Acyclovir safe in pregnancy
Acyclovir Safe for Treating Herpes Infection in Early Pregnancy
AMY CRAWFORD-FAUCHER, MD
Am Fam Physician. 2011;84(3):320-322
Background: The antiviral agents acyclovir (Zovirax), valacyclovir (Valtrex), and famciclovir (Famvir) are commonly used to treat initial and recurrent cases of herpes simplex virus infection, and to lessen the severity of herpes zoster virus infection. More than 1 percent of susceptible women acquire herpes simplex virus infection during the first trimester of pregnancy, and there is a high prevalence of recurrent infection; therefore, antiviral medication is recommended in a significant number of pregnant women. The U.S. Food and Drug Administration considers acyclovir, valacyclovir, and famciclovir category B drugs in pregnancy, but there are few data on early pregnancy exposure. Pasternak and Hviid investigated the risk of major birth defects in the infants of mothers who took antiviral medication in their first trimester.
The Study: This retrospective cohort study compiled data from three national registries in Denmark. A cohort of all live births between January 1996 and September 2008 was selected. The authors extracted data on the cohort mothers for prescriptions of oral acyclovir, valacyclovir, and famciclovir, as well as topical acyclovir and penciclovir (Denavir) that were filled from four weeks before conception through birth. The registry was not able to capture data from inpatient prescribing or for over-the-counter formulations of topical acyclovir and penciclovir.
The registry was not able to capture data from inpatient prescribing or for over-the-counter formulations of topical acyclovir and penciclovir.
Birth defects were identified through the National Patient Register, which lists all diagnoses assigned to persons during hospital admissions and emergency department and outpatient visits. Data for the study period were accessed, and major birth defect diagnoses were compiled using a surveillance classification system. Infants with chromosomal or genetic disorders, minor defects, birth defect syndromes with known causes, and congenital viral infections that can cause birth defects were excluded. To avoid confounding, the authors collected maternal information on medical conditions, including diabetes mellitus and immunodeficiency status, smoking status, history of sexually transmitted infections, exposure to corticosteroids and antibiotics in the first trimester, and history of birth defects in older siblings.
Results: A major birth defect in the first year of life was diagnosed in 19,960 of 837,795 live births (2. 4 percent). The rate of birth defects did not differ between the women who received antivirals (1,804 pregnancies with 40 birth defects; 2.2 percent) and those who did not (835,991 pregnancies with 19,920 birth defects; 2.4 percent). These results did not change when the analysis was restricted to acyclovir. Risk estimates were similar for acyclovir and valacyclovir; however, the authors caution that there were relatively few exposures reported for valacyclovir and famciclovir.
4 percent). The rate of birth defects did not differ between the women who received antivirals (1,804 pregnancies with 40 birth defects; 2.2 percent) and those who did not (835,991 pregnancies with 19,920 birth defects; 2.4 percent). These results did not change when the analysis was restricted to acyclovir. Risk estimates were similar for acyclovir and valacyclovir; however, the authors caution that there were relatively few exposures reported for valacyclovir and famciclovir.
Conclusion: Use of acyclovir in the first trimester does not increase birth defects, and it should be the antiviral drug of choice in early pregnancy.
Safety of antiviral medication for the treatment of herpes during pregnancy
Can Fam Physician. 2011 Apr; 57(4): 427–428.
Language: English | French
So-Hee Kang, RPh, Angela Chua-Gocheco, MD, Pina Bozzo, and Adrienne Einarson, RN
Copyright and License information Disclaimer
Question One of my patients is a pregnant woman in her first trimester with a history of recurrent genital herpes. She is concerned about whether use of her antiviral medication will adversely affect her baby. What should I tell her?
She is concerned about whether use of her antiviral medication will adversely affect her baby. What should I tell her?
Answer Studies have shown that the use of acyclovir or valacyclovir is not associated with an increase in birth defects. Limited data exist for famciclovir and therefore it would not be considered a first-line choice for treatment of herpes during pregnancy.
Question L’une de mes patientes enceintes en est à son premier trimestre de grossesse et elle a des antécédents d’herpès génital récurrent. Elle se demande si l’utilisation de ses médicaments antiviraux pourrait nuire à son bébé. Que devrais-je lui répondre?
Réponse Des études ont démontré que l’utilisation de l’acyclovir ou du valacyclovir n’est pas associée à une augmentation des anomalies congénitales. Les données concernant le famciclovir sont limitées et ce médicament ne devrait donc pas être considéré comme choix de traitement de première intention pour l’herpès durant la grossesse.
Herpes simplex virus (HSV) infections are common viral infections, with almost 40% of infected patients encountering frequent recurrence within the first year of disease onset.1 In Ontario the seroprevalence for HSV type 1 (HSV-1) and type 2 was 51.1% and 9.1%, respectively.2 A Canadian study revealed HSV type 2 seropositivity in pregnant women to be 17.3%, which raises the concern of potential viral transmission from mother to infant.3 It is also important to note that genital herpes due to HSV-1 infections has increased in frequency and is responsible for up to 30% to 50% of new genital HSV infections.3,4 Recent findings indicate that pregnant women who acquire HSV as a primary infection in the latter half of their pregnancies are at greatest risk of transmission to neonates.5
Neonatal HSV infections are considered more serious compared with adult infections, having consequences that include the following: skin, eye, and mouth infections; central nervous system diseases; disseminated infections; and death. The Canadian neonatal HSV surveillance data indicate that there are 5.9 cases per 100 000 live births, stressing the importance of antiviral treatment during pregnancy to reduce such complications.6 Treatment with antivirals in adults has established their efficacy and safety, but evidence of the safety of acyclovir, famciclovir, and valacyclovir during pregnancy is relatively lacking. It is important to discuss the use of topical antiviral preparations owing to the need to prevent potential orolabial to genital transmission of HSV-1.
The Canadian neonatal HSV surveillance data indicate that there are 5.9 cases per 100 000 live births, stressing the importance of antiviral treatment during pregnancy to reduce such complications.6 Treatment with antivirals in adults has established their efficacy and safety, but evidence of the safety of acyclovir, famciclovir, and valacyclovir during pregnancy is relatively lacking. It is important to discuss the use of topical antiviral preparations owing to the need to prevent potential orolabial to genital transmission of HSV-1.
Valacyclovir is well absorbed after oral administration; absorption of a single dose of 1000 mg is 54% higher than that achieved after single 200- or 800-mg doses of oral acyclovir.7 Valacyclovir is rapidly metabolized to acyclovir, the triphosphorylated form of which selectively inhibits human HSV DNA polymerase, reducing viral DNA replication. Similarly, famciclovir is the oral prodrug of its active form penciclovir; it is more stable than acyclovir triphosphate, as evidenced by longer half-lives intracellularly, which might account for the prolonged in vitro antiviral activity. 8 Acyclovir 5% and penciclovir 1% creams are used to treat orolabial herpes (HSV-1). Penciclovir was not detected in the plasma or urine of healthy volunteers after single or repeated application of the 1% cream.9 Systemic absorption of acyclovir following topical application is minimal or undetectable in adults.10
8 Acyclovir 5% and penciclovir 1% creams are used to treat orolabial herpes (HSV-1). Penciclovir was not detected in the plasma or urine of healthy volunteers after single or repeated application of the 1% cream.9 Systemic absorption of acyclovir following topical application is minimal or undetectable in adults.10
Although controlled studies evaluating the effectiveness of oral antivirals for recurrent HSV outbreaks in the mother and neonatal HSV infections at delivery do exist, they are limited by small sample sizes, the lack of fetal safety outcomes, and variation in timing of exposure.11 However, data on the safety of antiviral drugs during pregnancy have been collected in pregnancy registries, usually established by the manufacturers. The oldest registry (ie, Acyclovir Pregnancy Registry) was open from 1984 to 1998, evaluating the use of oral or intravenous acyclovir in pregnant women. In total, 1234 pregnancies were noted with 1246 outcomes from 24 countries; 756 exposed pregnancies were studied in the first trimester. The risk of birth defects was 3.2% (95% confidence interval 2.0% to 5.0%), which is similar to the baseline risk of birth defects in the general population. No unusual defects or patterns of defects were seen, but the limitation of the results was the high loss to follow-up (27% of registrants).12 During the years 1995 to 1999, the manufacturer of valacyclovir maintained its pregnancy registry, and 110 exposures were reported with 111 known outcomes. Within the first trimester, 1 birth defect was reported out of 28 exposures; 2 of 31 and 1 of 51 exposures resulted in defects in the second and third trimesters, respectively. Prenatal exposures were too limited to provide pregnancy outcomes. According to personal written correspondence from GlaxoSmithKline to Motherisk (January 2010), this registry was limited by the number of registrants and the length of monitoring.
The risk of birth defects was 3.2% (95% confidence interval 2.0% to 5.0%), which is similar to the baseline risk of birth defects in the general population. No unusual defects or patterns of defects were seen, but the limitation of the results was the high loss to follow-up (27% of registrants).12 During the years 1995 to 1999, the manufacturer of valacyclovir maintained its pregnancy registry, and 110 exposures were reported with 111 known outcomes. Within the first trimester, 1 birth defect was reported out of 28 exposures; 2 of 31 and 1 of 51 exposures resulted in defects in the second and third trimesters, respectively. Prenatal exposures were too limited to provide pregnancy outcomes. According to personal written correspondence from GlaxoSmithKline to Motherisk (January 2010), this registry was limited by the number of registrants and the length of monitoring.
A recent Danish population-based retrospective cohort study used data from its nationwide registry to examine live-born infants born between 1996 and 2008 who were exposed to antivirals during pregnancy and the rate of major birth defects within the first year of life. Of the 837 795 enrolled infants, 1804 pregnancies were exposed to acyclovir, valacyclovir, or famciclovir in the first trimester; prevalence odds ratios were considered no different between exposed and unexposed cohorts. Similar results were found when evaluating second and third trimester data. There was also no significant difference in the prevalence of malformations between exposed and unexposed groups when examining exposure during the first trimester to individual antivirals. The rate of malformations was 2.0% for acyclovir (32 of 1561 infants) and 3.1% for valacyclovir (7 of 229 infants). For famciclovir, exposure was uncommon with 1 infant of 26 exposed having a birth defect. As a supplementary analysis, the association between the use of dermatologic acyclovir and penciclovir creams and major birth defects was evaluated. The rate of malformations in those exposed to acyclovir cream and penciclovir cream in the first trimester (2.3%, 65 of 2850 infants, and 4.2%, 5 of 118 infants, respectively) was not different from the unexposed cohort; similar results were found in the second and third trimesters of pregnancy.
Of the 837 795 enrolled infants, 1804 pregnancies were exposed to acyclovir, valacyclovir, or famciclovir in the first trimester; prevalence odds ratios were considered no different between exposed and unexposed cohorts. Similar results were found when evaluating second and third trimester data. There was also no significant difference in the prevalence of malformations between exposed and unexposed groups when examining exposure during the first trimester to individual antivirals. The rate of malformations was 2.0% for acyclovir (32 of 1561 infants) and 3.1% for valacyclovir (7 of 229 infants). For famciclovir, exposure was uncommon with 1 infant of 26 exposed having a birth defect. As a supplementary analysis, the association between the use of dermatologic acyclovir and penciclovir creams and major birth defects was evaluated. The rate of malformations in those exposed to acyclovir cream and penciclovir cream in the first trimester (2.3%, 65 of 2850 infants, and 4.2%, 5 of 118 infants, respectively) was not different from the unexposed cohort; similar results were found in the second and third trimesters of pregnancy. 13
13
The accumulated evidence for the safety of oral acyclovir and valacyclovir, established from the manufacturer’s pregnancy registries and as a result of the Danish cohort study, does not demonstrate an increase in the rate of major birth defects when compared with the general population or an unexposed group. Data on the safety of famciclovir’s use during pregnancy is quite limited, and although it might not be expected to increase the risk of major malformations, it should not be the first choice of medication for treatment of HSV during pregnancy. In addition, topical antiviral preparations of acyclovir and penciclovir resulted in no increased rate of major birth defects during pregnancy. Limitations of the safety data on antivirals include a high lost-to-follow-up rate in the registries and the lack of prospective controlled studies. However, these data are reassuring, allowing physicians to offer pregnant patients either acyclovir or valacyclovir for treatment of primary or recurrent HSV infection, which not only treats the mother’s condition, but also reduces the likelihood of transmission to the neonate, without unduly compromising fetal safety.
Motherisk
Motherisk questions are prepared by the Motherisk Team at the Hospital for Sick Children in Toronto, Ont. Ms Kang is a doctoral candidate in the Faculty of Pharmacy at the University of Toronto. Dr Chua-Gocheco is a member of the Motherisk Program. At the time this paper was written, Ms Bozzo was a member and Ms Einarson was Assistant Director of the Motherisk Program. Ms Bozzo is now Assistant Director and Ms Einarson has retired but continues to be a member of the Motherisk Program.
Do you have questions about the effects of drugs, chemicals, radiation, or infections in women who are pregnant or breastfeeding? We invite you to submit them to the Motherisk Program by fax at 416 813-7562; they will be addressed in future Motherisk Updates.
Published Motherisk Updates are available on the Canadian Family Physician website (www. cfp.ca) and also on the Motherisk website (www.motherisk.org).
cfp.ca) and also on the Motherisk website (www.motherisk.org).
Competing interests
None declared
1. Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121(11):847–54. [PubMed] [Google Scholar]
2. Howard M, Sellors JW, Jang D, Robinson NJ, Fearon M, Kaczorowski J, et al. Regional distribution of antibodies to herpes simplex virus type 1 (HSV-1) and HSV-2 in men and women in Ontario, Canada. J Clin Microbiol. 2003;41(1):84–9. [PMC free article] [PubMed] [Google Scholar]
3. Patrick DM, Dawar M, Cook DA, Krajden M, Ng HC, Rekart ML. Antenatal seroprevalence of herpes simplex virus type 2 (HSV-2) in Canadian women: HSV-2 prevalence increases throughout the reproductive years. Sex Transm Dis. 2001;28(7):424–8. [PubMed] [Google Scholar]
4. Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–73. [PubMed] [Google Scholar]
JAMA. 2006;296(8):964–73. [PubMed] [Google Scholar]
5. Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Barucca V, Chiarini F, et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009;6:40. [PMC free article] [PubMed] [Google Scholar]
6. Kropp RY, Wong T, Cormier L, Ringrose A, Burton S, Embree JE, et al. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006;117(6):1955–62. [PubMed] [Google Scholar]
7. Ormrod D, Scott LJ, Perry CM. Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs. 2000;59(4):839–63. [PubMed] [Google Scholar]
8. Simpson D, Lyseng-Williamson KA. Famciclovir: a review of its use in herpes zoster and genital and orolabial herpes. Drugs. 2006;66(18):2397–416. [PubMed] [Google Scholar]
9. Denavir cream 1% [package insert] Parsippany, NJ: Novartis Consumer Health, Inc; 2002.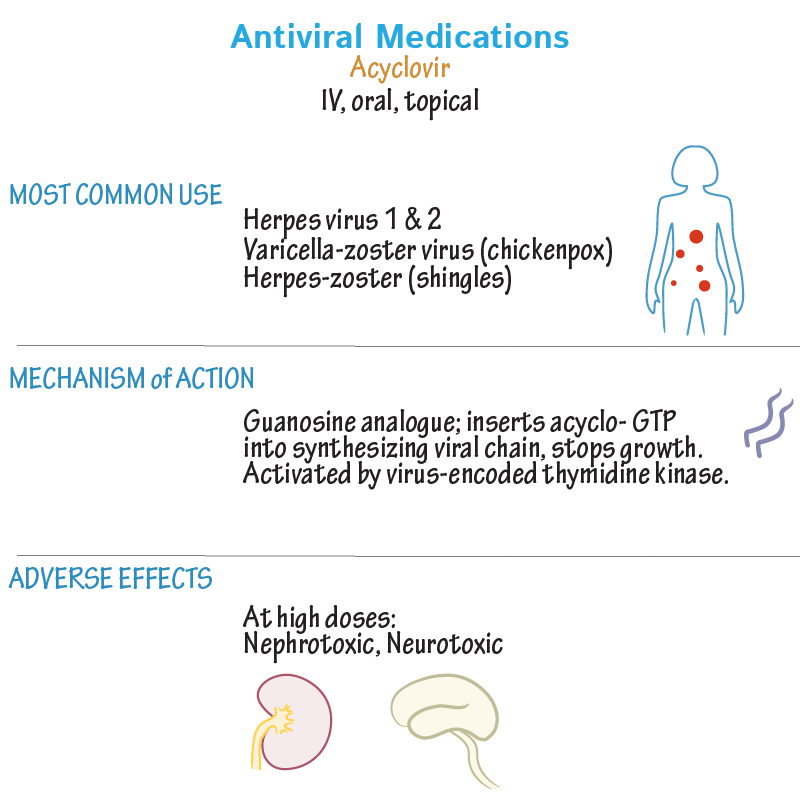 [Google Scholar]
[Google Scholar]
10. Zovirax cream 5% [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2002. [Google Scholar]
11. Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008;(1):CD004946. [PubMed] [Google Scholar]
12. GlaxoSmithKline . Acyclovir pregnancy registry and valacyclovir pregnancy registry [interim report] Research Triangle Park, NC: Glaxo Wellcome; 1997. [Google Scholar]
13. Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA. 2010;304(8):859–66. [PubMed] [Google Scholar]
use in the 1st, 2nd and 3rd trimesters
Pregnancy is a responsible and vulnerable period in a woman's life that requires careful preparation and care. Weakened immunity during this period of time becomes the cause of numerous diseases, which are often not possible to treat in the traditional way. In particular, during pregnancy, the risk of developing herpes is high.
In particular, during pregnancy, the risk of developing herpes is high.
The herpes virus is dangerous for the embryo, and the disease requires immediate treatment. One of the popular remedies against the herpes virus is the drug "Acyclovir". Whether treatment with Acyclovir is safe during pregnancy and how effective it is - this must be understood first of all.
Is Aciclovir recommended during pregnancy?
About 90% of people are carriers of at least one of the 8 known herpes viruses. However, this virus is actively manifested only in people with reduced immunity. They make up about 5% of all carriers. Ways of transmission of the virus are varied:
- household;
- sexual;
- airborne.
This intracellular parasite, having got on the mucous membranes of a person, is quickly sent to the bloodstream. It is impossible to expel the herpes virus from the body - it settles there for life. However, antibodies produced by any healthy and strong organism prevent its reproduction. In a latent form, herpes can stay for a long time, and at the time of weakening of the immune system, it immediately activates.
In a latent form, herpes can stay for a long time, and at the time of weakening of the immune system, it immediately activates.
Gestational period is one of the most favorable for its development. If the mother's body was infected with herpes long before pregnancy, a sufficient amount of antibodies accumulate in the blood that can protect the fetus. But when the body becomes infected with the virus after conception, the disease proceeds in a more severe form. Acyclovir is prescribed as treatment.
The antiviral drug "Acyclovir" works against all types of herpes, herpes zoster and chickenpox, and is also used in obstetric practice. It has been proven that Acyclovir is able to cross the placenta. It gets to the embryo, but it does not cause the development of abnormalities in the fetus. According to the results of a study conducted by scientists in Denmark, during which two groups of pregnant women, respectively, took and did not take Acyclovir, the percentage of anomalies in children born after taking the drug is much lower. Modern medicine claims that if we compare the risks of taking antiviral drugs and the consequences of developing herpes during pregnancy, then the latter will be much more dangerous. In addition to a direct effect on the developing organs of the child, the virus also affects the outcome of pregnancy, provoking early labor activity, which can end in miscarriage.
Modern medicine claims that if we compare the risks of taking antiviral drugs and the consequences of developing herpes during pregnancy, then the latter will be much more dangerous. In addition to a direct effect on the developing organs of the child, the virus also affects the outcome of pregnancy, provoking early labor activity, which can end in miscarriage.
Acyclovir is prescribed only in case of a serious course of the disease and taking into account the gestational age.
The use of Acyclovir in the first trimester
It is often up to the 12th week that a sharp decrease in immunity occurs and, as a result, herpes worsens. The same period is a contraindication to the use of antiviral agents. In the case of a severe illness, doctors risk prescribing a tablet form of Acyclovir. But in the vast majority of cases, treatment is limited to external application of the ointment to the affected areas of the skin. The injectable form of Acyclovir is completely contraindicated at this time.
Use of Acyclovir in the second trimester
In the second trimester, the embryo is already quite strong and partially formed, although the herpes virus can still cause irreparable damage to it. In this trimester, the use of the drug in tablets at a standard dosage is already allowed, symptomatic treatment by applying a gel to the area of rashes. Additionally, immunostimulants are prescribed.
Use of Acyclovir in the third trimester
Treatment of herpes virus in late pregnancy is advisable only in case of primary infection. As a rule, relapses of the chronic form of the disease already disappear by this time. Treatment involves the use of ointments and tablets, in severe cases, injections are acceptable.
Composition and action of the drug
The drug "Acyclovir" is a representative of antiviral agents. The main active ingredient is acyclovir. Auxiliary components differ depending on the forms of the drug. The tableted pharmaceutical form contains:
- silicon dioxide;
- capovidone;
- cellulose.

The composition of ointments includes:
- water;
- paraffin;
- propylene glycol;
- alcohol.
The active substance of the drug is incorporated into the DNA of the virus after it has entered the affected cell. This leads to disruption of the cycle of synthesis of new viral DNA. This is characterized by the absence of toxic effects on human cells.
It is worth mentioning the likelihood of tolerance with repeated use, as well as with long-term use. Viruses become insensitive to the drug, and the selection of new means to get rid of herpes is required.
Differences in the pharmaceutical forms of Acyclovir
The presence and use of various dosage forms is explained by the diversity of virus types. It causes rashes on different parts of the body of varying degrees of severity. Main pharmaceutical forms:
- ointment for external use;
- eye ointment;
- tablets;
- solution for injection.

The use of each of them during pregnancy differs in characteristic features.
Acyclovir eye ointment
During pregnancy, herpes often affects the eyes, resulting in herpetic keratitis (an inflammation of the cornea of the eye). To stop soreness and inflammation, as well as to prevent the spread of herpes to other parts of the body, an eye ointment with a 3% content of Acyclovir is used. This form is available in a metal tube, equipped with an applicator for laying the ointment directly into the conjunctival sac. The agent is quickly absorbed and distributed to all parts of the eyeball, including the vitreous body.
Even small doses of the drug cross the placental barrier and accumulate in breast milk. In most cases, the drug does not cause negative reactions of the body during pregnancy, but women who are allergic to medications should use Acyclovir with caution. As possible side effects, burning, allergic manifestations, conjunctivitis are noted.
Eye ointment treatment does not involve wearing contact lenses. You should also refrain from driving.
Acyclovir cream
Cream with 5% active ingredient is indicated for the treatment of rashes on the face and lips. Application to the mucous membranes of the mouth and genitals is not recommended. The cream is effective both before the appearance of bubbles and after, however, in the latter case, the treatment will take longer.
This form of the drug has only an external effect and does not penetrate into the bloodstream. Accordingly, the effect on the fetus is minimal.
Acyclovir tablets
The concentration of the active substance of acyclovir in the form of tablets varies:
- 200 mg;
- 400 mg;
- 800 mg.
This form of the drug is chosen when the treatment with an ointment or cream is proven to be unproductive, as well as in a severe form of the disease. In addition, during pregnancy, tablets may be prescribed to treat shingles and chicken pox.
Acyclovir injections
During pregnancy, injections are used in the fight against lichen and herpes strains that have developed immunity to other dosage forms of the drug. The agent is administered intravenously.
Dosage of Acyclovir during pregnancy and regimen
The choice of pharmaceutical form, dosage calculation and duration of the course are at the discretion of the specialist. This takes into account the duration of pregnancy and known information about the disease.
General recommendation for ophthalmic ointment: 4-5 applications per day, 4 hours apart, until symptoms disappear, plus three more days.
Cream treatment involves applying to rash areas 3-4 times a day. The course is 4-10 days.
The tablet form of the drug is used at a concentration of 200 mg 5 times a day for 5 days or more. An increase in concentration is permissible in severe manifestations of the disease.
Acyclovir injections are calculated taking into account the patient's weight - 5 mg per kilogram. Injections are made 3 times a day.
Injections are made 3 times a day.
Adverse reactions and contraindications
Contraindication is individual intolerance to any component of the drug.
The list of possible side effects is quite large and depends on the form of application:
- anemia;
- anaphylaxis;
- migraine;
- tremor;
- dizziness;
- hallucinations;
- convulsions;
- coma;
- diarrhea;
- abdominal pain;
- vomiting;
- arrester;
- hyperemia;
- itching.
Safe analogues of Acyclovir in pregnancy
There are enough drugs similar in properties and efficacy to Acyclovir. These include:
- Gerpevir;
- Zovirax;
- Virolex;
- Cyclovir and others.
The indications and method of use of these drugs are similar, they differ only in the concentration of the active substance and additional components.
Be sure to consult a specialist and weigh all the risks before taking medication.
Use and safety Aciclovir for pregnant and lactating women: can it be taken during pregnancy and breastfeeding
Can aciclovir be used during pregnancy to treat herpes and possible risks to the fetus
Aciclovir belongs to a class of antiviral drugs called synthetic nucleoside analogues. It works by stopping the spread of the herpes virus in the body. Used to reduce pain and speed up the healing of sores or blisters in people with chickenpox (chickenpox), shingles, a rash that can occur in people who have had chickenpox in the past, and outbreaks of genital herpes (occasionally causes ulcers around genitals and rectum).
In a study by the Centers for Disease Control and Prevention (CDC), aciclovir was found to cross the placenta, but teratogenicity (birth defects) was not observed, but there is still insufficient data to assess the risk of less common defects or to obtain reliable or final conclusions regarding the safety of this drug during pregnancy.
According to research by the Food and Drug Administration (FDA), it was noted that women taking acyclovir for the treatment of herpes simplex, even during the first trimester of pregnancy, did not demonstrate a risk to the fetus.
The Australian Medicines Administration (AU TGA) has studied a limited number of pregnant women and women of childbearing age who have taken oral acyclovir and found that it did not increase the incidence of malformations or other direct or indirect harmful effects on the mother's fetus. Animal studies have shown an increased incidence of fetal injury, the significance of which in humans is uncertain.
The American College of Obstetricians and Gynecologists (ACOG) recommends taking aciclovir for the treatment of genital herpes, especially recurrent ones, from the 36th week of pregnancy to avoid infection of the newborn baby. But since, as a result of not multiple studies, little information has been provided about it, it is advised to use it with extreme caution, strictly according to the doctor's prescription in the exact minimum dosage indicated as soon as possible.
Information was also provided to ACOG that suppressive therapy in late pregnancy reduces the rate of caesarean section in women with recurrent genital herpes by reducing the rate of relapse during pregnancy, but such treatment may not protect against transmission of the viral infection to the newborn in all cases .
The Centers for Disease Control and Prevention (CDC) in 2015 concluded that this drug can be used to treat herpetic lesions in women at all stages of pregnancy, but under the strict supervision of a doctor, following all his prescriptions for prescribed doses, frequency and duration of administration, as well as in case of the most urgent need and, as a rule, the benefit to the mother should exceed the risk to the fetus.
Breastfeeding use of acyclovir and exposure to infants
Food and Drug Administration (FDA) studies of acyclovir use in breastfeeding women have shown that it passes into breast milk, but even at the most At high maternal doses, the concentration of acyclovir in milk is only about 1% of the typical pediatric dose and is not expected to cause any side effects in breastfed infants. Local application of acyclovir to small areas of the mother's body away from the breast does not pose a risk to the infant.
Local application of acyclovir to small areas of the mother's body away from the breast does not pose a risk to the infant.
As a result of maternal herpesvirus infection of the infant during pregnancy, newborns are given aciclovir at doses of 20 to 30 mg/kg IV daily according to the CDC. Dosages obtained in breast milk at high maternal doses are only about 3-5% of this dose. Because the infant receives oral doses of breast milk and aciclovir is only about 20% orally bioavailable, the systemic dose received by the nursing infant is 1% or less of the typical pediatric dose, resulting in no reported adverse reactions in infants.
Several cases of oral aciclovir in breastfeeding mothers have also been reported to the CDC and FDA:
-
The mother of a 4-month-old infant did not notice any side effects in her breastfed infant when she took aciclovir 800 mg orally 5 times a day;
- 90,002 women who were 6 weeks postpartum received acyclovir 300 mg (5 mg/kg) intravenously three times a day for 5 days.