Why is fragile x more common in males
Fragile X Syndrome - NORD (National Organization for Rare Disorders)
Fragile X Syndrome
NORD gratefully acknowledges Jack C. Tarleton, PhD, FACMG, Fullerton Genetics Center, and Robert A. Saul, MD, Medical Director, General Pediatrics, Children's Hospital of Greenville Health System, for assistance in the preparation of this report.
Synonyms of Fragile X Syndrome
- fragile site, folic acid type, rare, Fra(X)(Q27.3)
- marker X syndrome
- Martin-Bell syndrome
General Discussion
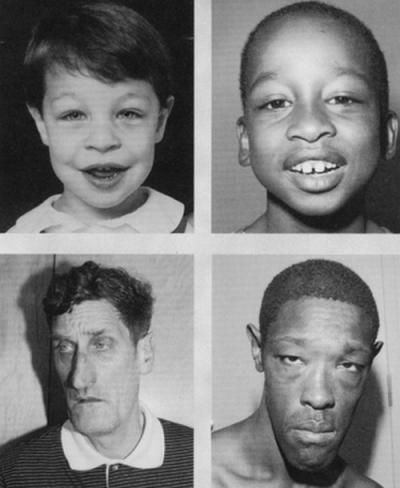
Fragile X syndrome is characterized by moderate intellectual disability in affected males and mild intellectual disability in affected females. Distinctive physical features are sometimes present in affected males including a large head, long face, prominent forehead and chin, protruding ears, loose joints and large testes, but these features develop over time and may not be obvious until puberty. Motor and language delays are usually present but also become more apparent over time. Behavioral abnormalities including autistic behaviors are common.
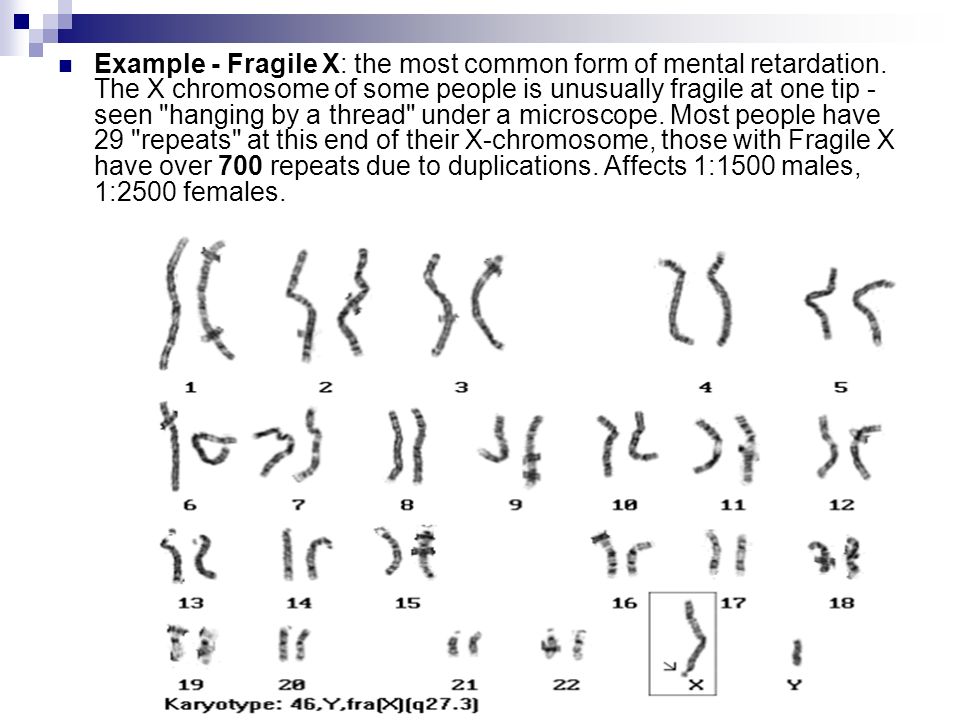
Fragile X syndrome has been found in all major ethnic groups and races, and is caused by an abnormality (mutation) in the FMR1 gene. FMR1 is a gene located on the X chromosome that produces a protein called FMRP needed for proper cell function. The syndrome became known as the fragile X syndrome because some individuals with the disorder were found to have a segment of their X chromosome that appeared to be broken or fragile (although not completely disconnected). Later it was learned that the FMR1 gene is located precisely where the X chromosome appears to be “fragile” in affected individuals.
Chromosomes, which are present in the nucleus of human cells, carry the genetic information for each individual. Human body cells normally have 46 chromosomes. Pairs of human chromosomes are numbered from 1 through 22 and the sex chromosomes are designated X and Y. Males have one X and one Y chromosome and females have two X chromosomes. Each chromosome has a short arm designated “p” and a long arm designated “q”. Chromosomes are further sub-divided into many bands that are numbered. For example, “chromosome Xq27.3” refers to band 27.3 on the long arm of the X chromosome where the FMR1 gene is located. The numbered bands specify the location of the thousands of genes that are present on each chromosome.
Each chromosome has a short arm designated “p” and a long arm designated “q”. Chromosomes are further sub-divided into many bands that are numbered. For example, “chromosome Xq27.3” refers to band 27.3 on the long arm of the X chromosome where the FMR1 gene is located. The numbered bands specify the location of the thousands of genes that are present on each chromosome.
X-linked dominant disorders such as fragile X syndrome are caused by an abnormal gene located on the X chromosome. Females with the abnormal gene may be affected by this disorder. Males are usually more severely affected than females).
It is the absence or severe reduction of the protein made by the FMR1 gene, FMRP, that causes fragile X syndrome. Mutation of the FMR1 gene causes the loss or reduction of FMRP. Nearly all affected individuals have an instability within the gene leading to an increased number of copies of a portion of the gene called the CGG repeat region (also sometimes called “trinucleotide” or “triplet” repeat region). When greater than 200 repeats are present, abnormal chemical changes occur in FMR1 called methylation. The expansion of the CGG repeat region to greater than 200 repeats accompanied by methylation of the gene, called a “full mutation”, causes the loss of FMRP leading to the fragile X syndrome. Fragile X syndrome occurs more often in males and results in more severe disorder in males.
When greater than 200 repeats are present, abnormal chemical changes occur in FMR1 called methylation. The expansion of the CGG repeat region to greater than 200 repeats accompanied by methylation of the gene, called a “full mutation”, causes the loss of FMRP leading to the fragile X syndrome. Fragile X syndrome occurs more often in males and results in more severe disorder in males.
Mutations in FMR1 are unusual when compared to mutations found in other genes. Some individuals carry between 55 – 200 CGG repeats called a “premutation,” usually without having symptoms associated with fragile X syndrome. These individuals are at risk for having children or grandchildren with fragile X syndrome, however, and also at risk for two adult onset disorders, fragile X tremor-ataxia syndrome (FXTAS) and primary ovarian insufficiency (POI). The conditions have been termed FMR1-Related Disorders. (Please see the Causes and Related Disorders sections of this report for a more detailed explanation regarding premutations and brief summaries of these FMR1-related disorders).
Signs & Symptoms
Fragile X syndrome is characterized by moderate intellectual disability in affected males and mild intellectual disability in affected females. The physical features in affected males are variable and may not be obvious until puberty. These symptoms can include a large head, long face, prominent forehead and chin, protruding ears, loose joints and large testes. Other symptoms can include flat feet, frequent ear infections, low muscle tone, a long narrow face, high arched palate, dental problems, crossed eyes (strabismus) and heart problems including mitral valve prolapse. Delayed motor development, hyperactivity, behavior problems, toe walking, and/or occasional seizures can also occur in some patients. Autistic behaviors such as poor eye contact, hand flapping, and/or self-stimulating behaviors are also common. Motor and language delays are usually present but become more apparent over time.
Causes
As mentioned above, fragile X syndrome is caused by a mutation in the FMR1 gene located on the X chromosome at Xq27. 3. Individuals with fragile X syndrome nearly always have (in greater than 99% of cases) a full mutation of the FMR1 gene which means that they have over 200 CGG repeats and abnormal methylation of the gene. Methylation is a chemical change to the DNA that carries the genetic code of a gene and the abnormal methylation associated with fragile X syndrome causes the gene to be unable to produce FMRP, the protein made by the FMR1 gene, needed for normal development. On rare occasions some patients with fragile X syndrome are partially or completely missing the FMR1 gene due to a deletion of the DNA on the X chromosome where FMR1 is located and have the syndrome because their cells do not produce FMRP. Ultra-rare patients with fragile X syndrome have been found to have a mutation in a single DNA base (called point mutations) resulting in absent or defective FMRP. FMRP is involved in making connections between neurons (nerve cells) in the brain. The absence or severe reduction of this protein leads to the symptoms of fragile X syndrome.
3. Individuals with fragile X syndrome nearly always have (in greater than 99% of cases) a full mutation of the FMR1 gene which means that they have over 200 CGG repeats and abnormal methylation of the gene. Methylation is a chemical change to the DNA that carries the genetic code of a gene and the abnormal methylation associated with fragile X syndrome causes the gene to be unable to produce FMRP, the protein made by the FMR1 gene, needed for normal development. On rare occasions some patients with fragile X syndrome are partially or completely missing the FMR1 gene due to a deletion of the DNA on the X chromosome where FMR1 is located and have the syndrome because their cells do not produce FMRP. Ultra-rare patients with fragile X syndrome have been found to have a mutation in a single DNA base (called point mutations) resulting in absent or defective FMRP. FMRP is involved in making connections between neurons (nerve cells) in the brain. The absence or severe reduction of this protein leads to the symptoms of fragile X syndrome.
Premutations have 55-200 CGG repeats and are potentially unstable. Individuals having a premutation do not have the fragile X syndrome but are at risk for having the adult onset FMR1 disorders FXTAS and POI.
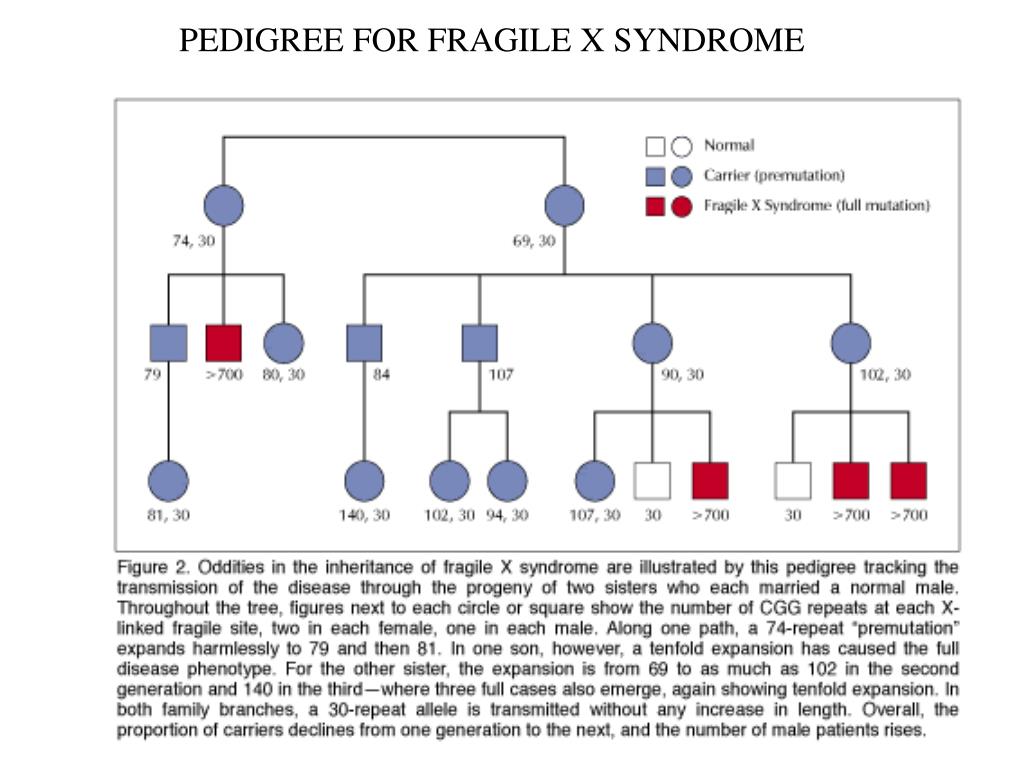
When passed from generation to generation premutations may be unstable and become full mutations, but the risk for instability is different depending upon whether a female or male premutation carrier is transmitting the premutation. Females with a premutation of the FMR1 gene are at risk to have children with fragile X syndrome because the number of CGG repeats can increase when the gene is passed into the next generation. The greater the number of copies of CGG in a premutation, the more likely these will increase to become a full mutation causing the fragile X syndrome in offspring.
When males with a premutation reproduce, their male offspring have no risk to inherit the premutation because fathers do not contribute an X chromosome to their sons. In contrast, female offspring whose fathers have a premutation always inherit it and thus grandchildren of males with the premutation are at risk to have fragile X syndrome. Because the premutation is relatively stable when transmitted from father to daughter, the daughters almost never are affected with fragile X syndrome. However, their children are at increased risk because the premutation may be unstable when transmitted to the next generation.
Because the premutation is relatively stable when transmitted from father to daughter, the daughters almost never are affected with fragile X syndrome. However, their children are at increased risk because the premutation may be unstable when transmitted to the next generation.
Normal FMR1 genes have approximately 5-44 CGG repeats and this number remains stable from generation to generation. Occasionally, in some individuals with 45-54 repeats there will be some minor instability such that these individuals will have several more (or less) repeats than their parents. A FMR1 repeat number between 45 and 54 is called “intermediate” or “gray zone”, but this minor instability does not lead to any symptoms of fragile X syndrome or the FMR1-related disorders. Having an intermediate number of CGG repeats is still considered as being in the normal range of repeat number.
Affected Populations
The fragile X syndrome affects about 1 in 4,000 males and 1 in 6,000 to 8,000 females in the USA; that is, it affects about twice as many males as it does females. However, about four times as many females appear to be carriers of the altered gene as do males (1:250 females and 1:1000 males). Fragile X syndrome has been found in all major ethnic groups and races.
However, about four times as many females appear to be carriers of the altered gene as do males (1:250 females and 1:1000 males). Fragile X syndrome has been found in all major ethnic groups and races.
Diagnosis
Over 99% of individuals with fragile X syndrome have a full mutation (over 200 CGG repeats and abnormal methylation) in the FMR1 gene. Molecular genetic testing is used to determine the number of CGG repeats in the FMR1 gene and testing to determine methylation status of the FMR1 gene is often used to follow up a finding of an expanded CGG region.
Chromosome analysis using special techniques to induce fragile sites in chromosomes was once used to diagnose fragile X syndrome, but is no longer used for this purpose. Fragile X syndrome is the name given to this condition because some affected individuals have an X chromosome that looked as if it had “broken” or was “fragile” and was held together by the slightest of ties. This technique is no longer used in the diagnosis of this syndrome because it is both less accurate and costlier than are molecular techniques.
Standard Therapies
Treatment
There are many treatments for fragile X syndrome that can improve the lives of affected individuals and their families. These include special education, speech, occupational, and sensory integration training, and behavior modification programs. With educational efforts, therapy, and support, all individuals with fragile X syndrome can make progress. Other treatment may depend on an affected individual’s specific symptoms. Genetic counseling is recommended for affected individuals and their families.
There are numerous Fragile X Clinics in the US and throughout the world. These clinics specialize in treatments, therapies, and support for individuals with fragile X syndrome and can guide parents to medication options to address specific symptoms. New medications are likely to become available to treat affected individual and the specialty clinics can assist parents with current information. For a detailed discussion of Treatments and Interventions please go to the National Fragile X Foundation website (https://fragilex. org/learn/treatment-and-intervention/).
org/learn/treatment-and-intervention/).
Investigational Therapies
The development of animal models for fragile X syndrome studies over the last two decades resulted in great anticipation that effective drug treatments could be discovered. Some drugs, originally developed to treat other disorders, were shown to be effective in treating symptoms of fragile X syndrome in the animal models but when used on human subjects appeared to be less effective. However, there are many researchers who are actively working on treatments for fragile X syndrome. FRAXA, an organization actively searching for a fragile X syndrome cure, raises funds and supports extensive efforts for fragile X research. The FRAXA website (www.fraxa.org) is an excellent source of current information about their research projects and clinical trials.
Information on current clinical trials is posted on www.clinicaltrials.gov.
All studies receiving U.S. government funding, and some supported by private industry, are posted on this government web site.
For information about clinical trials being conducted at the NIH Clinical Center in Bethesda, MD, contact the NIH Patient Recruitment Office:
Tollfree: (800) 411-1222
TTY: (866) 411-1010
Email: [email protected]
Some current clinical trials also are posted on the following page on the NORD website:
https://rarediseases.org/for-patients-and-families/information-resources/news-patient-recruitment/
For information about clinical trials sponsored by private sources, contact:
www.centerwatch.com
For information about clinical trials conducted in Europe, contact:
https://www.clinicaltrialsregister.eu/
References
TEXTBOOKS
Willemsen, R and Kooy RF, editors. Fragile X Syndrome: From Genetics to Targeted Treatment. Academic Press (Elsevier), London, UK, 2017. https://www.elsevier.com/books-and-journals
Kasper, DL, Fauci AS, Longo DL, et al. Eds. Harrison’s Principles of Internal Medicine.16th ed. McGraw-Hill Companies. New York, NY; 2005.
Berkow R., ed. The Merck Manual-Home Edition.2nd ed. Whitehouse Station, NJ: Merck Research Laboratories; 2003.
Rimoin D, Connor JM, Pyeritz RP, Korf BR. Eds. Emory and Rimoin’s Principles and Practice of Medical Genetics. 4th ed. Churchill Livingstone. New York, NY; 2002.
Gelehrter TD, Collins FS, Ginsburg D. Principles of Medical Genetics. 2nd ed. Lippincott Williams & Wilkins. Philadelphia, PA; 2002.
Beers MH, Berkow R., eds. The Merck Manual, 17th ed. Whitehouse Station, NJ: Merck Research Laboratories; 1999.
Hagerman RJ and Hagerman PJ (eds) Fragile X Syndrome: Diagnosis, Treatment, and Research, 3 ed. The Johns Hopkins University Press, Baltimore; 287-338.
JOURNAL ARTICLES
Erickson CA, Davenport MH, Schaefer TL, Wink LK, et al. Fragile X targeted pharmacotherapy: lessons learned and future directions. J Neurodev Disord 2017; 9:7.
Raspa M, Wheeler AC, Riley C. Public health literature review of fragile X syndrome. Pediatrics 2017; 139 (Suppl 3): S153-S171.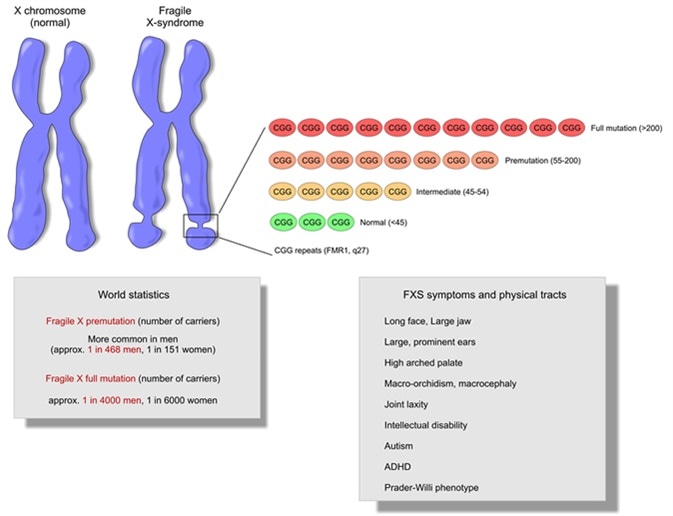
Quartier A, Poquet H, Gilbert-Dussardier B, Rossi M, et al. Intragenic FMR1 disease-causing variants: a significant mutational mechanism leading to Fragile-X syndrome. Eur J Hum Genet 2017; 25: 423-431.
Monaghan KG, Lyon E, Spector EB. ACMG Standards and guidelines for fragile X testing: a revision to the disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics and Genomics. Genet Med 2013 15:7: 575-586. (also available at http://www.acmg.net/docs/ACMG_SG_For_Fragile_X_Testing_7.24.13.PDF)
Van Esch H. The fragile X premutation: new insights and clinical consequences. Eur J Med Genet. 2006;49:1-8.
Glover TW, Arlt MF, Casper AM, Durkin SG. Mechanisms of common fragile site instability. Hum Mol Genet. 2005;14 Spec No. 2:R197-205.
Di Prospero NS, Fischbeck KH. Therapeutics development for triplet repeat expansion diseases. Nat Rev Genet. 2005;6:756-65.
Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet. 2005;6:743-55.
Diseases of unstable repeat expansion: mechanisms and common principles. Nat Rev Genet. 2005;6:743-55.
Willemsen R, Mientjes E, Oostra BA. FXTAS: a progressive neurological syndrome associated with fragile X premutation. Curr Neurol Neurosci Rep. 2005;5:405-10.
Vanderklish PW, Edelman GM. Differential translation and fragile X syndrome. Genes Brain Behav. 2005;4:360-84.
Terracciano A, Chiurazzi P. Neri G. Fragile X syndrome. Am J Med Genet C Semin Med Genet. 2005;137:32-37.
Visootsak J, Warren ST, Anido A, Graham JM Jr. Fragile X syndrome: an update and review for the primary care physician. Clin Pediatr (Phila). 2005;44:371-81.
Wattendorf DJ, Muenke M. Diagnosis and management of fragile X syndrome. Am Fam Physician. 2005;72:111-13.
INTERNET
The National Fragile X Foundation held an externally led patient-focused drug development meeting with the U.S. Food and Drug Administration (FDA) in March, 2021 and a Voice of the Patient report summarizes this meeting.
The National Fragile X Foundation maintains a comprehensive website of information about fragile X syndrome. Begin with www.fragilex.org and click on the “Learn” button to get information on various topics.
Saul RA, Tarleton JC. FMR1-Related Disorders. 1998 Jun 16 [Updated 2012 Apr 26]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1384/ Accessed September 13, 2017.
Fragile X syndrome. Genetics Home Reference. Last Comprehensive Review: April 2012. https://ghr.nlm.nih.gov/condition/fragile-x-syndrome Accessed September 13, 2017.
Online Mendelian Inheritance In Man (OMIM). McKusick VA, ed. The Johns Hopkins University. Fragile X Syndrome. Entry Number;3006240. Available at: https://www.omim.org/entry/300624?search=fragile%20x&highlight=x%20fragile Last Edit Date: 04/17/2017. Accessed September 13, 2017.
Jewell JA. Fragile X Syndrome. Medscape. Last Updated: April 6, 2016. http://emedicine.medscape.com/article/943776-overview Accessed September 13, 2017.
Fragile X Syndrome. Medscape. Last Updated: April 6, 2016. http://emedicine.medscape.com/article/943776-overview Accessed September 13, 2017.
Years Published
1988, 1989, 1990, 1991, 1994, 1995, 1997, 1998, 1999, 2006, 2010, 2017
The information in NORD’s Rare Disease Database is for educational purposes only and is not intended to replace the advice of a physician or other qualified medical professional.
The content of the website and databases of the National Organization for Rare Disorders (NORD) is copyrighted and may not be reproduced, copied, downloaded or disseminated, in any way, for any commercial or public purpose, without prior written authorization and approval from NORD. Individuals may print one hard copy of an individual disease for personal use, provided that content is unmodified and includes NORD’s copyright.
National Organization for Rare Disorders (NORD)
55 Kenosia Ave., Danbury CT 06810 • (203)744-0100
What is Fragile-X syndrome? – YourGenome
Genetics
- Fragile-X syndrome is caused by a mutation in a single gene, the Fragile-X mental retardation (FMR1) gene, which is located on the X chromosome.
- Normally, this gene contains a stretch of DNA in which a three base sequence (CGG) is repeated up to about 30 times in a row.
- In people with Fragile-X syndrome this three base sequence of DNA is repeated over 200 times. This abnormal repetition of bases creates the microscopic appearance of a gap on the long arm of the X chromosome (see illustration below), hence the name, ‘fragile’-X syndrome.
An illustration showing the appearance of normal and Fragile-X chromosomes.
Image credit: Genome Research Limited
Biology
- The FMR1 gene is responsible for providing instructions for making ‘Fragile-X mental retardation 1 protein’ (FMRP).
- This protein is required for the brain to develop normally. It plays a role in the development of synapses, specialised connections between our nerve cells which are critical for relaying nerve impulses.
- The Fragile-X mutation results in an FMR1 gene that is switched off, which means that FMRP is no longer produced, preventing normal neuronal development.

- Symptoms of Fragile-X syndrome are more severe in males compared with females. Because females have two X chromosomes, an inherited damaged FMR1 gene can be compensated for by an inherited healthy FMR1 gene. Unlike females, males only have one X chromosome, so an inherited damaged copy is their only copy.
Fragile X chromosome made visible by atomic force microscopy (AFM). The arrow indicates the fragile site.
Image credit: Dr Ben Oostra, Wellcome Images
Symptoms
- Fragile-X syndrome is the leading known genetic cause of autism (around 2-6 per cent of children with autism are diagnosed with Fragile-X syndrome).
- It is also the leading known cause of inherited intellectual disability.
Symptoms in boys
- Boys with Fragile-X syndrome have developmental delay, meaning they reach developmental milestones, such as sitting and talking, later than children without the condition.
- They show moderate to severe learning disabilities, combined with behavioural problems such as hyperactivity and autism.

- The physical signs of the disease become more pronounced as they get older. These include:
- a characteristic long face with jutting jaw
- large ears
- high forehead
- enlarged testicles
- flat feet
- unusually flexible finger joints.
Symptoms in girls
- Girls with Fragile-X syndrome have mild behavioural problems.
- 50 per cent of girls with the syndrome will show evidence of mental retardation.
- Like boys with the syndrome, girls tend to have large ears.
Diagnosis
- Testing for Fragile-X syndrome is recommended if:
- one or more of the disease symptoms are evident in a child
- the mother is known to carry a pre- or full mutation in the FMR1 gene. A pre-mutation is a FMR1 gene with 55 to 200 repeats of the CGG triplet. Women with the pre-mutation have an increased risk of having a child with Fragile-X syndrome.
- if there is a history of unexplained mental retardation in the family.
- Testing is carried out by looking directly at the FMR1 gene in cells obtained from a blood sample. The number of repeated CGG triplets is counted within the FMR1 gene to identify if there are more than 200 repeats.
- Prenatal diagnosis testing is also possible to determine whether or not the baby has inherited the abnormal gene.
Treatment
- There is currently no cure for Fragile-X syndrome.
- Drugs can be used to prevent seizures and treat some of the common behavioural problems.
- Children with Fragile-X syndrome often benefit from specialist education including occupational and speech therapy.
This page was last updated on 2021-07-21
DNA diagnosis of Martin-Bell syndrome (fragile X chromosome)
PRICES
*Prices for foreign citizens to be specified by phone or by e-mail
Dear patients, Prices for services may change and differ from the prices on the site, check the actual cost of services with the administrator.
Prenatal diagnosis
PRENATAL DNA diagnostics of neurosoenory non-rundromic hearing loss (by choriona villi or amniocytes)
5 410.00
PRENATAL DNA diagnostics of monciacidosis (according to choriona villi or amniocytes) 9000 9000 5 820.00
-Bell (fragile X chromosome) (by chorionic villi or amniocytes)
9,560.00
Prenatal DNA diagnosis of spinal muscular atrophy (by chorionic villi or amniocytes)
4 940.00
PRENATAL DNA diagnostics of Rhesus belonging to embryo (according to choriona villi or amniocytes)
4 990.00
PRENATAL DNA diagnostics of phenylketonuria (by choriona villi or amniocyte)
9000 5 260.00 9000 Prenatal sex determination of the fetus in X-linked diseases (by chorionic villi or amniocytes)1,610.00
DNA test for maternal contamination
4,740.00
Determination of the karyotype of amniocytes of a pregnancy that is developing
3 690.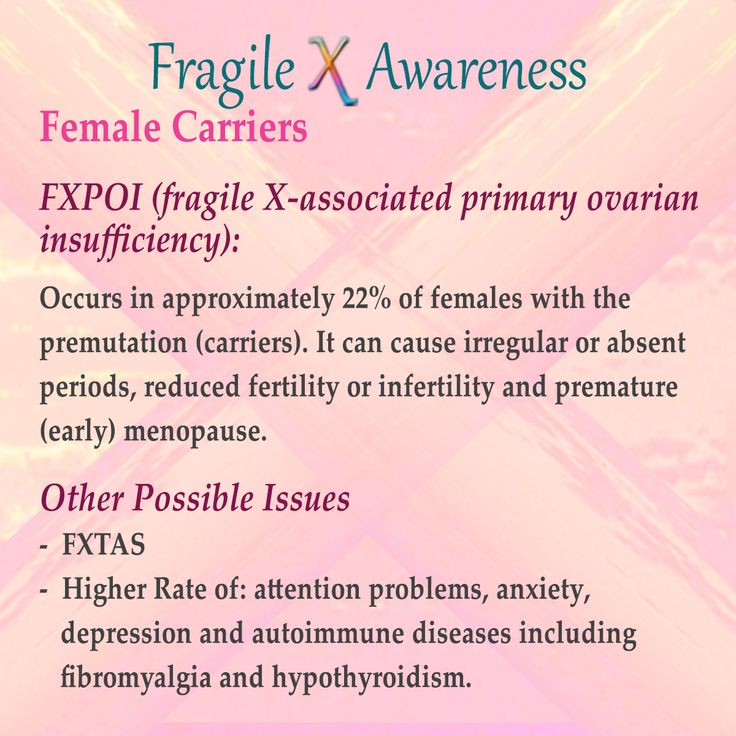 00
00
Determination of the carutation of developing pregnancy, Fish method
4 500.00
Determination of the Kariotip of the villi chorion in the abortive material
9000 3 600.00Determination of the Kariotip of the Vorsin of the Helpers of the Chorion, which develops
3 3 420.00
Determination of the karyotype of the chorionic villi of a developing pregnancy, FISH method
3,890.00
Determination of the fetal karyotype from cord blood
3040.00
Molecular diagnostic studies
DISCOUTION OF DNA from peripheral blood leukocytes
540.00
Determination of the zygity of the gene RHD
2 720.00
Determination of the mutations of genes BRCA1 and BRCA2 9000 9000 9000 3 500
DNA- DNA- diagnosis of an unidentified mutation (stage I)
14,000.00
DNA diagnosis of an unidentified mutation (stage II)
28 000.00
DNA diagnostics of an unidentified mutation (III Art. Difficulty)
Difficulty)
42 000.00
DNA diagnostics of microelements Y-chromosomes
1 940.00
DNA diagnostics of microstructural chromosome anomalies by comparative genomic genome hybridization
48,800.00
Preimplantation genetic diagnosis, comparative genomic hybridization, 24 chromosomes, number of embryos no more than 8 8, no blastocyst/blastomere biopsy
64,080.00
Pre-implantation genetic diagnosis: comparative genomic hybridization, 24 chromosomes, for each additional embryo over 8.
8 900.00
Predimplantation genetic diagnostics by the PCR method (I Art. Complexity)
14 000.00
Predictive genetic diagnostics by PCR (II Art. Complexity)
28 000.00
Predimplantation genetic diagnosis by the method PCR (III degree of complexity)
42 000.00
DNA diagnostics of cystic fiber
2 170.00
DNA diagnostics of neurosenoric non-runsrine hearing loss (GJB2 gene)
1 220. 00
00
DNA diagnostics of Martin-Bell syndrome (Lomka X-HiMosoma )
6520.00
DNA diagnostics of spinal muscular atrophy
1260.00
DNA diagnostics of phenylketonuria0006
8 040.00
PRENATAL DNA diagnostics of neurosenate non-rural heat loss (by choriona villi or amniocytes)
5 410.00
PRENATAL DNA diagnostics of chvicycidosis (by choriona villi or amniocytes)
9000 5 820.00 9000 9000 9000 9000 9000 9000 Prenatal DNA diagnosis of Martin-Bell syndrome or fragile X chromosome (by chorionic villi or amniocytes)9,560.00
Prenatal DNA diagnosis of spinal muscular atrophy (by chorionic villi or amniocytes)
4 940.00
PRENATAL DNA diagnostics of the embryo Rhesus (according to choriona villi or amniocytes)
4 990.00
PRENATAL DNA diagnostics of phenylketonuria (by choriona villi or amniocyte)
9000 5 260.00 Prenatal sex determination of the fetus in X-linked diseases (by chorionic villi or amniocytes) 1,610. 00
00
cystic fibrosis, DNA diagnosis of spinal muscular atrophy)
7,440.00
Program for future parents "Responsible Parenthood" EXPANDED (DNA diagnosis 289 genes)
15,750.00
HLA-typing (2 persons)
16,640.00
HLA-typing (1 person)
8425.00
blood of a pregnant woman)
16,000.00
VIEW ALL PRICES The partner of the clinic is the maternity hospital "Leleka"SIGN UP
"Fragile patient" or frailty syndrome
Senile frailty (or "fragility" - frailty) is a term presented in the international classification of diseases as R54, which combines about 85 different geriatric conditions and syndromes. However, most often it is:
- loss of body weight (sarcopenia) of at least 4.5 kg per year;
- decrease in the strength of the hands according to dynamometry;
- decrease in physical activity:
- slowing down the speed of movement of the patient;
- severe general weakness and increased fatigue.
The combination of at least three or more of the above criteria allows the patient to be diagnosed with SAS, and the presence of 1 or 2 symptoms is in favor of preasthenia.
The risk of developing senile asthenia syndrome (SSA) naturally increases in patients over 80 years of age, however, according to recent data, premature asthenia in patients aged 60-70 years is not ruled out.
There are predisposing risk factors in the genesis of SAS. These include age , gender (it is believed that frailty is significantly more common in women), education level (namely, in people with lower education), marital status (most often asthenia occurs in divorced patients or widowers, in persons who have never been married), socio-economic conditions (in Russia, 84% of the senile population has SAS), urbanization (residents of rural areas are more susceptible to the development of SAS ).
Below is an algorithm for diagnosing SAS according to the “age is not a hindrance” system for all patients over 65 years of age and the advisability of consulting a gerianthologist.
| Patient >= 60 years old → Age is no barrier screening | ||
| Question | Answer | |
| 1 | Have you lost weight for 6 or more months 900? (Weight) | Yes/No |
| 2 | Do you experience any limitations in daily life due to visual or hearing loss? | Yes/No |
| 3 | Have you had a fall-related injury in the last year? | Yes/No |
| 4 | Have you been feeling depressed, sad or anxious during the last weeks? (Mood) | Yes/No |
| 5 | Did you have problems with memory, understanding, orientation, or the ability to plan? | Yes/No |
| 6 | Do you suffer from urinary incontinence? | Yes/No |
| 7 | Do you have difficulty moving around the house or outside? (Walking up to 100 m or lifting to 1 staircase flight) | Yes/no |
| "Fragile" patients 9000 | 0 - very good; 0. 0.5 - medium; 0.75 - bad; 1, 0 point - very bad. | ||
| 2 | the presence, according to the patient, of at least one of the following diseases: arthrosis, history of stroke, coronary artery disease, diabetes mellitus, COPD, bronchial asthma, anxiety-depressive syndrome, arterial hypertension, cataract 9006 | 0 - no diseases; 1, 0 — diseases are present activity; the ability to stretch your arms forward and hold them; the ability to concentrate; walk for a long time; the ability to wash, dress, work independently; take a bath, use the toilet, carry objects, the safety of the emotional sphere) | 0 - no problem; 0, 25 - slight decrease in abilities; 0.5 — moderate decrease; 0.75 - pronounced decrease; BMI >18.5 1.0 - BMI < 18.5 |
| 5 | muscle strength during carpal dynamometric test | 0 — no weakness; 1, 0 — presence of weakness | |
| 6 | ability to walk fast | 0 points — preserved; 1. |
The higher the frailty index, the more the patient needs outside care:
0 - 0.2 points - no signs of frailty.
0.2 - 0.4 points - moderately pronounced signs of senile asthenia.
0.4 points and above - pronounced senile asthenia.
The first letters of the English term "senile asthenia" FRAILTY combine the following principles of prevention:
F (food intake maintenance) - implies the control of food intake and diet regulation;
R (resistance exercises) - physical activity;
A (atherosclerosis prevention) - prevention of the development of atherosclerosis;
I (isolation avoidance) - avoid social isolation;
L (limit pain) - stop pain;
T (tai-chi or other balance exercises)
Y (yearly functional checking) - regular medical check-ups.
Thus, "fragile" patients with frailty syndrome need special attention from doctors and medical personnel, an individual approach.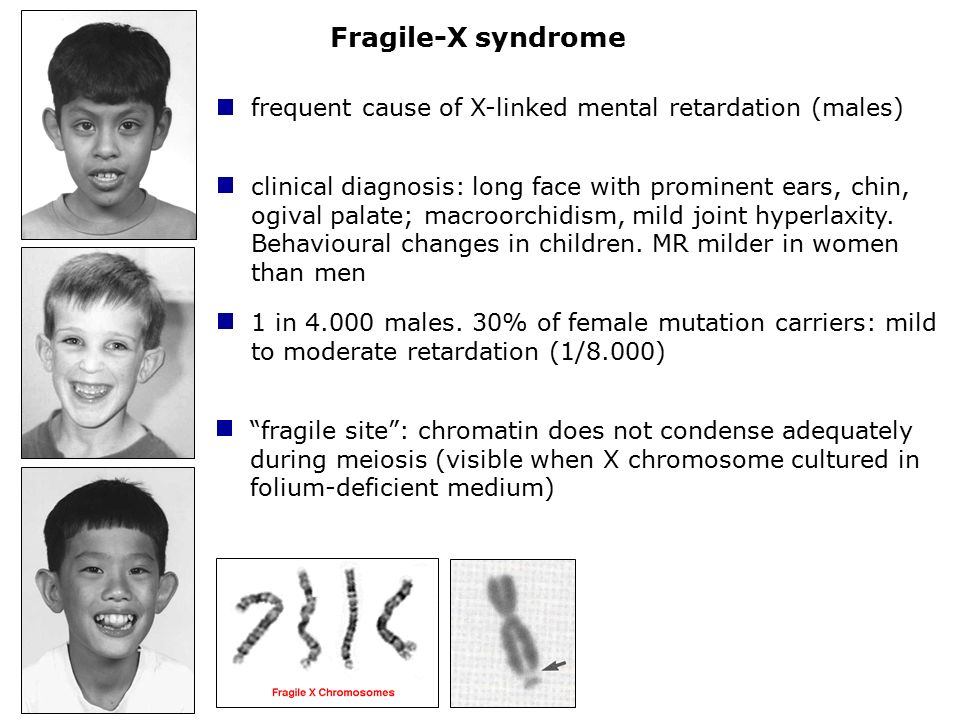
 25 - very good;
25 - very good; 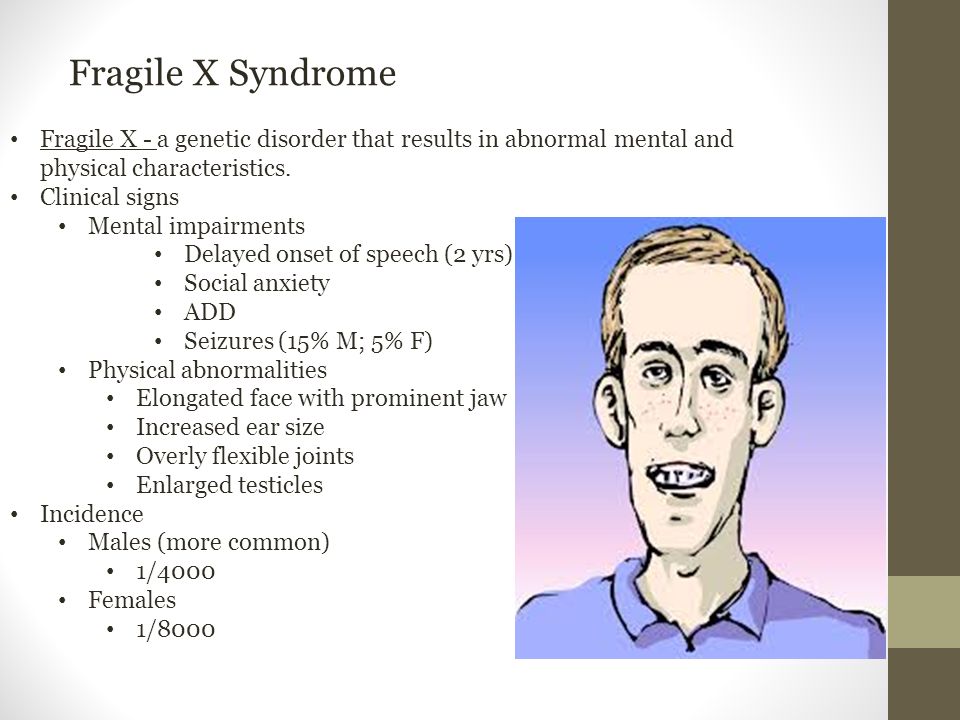 0 point - not saved
0 point - not saved