Where is the umbilical cord attached to
What is the umbilical cord?
The umbilical cord is a lifeline that connects you to your baby during pregnancy. It has three blood vessels: One vein delivers nutrients and oxygen-rich blood to your baby, and two arteries carry waste from your baby back to you. The umbilical cord is cut soon after birth, and your baby's remaining umbilical cord stump will dry and fall off one to three weeks after birth. Your baby will always carry a reminder of the time they spent attached to you: their belly button!
What is the umbilical cord?
During pregnancy, the umbilical cord is your baby’s lifeline to the placenta, the pancake-shaped organ attached to your uterus. It delivers vital nutrients from your body to your baby, and ferries away the waste products your baby produces. The umbilical cord starts to form at about 4 weeks of pregnancy.
The umbilical cord is surprisingly thick and tough, and typically measures about 20 inches long and 1 inch in diameter. It contains three blood vessels: one vein that carries nutrients and oxygen-rich blood from your circulatory system to your baby, and two arteries that deliver waste products and oxygen-depleted blood back to you. Your kidneys process your baby’s wastes along with yours for disposal and your lungs replenish the blood with oxygen.
Cutting the umbilical cord
Soon after your baby is delivered, it’s time to clamp and cut the cord. Your partner or labor support person can cut the cord themselves, if they'd like.
First, your practitioner uses two special clamps to close the cord shut, usually in two places about one inch apart. It takes a bit of effort to cut through the thick, rope-like cord, but not to worry: There aren’t any nerves in the cord, so this procedure is painless for your baby (and for you).
When does the umbilical cord fall off?
Once the cord is cut, a small umbilical cord stump is left behind. This piece of tissue eventually dries up and falls off in one to three weeks. Until it does, you’ll need to keep it clean and dry. Find out more about caring for your baby's umbilical cord stump.
Of course, your baby will carry a small remnant of the time they spent attached to you: their belly button! You have no control over whether your baby ends up with an innie or an outie.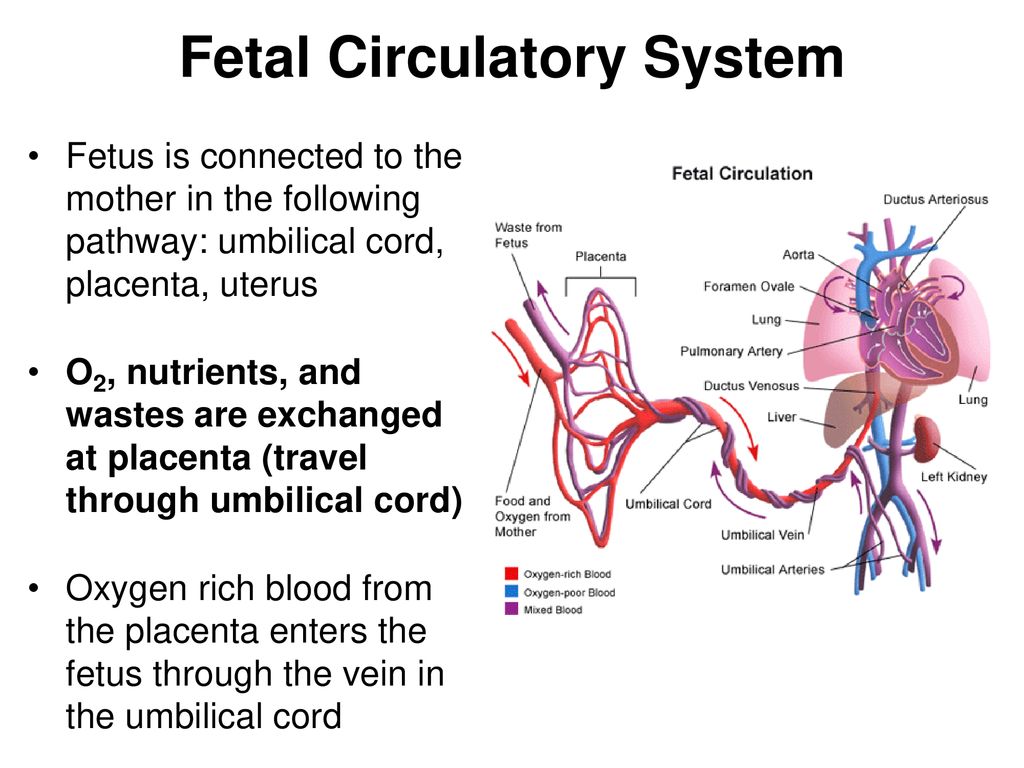 How your baby's belly button looks depends on how the umbilical cord was attached to your baby’s belly during pregnancy, and it can change over time as your child grows.
How your baby's belly button looks depends on how the umbilical cord was attached to your baby’s belly during pregnancy, and it can change over time as your child grows.
Delayed cord clamping
Traditionally, practitioners in the United States cut the baby's umbilical cord almost immediately after birth, but research shows that delayed umbilical cord clamping has health benefits, particularly if your baby is born prematurely.
Waiting to clamp the cord allows the blood in the cord to continue to flow to your baby, lowering the risk of newborn anemia and iron deficiency in infancy.
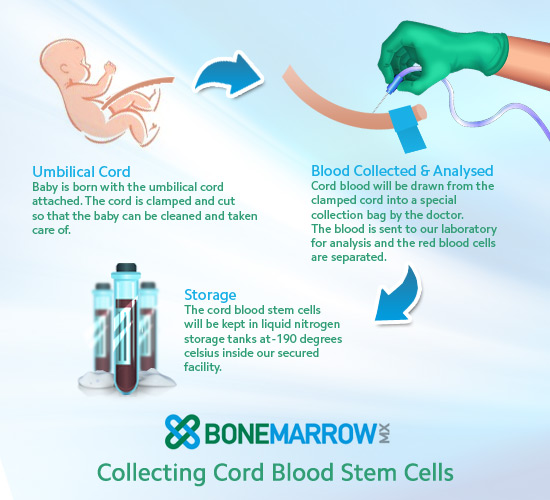
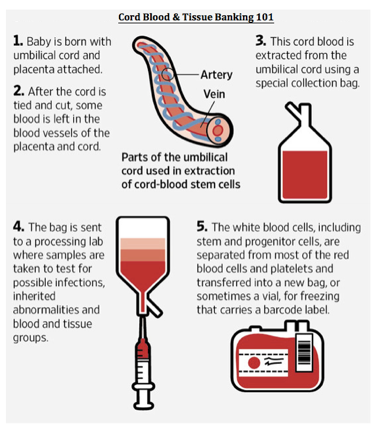
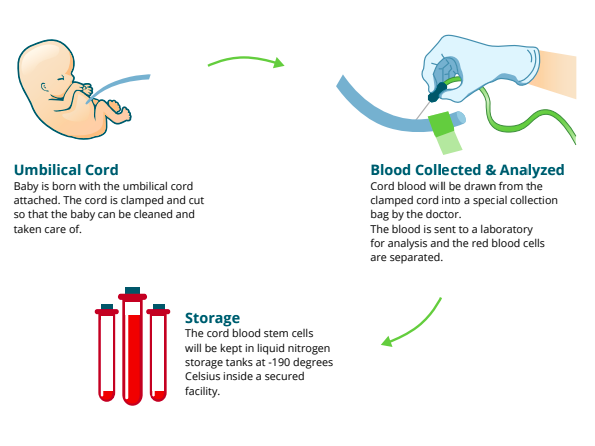
Umbilical cord blood banking
You might want to consider banking your baby's cord blood. Cord blood contains stem cells that can grow into different kinds of body cells and have been used to treat certain diseases, including some cancers and blood disorders.
Advertisement | page continues below
For a yearly fee (plus the cost of collecting the cord blood), you can store some of your baby’s cord blood in a private bank for future use by her or other family members.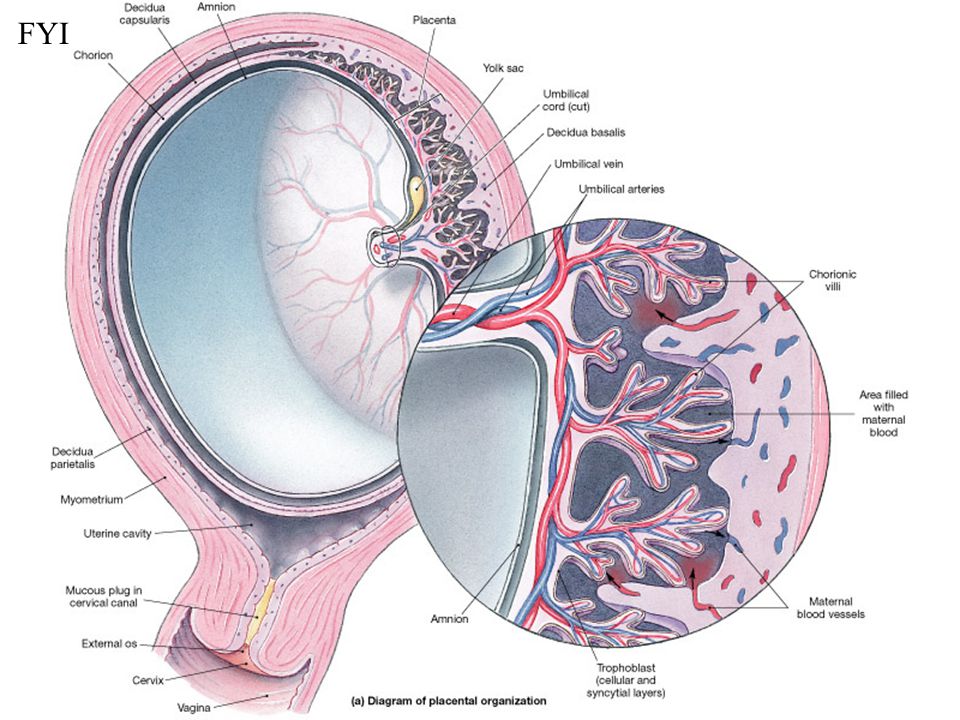 Or you can donate your baby’s cord blood to a public bank, where it remains available to anyone who needs it and who matches your baby’s blood type.
Or you can donate your baby’s cord blood to a public bank, where it remains available to anyone who needs it and who matches your baby’s blood type.
Keep in mind that you’ll need to decide what you want to do well in advance of your baby’s birth so that all the necessary arrangements can be made. Here's a rundown of everything you need to know about cord blood banking.
Umbilical cord abnormalities
Some conditions can arise during pregnancy and delivery that affect the umbilical cord. Most of the time, they resolve on their own and don’t harm your baby. A few, however, can be serious. The most common umbilical cord conditions include the following:
Nuchal cord
The umbilical cord may be wrapped around the baby’s neck during delivery. This condition is actually quite common. In most cases, the cord is simply untangled from the baby’s neck once the head is out, and delivery proceeds normally. But if the cord is wrapped very tightly, your healthcare provider may need to clamp and cut it before the baby’s shoulders are delivered.
Umbilical cord compression
This occurs when the cord becomes constricted, cutting off a baby’s oxygen supply. The cord may have become knotted or tangled, which can happen if it’s unusually long or if the baby’s head is pressing on it during delivery. Umbilical cord compression usually can be detected during labor by changes in the baby’s heart rate. A technique called amnioinfusion, in which fluid is inserted into the uterus through a thin tube, may help take the pressure off the cord and allow delivery to proceed normally.
Umbilical cord prolapse
This is a serious delivery complication: A cord prolapse means the umbilical cord comes out of the vagina as the baby’s head is delivered, which can cut off blood flow to the baby. An emergency c-section is usually performed in such cases.
Single umbilical artery
A very small number of babies have only one umbilical artery instead of two. This condition occurs more often when the woman is carrying two or more babies. Having only one umbilical artery can cause problems for the baby’s digestive organs, kidneys, and heart. If you’re diagnosed with this condition during pregnancy, you’ll have special tests to monitor your baby’s health along the way.
Having only one umbilical artery can cause problems for the baby’s digestive organs, kidneys, and heart. If you’re diagnosed with this condition during pregnancy, you’ll have special tests to monitor your baby’s health along the way.
Umbilical cord cysts
These are pockets of fluid that form in the cord. One type, called a true cyst, is harmless and often goes away on its own before the baby is born. It’s usually discovered during a first-trimester ultrasound. However, cysts that are found later in the pregnancy may indicate a genetic condition or other problem. If this type of cyst is found, your healthcare provider will likely recommend a detailed ultrasound exam and genetic testing.
Velamentous cord insertion
Velamentous cord insertion and marginal cord insertion mean that the umbilical cord is attached to the amniotic membrane or the side of the placenta rather than going right into the center of the placenta as usual. This leaves the vessels of the cord more exposed than they would be if they were attached directly into the placenta, where they would be protected by a gel-like substance called Wharton's jelly.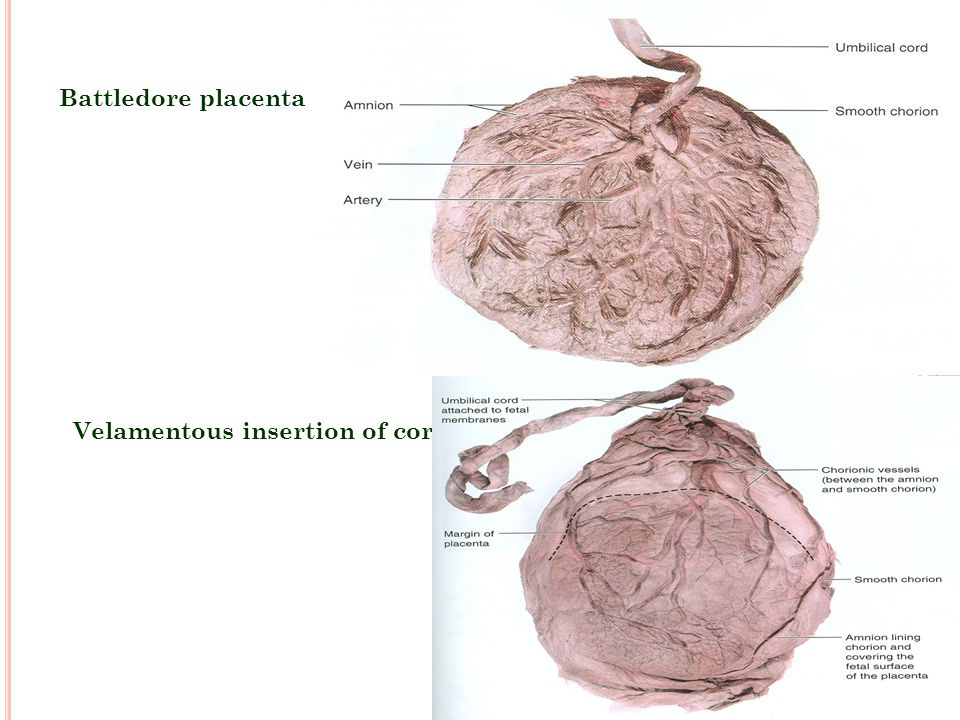
Vasa previa
In vasa previa, some of the fetal blood vessels are exposed and cross over the cervical opening, instead of being contained in the umbilical cord. When contractions happen, these blood vessels stretch and may rupture, resulting in catastrophic fetal blood loss and fetal distress. This is a very serious condition and may require prolonged monitoring in the hospital.
Embryology, Umbilical Cord - StatPearls
Introduction
The umbilical cord is the vital connection between the fetus and the placenta. Umbilical cord development begins in the embryologic period around week 3 with the formation of the connecting stalk. By week 7, the umbilical cord has fully formed, composed of the connecting stalk, vitelline duct, and umbilical vessels surrounding the amniotic membrane. The umbilical vessels carry the fetal blood back and forth to the placenta, with the umbilical vein carrying oxygenated blood with nutrients from the placenta to the fetus and the umbilical arteries transporting deoxygenated blood with waste products from the fetus to the placenta.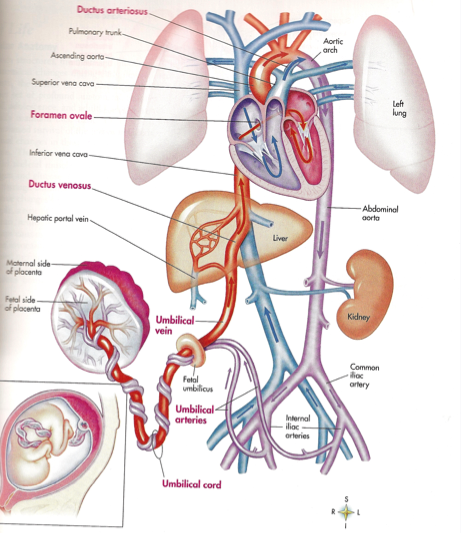 Embryonic structures regress near the end of the first trimester, leaving the umbilical cord composed of two umbilical arteries and one umbilical vein surrounding by a gelatin-like extracellular matrix known as Wharton’s jelly. Elongation of the umbilical cord occurs primarily in the second trimester. The average umbilical cord is 50 to 60 centimeters in length, 2 centimeters in diameter, with up to 40 helical turns. Abnormalities of the umbilical cord can lead to increased morbidity and mortality of the fetus.
Embryonic structures regress near the end of the first trimester, leaving the umbilical cord composed of two umbilical arteries and one umbilical vein surrounding by a gelatin-like extracellular matrix known as Wharton’s jelly. Elongation of the umbilical cord occurs primarily in the second trimester. The average umbilical cord is 50 to 60 centimeters in length, 2 centimeters in diameter, with up to 40 helical turns. Abnormalities of the umbilical cord can lead to increased morbidity and mortality of the fetus.
Development
The development of the umbilical cord begins in the third week of embryologic formation. The developing embryo consists of a trilaminar disc attached to the decidua basalis by the connecting stalk, the primitive umbilical cord.[1][2] The connecting stalk is a thick stalk of the extraembryonic membrane extending from the caudal end of the embryo to the center of the developing placenta on the decidua basalis.[3] The process of body folding occurs during week four with rapid growth amnion and embryonic disc compared to the yolk sac. Cranial caudal folding causes approximation of the connecting stalk and yolk sac on the ventral surface of the embryo.[1][2] The amnion expands to cover the entire embryo except for the rudimentary umbilical ring, where the connecting stalk and yolk sac emerge.[1][2] During this time, the allantois, an outpouching of the endodermal hindgut, forms and extends into the connecting stalk.[1][2][4] Between the fourth and eighth weeks, there is an increase in amniotic fluid production, which causes the amniotic cavity to swell and fill the chorionic space. This increase in the amniotic fluid also causes elongation of the connecting stalk, and the yolk sac is compressed down within the connecting stalk to form the omphalomesenteric or vitelline duct.[1][2][5] The expansion of the amniotic cavity causes the amnion and the chorion to come into contact, and the extraembryonic mesoderm covering these two layers fuses. As such, the chorionic cavity disappears, leaving the umbilical cord, the composite of the connecting stalk and vitelline duct surrounded by the amnion, floating in the amniotic fluid.
Cranial caudal folding causes approximation of the connecting stalk and yolk sac on the ventral surface of the embryo.[1][2] The amnion expands to cover the entire embryo except for the rudimentary umbilical ring, where the connecting stalk and yolk sac emerge.[1][2] During this time, the allantois, an outpouching of the endodermal hindgut, forms and extends into the connecting stalk.[1][2][4] Between the fourth and eighth weeks, there is an increase in amniotic fluid production, which causes the amniotic cavity to swell and fill the chorionic space. This increase in the amniotic fluid also causes elongation of the connecting stalk, and the yolk sac is compressed down within the connecting stalk to form the omphalomesenteric or vitelline duct.[1][2][5] The expansion of the amniotic cavity causes the amnion and the chorion to come into contact, and the extraembryonic mesoderm covering these two layers fuses. As such, the chorionic cavity disappears, leaving the umbilical cord, the composite of the connecting stalk and vitelline duct surrounded by the amnion, floating in the amniotic fluid. [1][2][4]
[1][2][4]
Starting in week three, endothelial precursor cells in the mesoderm surrounding the allantois coalesce to form small capillaries. Vasculogenesis continues, and by the end of the third week, the capillaries have grown to establish a functional vascular network within the connecting stalk. During the same period, the arterial and venous systems within the embryo are developing. The arterial system is initially established as the paired dorsal aortae from which the aortic arches originate. The primitive venous system is initially made up of the umbilical, vitelline, and cardinal systems. Early in the fourth week, two umbilical arteries branch from the paired dorsal aortae to become connected to the vascular network of the umbilical cord.[1] During the fifth week, this connection is obliterated as the umbilical arteries develop their connection to a branch of the fifth pair of lumbar intersegmental arteries that will later become the internal iliac arteries.[1][2][4] The umbilical veins are originally bilateral and drain into the right and left sinus horns of the sinus venosus.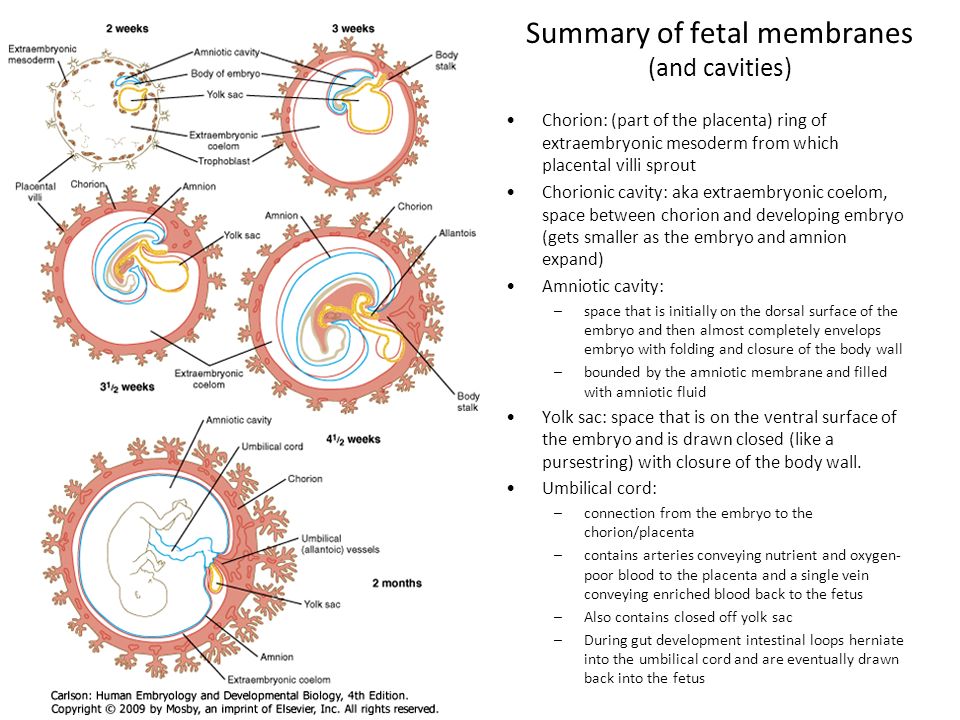 The connections of the umbilical veins to the sinus horns regress in the second month with complete regression of the right umbilical vein as the left umbilical vein persists and forms its connection to the ductus venosus within the developing liver.[2][4] With the initiation of fetal heart pumping around week four, the umbilical arteries carry deoxygenated blood to the placenta, and the umbilical vein carries oxygenated blood back to the fetus from the placenta.[4]
The connections of the umbilical veins to the sinus horns regress in the second month with complete regression of the right umbilical vein as the left umbilical vein persists and forms its connection to the ductus venosus within the developing liver.[2][4] With the initiation of fetal heart pumping around week four, the umbilical arteries carry deoxygenated blood to the placenta, and the umbilical vein carries oxygenated blood back to the fetus from the placenta.[4]
By week seven, the intestines begin to herniate out of the embryo through the umbilical ring and into the umbilical cord.[4][6][2] This physiologic herniation is necessary for proper rotation of the intestines and adequate growth of the fetus to house the expanding intestines.[6] The rapid development of the intestines causes elongation of the umbilical cord.[6] Between weeks ten and twelve, the intestines leave the umbilical cord and return to the abdominal cavity.[4][2][6] During this time, the extraembryonic mesoderm develops a rich extracellular matrix to protect the cord called Wharton’s jelly.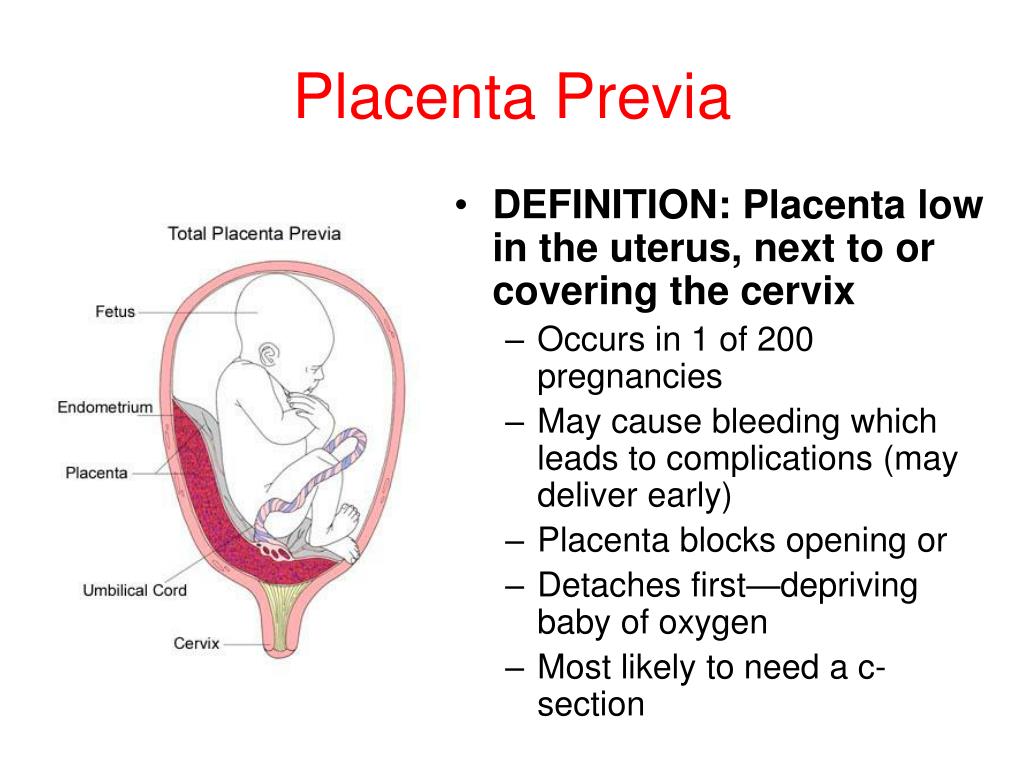 [2][7] The umbilical cord continues to elongate during the second trimester with a length comparable to the crown-rump length of the fetus.[1][8] By term, the vitelline duct and allantois have typically completely involuted.[1][5] However, in some cases, remnants of the allantois and vitelline duct can be found in the umbilical cord proximal to the neonate.[2][5] At birth, the cord typically measures an average of 50 to 60 cm in length and 2 cm in diameter with up to 40 helical turns.[4][8] After the birth of the neonate, the umbilical cord is clamped and then cut as the neonate now breathes on its own, and the remainder of the umbilical cord is delivered along with the placenta.
[2][7] The umbilical cord continues to elongate during the second trimester with a length comparable to the crown-rump length of the fetus.[1][8] By term, the vitelline duct and allantois have typically completely involuted.[1][5] However, in some cases, remnants of the allantois and vitelline duct can be found in the umbilical cord proximal to the neonate.[2][5] At birth, the cord typically measures an average of 50 to 60 cm in length and 2 cm in diameter with up to 40 helical turns.[4][8] After the birth of the neonate, the umbilical cord is clamped and then cut as the neonate now breathes on its own, and the remainder of the umbilical cord is delivered along with the placenta.
Cellular
Mesenchymal stem cells found within the Wharton’s jelly of the umbilical cord express c-kit and telomerase activity, consistent with the markers of stem cells.[9][10][11] These cells can be easily extracted after delivery and offer a source of stem cells with fewer ethical considerations than other sources. [7][10] These cells have shown the ability to differentiate into neurons and glia when exposed to specific growth factors.[9] Additionally, these cells may play a role in treating autoimmune disease due to their ability to suppress the secretion of interferon-gamma and transform growth factor-beta1.[10] A more recent study completed on rats showed that transplantation of Wharton’s jelly into the site of traumatic brain injury could reduce the amount of brain damage by reducing brain edema and increasing expression of brain-derived neurotrophic factor.[7] Researchers continue to investigate Wharton’s jelly and mesenchymal stem cells for their potential therapeutic and technological roles.[10][12][13]
[7][10] These cells have shown the ability to differentiate into neurons and glia when exposed to specific growth factors.[9] Additionally, these cells may play a role in treating autoimmune disease due to their ability to suppress the secretion of interferon-gamma and transform growth factor-beta1.[10] A more recent study completed on rats showed that transplantation of Wharton’s jelly into the site of traumatic brain injury could reduce the amount of brain damage by reducing brain edema and increasing expression of brain-derived neurotrophic factor.[7] Researchers continue to investigate Wharton’s jelly and mesenchymal stem cells for their potential therapeutic and technological roles.[10][12][13]
Biochemical
The Wharton’s jelly of the umbilical cord is a gelatin-like structure rich in proteoglycans, specifically hyaluronic acid and chondroitin sulfate.[11] Hyaluronic acid is present throughout the body in connective and epithelial tissues.[14] It is a disaccharide polymer made of alternating glycosidic bonds between D-glucuronic acid and N-acetyl-D-glucosamine. [14][15] The number of repeats differs between tissues, but hyaluronic acid purified from the umbilical cord is 3,140,000 Da in size.[16] Chondroitin sulfate is a disaccharide consisting of repeats of N-acetylgalactosamine and iduronic acid.[15] Together, these compounds contribute to cellular hydration and the scaffolding of the umbilical cord.[11][15]
[14][15] The number of repeats differs between tissues, but hyaluronic acid purified from the umbilical cord is 3,140,000 Da in size.[16] Chondroitin sulfate is a disaccharide consisting of repeats of N-acetylgalactosamine and iduronic acid.[15] Together, these compounds contribute to cellular hydration and the scaffolding of the umbilical cord.[11][15]
Molecular
Wharton’s jelly is the gelatinous extracellular matrix contained within the umbilical cord that serves as protection for the umbilical vessels.[9][17][4] It prevents the umbilical cord from compressing and provides flexibility to allow for fetal movement within the amniotic cavity.[4][17] It originates from the extraembryonic mesoderm and contains proteoglycans, specifically hyaluronic acid and chondroitin sulfate.[11][17][18] As opposed to other tissues in the body, Wharton’s jelly contains no capillaries.[11] When exposed to temperature changes, such as after delivery of the fetus, the structure of Wharton’s jelly collapses, contributing to the physiological clamping of the cord.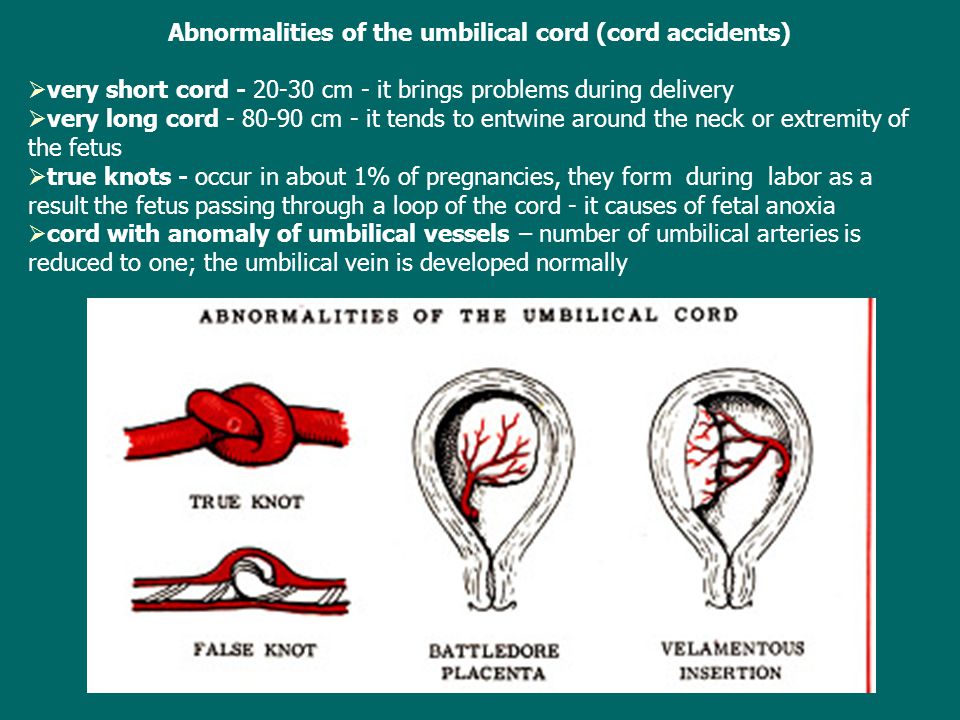 [19]
[19]
Function
The main function of the umbilical cord is to house the umbilical vessels, which circulate blood between the embryo and the placenta. The umbilical arteries and veins are the vital connection carrying blood between the growing fetus and the placenta.[4] Without this connection to the placenta, the fetus would have no way to receive oxygen and other nutrients or filter out carbon dioxide, urea, and other waste products.[4][20] With the expansion of the amniotic cavity and elongation of the umbilical cord, the fetus has ample space for movement and growth.[4][21][17] During this time in utero, Wharton’s jelly protects the umbilical vessels so the fetus can move and turn without compression of its blood supply.[7][17][22]
Mechanism
The umbilical vein carries fetal blood from the placenta to the fetus, providing the necessary oxygen and nutrients.[4] Normally found at the 12 o'clock position when facing the umbilicus of the fetus, the umbilical vein is recognizable by its thinner wall and larger lumen in comparison to the arteries.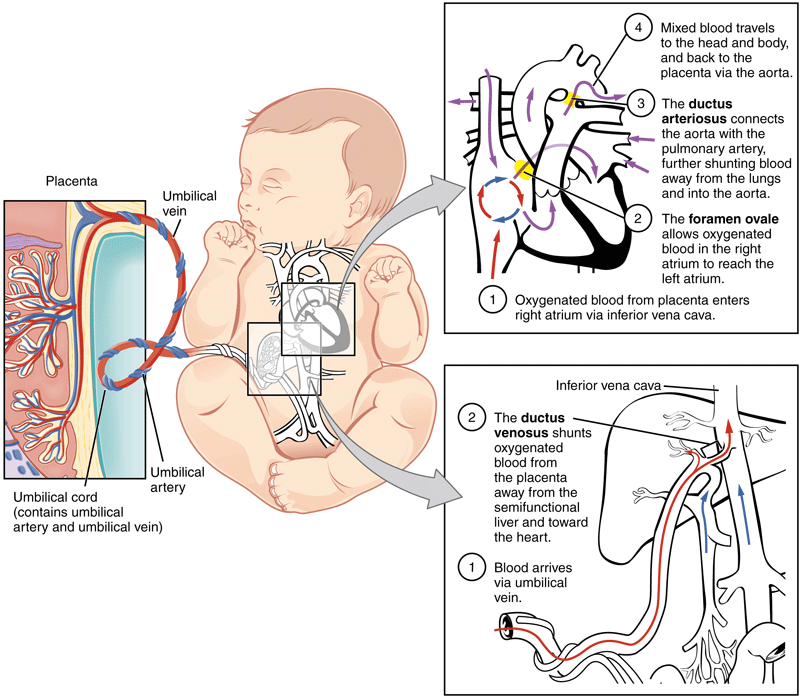 [8][23][2] Blood flowing through the umbilical vein enters the fetus through the umbilical ring and passes through ductus venosus before entering the inferior vena cava.[8][23] In return, the two umbilical arteries carry deoxygenated fetal blood containing waste products from the internal iliac arteries back to the placenta.[4][23] The exchange of these materials happens in the intervillous spaces of the placenta between the maternal and fetal blood supplies.[4][20] Wharton's jelly, the gelatin-like extracellular matrix surrounding the umbilical vessels, provides an elastic cushioning resistant to compression and twisting, allowing for continued blood flow with fetal movement.[7][17][21][22][4] There are several hypotheses regarding how the umbilical cord develops its helices, including differential flow through umbilical arteries and twisting of the intestines within the cord. The belief is that adequate coiling contributes to the strength of Wharton's jelly in protecting the umbilical vessels from compression.
[8][23][2] Blood flowing through the umbilical vein enters the fetus through the umbilical ring and passes through ductus venosus before entering the inferior vena cava.[8][23] In return, the two umbilical arteries carry deoxygenated fetal blood containing waste products from the internal iliac arteries back to the placenta.[4][23] The exchange of these materials happens in the intervillous spaces of the placenta between the maternal and fetal blood supplies.[4][20] Wharton's jelly, the gelatin-like extracellular matrix surrounding the umbilical vessels, provides an elastic cushioning resistant to compression and twisting, allowing for continued blood flow with fetal movement.[7][17][21][22][4] There are several hypotheses regarding how the umbilical cord develops its helices, including differential flow through umbilical arteries and twisting of the intestines within the cord. The belief is that adequate coiling contributes to the strength of Wharton's jelly in protecting the umbilical vessels from compression. [4][17]
[4][17]
After birth, closure of the umbilical arteries initiates by the contraction of circularly arranged smooth muscle within the vascular wall.[19][2] Physiologic closure of the umbilical vein occurs after the umbilical arteries, allowing for prolonged communication and possible transfusion of the remaining placental blood to the neonate.[2][8] The remnants of the umbilical arteries within the neonate become the medial umbilical ligaments, found on the anterior abdominal wall running from the umbilicus inferiorly to the pelvis.[2][19] The remnant of the umbilical vein becomes the ligamentum teres hepatis, which extends superiorly from the umbilicus to connect to the falciform ligament of the liver.[2][8]
Testing
The most useful investigational tool in utero is the ultrasound.[4] It can be useful in the evaluation of fetal anatomy, measuring amniotic fluid levels, watching for fetal movement, and visualizing fetal blood flow. Continuous Doppler ultrasound can visualize the blood flow through the umbilical artery.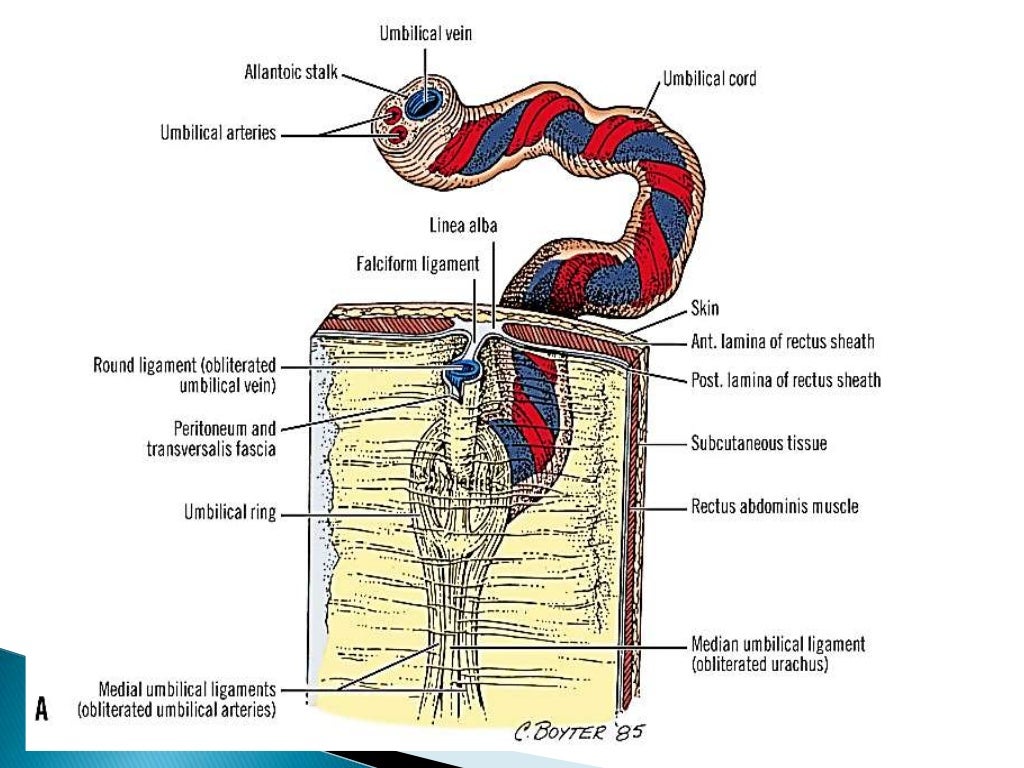 [4][24][25] This information is then used to create a velocity waveform that can predict the amount of vascular resistance in the placenta.[4] A high placental vascular resistance is associated with intrauterine growth restriction, and the presence of abnormal reverse flow through the umbilical artery can help to determine the need for early delivery.[4][24] When assessing high-risk pregnancies, the evaluation of umbilical artery Doppler velocimetry is utilized to decrease perinatal mortality.[25] Additionally, during high-risk deliveries and in cases of neonatal depression, umbilical cord arterial blood gases should be collected.[23] Umbilical artery pH and gas analysis are the most reliable tests to assess fetal oxygenation and acid-base in the perinatal period directly. A normal pH and gas analysis at delivery rules out intrapartum asphyxia.[23]
[4][24][25] This information is then used to create a velocity waveform that can predict the amount of vascular resistance in the placenta.[4] A high placental vascular resistance is associated with intrauterine growth restriction, and the presence of abnormal reverse flow through the umbilical artery can help to determine the need for early delivery.[4][24] When assessing high-risk pregnancies, the evaluation of umbilical artery Doppler velocimetry is utilized to decrease perinatal mortality.[25] Additionally, during high-risk deliveries and in cases of neonatal depression, umbilical cord arterial blood gases should be collected.[23] Umbilical artery pH and gas analysis are the most reliable tests to assess fetal oxygenation and acid-base in the perinatal period directly. A normal pH and gas analysis at delivery rules out intrapartum asphyxia.[23]
Pathophysiology
A single umbilical artery occurs in less than 1% of all pregnancies due to primary agenesis or secondary atrophy. Over half of these are isolated single umbilical arteries, but the anomaly is also associated with an increased risk of congenital and chromosomal abnormalities. Additionally, a single umbilical artery correlates with prematurity and intrauterine growth restriction.[4][26][27][1][28]
Over half of these are isolated single umbilical arteries, but the anomaly is also associated with an increased risk of congenital and chromosomal abnormalities. Additionally, a single umbilical artery correlates with prematurity and intrauterine growth restriction.[4][26][27][1][28]
When the umbilical cord inserts near the margin of the placenta instead of the center, it is referred to as marginal cord insertion or battledore placenta; this occurs at a rate of 9% in singleton pregnancies, with an increased rate in twin pregnancies (24 to 33%). Marginal cord insertion is associated with intrauterine growth restriction, preterm labor, and fetal distress.[3][4][28]
Velamentous cord insertion is a type of abnormal insertion occurring in 1 to 2% of pregnancies in which the umbilical vessels begin to spread out before reaching their normal insertion site at the center of the placenta. In this anomaly, the vessels travel separately between the amnion and chorion before reaching the placenta.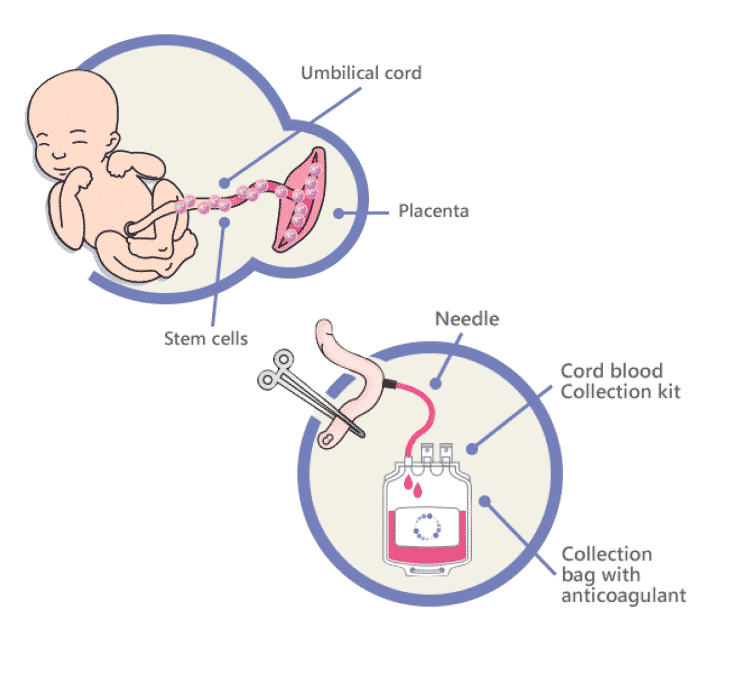 This area lacks the normal protection by Wharton’s jelly, leaving it susceptible to compression and rupture. Velamentous cord insertion increases the risk of adverse outcomes in the perinatal period due to vasa previa and placental abruption.[3][4][28][29]
This area lacks the normal protection by Wharton’s jelly, leaving it susceptible to compression and rupture. Velamentous cord insertion increases the risk of adverse outcomes in the perinatal period due to vasa previa and placental abruption.[3][4][28][29]
Vasa previa occurs in about 0.04% of pregnancies when fetal vessels are located between the cervix and the fetal presenting part and can result from velamentous cord insertion or vessels traveling between lobes of the placenta. If pregnancy progresses to rupture of membranes, vasa previa presents with the combination of painless vaginal bleeding and fetal heart tones showing signs of distress.[4][29][30][28]
Loss of Wharton’s jelly occurs most commonly near the fetal insertion site but also presents near the placental insertion site. The loss of this protective material leaves the umbilical cord vessels susceptible to compression due to twisting and knotting. The absence of Wharton’s jelly at any location on the umbilical cord increases the risk of intrauterine fetal demise as well as adverse perinatal outcomes due to compression of vessels during labor.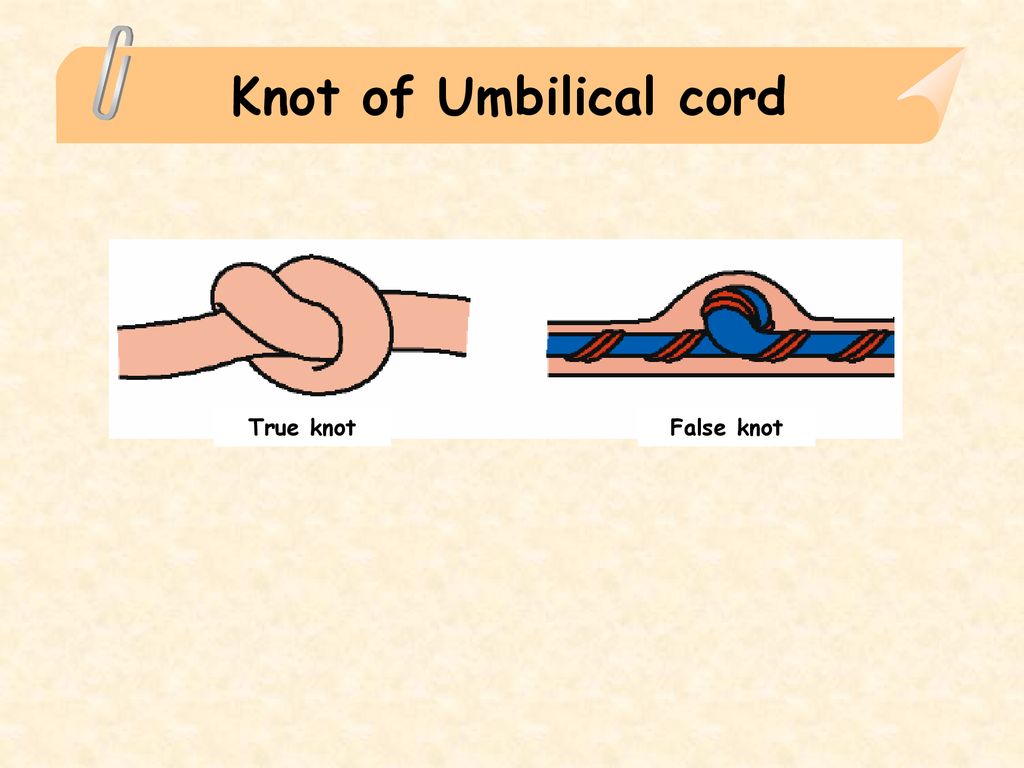 Loss of Wharton’s jelly may be diagnosable before delivery, with a decreased diameter of the umbilical cord visualized by ultrasound.[4][22]
Loss of Wharton’s jelly may be diagnosable before delivery, with a decreased diameter of the umbilical cord visualized by ultrasound.[4][22]
When the vitelline duct fails to regress during the embryonic period completely, it can lead to the formation of an abnormal outpouching of the intestines referred to as a Meckel’s diverticulum. This outpouching persists in about 2% of neonates, usually measures around 2 inches in length, and is typically located in the ileum about 2 feet from the ileocecal valve (following the rule of 2’s). Partial regression of the duct can lead to a vitelline fistula, a fibrous band-like connection between the umbilicus and intestine, or a vitelline cyst, which is an abnormal collection of fluid within the remaining duct. In most cases, these anomalies are asymptomatic, but the abnormal connection is known to increase the risk of internal hernia, volvulus, and intussusception.[2][5]
Funisitis is the migration of fetal neutrophils out of the bloodstream and into the umbilical cord. This migration process initiates with the release of neutrophil chemokines, such as interleukin-8 and granulocyte chemotactic protein. Funisitis is most commonly present in the setting of intraamniotic infection, specifically chorioamnionitis, and is part of the fetal inflammatory response syndrome, which indicates a high risk of preterm labor and increased neonatal morbidity. This process is identified microscopically after delivery, but due to the need for mature neutrophils within fetal blood, it is not typically present before 20 weeks of gestation.[31]
This migration process initiates with the release of neutrophil chemokines, such as interleukin-8 and granulocyte chemotactic protein. Funisitis is most commonly present in the setting of intraamniotic infection, specifically chorioamnionitis, and is part of the fetal inflammatory response syndrome, which indicates a high risk of preterm labor and increased neonatal morbidity. This process is identified microscopically after delivery, but due to the need for mature neutrophils within fetal blood, it is not typically present before 20 weeks of gestation.[31]
If the umbilical cord becomes too long in utero, there is an increased risk that it can become wound around the fetus or even become tied into a knot due to fetal movement. If the cord becomes wound around the fetal neck, it is referred to as a nuchal cord. The incidence of the nuchal cord is estimated at up to 29% at term, with the incidence increasing relative to gestational age. When the fetus descends during labor, increased torsional forces on the umbilical cord can decrease blood flow through the umbilical vessels and lead to signs of fetal distress and acidosis. When discovered, expedient reduction of the nuchal cord is important to return proper blood flow to the fetus and avoid prolonged asphyxia.[4][17]
When discovered, expedient reduction of the nuchal cord is important to return proper blood flow to the fetus and avoid prolonged asphyxia.[4][17]
Similarly, knots that form in utero are associated with longer umbilical cords. Loose umbilical knots present no danger to the fetus on their own, but when the knot tightens, the increased compression on the cord initially collapses the thin-walled vein before the thicker-walled arteries. This tightening of the knot can happen in utero and during labor, leading to signs of fetal distress, asphyxia, or even intrauterine fetal demise.[4][17][21]
Additionally, longer umbilical cords are more likely to lie between the cervix and the presenting fetal part during delivery leading to possible umbilical cord prolapse with rupture of membranes. Umbilical cord prolapse is diagnosed by palpation of the umbilical cord within the vagina along with changes in the fetal heart tracing indicating fetal distress, such as recurrent and prolonged decelerations.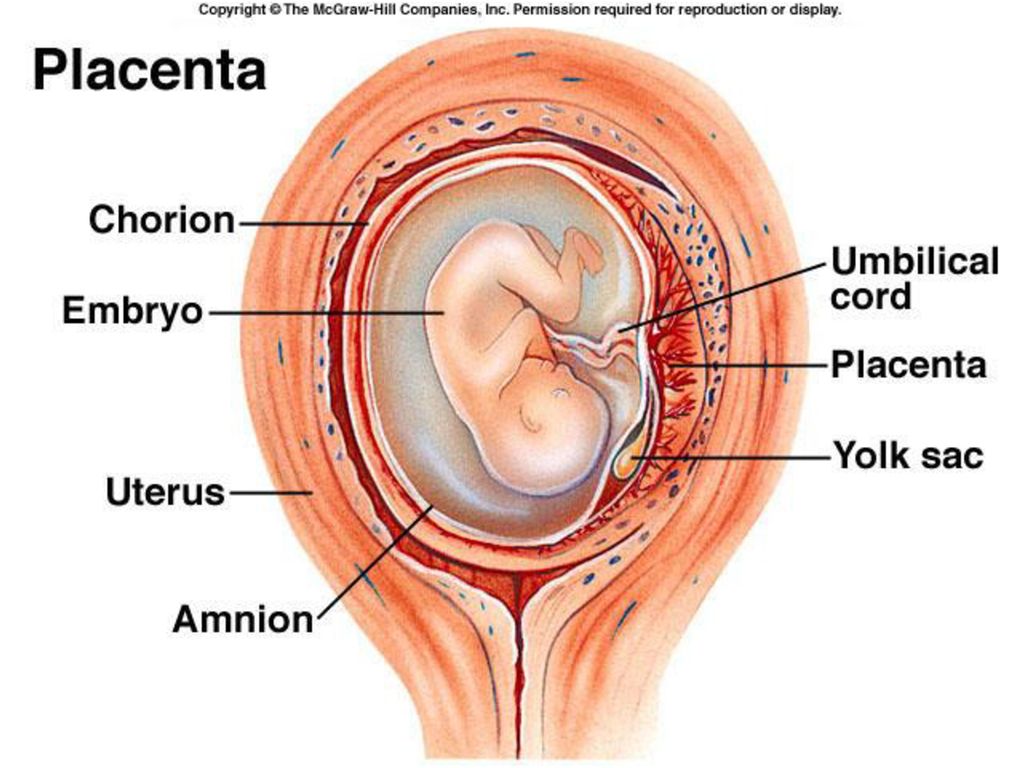 Management of this condition is expedient delivery of the baby, mostly via cesarean section, but operational vaginal delivery may be performed if that is determined to be the faster route.[4][32]
Management of this condition is expedient delivery of the baby, mostly via cesarean section, but operational vaginal delivery may be performed if that is determined to be the faster route.[4][32]
In monochorionic monoamniotic twin gestations, the fetuses share the same amniotic cavity within the uterus leading to possible cord entanglement. Similar to a knotted cord, tightening of the cord entanglement and increasing entanglement can cause compression of fetal vessels leading to intrauterine fetal demise. Cord entanglement can be detected in utero by ultrasound, but studies suggest that prenatal diagnosis has not shown improved neonatal outcomes.[4][28]
Other rare abnormalities include persistent right umbilical vein, umbilical artery aneurysm, umbilical cord cyst, umbilical hemangioma, and umbilical teratoma.[4]
Clinical Significance
The umbilical cord is the vital connection between the fetus and the placenta. Without this connection, the fetus would be unable to receive oxygen and nutrients from the mother or remove carbon dioxide and other waste products. [4] Sonographic analysis of the umbilical cord in the antepartum period is important for the early diagnosis of umbilical abnormalities.[4][2][25] Early recognition of abnormalities, such as velamentous cord insertion and nuchal cord, can improve perinatal outcomes.[4][17][29] Detection of any abnormality early in pregnancy should lead to serial ultrasound examinations to evaluate the patient for any associated complications.[4]
[4] Sonographic analysis of the umbilical cord in the antepartum period is important for the early diagnosis of umbilical abnormalities.[4][2][25] Early recognition of abnormalities, such as velamentous cord insertion and nuchal cord, can improve perinatal outcomes.[4][17][29] Detection of any abnormality early in pregnancy should lead to serial ultrasound examinations to evaluate the patient for any associated complications.[4]
Possible adverse outcomes of umbilical cord abnormalities include intrauterine growth restriction, preterm labor, fetal distress and asphyxia, and even intrauterine fetal demise.[4] After delivery, the portion of the cord remaining attached to the fetus may be useful for intravenous access by umbilical vein catheterization.[2][8] This can be used for transfusions and resuscitation of the neonate while the vessel is still patent, up to 14 days.[8] Additionally, umbilical cord blood has been used as an alternative source for bone marrow transplants since 1988 due to the presence of hematopoietic stem cells. [33] Umbilical cord blood transplantation has successfully helped to cure patients with hematologic diseases through the transplantation of allogeneic hematopoietic stem cells.[33] Furthermore, the therapeutic roles of Wharton’s jelly and the stem cells found within the umbilical cord are still under investigation.[10][12][13]
[33] Umbilical cord blood transplantation has successfully helped to cure patients with hematologic diseases through the transplantation of allogeneic hematopoietic stem cells.[33] Furthermore, the therapeutic roles of Wharton’s jelly and the stem cells found within the umbilical cord are still under investigation.[10][12][13]
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
Figure
Development of the Fetal membrane and the Placenta, Diagram illustrating a later stage in the development of the umbilical cord, Placental villi, Umbilical cord, Allantois, Heart, Embryo. Contributed by Gray's Anatomy Plates
Figure
Development of the Fetal Membranes and Placenta, Fetus of about eight weeks; enclosed in the amnion, Umbilical cord, Chorionic, Placenta. Contributed by Gray's Anatomy Plates
Figure
Development of Fetal Membranes and Placenta, Fetus in utero; between fifth and sixth months, Umbilical cord, Cervix uteri. Contributed by Gray's Anatomy Plates
Contributed by Gray's Anatomy Plates
Figure
The Branchial Region, Embryo of about six weeks, Umbilical cord, Embryology. Contributed by Gray's Anatomy Plates
Figure
Umbilical cord. Image courtesy S Bhimji MD
References
- 1.
Persutte WH, Hobbins J. Single umbilical artery: a clinical enigma in modern prenatal diagnosis. Ultrasound Obstet Gynecol. 1995 Sep;6(3):216-29. [PubMed: 8521073]
- 2.
Hegazy AA. Anatomy and embryology of umbilicus in newborns: a review and clinical correlations. Front Med. 2016 Sep;10(3):271-7. [PubMed: 27473223]
- 3.
Rathbun KM, Hildebrand JP. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Oct 5, 2022. Placenta Abnormalities. [PubMed: 29083591]
- 4.
Moshiri M, Zaidi SF, Robinson TJ, Bhargava P, Siebert JR, Dubinsky TJ, Katz DS. Comprehensive imaging review of abnormalities of the umbilical cord. Radiographics. 2014 Jan-Feb;34(1):179-96.
 [PubMed: 24428290]
[PubMed: 24428290]- 5.
An J, Zabbo CP. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 26, 2022. Meckel Diverticulum. [PubMed: 29763135]
- 6.
Malone JC, Shah AB. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Feb 10, 2022. Embryology, Midgut. [PubMed: 31985949]
- 7.
Cheng T, Yang B, Li D, Ma S, Tian Y, Qu R, Zhang W, Zhang Y, Hu K, Guan F, Wang J. Wharton's Jelly Transplantation Improves Neurologic Function in a Rat Model of Traumatic Brain Injury. Cell Mol Neurobiol. 2015 Jul;35(5):641-9. [PMC free article: PMC4481175] [PubMed: 25638565]
- 8.
Lewis K, Spirnak PW. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 26, 2022. Umbilical Vein Catheterization. [PubMed: 31751059]
- 9.
Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S.
 Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21(1):50-60. [PubMed: 12529551]
Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21(1):50-60. [PubMed: 12529551]- 10.
Zhou C, Yang B, Tian Y, Jiao H, Zheng W, Wang J, Guan F. Immunomodulatory effect of human umbilical cord Wharton's jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol. 2011;272(1):33-8. [PMC free article: PMC3235326] [PubMed: 22004796]
- 11.
Safari F, Fani N, Eglin D, Alini M, Stoddart MJ, Baghaban Eslaminejad M. Human umbilical cord-derived scaffolds for cartilage tissue engineering. J Biomed Mater Res A. 2019 Aug;107(8):1793-1802. [PubMed: 30983084]
- 12.
Corsello T, Amico G, Corrao S, Anzalone R, Timoneri F, Lo Iacono M, Russo E, Spatola GF, Uzzo ML, Giuffrè M, Caprnda M, Kubatka P, Kruzliak P, Conaldi PG, La Rocca G. Wharton's Jelly Mesenchymal Stromal Cells from Human Umbilical Cord: a Close-up on Immunomodulatory Molecules Featured In Situ and In Vitro. Stem Cell Rev Rep. 2019 Dec;15(6):900-918.
 [PubMed: 31741193]
[PubMed: 31741193]- 13.
Nishida F, Zappa Villar MF, Zanuzzi CN, Sisti MS, Camiña AE, Reggiani PC, Portiansky EL. Intracerebroventricular Delivery of Human Umbilical Cord Mesenchymal Stem Cells as a Promising Therapy for Repairing the Spinal Cord Injury Induced by Kainic Acid. Stem Cell Rev Rep. 2020 Feb;16(1):167-180. [PubMed: 31760626]
- 14.
Walker K, Basehore BM, Goyal A, Zito PM. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Aug 25, 2022. Hyaluronic Acid. [PubMed: 29494047]
- 15.
Casale J, Crane JS. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Mar 27, 2022. Biochemistry, Glycosaminoglycans. [PubMed: 31335015]
- 16.
Saari H, Konttinen YT, Friman C, Sorsa T. Differential effects of reactive oxygen species on native synovial fluid and purified human umbilical cord hyaluronate. Inflammation. 1993 Aug;17(4):403-15. [PubMed: 8406685]
- 17.
Peesay M.
 Nuchal cord and its implications. Matern Health Neonatol Perinatol. 2017;3:28. [PMC free article: PMC5719938] [PubMed: 29234502]
Nuchal cord and its implications. Matern Health Neonatol Perinatol. 2017;3:28. [PMC free article: PMC5719938] [PubMed: 29234502]- 18.
Gupta A, El-Amin SF, Levy HJ, Sze-Tu R, Ibim SE, Maffulli N. Umbilical cord-derived Wharton's jelly for regenerative medicine applications. J Orthop Surg Res. 2020 Feb 13;15(1):49. [PMC free article: PMC7017504] [PubMed: 32054483]
- 19.
Meyer WW, Rumpelt HJ, Yao AC, Lind J. Structure and closure mechanism of the human umbilical artery. Eur J Pediatr. 1978 Jul 19;128(4):247-59. [PubMed: 668732]
- 20.
Kapila V, Chaudhry K. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Jul 25, 2022. Physiology, Placenta. [PubMed: 30855916]
- 21.
Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000 Mar;79(3):157-9. [PubMed: 10716294]
- 22.
Damasceno EB, de Lima PP. Wharton's jelly absence: a possible cause of stillbirth. Autops Case Rep.
 2013 Oct-Dec;3(4):43-47. [PMC free article: PMC5453660] [PubMed: 28584806]
2013 Oct-Dec;3(4):43-47. [PMC free article: PMC5453660] [PubMed: 28584806]- 23.
Thorp JA, Rushing RS. Umbilical cord blood gas analysis. Obstet Gynecol Clin North Am. 1999 Dec;26(4):695-709. [PubMed: 10587963]
- 24.
Fleischer A, Schulman H, Farmakides G, Bracero L, Blattner P, Randolph G. Umbilical artery velocity waveforms and intrauterine growth retardation. Am J Obstet Gynecol. 1985 Feb 15;151(4):502-5. [PubMed: 3976751]
- 25.
Divon MY. Umbilical artery Doppler velocimetry: clinical utility in high-risk pregnancies. Am J Obstet Gynecol. 1996 Jan;174(1 Pt 1):10-4. [PubMed: 8571990]
- 26.
Murphy-Kaulbeck L, Dodds L, Joseph KS, Van den Hof M. Single umbilical artery risk factors and pregnancy outcomes. Obstet Gynecol. 2010 Oct;116(4):843-850. [PubMed: 20859147]
- 27.
Ramesh S, Hariprasath S, Anandan G, Solomon PJ, Vijayakumar V. Single umbilical artery. J Pharm Bioallied Sci. 2015 Apr;7(Suppl 1):S83-4.
 [PMC free article: PMC4439720] [PubMed: 26015760]
[PMC free article: PMC4439720] [PubMed: 26015760]- 28.
Hubinont C, Lewi L, Bernard P, Marbaix E, Debiève F, Jauniaux E. Anomalies of the placenta and umbilical cord in twin gestations. Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S91-S102. [PubMed: 26428508]
- 29.
Rocha J, Carvalho J, Costa F, Meireles I, do Carmo O. Velamentous cord insertion in a singleton pregnancy: an obscure cause of emergency cesarean-a case report. Case Rep Obstet Gynecol. 2012;2012:308206. [PMC free article: PMC3517836] [PubMed: 23243528]
- 30.
Derbala Y, Grochal F, Jeanty P. Vasa previa. J Prenat Med. 2007 Jan;1(1):2-13. [PMC free article: PMC3309346] [PubMed: 22470817]
- 31.
Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015 Oct;213(4 Suppl):S29-52. [PMC free article: PMC4774647] [PubMed: 26428501]
- 32.
Sayed Ahmed WA, Hamdy MA. Optimal management of umbilical cord prolapse. Int J Womens Health. 2018;10:459-465. [PMC free article: PMC6109652] [PubMed: 30174462]
- 33.
Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013 Jul 25;122(4):491-8. [PMC free article: PMC3952633] [PubMed: 23673863]
Umbilical cord - norm and pathology
The umbilical cord is a spiral tube that connects the fetus to the placenta. Outside, the umbilical cord is covered with fetal membranes. It contains two arteries and one vein.
Arterial blood flows through the vein of the umbilical cord, carrying oxygen to the organs of the fetus. The umbilical arteries carry venous blood from the fetus to the placenta, this blood contains the metabolic products of the fetus. The vessels of the umbilical cord are in a special gelatinous substance that fixes them and protects them from injury, and also exchanges substances between the fetal blood and amniotic fluid.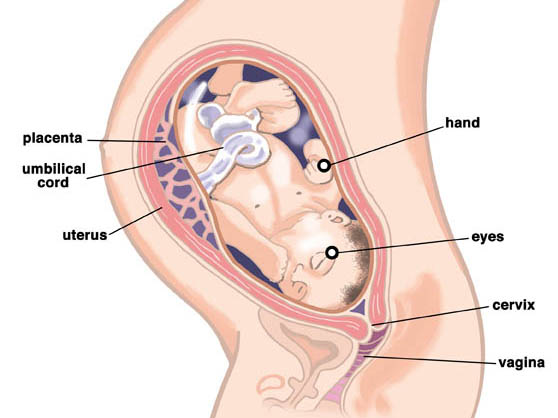 The umbilical cord begins to form from 2-3 weeks of pregnancy and grows with the baby. By the time of birth, its length is 45–60 cm (the length of the umbilical cord, on average, corresponds to the height of the child), and its diameter is 1.5–2 cm. nine0003
The umbilical cord begins to form from 2-3 weeks of pregnancy and grows with the baby. By the time of birth, its length is 45–60 cm (the length of the umbilical cord, on average, corresponds to the height of the child), and its diameter is 1.5–2 cm. nine0003
The umbilical cord can attach to the placenta in different ways. In some cases, attachment occurs in the center of the placenta (central attachment), in others - on the side (lateral attachment). Sometimes the umbilical cord is attached to the membranes, not reaching the placenta itself (shell attachment). In these cases, the vessels of the umbilical cord approach the placenta between the membranes. Such attachment of the placenta is a risk factor for the occurrence of fetal placental insufficiency. nine0003
The umbilical cord may also have features such as true and false nodes. False nodes are local thickening of the umbilical cord due to varicose veins of the umbilical cord or accumulation of Wharton's jelly. They do not affect the development of the fetus and the process of childbirth.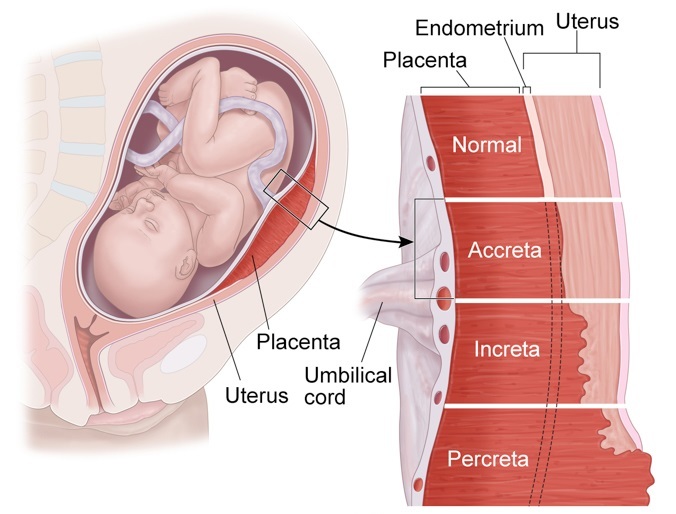 True umbilical cord knots form early in pregnancy, when the fetus is still small, allowing it to slip through the umbilical cord loop. True umbilical cord knots can affect the outcome of labor. When the umbilical cord is pulled, the knot is tightened, the flow and outflow of blood through the vessels stop. In this case, acute fetal hypoxia occurs. nine0003
True umbilical cord knots form early in pregnancy, when the fetus is still small, allowing it to slip through the umbilical cord loop. True umbilical cord knots can affect the outcome of labor. When the umbilical cord is pulled, the knot is tightened, the flow and outflow of blood through the vessels stop. In this case, acute fetal hypoxia occurs. nine0003
The pathology of the development of the umbilical cord is also a condition in which only one umbilical artery is formed instead of two; in some fetuses with one umbilical artery, various malformations are observed. The reason for this formation of the umbilical cord can be factors that cause fetal malformations - the so-called teratogenic factors (chemicals, certain drugs, ionizing radiation, genetic diseases of the parents).
Some problems may arise in connection with the shortening of the umbilical cord. Shortening of the umbilical cord can be divided into absolute and relative. With an absolute shortening of the umbilical cord, the length of the umbilical cord is less than 45 cm.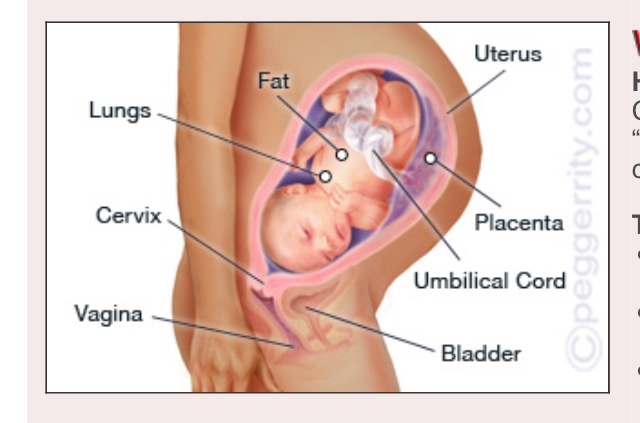 During pregnancy, this condition does not affect the development of the baby. During childbirth, both with relative and absolute shortness of the umbilical cord, due to its tension, the placenta can prematurely exfoliate, which the umbilical cord pulls along, which creates a direct threat to the life of the fetus. nine0003
During pregnancy, this condition does not affect the development of the baby. During childbirth, both with relative and absolute shortness of the umbilical cord, due to its tension, the placenta can prematurely exfoliate, which the umbilical cord pulls along, which creates a direct threat to the life of the fetus. nine0003
False shortening of the umbilical cord occurs when the umbilical cord is wrapped around the neck and torso of the fetus. The cause of umbilical cord entanglement may be its excessively long length (more than 70 cm), as well as increased fetal motor activity, which may be associated with chronic intrauterine fetal hypoxia. The causes of chronic lack of oxygen are different - these are maternal diseases, fetal diseases, and placental pathology. The entanglement of the umbilical cord can be single, double or even triple. During pregnancy, this condition usually does not affect the fetus, but problems can occur during childbirth. Tension or clamping of the vessels of the umbilical cord leads to disruption of blood flow. nine0003
nine0003
Conditions such as shortening of the umbilical cord (absolute and relative) and true knots of the umbilical cord during childbirth can lead to acute intrauterine fetal hypoxia. It is manifested by a change in the number of heartbeats. (The normal fetal heart rate is 120-160 beats per minute.) When acute intrauterine fetal hypoxia occurs, primordial feces (meconium) appears in the amniotic fluid, the water turns green. The appearance of all these symptoms requires emergency treatment. First of all, it is necessary to eliminate the cause of hypoxia, which is achieved by early delivery. The method of delivery depends on the period of labor and how far the presenting part of the fetus (head or pelvic end) has advanced along the birth canal. If acute hypoxia occurs during pregnancy or in the first stage of childbirth, the woman is given a caesarean section. In the second stage of labor, when the head or pelvic end is already close to the exit from the small pelvis, various obstetric aids are used to speed up the completion of the second stage of labor.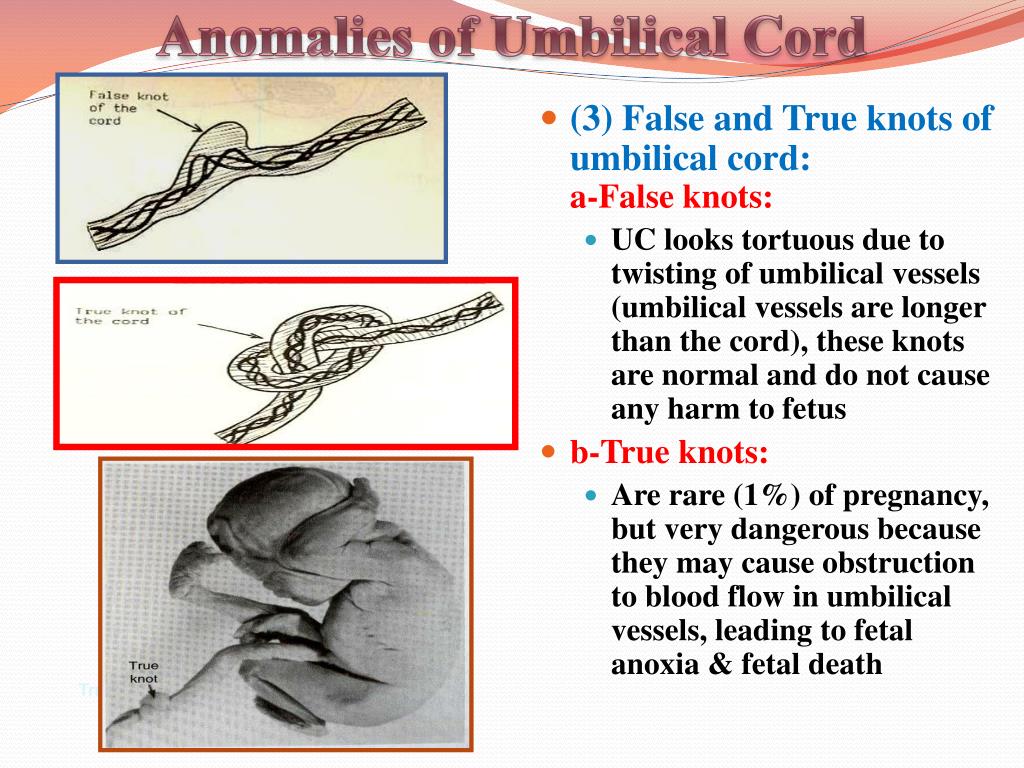 nine0003
nine0003
Fortunately, this condition does not occur often. Therefore, entanglement of the umbilical cord and knots of the umbilical cord are not an absolute indication for a planned caesarean section (it is impossible to diagnose the absolute shortness of the umbilical cord before delivery). These states are relative indications for surgery, i.e. caesarean section is done only in cases where, in addition to them, there are also other relative indications for surgery (the age of the woman is over 30 years old, mild forms of preeclampsia, etc.). nine0003
The only method that allows us to assume the pathology of the umbilical cord is an ultrasound scan. With the help of ultrasound, abnormalities of the umbilical cord can be detected, such as abnormal development of blood vessels (the only artery of the umbilical cord), true and false knots of the umbilical cord, entanglement of the umbilical cord. But the length of the umbilical cord during pregnancy is almost impossible to determine.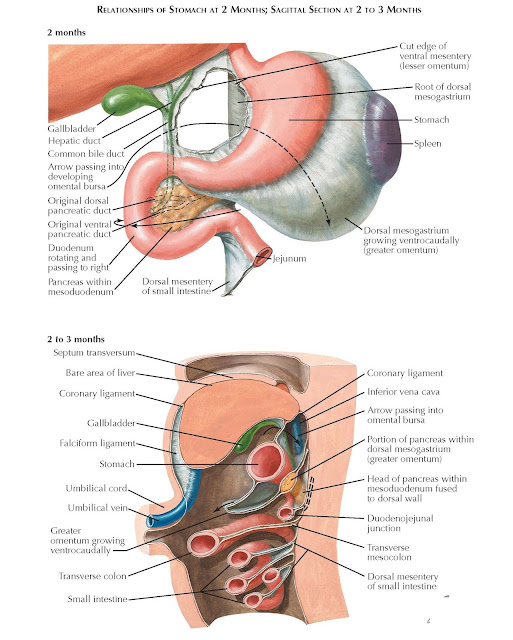
Special mention should be made of the diagnosis of entanglement of the umbilical cord. Sometimes, during examination, only loops of the umbilical cord in the neck are visible, but it is impossible to determine whether they wrap around the neck. In these cases, a Doppler study helps, during which it is possible to study the movement of blood through the vessels, including the umbilical cord. In addition, during childbirth, the cardiotocography method is used, which allows you to monitor the number of heartbeats, or listen to the fetal heartbeat with a stethoscope. nine0003
Thus, during pregnancy, the pathology of the umbilical cord can only be suspected. However, timely ultrasound examinations will help doctors make your birth safe.
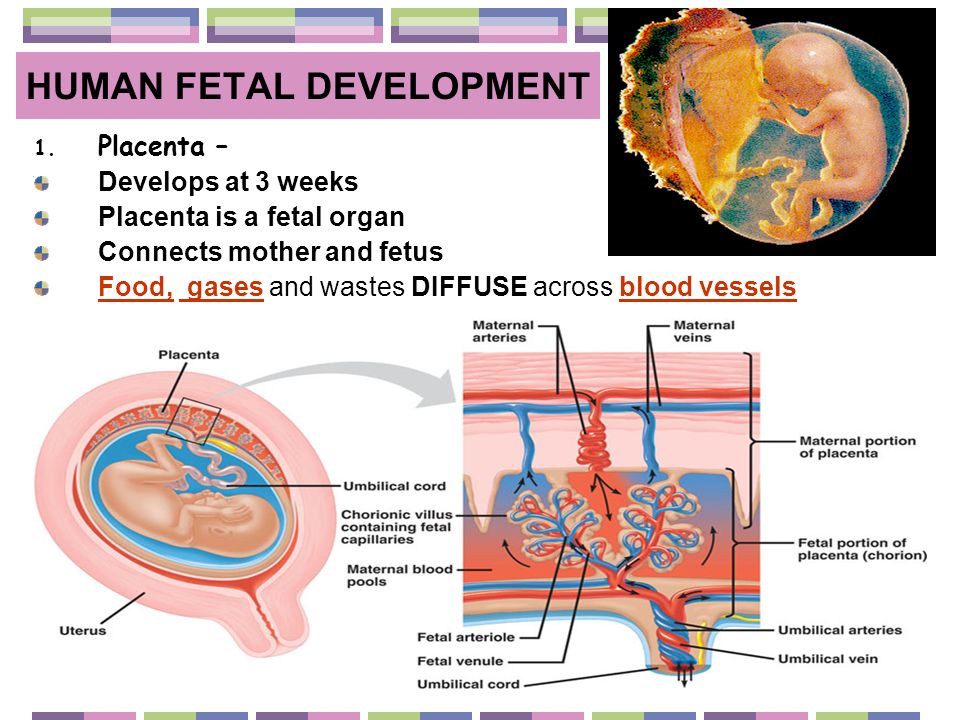
Placenta and umbilical cord: what is important to know?
9 monthsHealth
By the 12-14th week of pregnancy, the placenta and umbilical cord are finally formed - structures that exist only during pregnancy and perform the functions of exchange between mother and baby. What are these temporary organs and how do they affect the development and condition of the baby? nine0003
What are these temporary organs and how do they affect the development and condition of the baby? nine0003
Placenta
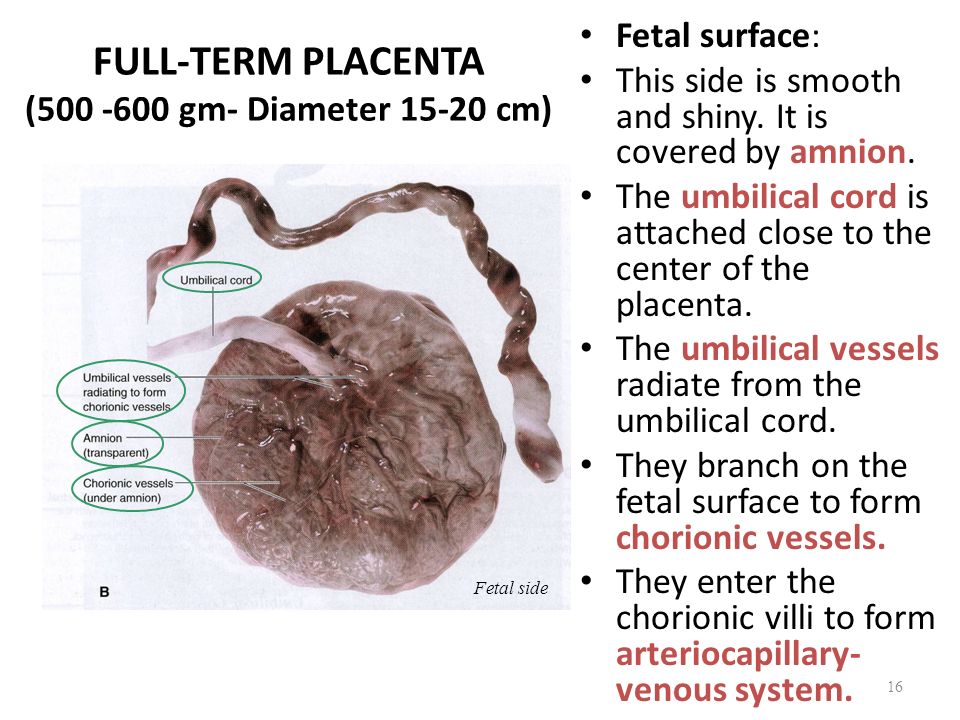
Placenta (from Latin placenta – “pie, cake, pancake”) plays an invaluable role in the development of the fetus, ensuring its growth, development, nutrition, respiration and excretion of waste metabolic products, as well as protecting the fetus from all kinds of harmful impacts.
The placenta is a spongy oval or semicircular organ with a lobed structure. Each lobule of the placenta contains many small vessels. With a normal full-term pregnancy and a fetal weight of 3300-3400 g, the diameter of the placenta is from 15 to 25 cm, thickness 2-4 cm, weight 500 g.
Two systems of blood vessels converge in this organ: one of them (maternal) connects the placenta with the vessels of the uterus, the other (fetal) with the umbilical cord of the fetus. The baby's blood vessels come very close to the mother's, but the maternal and fetal blood streams never mix.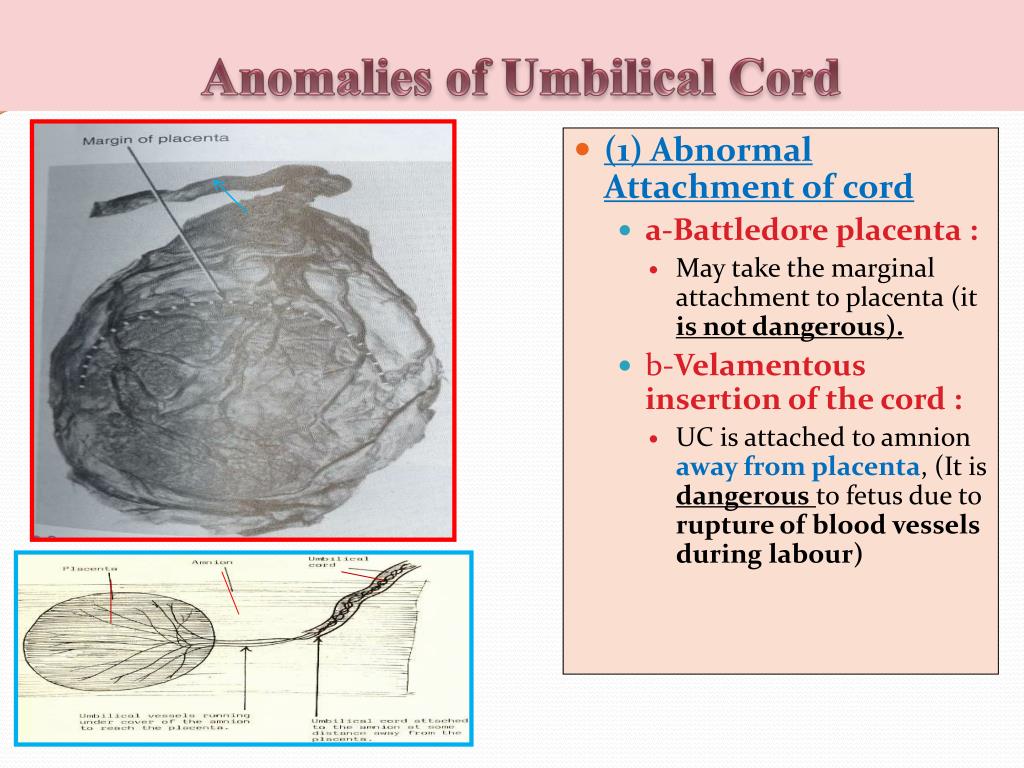 Between the two vascular systems there is a barrier membrane (one layer of cells) - the "customs and border service" of the placenta. Through it, the exchange of substances between mother and baby takes place: the maternal circulatory system brings oxygen and nutrients to the placenta and removes waste products and carbon dioxide from the placenta. The placental barrier allows the protective proteins (antibodies) of the mother to the child, ensuring its protection, and at the same time delays the cells of the woman's immune system that can cause a rejection reaction of the fetus, recognizing a foreign object in it. It is impenetrable to many harmful substances, viruses, bacteria. In addition, the placenta produces hormones that are important for successful pregnancy, and enzymes that destroy harmful substances. nine0003
Between the two vascular systems there is a barrier membrane (one layer of cells) - the "customs and border service" of the placenta. Through it, the exchange of substances between mother and baby takes place: the maternal circulatory system brings oxygen and nutrients to the placenta and removes waste products and carbon dioxide from the placenta. The placental barrier allows the protective proteins (antibodies) of the mother to the child, ensuring its protection, and at the same time delays the cells of the woman's immune system that can cause a rejection reaction of the fetus, recognizing a foreign object in it. It is impenetrable to many harmful substances, viruses, bacteria. In addition, the placenta produces hormones that are important for successful pregnancy, and enzymes that destroy harmful substances. nine0003
Umbilical cord
From the fetal part of the placenta come vessels that unite into larger ones, which eventually form the umbilical cord. The umbilical cord is a long cord (normally from 40 to 60 cm), up to 2 cm thick, consisting of connective tissue, inside of which there are two arteries and one vein. Despite the apparent discrepancy, the vessel called the venous carries arterial blood, while the two arterial vessels carry venous blood. These large vessels are surrounded by a special protective jelly-like substance - Wharton's jelly, which, due to its consistency, plays an important protective role - it protects the vessels from squeezing. The umbilical cord connects the placenta and the baby through the umbilical ring. The single umbilical vein that leaves the placenta enters the fetal abdomen through the umbilical ring and carries oxygenated blood, nutrients, and drugs that have passed the placental barrier. Through the arteries of the umbilical cord, the baby's blood flows back to the placenta, it carries waste products of decay and carbon dioxide. This is how fetal-placental blood flow occurs. After the birth of the baby, when the umbilical cord is cut, the connection between the blood circulation of the fetus and the blood flow of the mother stops, and only the navel reminds of their "blood" connection.
Despite the apparent discrepancy, the vessel called the venous carries arterial blood, while the two arterial vessels carry venous blood. These large vessels are surrounded by a special protective jelly-like substance - Wharton's jelly, which, due to its consistency, plays an important protective role - it protects the vessels from squeezing. The umbilical cord connects the placenta and the baby through the umbilical ring. The single umbilical vein that leaves the placenta enters the fetal abdomen through the umbilical ring and carries oxygenated blood, nutrients, and drugs that have passed the placental barrier. Through the arteries of the umbilical cord, the baby's blood flows back to the placenta, it carries waste products of decay and carbon dioxide. This is how fetal-placental blood flow occurs. After the birth of the baby, when the umbilical cord is cut, the connection between the blood circulation of the fetus and the blood flow of the mother stops, and only the navel reminds of their "blood" connection.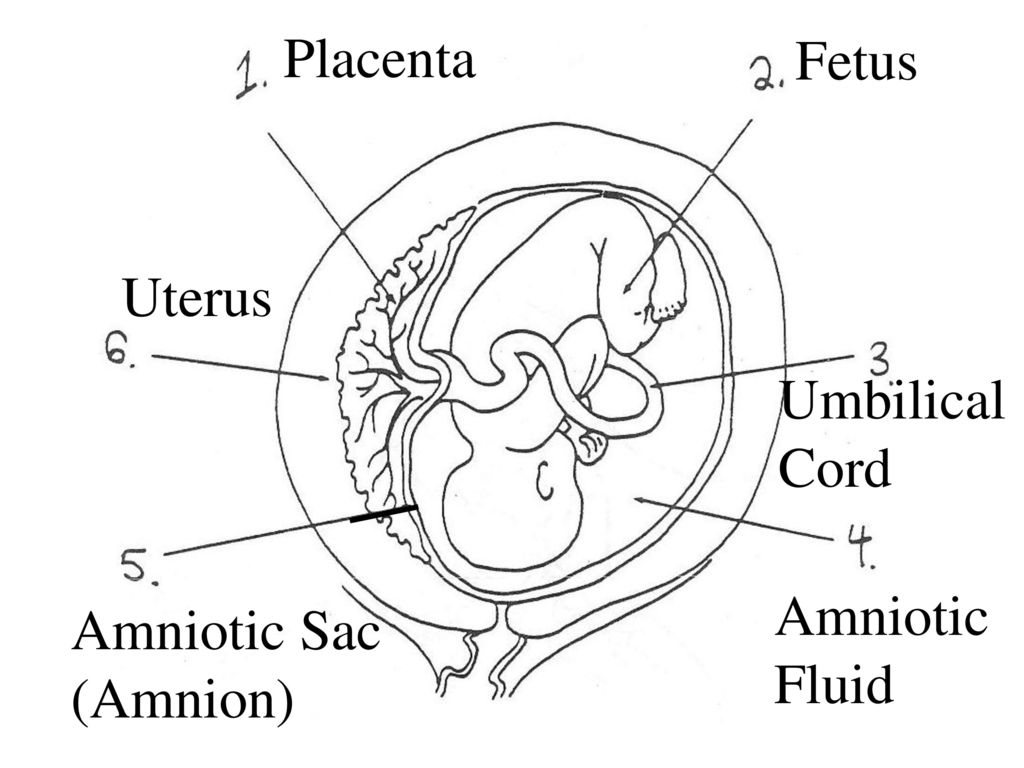 nine0003
nine0003
Disorders of the placenta
A change in the size (diameter and thickness) of the placenta, detected by ultrasound, does not always indicate that the pregnancy is unfavorable. Most often, such "deviations" are only an individual feature and do not affect the development of the fetus. Only significant deviations deserve attention.
Small placenta, or placental hypoplasia. Such a diagnosis is valid only with a significant decrease in the size of the placenta. The cause of this condition is most often genetic abnormalities, while the fetus often lags behind in development and has other malformations. nine0003
A thin placenta is considered to be a placenta with insufficient weight, but at the same time of normal size. Sometimes a thin placenta accompanies placental insufficiency and is therefore a risk factor for intrauterine growth retardation and serious problems in the neonatal period.
An increase in the thickness and size of the placenta can also be the result of an abnormal pregnancy.