Mmr during pregnancy
Vaccine Safety for Moms-to-Be | CDC
Pregnant women may safely receive inactivated vaccines (Tdap and flu), mRNA (Moderna and Pfizer), and viral vector vaccines (J&J).
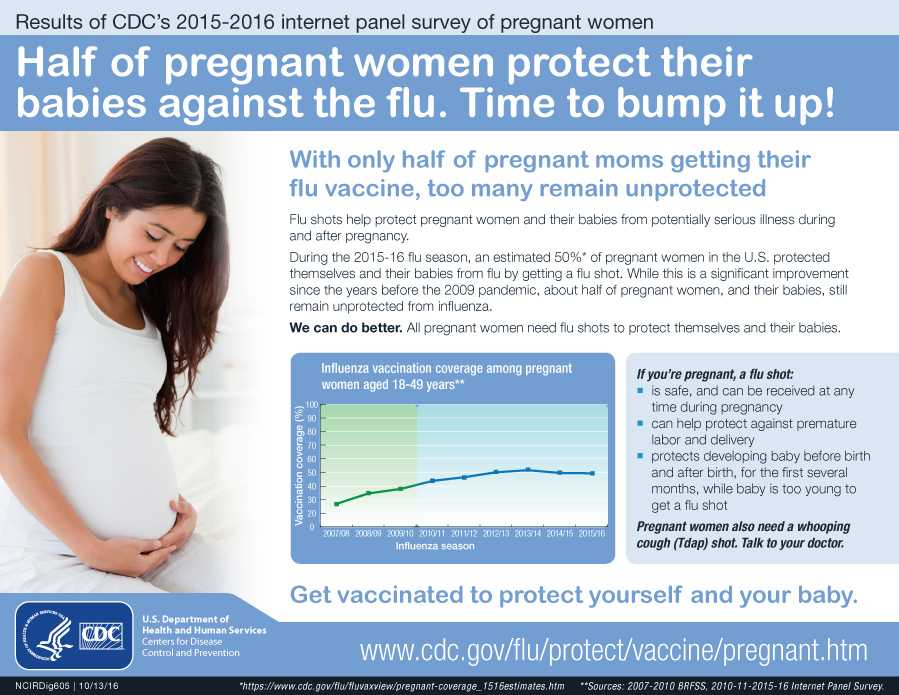
Vaccines help protect pregnant people and babies against serious diseases
Pregnant people share everything with their babies. That means when a pregnant person gets vaccines, she isn’t just protecting herself— they are giving the baby some early protection too.
CDC has recommendations for the vaccines needed before, during, and after pregnancy. Currently, CDC routinely recommends Tdap and flu shots during pregnancy.
- Get the Tdap vaccine (to help protect against whooping cough), during pregnancy.
- The flu shot can be given before or during pregnancy, depending on whether or not it is flu season during a pregnancy.
- It is safe for pregnant people to receive vaccines right after giving birth, even while breastfeeding.
- Some vaccines, such as the measles, mumps, rubella (MMR) vaccine, should be given a month or more before pregnancy if a pregnant person didn’t get the vaccine as a child.
Live virus vaccines, such as the MMR and chickenpox, should not be given to pregnant people, but should be given to them before or after pregnancy, if indicated. Talk to your doctor about the MMR, Tdap, and flu vaccines before getting vaccinated. The COVID-19 vaccine is also recommended for pregnant people. The authorized and recommended COVID-19 vaccines for pregnant people are the mRNA Moderna and Pfizer-BioNTech vaccines, which contain no live virus, and the J&J/Janssen viral vector vaccine, meaning it uses a modified version of a different virus (the vector) to deliver important instructions to our cells. If you have any questions about these vaccines, talk to your doctor.
Vaccine safety before, during, and after pregnancy
It’s important to know that the Tdap and flu vaccines are safe for a pregnant person and their baby. Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
- The Tdap and flu vaccines are inactivated vaccines, which means they are made by inactivating or killing the germ during the process of making the vaccine.
- Studies done on the Tdap vaccine have concluded that it is safe and effective for pregnant people and babies.
- Similarly, results from multiple studies on the flu shot continue to support the safety and effectiveness of the vaccine during pregnancy.
- There is limited information available about the safety of the COVID-19 vaccines for people who are pregnant; however, based on how these vaccines work in the body, experts believe they are unlikely to pose risk for pregnant people.
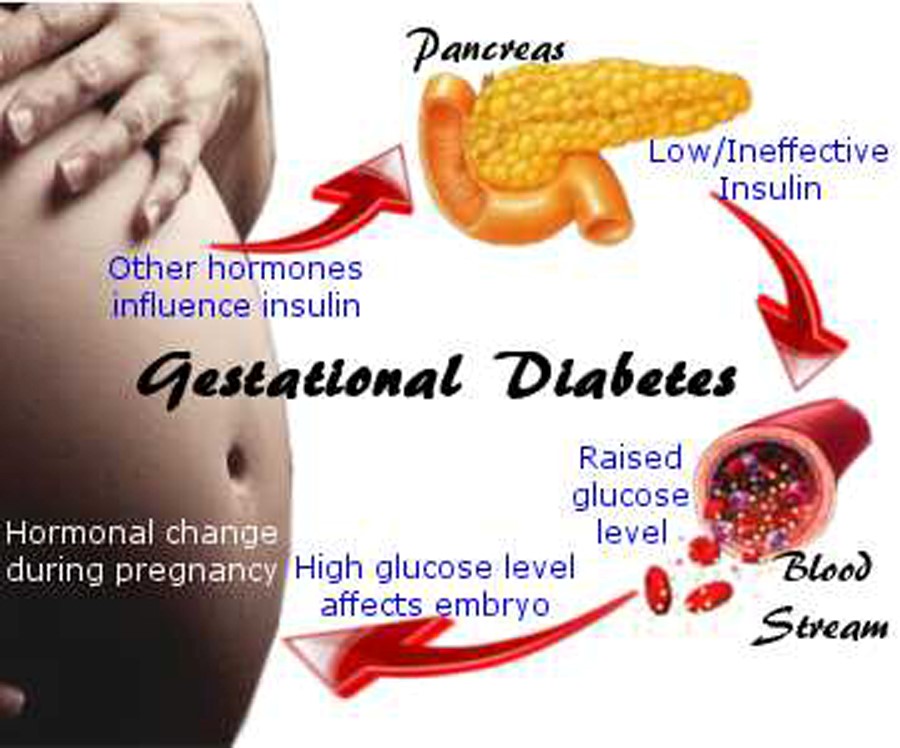
It is important to get MMR before becoming pregnant to reduce the risk of becoming infected with rubella which can pass on to the unborn child, causing Congenital Rubella Syndrome (CRS). CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
- Live vaccines are generally not recommended during pregnancy.
- If a pregnant person did not get MMR as a child, she should get the vaccine before pregnancy.
All vaccines are held to the highest standards of safety—meaning they are carefully studied and monitored for side effects. Vaccines are like any medicine, which means they can have some side effects. However, most people who get vaccinated have no side effects or only mild side effects. CDC continually monitors vaccine safety, and the most common side effects seen are mild and go away quickly on their own (redness, swelling, and tenderness at the site where the shot was given. Other possible side effects associated with the COVID-19 vaccine are tiredness, headache, muscle pain, chills, fever, and nausea. ).
).
For more studies, the FDA also has a pregnancy exposure registry,external icon which is a study that collects health information from pregnant persons who take medicines or vaccines when they are pregnant.
Rubella Information For Healthcare Professionals
- Clinical Overview
- Diagnosis and Treatment
- Vaccination
- Isolation
Clinical Overview
Clinical Features
Rubella is a viral illness that can lead to complications and death. It is characterized by a mild, maculopapular rash along with lymphadenopathy, and a slight fever. The rash usually starts on the face, becomes generalized within 24 hours, and lasts a median of 3 days; it occurs in 50% to 80% of infected people, Lymphadenopathy, which may precede rash, often involves posterior auricular or suboccipital lymph nodes, can be generalized, and lasts between 5 and 8 days. About 25% to 50% of infections are asymptomatic.
Clinical diagnosis of rubella virus is unreliable and should not be considered in assessing immune status. Up to half of all infections may be subclinical or unapparent. Many rubella infections are not recognized because the rash resembles many other rash illnesses.
Up to half of all infections may be subclinical or unapparent. Many rubella infections are not recognized because the rash resembles many other rash illnesses.
The Virus
Rubella virus is an enveloped, positive-stranded RNA virus classified as a Rubivirus in the Matonaviridae family.
Background
Before the rubella vaccine was licensed in the United States in 1969, rubella was a common disease that occurred primarily among young children. Epidemics occurred every 6 to 9 years, with the highest number of cases during the spring.
Rubella was declared eliminated (the absence of endemic transmission for 12 months or more) from the United States in 2004. However, it is still commonly transmitted in many parts of the world. As a result, less than 10 cases (primarily import-related) have been reported annually in the United States since elimination was declared. Rubella incidence in the United States has decreased by more than 99% from the pre-vaccine era.
Because rubella continues to circulate in other parts of the world, an estimated 100,000 infants are born with congenital rubella syndrome (CRS) annually worldwide.
Complications
Arthralgia or arthritis may occur in up to 70% of adult women with rubella. Rare complications include thrombocytopenic purpura and encephalitis.
When rubella infection occurs during pregnancy, especially during the first trimester, serious consequences can result. These include miscarriages, fetal deaths/stillbirths, and severe birth defects known as CRS. The most common congenital defects are cataracts, heart defects, and hearing impairment.
Transmission
Rubella is transmitted primarily through direct or droplet contact from nasopharyngeal secretions. Humans are the only natural hosts. In temperate climates, infections usually occur during late winter and early spring.
The average incubation period of rubella virus is 17 days, with a range of 12 to 23 days. People infected with rubella are most contagious when the rash is erupting, but they can be contagious from 7 days before to 7 days after the rash appears.
Diagnosis and Treatment
Healthcare providers should
- consider rubella in unvaccinated patients with febrile rash illness and other rubella symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness
- promptly isolate people suspected to have rubella and report them to the local health department
- collect throat (best source), nasal, or urine specimens for viral detection by polymerase chain reaction (PCR) testing and molecular typing, and blood for serologic testing.

See information on laboratory testing here.
There is no specific antiviral therapy for rubella infection.
Evidence of Immunity
Acceptable presumptive evidence of immunity against rubella includes at least one of the following:
- written documentation of vaccination with one dose of live rubella virus-containing vaccine administered on or after the first birthday,
- laboratory evidence of immunity,
- laboratory confirmation of rubella disease, or
- birth before 1957
Healthcare providers should not accept verbal reports of vaccination without written documentation as presumptive evidence of immunity. For additional details about evidence of immunity criteria, see Table 3 in Prevention of Measles, Rubella, Congenital Rubella Syndrome, and Mumps, 2013: Summary Recommendations of the Advisory Committee on Immunization Practices (ACIP).
Top of Page
Vaccination
Rubella can be prevented with rubella-containing vaccine, which is primarily administered as the combination measles-mumps-rubella (MMR) vaccine. The combination measles-mumps-rubella-varicella (MMRV) vaccine can be used for children aged 12 months through 12 years for protection against measles, mumps, rubella and varicella. Single-antigen rubella vaccine is not available.
The combination measles-mumps-rubella-varicella (MMRV) vaccine can be used for children aged 12 months through 12 years for protection against measles, mumps, rubella and varicella. Single-antigen rubella vaccine is not available.
One dose of MMR vaccine is about 97% effective at preventing rubella if exposed to the virus.
Vaccine Recommendations
Children
CDC recommends routine childhood immunization for MMR vaccine starting with the first dose at 12 through 15 months of age, and the second dose at 4 through 6 years of age or at least 28 days following the first dose.
Adults
Adults born during or after 1957 should receive at least one dose of rubella-containing vaccine. These people include students attending colleges or other post high school educational institutions, healthcare personnel, international travelers, and non-pregnant women of childbearing age. Healthcare providers should routinely assess women of childbearing age for evidence of immunity (see section above) and vaccinate those who lack acceptable evidence of immunity and who are not pregnant. Pregnant women who do not have evidence of immunity should be vaccinated immediately after giving birth.
Pregnant women who do not have evidence of immunity should be vaccinated immediately after giving birth.
For more information, see rubella vaccination recommendations.
Some people should not get MMR vaccine. For information about contraindications, see who should NOT get vaccinated with MMR vaccine.
Isolation
Patients with rubella should be isolated for 7 days after they develop rash. In settings where pregnant women may be exposed, outbreak control measures should begin as soon as rubella is suspected and should not be postponed until laboratory confirmation of cases.
People at risk who cannot readily provide acceptable evidence of rubella immunity should be considered susceptible and should be vaccinated. People without evidence of immunity who are exempt from rubella vaccination for medical, religious, or other reasons should be excluded from affected institutions in the outbreak area until 23 days after the onset of rash in the last case of rubella. Unvaccinated people who receive MMR vaccine as part of rubella outbreak control may immediately return to school provided all people without documentation of rubella immunity have been excluded.
Unvaccinated people who receive MMR vaccine as part of rubella outbreak control may immediately return to school provided all people without documentation of rubella immunity have been excluded.
Top of Page
- CDC – Surveillance of Rubella – Chapter 14 – Vaccine-Preventable Diseases
- Rubella – Red Book, American Academy of Pediatrics, 2015
- Provider Resources for Vaccine Conversations with Parents
MMR vaccine II: measles, rubella, mumps
MMR II vaccine is not currently available. We vaccinate children against measles, rubella and mumps with vaccines produced in the Russian Federation.
We stand for safe, responsible vaccination! All vaccination rules are strictly observed. We have anti-stress vaccinations in our clinic: interactive toys, soap bubbles, a special Buzzy Ladybug, virtual reality glasses with 3D cartoons!
Make an appointment via WhatsApp
Video Prices Doctors
The first children's clinic of evidence-based medicine in Moscow
No unnecessary examinations and drugs! We will prescribe only what has proven effective and will help your child.
Treatment according to world standards
We treat children with the same quality as in the best medical centers in the world.
Fantasy has the best team of doctors!
Pediatricians and subspecialists Fantasy - highly experienced doctors, members of professional societies. Doctors constantly improve their qualifications, undergo internships abroad.
Ultimate safety of treatment
We have made children's medicine safe! All our staff work according to the strictest international standards JCI
We have fun, like visiting best friends
Game room, cheerful animator, gifts after the reception. We try to make friends with the child and do everything to make the little patient feel comfortable with us.
You can make an appointment by calling or by filling out the form on the site
Other vaccinations
- Imported vaccines in stock!
- Adasel - vaccination against diphtheria, tetanus and whooping cough
- BiVac polio polio vaccine
- Vactrivir Measles, rubella, mumps vaccine
- DTP vaccine Whooping cough, diphtheria, tetanus vaccine
- Vaccine Infanrix Vaccination against whooping cough, diphtheria, tetanus
- Vaccine Infanrix Hexa
- Vaccine Pentaxim Vaccination against polio, diphtheria, tetanus, whooping cough, Haemophilus influenzae type b (Hib)
- Rabies vaccination
- Varilrix - chickenpox vaccine
- Polimilex polio vaccine
- Chickenpox vaccine Varivax
- HPV (human papillomavirus) vaccine
- Hepatitis A vaccination
- Hepatitis B vaccination
- Tick-borne encephalitis vaccine
- Measles, rubella and mumps vaccine
- Vaccination against meningococcal infection
- Vaccination against pneumococcal infection
- Vaccination against rotavirus infection
- Ultrix Quadri or Influvac.
 Flu shot for the whole family
Flu shot for the whole family - Hiberix: Haemophilus influenzae type b (Hib) vaccine
Online payment
Documents online
Online services
MMR vaccine
DocDeti pediatrician Svetlana Savelyeva prepared an article about the MMR-II vaccine.
The American-made vaccine is designed to protect against 3 diseases: measles, rubella, mumps.
1. Measles is a highly contagious viral disease (one patient can infect 9out of 10 who contacted him), the mortality rate is also quite high.
The first measles vaccine was developed in 1963. It contained killed viruses and was not effective enough. In 1968, they decided to replace it with another vaccine containing attenuated viruses.
Before the mass use of the vaccine against this disease, approximately 2. 5 million people died every year!
5 million people died every year!
Thanks to the use of the vaccine, the number of deaths decreased by 84% (between 2000 and 2016).
According to the World Health Organization, in 2018, approximately 140 thousand people died worldwide, mostly children under 5 years old. And this means that the problem has not lost its relevance today.
2. Pregnant women should be wary of contracting rubella, for them it is especially dangerous. Infected with this virus in the 1st trimester of pregnancy, you can lose a child.
3. Parotitis or "mumps" is a viral infection leading to the defeat of the salivary glands. It can lead to complications such as:
- Orchitis (inflammation of the testicles) - which can lead to male infertility.
- Meningitis and encephalitis - inflammation of the brain and meninges
- Pancreatitis - inflammation of the pancreas
- Hearing loss
In 1967 and 1969, a mumps and rubella vaccine was developed.
- One vaccine, not two or three (we are talking about domestic analogues),
- Minimal risk of complications after vaccination
- Suitable for emergency vaccinations. Administering the vaccine within the first 72 hours of exposure to a person with measles will either reduce the severity of the condition during the illness or protect against infection.
A vaccinated person cannot infect anyone.
Vaccination is contraindicated in:
- Exacerbation of a chronic or acute disease
- Immunodeficiency, immunosuppressive therapy
- Anaphylactic reaction to neomycin and previous vaccination with this drug
- Pregnancy. And it is not recommended to become pregnant for three months after the introduction of the vaccine.
Vaccination is carried out according to the following scheme:
- First vaccination at 1 year
- Revaccination at 6 years
If the epidemiological situation is unfavorable, then vaccination can be started from 9 months.