early signs of sepsis include respiratory disorders (apnea, moaning, tachy- and dyspnea) and impaired skin perfusion (grayish pallor, marbling), as well as tachycardia and arterial hypotension. These clinical signs of sepsis are, however, non-specific, and the classic blood acute phase reactions are delayed, so that the diagnosis is often made rather late. Respiratory failure (Atemnotsyndrome) and septic shock often necessitate intubation, artificial respiration and circulatory therapy. Sometimes the course is fulminant up to multiple organ failure (multiple organ failure).
The more immature the newborn, the more often the GBS infection proceeds as severe sepsis. Term neonates are more likely to develop pneumonia with sometimes severe respiratory failure up to persistent pulmonary hypertension of the newborn (PPHN).
The late form of GBS infections often occurs in the form of meningitis.
 Often a brief period of fever, thirst, restlessness, and sensitivity to touch is misinterpreted. Later, a full picture of meningitis develops with fontanelle tension, fever, lethargy up to coma, and tonic-clonic convulsions. The course is often less frustrating than in early sepsis, but neurologic residual damage is extremely common. nine0005
Often a brief period of fever, thirst, restlessness, and sensitivity to touch is misinterpreted. Later, a full picture of meningitis develops with fontanelle tension, fever, lethargy up to coma, and tonic-clonic convulsions. The course is often less frustrating than in early sepsis, but neurologic residual damage is extremely common. nine0005 Epidemiology
It is well known that in 10-30% of pregnant women the gastrointestinal tract and genital tract are colonized with GBS. However, it is still unclear when in what period of childhood and adolescence the colonization of the gastrointestinal tract begins and whether colonization has sex-specific dynamics. It has recently been shown that the occupancy rate of healthy male co-students in the US was almost the same as that of healthy non-pregnant female students (31% vs. 34%). After vaginal delivery, almost half of the children of colonized mothers also colonize the skin and mucous membranes with GBS, the risk of disease in full-term children is about 0.
 2%. Based on a cohort of 800,000 children per year in Germany, an established rate of colonization of pregnant women of 10%, and a risk of colonization of children of 0.2%, and on the other hand, from an established incidence of blood-positive cases of sepsis of 0.5/1000, one can expect the development of 400 up to 800 cases of sepsis in Germany. nine0005
2%. Based on a cohort of 800,000 children per year in Germany, an established rate of colonization of pregnant women of 10%, and a risk of colonization of children of 0.2%, and on the other hand, from an established incidence of blood-positive cases of sepsis of 0.5/1000, one can expect the development of 400 up to 800 cases of sepsis in Germany. nine0005 ESPED study
On April 1, 2001, a two-year ESPED (German Rare Disease Detection) study was launched in Germany with federal coverage of invasive infections caused by GBS. The aim of the study was to obtain data on the incidence of invasive GBS in Germany, infections of newborns and infants up to 3 months of age, identification of risk factors, clinical course, as well as phenotypic and genotypic characteristics of the isolates. At the ESPED central office, positive results of isolations on cultures from blood and cerebrospinal fluid of cases of GBS disease were recorded monthly. In parallel with the report to ESPED, data from German microbiological laboratories on the release of GBS in blood and cerebrospinal fluid cultures were sent monthly to the Koch-Institut.
 nine0005
nine0005 For the first year of observation from April 1, 2001 to March 31, 2002, only the results of ESPED detection were available, so it was not possible to obtain an accurate frequency calculation using the Capture-recapture method. The actual frequency could be almost twice as high as the number of reports made to ESPED, which was 231 in 1 year of follow-up. without isolation of GBS, 4 reports with incorrect dates) the total number was 161. GBS was detected in 122 cases on blood culture, in 10 cases in CSF and in 2 cases from punctures. In 30 other cases, the microorganism was isolated from both blood culture and CSF culture, 2/3 of the reported cases were newborns with early onset of sepsis, 34% of children fell ill at the age of 7 days to 3 months postnatally (late onset), in 62% of cases were full-term newborns, 30% were premature, in the remaining cases (8%) there was no data on gestational age. In 73% of cases, a complete cure occurred, and about 6% died. nine0005
Using the epidemiological "capture-recapture" method, after receiving all data from ESPED and RKI over a two-year period, it will be possible to draw reliable conclusions about the frequency of invasive GBS infections in Germany.

Based on single blood culture-based recording of episodes, the incidence of GBS associated sepsis is largely underestimated.
It should be many times higher when taking into account a large number of blood-negative, so-called clinical cases of sepsis. The number of these clinical cases of sepsis must therefore be taken into account additionally in some smaller Standorten (Strepss-Net) in order to make it possible to recalculate the actual frequency in Germany. nine0005
Bacteriology
Exact bacteriological species name Streptococcus agalactiae ; in clinical speech included the concept of group B streptococci. We are talking about facultative gram-positive cocci arranged in a chain. On sheep blood agar they show a characteristic growth as flat gray colonies surrounded by a narrow b-hemolytic yard with a somewhat indistinct margin. It is very rare to find serogroup B streptococci that lack the characteristic b-hemolytic appearance, but without this having any effect on virulence.
 nine0268 *-
nine0268 *-
Streptococcus agalactiae has - like other hemolyzing streptococci - a well-established carbohydrate antigen, strongly associated with the cell wall and defining group affiliation (group B). In addition, there is a capsule of type-specific carbohydrates, which are cavalently bound to the peptidoglycan of the cell wall and determine belonging to certain serotypes. The most common serotypes are Ia, Ib, II, III, IV, V, VI and VIII. However, there are a number of untyped strains. In the ESPED-RKI study, almost 2/3 of the isolates belong to serotype III. In second place in terms of frequency of occurrence are serotypes Ia and V, and the latter in the United States has already surpassed serotype III in frequency. In Germany, serotype V did not play any role at all until recently, but it is becoming increasingly important. nine0005While polysaccharide antigens are unequivocally identified as virulence factors, the pathogenetic significance of GBS protein antigens remains less understood.
 Proteins that have been attributed pathogenetic significance include the protein complex of the so-called C-protein, which are involved in resistance against opsonization and intracellular death. In particular, the C-protein b-antigen, which has the ability to bind to the Fc portion of human IgA (as well as IgA1 and IgA2), suggests a specific association with pathogenicity. nine0005
Proteins that have been attributed pathogenetic significance include the protein complex of the so-called C-protein, which are involved in resistance against opsonization and intracellular death. In particular, the C-protein b-antigen, which has the ability to bind to the Fc portion of human IgA (as well as IgA1 and IgA2), suggests a specific association with pathogenicity. nine0005 By binding human IgA to the bacterial cell surface, GBS is able to bind opsonizing antibodies or mask other antigens on the cell surface and inhibit phagocytosis. Along with capsular polysaccharyls and C-protein, other virulence factors are also associated with the pathogenesis of GBS infections. These include the CAMP factor, hemolysin, and a recently identified protein (laminin binding protein, Imb) that mediates GBS binding to laminin, the main constituent of the basement membrane. It is hypothesized that the damage caused by b-hemolysin to the lung epithelium leads to the exposure of the basement membrane and, through Imp-mediated attachment, allows the invasion of bacteria into the circulation.
 nine0005
nine0005 Pathogenesis
Conditions of infection
The polysaccharide capsule, which determines the serotype and protects against phagocytosis and complement attack, is one of the most important signs of the pathogenicity of the pathogen. Maternal specific antibodies directed against the capsule's polysaccharide antigen act opsonically. It can be shown that peak maternal antibody levels are strongly correlated with neonatal protection from invasive infection.This means that the absence of transplacental transmission of maternal serotype-specific antibodies is a decisive precondition for infection in the newborn. nine0005
Significant transfer of immunoglobulins across the placenta is noted only in the 3rd trimester of pregnancy, so that extremely preterm infants a priori receive a very small amount of specific antibodies transplacentally.
In addition, it is certain that not all pregnant women produce a sufficient amount of specific antibodies against the capsular antigens of the pathogen.
 - despite the existing massive rectovaginal colonization. It is possible that this is based on a partial maternal deficiency, to form T-cell-independent polysaccharide-specific antibodies. In the absence of correspondingly high titers of antibodies, direct opsonization of the pathogen after invasion is impossible. The pool of neutrophilic granulocytes, respectively, is not able to quickly and completely eliminate pathogens. nine0005
- despite the existing massive rectovaginal colonization. It is possible that this is based on a partial maternal deficiency, to form T-cell-independent polysaccharide-specific antibodies. In the absence of correspondingly high titers of antibodies, direct opsonization of the pathogen after invasion is impossible. The pool of neutrophilic granulocytes, respectively, is not able to quickly and completely eliminate pathogens. nine0005 Recruitment and rapid consumption of granulocytes without elimination of the pathogen eventually leads to the depletion of granulocyte reserves and, at the same time, as a consequence of the neutropenia often observed in the context of GBS infection. The monocyte-macrophage system, whose immunological competence is retained in sufficient volume, even leads to the maximum activation of the inflammatory reaction, which is clinically manifested by the sepsis syndrome, while sufficient elimination of the pathogen, however, cannot be achieved without prompt adequate therapy.
 nine0005
nine0005 Immunoactivation induced by GBS
The production of a pro-inflammatory cytokine like TNF-α after stimulation of umbilical cord blood mononuclear cells (CBMNC) by GBS is extremely pronounced, objectively stronger than after stimulation by other pathogens or LPS. GBS induce strong translocation of the nuclear transcription factor NF-kB, increased secretion of TNF-a, and production of nitric oxide (NO). GBS activate macrophages, as part of the innate immune system (innate immunity), through the so-called "pattern recognition receptors (PRR)". These receptors include Toll-like receptors (TLRs), which play a key role in inducing immune and inflammatory responses. The intracellular adapter molecule MyD88, along with other elements, performs a crucial function in TLR-mediated intracellular signal transduction, which again, in turn, enters the pro-inflammatory cytokine response. nine0005
Using the corresponding knockout models, it was possible to demonstrate that immune activation by heat-inactivated whole cell preparation of GBS unequivocally depends on MyD88 and, at the same time, on TLR (Fig.
 7). The responsible TLR, however, has yet to be identified. As an activating principle, GBS is assumed to be an integral part of the gram-positive bacterial cell wall, to which polysaccharides, peptidlycan and lipoteichonic acids belong.
7). The responsible TLR, however, has yet to be identified. As an activating principle, GBS is assumed to be an integral part of the gram-positive bacterial cell wall, to which polysaccharides, peptidlycan and lipoteichonic acids belong. However, none of these components has an immuno-activating potential comparable to LPS. Extremely strong immuno-activation - measured by the production of TNF-a - in contrast, is induced by GBS securable soluble factor (GBS-F). This additional activation of GBS-F is apparently also mediated through various PRRs. It is believed that CD14, TLR2 and TLR6 act as co-receptors for GBS-F. GBS-F appears to be a potent pro-inflammatory toxin. nine0005
Another hypothesis explaining the pronounced immunostimulatory potency of GBS concerns a TLR-independent activation pathway. In an animal model, as well as in cell cultures, following stimulation with GBS-secured proteins, oligoclonal expansion of certain families of T-cells appears, the expression of which is very similar to the state of immunoactivation by superantigen.
Superantigens are characterized by strong activation of CD4+ or CD8+ T cells without being recognized as a specific antigen by T cell receptors. In newborns with GBS sepsis, preliminary results indicate that selective expansion of certain families of T-cell receptors occurs with GBS sepsis. Hence, the clinically manifest toxic-shock-like-syndrome would very well correspond to the frequently observed fulminant neonatal early sepsis. The "cytokine assault" confirmed by many studies could indicate a massive activation of the immune system by the superantigen. nine0005
Immunoactivation by various clinical isolates
As has been shown, one of the characteristics of GBS is the ability in vivo and under experimental conditions to induce high cytokine expression in CBMNC. It is a misunderstood clinical phenomenon that many newborns have their entire skin and mucous membranes colonized by GBS after birth, but remain healthy, while others develop a hyperacute septic pattern.
 Regardless of the risk factors associated with extreme prematurity or severe amnion infection syndrome with systemic hematogenous damage, as well as the height of the level of specific maternal antibodies, there are few points on the part of the pathogen that could convincingly explain the various courses. nine0005
Regardless of the risk factors associated with extreme prematurity or severe amnion infection syndrome with systemic hematogenous damage, as well as the height of the level of specific maternal antibodies, there are few points on the part of the pathogen that could convincingly explain the various courses. nine0005 Comparing clinically and microscopically quite characteristic GBS isolates from healthy colonized and septic neonates in terms of their potential to induce the expression of various pro-inflammatory cytokines, after stimulation with septic isolates, greater cytokine expression is elicited than isolates from conventional swabs. This allows one to interpret in the direction that the amount of immunoactivation - especially of neonatal monocytes or macrophages - represents a strain-specific characteristic. Isolates that cause systemic infection and in vivo cause high secretion of cytokines are also characterized in vitro by a higher immunoactivating potential. Other experimental studies are needed to identify those features of the pathogen that cause this difference.
 nine0005
nine0005 The goal of future vaccination strategies might be to induce protective immunity against only those GBS strains that exhibit virulence factors that lead to the development of a septic picture. It is possible that soluble proteins of the pathogen could become these candidates for the vaccine, naturally creating a higher immunogenicity than that produced by the polysaccharide antigens of the bacterial capsule.
Diagnosis
Evidence for the establishment of GBS infection is the isolation of the pathogen in a culture of blood or cerebrospinal fluid or from other initially sterile body fluids. The fact that in newborns with a clinical picture of sepsis, isolation of the pathogen through a blood culture cannot be achieved is a daily clinical problem. The reasons for the small number of isolates from blood cultures or cerebrospinal fluid in neonatal GBS sepsis are manifold. They consist, among other things, in the fact that bacteremia is not constant and the density of the pathogen in the blood of a newborn is low; in addition, in this group of patients it is possible to obtain a one-time small volume of blood and use it for diagnostics on culture.
 nine0005
nine0005 Last but not least, it prevents the growth of pathogens from the blood of the child and the treatment of antibiotics by the mother before and during childbirth. Sometimes it is possible to grow bacteria from meconium, stomach secretions, or swabs from the ear and umbilical cord. However, this only proves the presence of settlement (colonization). From which it follows that an exceptional indication for the treatment of a newborn occurs only when, in addition to this, the infection is confirmed by clinical symptoms and laboratory parameters. nine0005
Many newborns are colonized with GBS without clinical manifestations.
As a surrogate parameter in recent years in pediatric practice, along with the differential blood picture and CRP, which, however, have a very limited sensitivity, the determination of pro-inflammatory cytokines has justified itself, of which interleukin (IL)-6 and/or IL-8.
It is very rational and desirable to screen all pregnant women in the last trimenone for colonization (colonization) of GBS (see below), as this opens up the possibility of targeted antibacterial prophylaxis during childbirth.
 A combined smear from Introitus vaginae and Anorectum can serve as a material for research. The standard method now, as before, is the detection of GBS in culture, moreover, on special media, which, in a positive case, will change color, which will significantly reduce the time to carry out the test. Enzymatic speed test and PCR method have not shown themselves well enough in practice. nine0005
A combined smear from Introitus vaginae and Anorectum can serve as a material for research. The standard method now, as before, is the detection of GBS in culture, moreover, on special media, which, in a positive case, will change color, which will significantly reduce the time to carry out the test. Enzymatic speed test and PCR method have not shown themselves well enough in practice. nine0005 Therapy
GBS still has good sensitivity to b-lact antibiotics, including penicillin. Sensitivity to penicillin in vitro appears to be almost a factor 10 lower than that of group A streptococci. there is synergy. It is likely that the action of β-lactam on the bacterial cell wall mediates the penetration and, at the same time, the intracellular activity of aminoglycosides. Therefore, a recommendation was made to treat GBS infections with penicillin (300,000 IU/kg KG day) or ampicillin (200 mg/kg KG day) for at least 5 days in combination with an aminoglycoside. nine0005
Combination treatment also aims to destroy tolerant bacteria.
 In meningitis, the dose of b-lactam antibiotics naturally increases, for penicillin up to 500,000 IE/kg KG a day, for ampicillin up to 300 mg/kg KG a day.
In meningitis, the dose of b-lactam antibiotics naturally increases, for penicillin up to 500,000 IE/kg KG a day, for ampicillin up to 300 mg/kg KG a day. The required total duration of antibiotic therapy for GBS sepsis has not been clearly established. The recommended duration of therapy is 7-10 days with a positive blood culture; with the so-called clinical sepsis without isolating the pathogen from the blood, 5-7 days are probably enough, depending on the clinical picture and the severity of the course. Treatment of meningitis should continue for 14 days after the time of establishing the sterility of the CSF. Cephalosporins can be used in justified individual cases, however, they do not offer any advantage over penicillin or ampicillin. It does not seem rational to combine a cephalosporin and penicillin or ampicillin in the treatment of GBS sepsis. nine0005
GBS recurrence may occur even after treatment for invasive GBS infection has been completed, with an estimated incidence of 1%.
It should be assumed that colonization by the same pathogen persists despite parenteral therapy. Molecular biological typing of a pair of isolates, as a rule, confirms this. An attempt to carry out eradication treatment with rifampicin with connection to the final parenteral treatment with effective antibiotics - similarly for infections with meningococcus and Haemophilus influenzae - does not lead, despite the confirmed sensitivity of the pathogen, to the elimination of relapses of the disease. nine0005
This means that antibiotic treatment of asymptomatic colonized neonates to prevent subsequent invasive infection is not rational and therefore not indicated.
Antibiotic resistance
In recent years, GBS - like other strains of streptococci - has rapidly developed resistance to macrolides and the closely related class of lincosamides (clindamycin). Although both groups of substances are not used in the treatment of neonatal sepsis, nevertheless, this observation is not only of purely academic significance.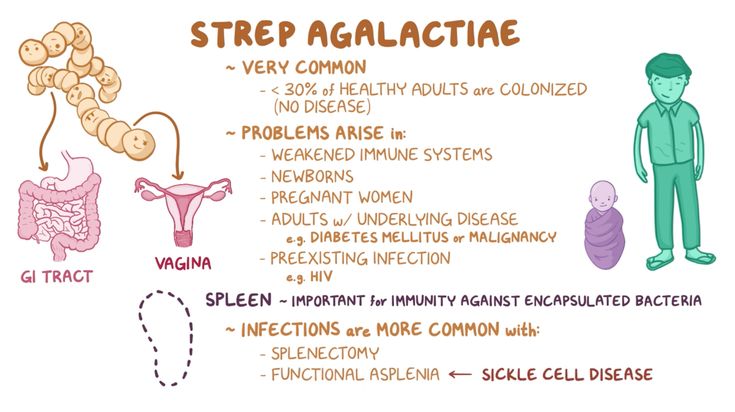 Macrolides and clindamycin are recommended as alternative agents for penicillin intolerance in peripartum prophylaxis. In full agreement with publications from other countries, which indicate a resistance rate of more than 30%, can also be demonstrated on the basis of local epidemiology in Freiburg since 1997 to 1999 doubling the level of resistance to macrolyls and clindamycin.
Macrolides and clindamycin are recommended as alternative agents for penicillin intolerance in peripartum prophylaxis. In full agreement with publications from other countries, which indicate a resistance rate of more than 30%, can also be demonstrated on the basis of local epidemiology in Freiburg since 1997 to 1999 doubling the level of resistance to macrolyls and clindamycin. The rise in resistance has been associated with the rise of serotype V, which has so far been isolated quite rarely in Germany, unlike in the USA and Japan. Using genetic fingerprint analysis, it can be shown that resistant isolates are identical to serotype V, and together demonstrate an increase in resistance due to the spread of individual resistant clones.
With regard to the increasing resistance of pneumococci and A-streptococci, as well as the increasing selection pressure due to outpatient antibiotic therapy and maternal prophylaxis, a further increase in resistance also to GBS should be feared.
 nine0005
nine0005 Prevention and prevention
In the US, the incidence of neonatal early-onset sepsis and meningitis due to GBS has decreased by 67% since the introduction of the CDC national intrapartum chemoprevention guidelines in 1996, from 1.7 to 0 .6/1000 live births. Basically, 2 alternative strategies for intrapartum prevention are used, which were also recommended by the specialist societies and for Germany (AWMF): one of which is "risk-based" and the other is "screen-based" prevention strategies. nine00051. Risk-based strategy
In the "risk-based" strategy, mothers receive antibiotics in labor when one or more of the following are present:
- Previous child with invasive GBS6
- Bacteriuria during pregnancy,
- Threat of preterm labor before completion of 37 weeks,
- High density of GBS bacteria in the mother's urogenital tract at the time of delivery,
- The time interval from rupture of membranes and delivery exceeds 18 hours.

- Peripartum fever >38oC
If GBS is isolated, antibiotic prophylaxis is recommended. Regardless of screening result Antibiotics are given if one of the 3 main risk factors is present :
- Previous child with invasive GBS infection,
- Bacteriuria or
- Threatened preterm birth before 37 weeks gestation.
In the latter case, prophylaxis is not necessary if the culture is negative for GBS between 35 and 37 weeks of gestation.
Antibiotic prophylaxis with penicillin (initially 5 Mega I.E. passing through the placenta) or erythromycin (poorly passing through the placenta). Prophylactic administration of antibiotics even before the onset of straining and/or opening of the membranes in pregnant women with GBS colonization has not shown itself to be effective, since 70% of treated women again colonize GBS by the time of delivery. nine0005
Despite advances in maternal antibiotic prophylaxis, there continues to be a vigorous debate about the best prophylactic method and the intermediate and long-term risks of widespread antibiotic use.
 A recent but retrospective study from the USA was able to show that a "screen-based" prevention approach appears to have clear advantages over a "risk-based" strategy (RR 0.46, 95 % - confidential interval 0.36-0.6). nine0005
A recent but retrospective study from the USA was able to show that a "screen-based" prevention approach appears to have clear advantages over a "risk-based" strategy (RR 0.46, 95 % - confidential interval 0.36-0.6). nine0005 With regard to the basis of the discussion about intrapartum prophylaxis, the following questions seem to be relevant: It has not yet been unambiguously proven whether prophylaxis actually leads to a reduction in the incidence of neonatal GBS sepsis, which is a priori quite probable, or more exclusively reduces the magnitude cases confirmed by positive blood cultures.
This is a known problem as there are many clinical cases of sepsis in which the blood culture remains sterile. Another open question concerns the prevention of GBS-induced preterm labor. In an analysis of a large cohort of 13,646 pregnant women, it was possible to show that high GBS population density at 23-26 weeks of gestation is associated with a high risk of preterm birth. The problem of preterm birth, as well as the incidence of late-onset sepsis, is not addressed by the recommendations for prevention. In addition, concerns have been raised that the widespread use of antibiotics increases the likelihood of developing allergic reactions, antibiotic-induced pseudomembranous colitis, and the problem of developing resistance. In many studies, pre-treatment antibiotic prophylaxis appears to increase the incidence of neonatal sepsis, especially in preterm infants, caused by resistant Escherichia coli. nine0005
In addition, concerns have been raised that the widespread use of antibiotics increases the likelihood of developing allergic reactions, antibiotic-induced pseudomembranous colitis, and the problem of developing resistance. In many studies, pre-treatment antibiotic prophylaxis appears to increase the incidence of neonatal sepsis, especially in preterm infants, caused by resistant Escherichia coli. nine0005 These limitations to the otherwise generally successful prevention of GBS objectively indicate that it would be prudent to consider alternative prevention strategies.
Vaccine development
Due to the high morbidity of GBS sepsis, vaccine development has been moving forward for several years. Studies dating back to the 1970s, as well as modern studies, show that the absence of maternal antibodies against GBS serotype-specific polysaccharide capsular antigens correlates with increased neonatal susceptibility to invasive infections. To lead to a reduction in the risk of disease up to 90%, serum serotype-specific antibodies should be >5 mg/ml (vs.

).Many of the vaccine candidates have already been studied in a number of clinical and preclinical studies, such as Baker et al. antibodies with good opsophagocytosis activity It is problematic that vaccines and their protective effect are serotype-specific Of the nearly 8 GBS serotypes, only a few are responsible for the majority of invasive infections in newborns, while there are significant regional and national differences in serotype distributions.
Although potential future vaccines are expected to be multivalent, it should not be expected to cover all available serotypes. This calls even more attention to the knowledge of national and regional distributions of serotypes of GBS infections.
Summary for practice
Group B streptococci today, as before, is the most common causative agent of neonatal sepsis and meningitis. The frequency in Germany is estimated at almost 0.5/1000 live births, and the number of blood culture-negative clinical cases of sepsis may be several times higher.
 nine0005
nine0005 The likelihood of encountering neonatal GBS-sepsis for clinical and practical pediatricians is very high. The fulminance of early-onset sepsis, as well as the imperceptible gradual onset of late-onset sepsis, make it vital to be able to quickly make a diagnosis and diagnosis of suspicion, as well as to conduct adequate therapy. It is for the diagnosis of early-onset sepsis in clinical practice that the determination of the cytokine IL-6 or IL-8, for example, in the blood of the umbilical cord, is of great help. nine0005
Despite increasing antibiotic resistance, nothing has changed in penicillin and aminoglycoside therapy over the past 30 years. Prevention is of particular importance today.
National and international specialist societies recommend intrapartum antibiotic prophylaxis in the mother based on screening tests in late pregnancy and in the presence of risk factors. Since these prevention strategies, although effective, are not without their problems, it is possible that in the future, immunization will be carried out by vaccination of the mother.