Level 2 ultrasound down syndrome
Influence of Second-Trimester Ultrasound Markers for Down Syndrome in Pregnant Women of Advanced Maternal Age
J Pregnancy. 2014; 2014: 785730.
Published online 2014 Mar 25. doi: 10.1155/2014/785730
,,*,, and
Author information Article notes Copyright and License information Disclaimer
The objective of the present study was to evaluate the influence of second-trimester ultrasound markers on the incidence of Down syndrome among pregnant women of advanced maternal age. This was a retrospective cohort study on 889 singleton pregnancies between the 14th and 30th weeks, with maternal age ≥ 35 years, which would undergo genetic amniocentesis. The second-trimester ultrasound assessed the following markers: increased nuchal fold thickness, cardiac hyperechogenic focus, mild ventriculomegaly, choroid plexus cysts, uni- or bilateral renal pyelectasis, intestinal hyperechogenicity, single umbilical artery, short femur and humerus length, hand/foot alterations, structural fetal malformation, and congenital heart disease. To investigate differences between the groups with and without markers, nonparametric tests consisting of the chi-square test or Fisher's exact test were used. Moreover, odds ratios with their respective 95% confidence intervals were calculated. Out of the 889 pregnant women, 131 (17.3%) presented markers and 758 (82.7%) did not present markers on the second-trimester ultrasound. Increased nuchal fold (P < 0.001) and structural malformation (P < 0.001) were the markers most associated with Down syndrome. The presence of one marker increased the relative risk 10.5-fold, while the presence of two or more markers increased the risk 13.5-fold. The presence of markers on the second-trimester ultrasound, especially thickened nuchal fold and structural malformation, increased the risk of Down syndrome among pregnant women with advanced maternal age.
Although chromosomal abnormalities occur at low frequency in the population, around 0.5% to 2% [1], they contribute significantly to increased perinatal morbidity and mortality [2]. Trisomy is the most frequent chromosomal abnormality, especially of chromosome 21, that is, Down syndrome. Since Down syndrome is difficult to diagnose during the prenatal period and because there is the possibility of survival after birth, it contributes to increasing the statistics of cases of mental retardation. Thus, prenatal screening is important, especially among women of advanced maternal age, that is, greater than or equal to 35 years [3].
Trisomy is the most frequent chromosomal abnormality, especially of chromosome 21, that is, Down syndrome. Since Down syndrome is difficult to diagnose during the prenatal period and because there is the possibility of survival after birth, it contributes to increasing the statistics of cases of mental retardation. Thus, prenatal screening is important, especially among women of advanced maternal age, that is, greater than or equal to 35 years [3].
The presence of certain alterations on the second-trimester ultrasound, called markers, enables increased sensitivity in screening for trisomy 21. This can reach up to 84% and possibly surpass 90% when heart markers are included [4–7].
The challenge is to distinguish the presence of these small alterations on the second-trimester ultrasound, between chromosomally abnormal and normal fetuses, considering that the latter may also present these markers at a rate of around 13% to 17%, which can be considered to be a high percentage of false positives [8].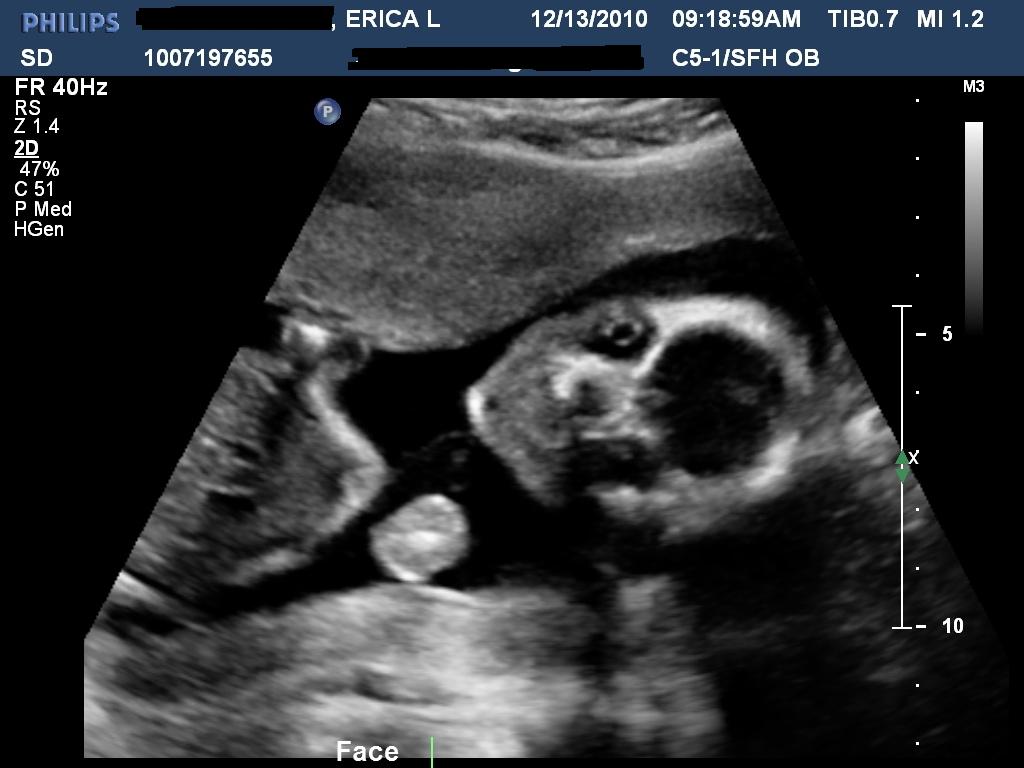 The importance of this challenge is greater among pregnant women of advanced maternal age, when the relationship with Down syndrome becomes closer [9]. If, on the one hand, these markers enable good detection of aneuploidy, on the other, they lead to an unacceptably high rate of invasive procedures, thereby exposing chromosomally normal fetuses to a risk of death or unnecessary complications [10]. Thus, second-trimester ultrasound can be used as a method to assist in evaluating the risk of fetal aneuploidy, thereby making it possible for the couple to better evaluate the need for an invasive examination to assess the fetal karyotype [11].
The importance of this challenge is greater among pregnant women of advanced maternal age, when the relationship with Down syndrome becomes closer [9]. If, on the one hand, these markers enable good detection of aneuploidy, on the other, they lead to an unacceptably high rate of invasive procedures, thereby exposing chromosomally normal fetuses to a risk of death or unnecessary complications [10]. Thus, second-trimester ultrasound can be used as a method to assist in evaluating the risk of fetal aneuploidy, thereby making it possible for the couple to better evaluate the need for an invasive examination to assess the fetal karyotype [11].
The objective of the present study was to determine associations that might exist between some second-trimester ultrasound markers and Down syndrome, among pregnant women of advance maternal age, who underwent fetal chromosomal analysis through amniocentesis.
This was a retrospective cohort study on 889 singleton pregnant women aged ≥ 35 years, who underwent genetic amniocentesis. The present study was approved by the Research Ethics Committee of the Federal University of São Paulo (UNIFESP). All the patients who voluntarily agreed to participate signed a consent form.
The present study was approved by the Research Ethics Committee of the Federal University of São Paulo (UNIFESP). All the patients who voluntarily agreed to participate signed a consent form.
The present study was carried out at the Department of Obstetrics of UNIFESP and at Santa Joana Hospital, São Paulo, SP, Brazil. Singleton pregnancies between their 14th and 30th weeks that would undergo genetic amniocentesis because of maternal age ≥ 35 years, with or without markers for Down syndrome on the second-trimester ultrasound, were selected. Pregnant women with a history of genital bleeding over the past seven days, suspected premature rupture of membranes, fetal heart rate abnormalities, suspected congenital infection (from ultrasound alterations), or use of potentially teratogenic drugs were excluded.
The second-trimester ultrasound was performed before the genetic amniocentesis, using the Logic 500 device (General Electric Medical System, Milwaukee, WI, USA) equipped with a convex transducer (3–5 MHz).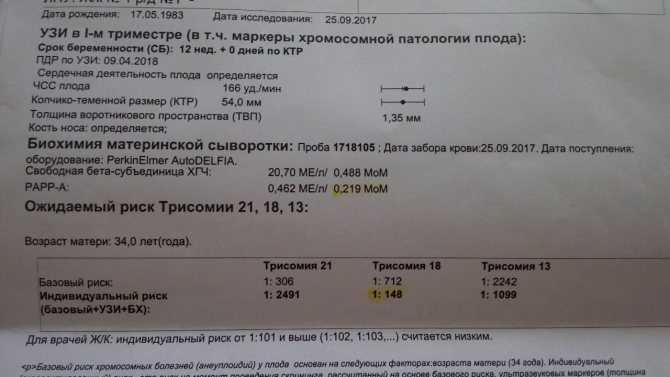 In this examination, the presence of the following markers was evaluated: increased nuchal fold thickness (≥6 mm), mild ventriculomegaly (atrium of the lateral ventricle measuring between 12 and 15 mm), choroid plexus cysts, cardiac hyperechogenic focus, unilateral or bilateral renal pyelectasis (renal pelvis measuring ≥4 mm between the 15th and 20th weeks and ≥5 mm between the 21st and 30th weeks), intestinal hyperechogenicity, single umbilical artery, short femur length (observed/expected measurement < 0.90), short humerus length (observed/expected measurement < 0.89), alterations at the extremities such as the shape or position of hands or feet, structural malformations, or congenital heart diseases.
In this examination, the presence of the following markers was evaluated: increased nuchal fold thickness (≥6 mm), mild ventriculomegaly (atrium of the lateral ventricle measuring between 12 and 15 mm), choroid plexus cysts, cardiac hyperechogenic focus, unilateral or bilateral renal pyelectasis (renal pelvis measuring ≥4 mm between the 15th and 20th weeks and ≥5 mm between the 21st and 30th weeks), intestinal hyperechogenicity, single umbilical artery, short femur length (observed/expected measurement < 0.90), short humerus length (observed/expected measurement < 0.89), alterations at the extremities such as the shape or position of hands or feet, structural malformations, or congenital heart diseases.
To carry out the amniocentesis, a 20 mL syringe and a 20 G needle were used after applying local anesthesia consisting of 2% lidocaine. 20 mL of clear liquid were collected, without blood contamination, and were immediately forwarded to the cytogenetic laboratory. Pregnant women with a negative Rh blood type, negative indirect Coombs test, or a Rh-positive partner received anti-D immunoglobulin within 72 hours after the procedure, so as to avoid maternal sensitization.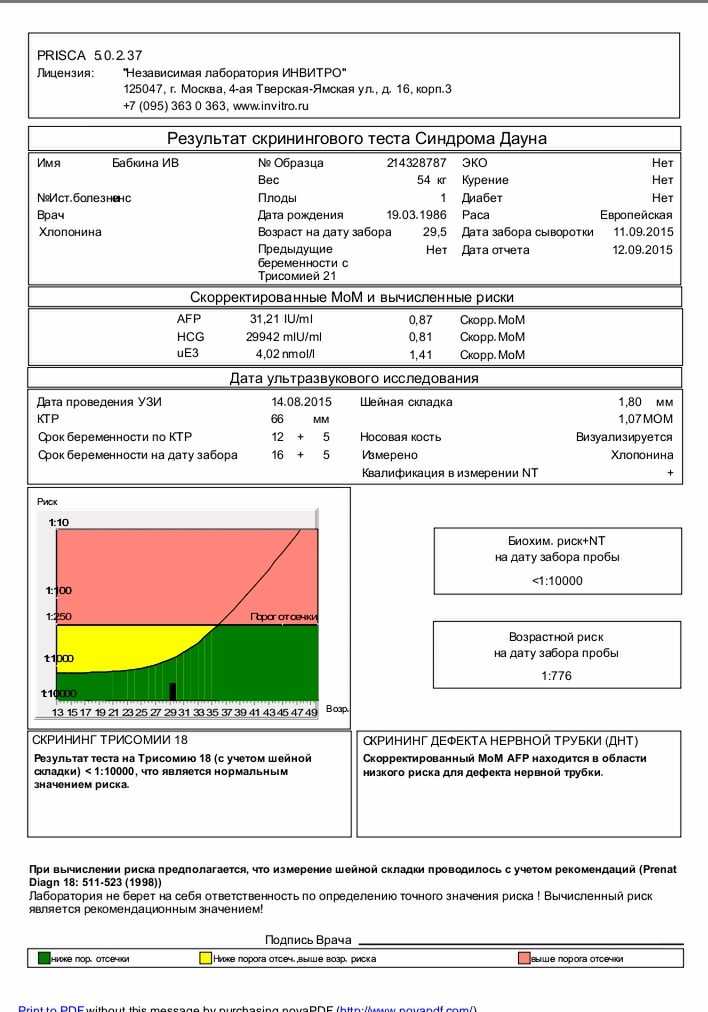
The data were transferred to a worksheet in the Excel 2003 software (Microsoft Corp., Redmond, WA, USA) and were analyzed using the SPSS version 13.0 software for Windows (SPSS Inc., Chicago, IL, USA). Probability calculations were used to search for any relationships between fetal malformations and/or second-trimester ultrasound markers for detecting Down syndrome in women of advanced maternal age, so as to observe whether there were any associations between the presence of markers (singly or in combination) and the presence of Down syndrome and, for each of the markers found, the association of each type of marker with the syndrome. Initially, a descriptive analysis was performed, presenting the data as absolute frequencies (N) and relative frequencies (%) for each category of the qualitative variables of the patients' profile. For the quantitative variables, means, standard deviations, and minimum and maximum values were calculated. To investigate possible associations between pairs of characteristics or qualitative variables, nonparametric tests consisting of the chi-square test or Fisher's exact test when necessary were used, with a significance level of 5%. Combined associations of the variables were assessed in a multivariate manner, by means of correspondence analysis. The relative risk calculations and odds ratios were considered significant when the data were within the 95% confidence interval.
Combined associations of the variables were assessed in a multivariate manner, by means of correspondence analysis. The relative risk calculations and odds ratios were considered significant when the data were within the 95% confidence interval.
The maternal age ranged from 35 to 47 years, with a mean of 38.8 ± 2.9 years for the 31 pregnant women with fetuses with Down syndrome and 38.6 ± 2.6 years for the 858 pregnant women with chromosomally normal fetuses, without any statistical difference. Similarly, gestational age at the time of amniocentesis did not present any significant difference between the two groups, with a mean of 17.7 ± 3.1 weeks for the Down syndrome cases and 17.3 ± 2.4 weeks for the chromosomally normal fetuses.
Among the 889 pregnant women, 131 (17.3%) presented markers and 758 (82.7%) did not present markers on the second-trimester ultrasound.
presents the distribution of the 31 fetuses with Down syndrome and their correlation with the ultrasound markers, which presented a statistically significant difference (P < 0.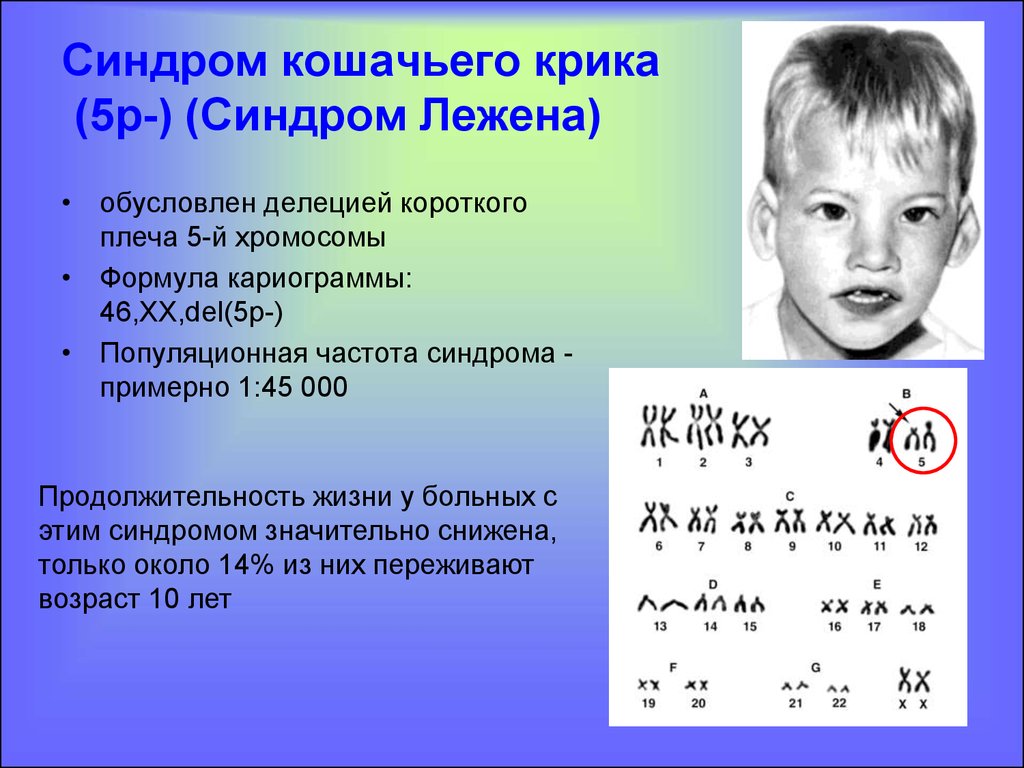 001).
001).
Table 1
Description of the cases of Down syndrome according to the groups with and without ultrasound markers (P < 0.001).
| Group of pregnant women | Down syndrome | |||||
|---|---|---|---|---|---|---|
| Present | Absent | Total | ||||
| With markers | 19 | 14.6% | 112 | 85.5% | 131 | 100.0% |
| Without markers | 12 | 1. 6% 6% | 746 | 98.4% | 758 | 100.0% |
| | ||||||
| Total | 31 | 3.5% | 858 | 96.5% | 889 | 100.0% |
Open in a separate window
presents the distribution of the Down syndrome cases, between the two groups studied, with and without markers, according to maternal age, in which there was a statistically significant difference for the maternal ages of 37, 38, and ≥40 years. The odds ratio calculation did not show any increased occurrence of Down syndrome among women ≥40 years of age in the presence of ultrasound markers (OR = 1. 74; 95% CI: 0.84–3.57).
74; 95% CI: 0.84–3.57).
Table 2
Distribution of the cases of Down syndrome according to maternal age and ultrasound markers.
| Maternal age (years) | With markers | Without markers | Statistical analysis (P < 0.05) | ||
|---|---|---|---|---|---|
| Present | Absent | Present | Absent | ||
| 35 | 4 | 46 | 2 | 89 | P = 0.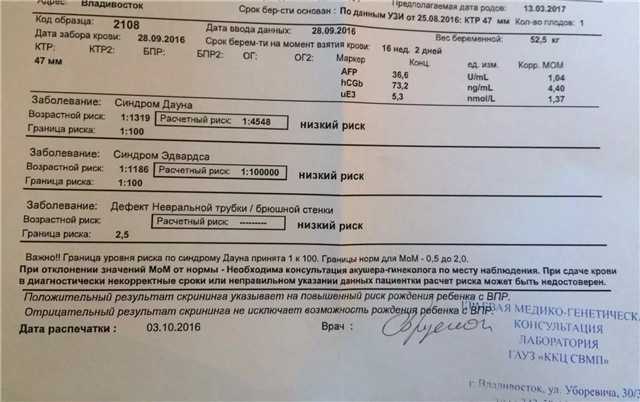 11 11 |
| 36 | 2 | 19 | 1 | 91 | P = 0.08 |
| 37 | 2 | 13 | 1 | 112 | P = 0.03 |
| 38 | 2 | 9 | 1 | 101 | P = 0.02 |
| 39 | 1 | 8 | 1 | 94 | P = 0. 16 16 |
| ≥40 | 8 | 17 | 6 | 259 | P < 0.001 |
| | |||||
| Total | 19 | 112 | 12 | 746 | |
Open in a separate window
presents the correlation between some second-trimester ultrasound markers and Down syndrome, with positive correlations with thickened nuchal fold, short femur, and structural malformations. presents the odds ratios for some ultrasound markers in relation to Down syndrome, showing increased occurrence of this syndrome in the presence of thickened nuchal fold and short femur length.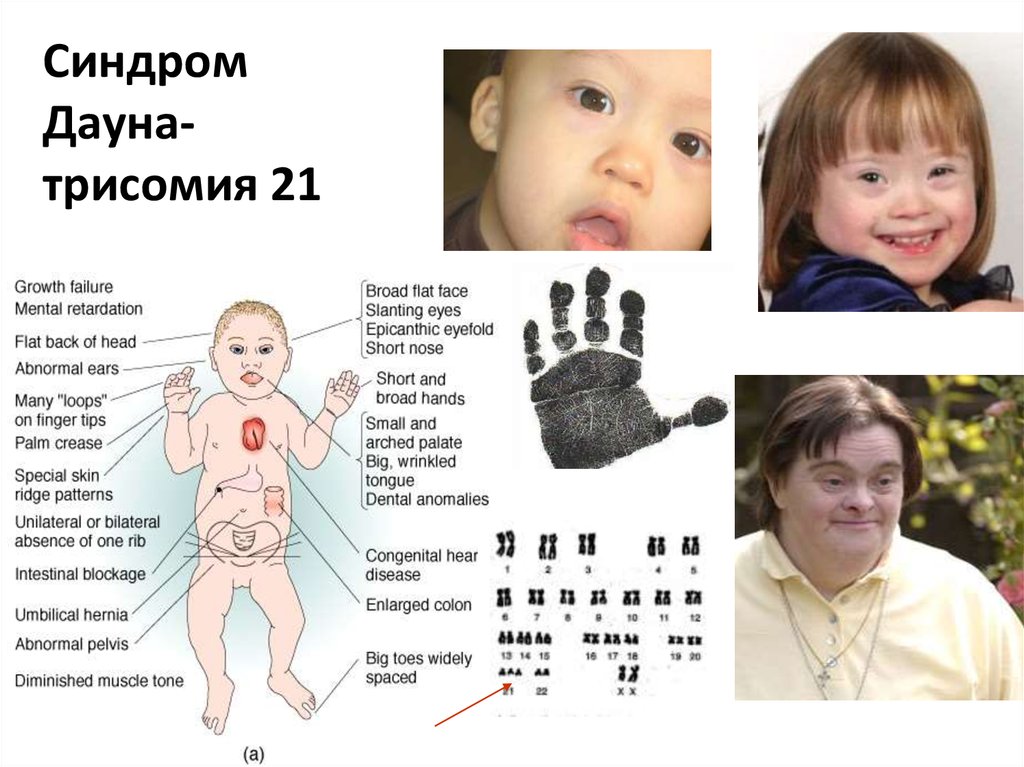 presents the sensitivity, specificity, and positive and negative predictive values for the ultrasound markers in occurrences of Down syndrome.
presents the sensitivity, specificity, and positive and negative predictive values for the ultrasound markers in occurrences of Down syndrome.
Table 3
Correlation between ultrasound markers and Down syndrome.
| Marker | Down syndrome | Statistical analysis | |
|---|---|---|---|
| Present | Absent | ||
| Nuchal fold thickness ≥ 6 mm | 12/31 | 35/858 | P < 0.001 |
| Intracardiac hyperechogenic focus | 4/31 | 35/858 | P = 0.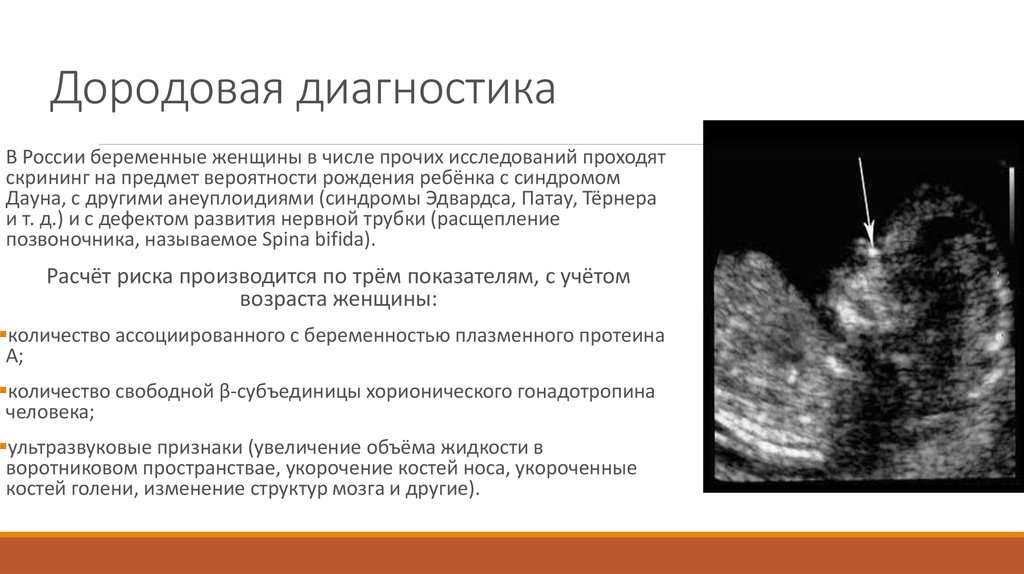 05 05 |
| Short femur length | 1/31 | 2/858 | P = 0.10 |
| Structural malformation | 6/31 | 26/858 | |
| Duodenal Atresia | 1 | 5 | P < 0.001 |
| Esophageal Atresia | 1 | 4 | |
| Cystic Hygroma | 3 | 0 | |
| Meningomyelocele | 0 | 6 | |
| Spina Bifida | 0 | 4 | |
| Congenital heart disease | 1 | 2 | |
| Hydrocephalus | 0 | 5 | |
Open in a separate window
Table 4
Odds ratios for the ultrasound markers for Down syndrome.
| Marker | Down syndrome | Odds ratio | Confidence interval (95%) | ||
|---|---|---|---|---|---|
| Absent | Present | ||||
| Nuchal fold thickness ≥ 6 mm | Present | 35 | 12 | 14.9 | 6.7–33.0 |
| Absent | 823 | 19 | |||
| | |||||
| Intracardiac hyperechogenic focus | Present | 35 | 4 | 3.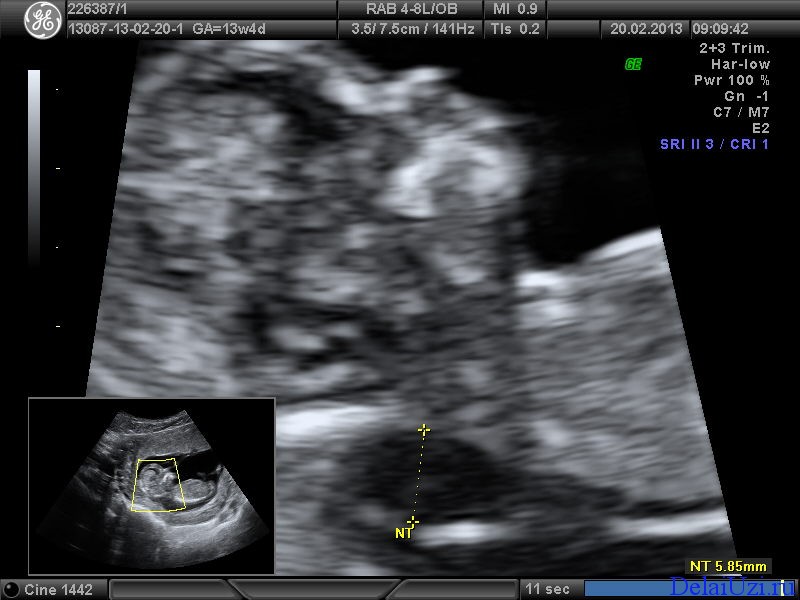 5 5 | 1.1–10.5 |
| Absent | 823 | 27 | |||
| | |||||
| Short femur length | Present | 2 | 1 | 14.2 | 1.3–161.7 |
| Absent | 856 | 30 | |||
| | |||||
| Structural fetal malformation | Present | 26 | 6 | 7.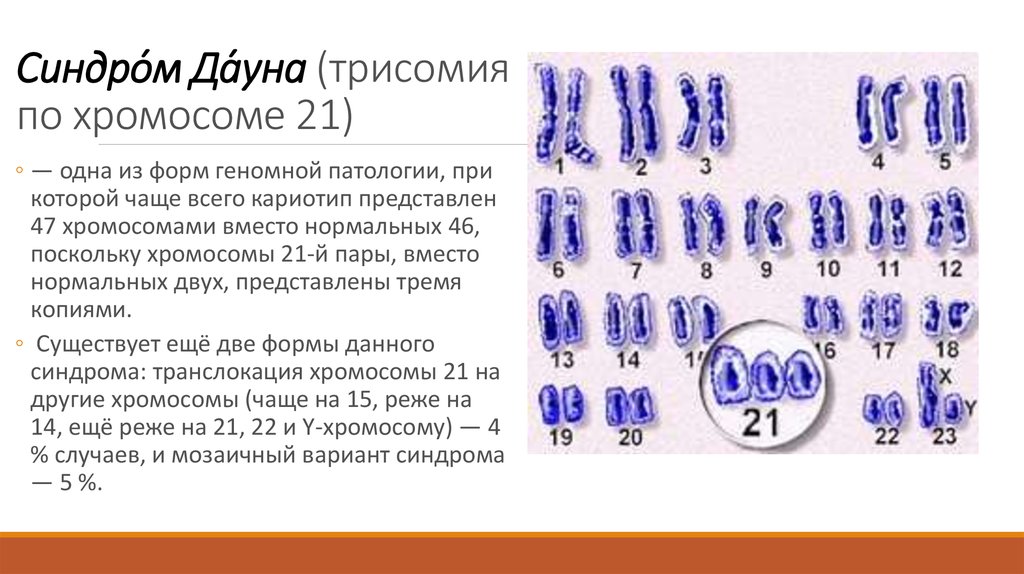 7 7 | 2.9–20.3 |
| Absent | 832 | 25 | |||
Open in a separate window
Table 5
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the ultrasound markers for detecting Down syndrome.
| Marker | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Nuchal fold thickness ≥ 6 mm | 38.7 | 95.9 | 25.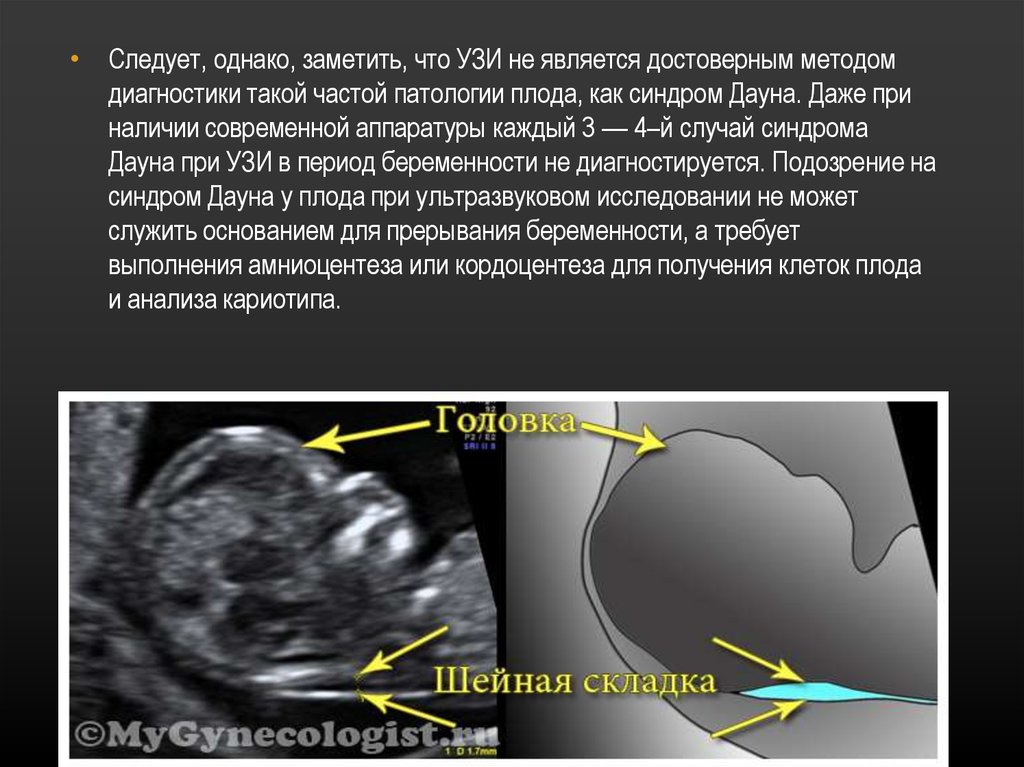 5 5 | 97.7 |
| Short femur length | 32.3 | 99.8 | 33.3 | 96.6 |
| Intracardiac hyperechogenic focus | 12.9 | 95.9 | 10.3 | 96.8 |
| Structural fetal malformation | 19.3 | 97.0 | 18.8 | 97.1 |
Open in a separate window
presents the calculation of chances between the number of ultrasound markers and Down syndrome. We observed that the greater the number of markers was, the higher the chance of Down syndrome was.
Table 6
Odds ratios between the number of ultrasound markers and Down syndrome.
| Number of markers | Down syndrome | Odds ratio | Confidence interval (95%) | |||
|---|---|---|---|---|---|---|
| Present | Absent | |||||
| 0 | 12 | 38.7 | 746 | 86.9 | 0.1 | 0.0–0.2 |
| 1 | 14 | 45. | 89 | 10.4 | 10.5 | 4.1–23.4 |
| ≥2 | 5 | 16.1 | 23 | 2.7 | 13.5 | 3.8–46.2 |
Open in a separate window
Amniocentesis has traditionally been offered during the second trimester, to patients with a high risk of Down syndrome, that is, pregnant women with advanced maternal age. However, using only this parameter for screening, only around 31% of the cases of trisomy 21 are diagnosed, with false positive rates of 13% to 14% [12]. Investigation of aneuploidy markers on second-trimester ultrasound presents high rates of false positives (12% to 15%) due to the high frequency of these markers in low-risk populations and, especially, due to the low sensitivity when these markers are present separately [13]. Therefore, it is important to define and study this type of population separately, so as to achieve screening of greater reliability, with better results for detecting chromosomal abnormalities [10].
It has been suggested that a normal second-trimester ultrasound examination reduces the initial risk of Down syndrome by 50% to 80% [11, 14, 15]. The present study obtained a similar result, in which there was a 90% decrease in risk in the absence of ultrasound markers and/or structural malformations.
According to Nyberg et al. [8], in the presence of at least one marker, the risk of Down syndrome increased two-fold from the initial risk; in the presence of two markers, the risk increased tenfold and, when three or more markers were present, the risk increased more than a hundredfold. On the other hand, while Benacerraf [16] also showed that in the presence of one marker the risk increased twofold, they showed that in the presence of two or more markers, the risk increased 23-fold. In the present study, in the presence of one marker, the risk increased 10.5-fold and when two or more markers were present, the risk increased 13.5-fold.
Increased nuchal fold (≥6 mm) is without doubt the marker most correlated with Down syndrome during the second trimester, and it is present in approximately 39% to 45% of the cases [16]. In the present study, we observed nuchal fold sensitivity of 39% with specificity of 96%, which was statistically significant. Although specificity of this marker was similar to that found by DeVore [17] and Aagaard-Tillery et al. [18] (99.2%, 99%, resp.) the same did not occur with sensitivity (28.8% and 18%, resp.). The variability of specificity may be accompanied by an even greater variability in likelihood ratio (LLR) with these previous studies finding rates of 53.4 and 49, respectively, whereas the present study determined a LLR of 15. Other studies, on the other hand, showed LLR varying from 10 to 17 [8, 15, 19].
The presence of structural malformation has, over the years, remained a good parameter for diagnosing Down syndrome [19]. In the present study, structural abnormalities showed sensitivity of 19%, with specificity of 97%. The most frequent type of malformation in the syndrome cases was cystic hygroma (50%). These results are in agreement with those reported by other authors, such as Sohl et al. [20] and Bromley et al. [15], who described structural malformations presenting sensitivities of 16.4% to 19.5% in screening for this syndrome, respectively.
It seems that shorter femur is not a good marker for Down aneuploidy. Shorter femur was shown by just 1 out of the 31 of Down cases. Furthermore, previous studies report a high frequency of this marker among euploid individuals [21, 22]. In the present study, the risk increased 14-fold, with a very wide confidence interval, reflecting in low accuracy. Similarly, Cho et al. [23] observed that short femur length was a poor marker for Down syndrome in a Korean population.
The intracardiac hyperechogenic focus is a controversial marker, because it is a common finding among the normal population during the second trimester [24]. Other studies have correlated this marker with Down syndrome in proportions of 16% to 18% [25, 26]. In the present study, this marker was present in 10.3% of the chromosomally altered cases. Most were present together with other ultrasound markers, thus presenting a statistically significant tendency towards an association with increased risk. However, when present separately, this marker did not present any significant association with Down syndrome.
The congenital heart disease (CHD) was present in one fetus with Down syndrome (1/31 = 3.2%) and in two euploid fetuses (2/858 = 0.23%). We included the cases of CHD in the group of structural malformations, because the number cases were insufficient to correlate them with the Down syndrome. It has occurred because of the low gestational age at the moment of ultrasound exam (17 weeks), when the CHD rate detection is smaller than after 20 weeks [27]. Furthermore, the ultrasound exams were realized by sonographers without expertise in fetal echocardiography, justifying the low rate detection. The main structural CHD in fetuses with Down syndrome is atrioventricular septal defect [28]; however, the detection rate of this CHD during prenatal ultrasound is low [29].
In the present study, we performed a statistical calculation comparing the diagnosis of Down syndrome in the presence and absence of ultrasound markers. In considering the calculation with two markers, the sensitivity reduced markedly to 29.4%, but with an increase in specificity to 97%. Thus, we considered that second-trimester ultrasound had a high chance of screening for Down syndrome in the presence of at least two markers in the population of pregnant women with advanced maternal age.
The use of ultrasound markers during the second trimester to screen for Down syndrome is subject to bias, since there are still no definitive studies regarding the sensitivity and specificity of each marker or set of markers [30]. However, through appropriate standardization of the types of populations and the studies carried out, the analyses on these markers will become better, because the interactions of each marker, separately or in association with other markers, will be evaluated, thereby resulting in a second-trimester screening for Down syndrome that is more reliable overall [31].
The accuracy of ultrasound as Down syndrome screening examination has been improved by combining its findings in the first trimester of pregnancy with biochemical screening in the first and second trimester (FASTER trial). Currently, a much more accurate screening tool is available. Noninvasive prenatal testing (NIPT) is able to detect trisomy 21 directly on free fetus DNA fragments in mother's blood stream. It has high accuracy; however, it is associated with higher costs and equipment availability, which is not universally provided, mainly in developing countries as Brazil. This makes genetic ultrasound a relatively reliable screening tool, thus supporting investigative work undertaken to better use this tool.
In summary, increased nuchal fold and presence of structural malformation presented significant associations with Down syndrome, especially when associated with other ultrasound markers, among pregnant women of advanced maternal age.
The authors declare that there is no conflict of interests regarding the publication of this paper.
1. Baird PA, Anderson TW, Newcombe HB, Lowry RB. Genetic disorders in children and young adults: a population study. The American Journal of Human Genetics. 1988;42(5):677–693. [PMC free article] [PubMed] [Google Scholar]
2. Sprigg A. Fetal malformations diagnosed antenatally 1: general principles. British Journal of Hospital Medicine. 1995;54(8):387–390. [PubMed] [Google Scholar]
3. Snijders RJM, Sundberg K, Holzgreve W, Henry G, Nicolaides KH. Maternal age and gestation-specific risk for trisomy 21. Ultrasound in Obstetrics and Gynecology. 1999;13(3):167–170. [PubMed] [Google Scholar]
4. Benacerraf BR, Neuberg D, Bromley B, Frigoletto FD., Jr. Sonographic scoring index for prenatal detection of chromosomal abnormalities. Journal of Ultrasound in Medicine. 1992;11(9):449–458. [PubMed] [Google Scholar]
5. Nyberg DA, Luthy DA, Cheng EY, Sheley RC, Resta RG, Williams MA. Role of prenatal ultrasonography in women with positive screen for Down syndrome on the basis of maternal serum markers. The American Journal of Obstetrics and Gynecology. 1995;173(4):1030–1035. [PubMed] [Google Scholar]
6. DeVore GR, Romero R. Genetic sonography: an option for women of advanced maternal age with negative triple-marker maternal serum screening results. Journal of Ultrasound in Medicine. 2003;22(11):1191–1199. [PubMed] [Google Scholar]
7. Wellesley D, de Vigan C, Baena N, et al. Contribution of ultrasonographic examination to the prenatal detection of trisomy 21: experience from 19 European registers. Annales de Genetique. 2004;47(4):373–380. [PubMed] [Google Scholar]
8. Nyberg DA, Souter VL, El-Bastawissi A, Young S, Luthhardt F, Luthy DA. Isolated sonographic markers for detection of fetal Down syndrome in the second trimester of pregnancy. Journal of Ultrasound in Medicine. 2001;20(10):1053–1063. [PubMed] [Google Scholar]
9. Vintzileos AM, Campbell WA, Guzman ER, Smulian JC, Mclean DA, Ananth CV. Second-trimester ultrasound markers for detection of trisomy 21: which markers are best? Obstetrics and Gynecology. 1997;89(6):941–944. [PubMed] [Google Scholar]
10. Nyberg DA, Luthy DA, Resta RG, Nyberg BC, Williams MA. Age-adjusted ultrasound risk assessment for fetal Down’s syndrome during the second trimester: description of the method and analysis of 142 cases. Ultrasound in Obstetrics and Gynecology. 1998;12(1):8–14. [PubMed] [Google Scholar]
11. Nyberg DA, Souter VL. Use of genetic sonography for adjusting the risk for fetal Down syndrome. Seminars in Perinatology. 2003;27(2):130–144. [PubMed] [Google Scholar]
12. Egan JFX, Benn P, Borgida AF, Rodis JF, Campbell WA, Vintzileos AM. Efficacy of screening for fetal Down syndrome in the United States from 1974 to 1997. Obstetrics and Gynecology. 2000;96(6):979–985. [PubMed] [Google Scholar]
13. Hobbins JC, Lezotte DC, Persutte WH, et al. An 8-center study to evaluate the utility of midterm genetic sonograms among high-risk pregnancies. Journal of Ultrasound in Medicine. 2003;22(1):33–38. [PubMed] [Google Scholar]
14. Vintzileos AM, Ananth CV, Smulian JC, Day-Salvatore DL, Beazoglou T, Knuppel RA. Cost-benefit analysis of prenatal diagnosis for Down syndrome using the British or the American approach. Obstetrics and Gynecology. 2000;95(4):577–583. [PubMed] [Google Scholar]
15. Bromley B, Lieberman E, Shipp TD, Benacerraf BR. The genetic sonogram: a method of risk assessment for Down syndrome in the second trimester. Journal of Ultrasound in Medicine. 2002;21(10):1087–1096. [PubMed] [Google Scholar]
16. Benacerraf BR. Use of sonographic markers to determine the risk of Down syndrome in second-trimester fetuses. Radiology. 1996;201(3):619–620. [PubMed] [Google Scholar]
17. DeVore GR. Genetic sonography: the historical and clinical role of fetal echocardiography. Ultrasound in Obstetrics and Gynecology. 2010;35(5):509–521. [PubMed] [Google Scholar]
18. Aagaard-Tillery KM, Malone FD, Nyberg DA, et al. Role of second-trimester genetic sonography after Down syndrome screening. Obstetrics and Gynecology. 2009;114(6):1189–1196. [PMC free article] [PubMed] [Google Scholar]
19. Smith-Bindman R, Chu P, Goldberg JD. Second trimester prenatal ultrasound for the detection of pregnancies at increased risk of Down syndrome. Prenatal Diagnosis. 2007;27(6):535–544. [PubMed] [Google Scholar]
20. Sohl BD, Scioscia AL, Budorick NE, Moore TR. Utility of minor ultrasonographic markers in the prediction of abnormal fetal karyotype at a prenatal diagnostic center. The American Journal of Obstetrics and Gynecology. 1999;181(4):898–903. [PubMed] [Google Scholar]
21. Shipp TD, Bromley B, Mascola M, Benacerraf B. Variation in fetal femur length with respect to maternal race. Journal of Ultrasound in Medicine. 2001;20(2):141–144. [PubMed] [Google Scholar]
22. Kovac CM, Brown JA, Apodaca CC, et al. Maternal ethnicity and variation of fetal femur length calculations when screening for Down syndrome. Journal of Ultrasound in Medicine. 2002;21(7):719–722. [PubMed] [Google Scholar]
23. Cho HJ, Won HS, Ju DH, Roh HJ, Lee PR, Kim A. Evaluation of the usefulness of the fetal femur length with respect to gestational age to detect Down syndrome in Korean subjects. Prenatal Diagnosis. 2010;30(8):734–738. [PubMed] [Google Scholar]
24. Shipp TD, Bromley B, Lieberman E, Benacerraf BR. The frequency of the detection of fetal echogenic intracardiac foci with respect to maternal race. Ultrasound in Obstetrics and Gynecology. 2000;15(6):460–462. [PubMed] [Google Scholar]
25. Manning JE, Ragavendra N, Sayre J, et al. Significance of fetal intracardiac echogenic foci in relation to trisomy 21: a prospective sonographic study of high-risk pregnant women. The American Journal of Roentgenology. 1998;170(4):1083–1084. [PubMed] [Google Scholar]
26. Wax JR, Cartin A, Pinette MG, Blackstone J. Are intracardiac echogenic foci markers of congenital heart disease in the fetus with chromosomal abnormalities? Journal of Ultrasound in Medicine. 2004;23(7):895–898. [PubMed] [Google Scholar]
27. DeVore GR. The role of fetal echocardiography in genetic sonography. Seminars in Perinatology. 2003;27(2):160–172. [PubMed] [Google Scholar]
28. Mogra R, Zidere V, Allan LD. Prenatally detectable congenital heart defects in fetuses with Down syndrome. Ultrasound in Obstetrics and Gynecology. 2011;38(3):320–324. [PubMed] [Google Scholar]
29. ter Heide H, Thomson JDR, Wharton GA, Gibbs JL. Poor sensitivity of routine fetal anomaly ultrasound screening for antenatal detection of atrioventricular septal defect. Heart. 2004;90(8):916–917. [PMC free article] [PubMed] [Google Scholar]
30. Graupe MH, Naylor CS, Greene NH, Carlson DE, Platt L. Trisomy 21: second-trimester ultrasound. Clinics in Perinatology. 2001;28(2):303–319. [PubMed] [Google Scholar]
31. Schluter PJ, Pritchard G. Mid trimester sonographic findings for the prediction of Down syndrome in a sonographically screened population. The American Journal of Obstetrics and Gynecology. 2005;192(1):10–16. [PubMed] [Google Scholar]
Significance of second trimester markers for Down's syndrome revealed -- ScienceDaily
Science News
from research organizations
- Date:
- January 30, 2013
- Source:
- Wiley
- Summary:
- A new analysis has found that some second trimester markers for Down's syndrome that are detected by ultrasound are more telling than others. The study's results will help adjust pregnant women's risks for having a child with the condition.
- Share:
-
Facebook Twitter Pinterest LinkedIN Email
FULL STORY
A new analysis has found that some second trimester markers for Down's syndrome that are detected by ultrasound are more telling than others. Published early online in Ultrasound in Obstetrics & Gynecology, the study's results will help adjust pregnant women's risks for having a child with the condition.
advertisement
Screening for Down's syndrome is offered to all pregnant women, who start out with a background risk based on their age. Certain features detected during a second trimester ultrasound exam are potential markers for Down's syndrome, and they include dilated brain ventricles, absent or small nose bone, increased thickness of the back of the neck, an abnormal artery to the upper extremities, bright spots in the heart, 'bright' bowels, mild kidney swelling, and shortening of an arm bone or thigh bone.
To determine how these markers affect risk, Kypros Nicolaides, MD, of the Harris Birthright Research Centre for Fetal Medicine at King's College London in England, and his colleagues analyzed all published studies that reported results on second trimester markers for Down's syndrome between 1995 and 2012.
The researchers identified 48 studies, and they discovered that most single markers have only a small effect on modifying the odds for Down's syndrome. . This finding could have important clinical implications because currently in the United States, when a marker such as a short arm or thigh bone is detected, women are told that they are at high risk of having a child with Down's syndrome. Dr. Nicolaides and his team found that a few markers do carry increased risks, though.
Dilated brain ventricles, increased thickness of the back of the neck, and an abnormal artery to the upper extremities increase the risk by three- to four-fold, and an absent or small nose bone increases the risk by six- to seven-fold.
"The detection of any one of the findings during the scan should prompt the sonographer to look for all other markers or abnormalities," said Prof. Nicolaides. He added that the study also revealed that if a detailed second trimester ultrasound exam demonstrates the absence of all major markers, the risk of having a baby affected by Down's syndrome is reduced by more than seven-fold.
The findings indicate that the relative importance of ultrasound markers is very different from what has been previously assumed. Prof. Nicolaides noted that the results from this study will be incorporated in obstetric ultrasound scan software that adjusts women's risks for having a child with Down's syndrome.
advertisement
Story Source:
Materials provided by Wiley. Note: Content may be edited for style and length.
Journal Reference:
- M. Agathokleous, P. Chaveeva, L. C. Y. Poon, P. Kosinski, K. H. Nicolaides. Meta-analysis of second-trimester markers for trisomy 21. Ultrasound in Obstetrics & Gynecology, 2013; DOI: 10.1002/uog.12364
Cite This Page:
- MLA
- APA
- Chicago
Wiley. "Significance of second trimester markers for Down's syndrome revealed." ScienceDaily. ScienceDaily, 30 January 2013. <www.sciencedaily. com/releases/2013/01/130130101839.htm>.
Wiley. (2013, January 30). Significance of second trimester markers for Down's syndrome revealed. ScienceDaily. Retrieved April 19, 2023 from www.sciencedaily.com/releases/2013/01/130130101839.htm
Wiley. "Significance of second trimester markers for Down's syndrome revealed." ScienceDaily. www.sciencedaily.com/releases/2013/01/130130101839.htm (accessed April 19, 2023).
Ultrasound diagnosis of Down syndrome and other chromosomal abnormalities
- Fetal ultrasound is the main method for diagnosing the likelihood of Down syndrome
- Basic ultrasound markers of Down syndrome
- Signs of Down syndrome with ultrasound in the second trimester of pregnancy
- How else can you determine the risk of having a baby with Down syndrome
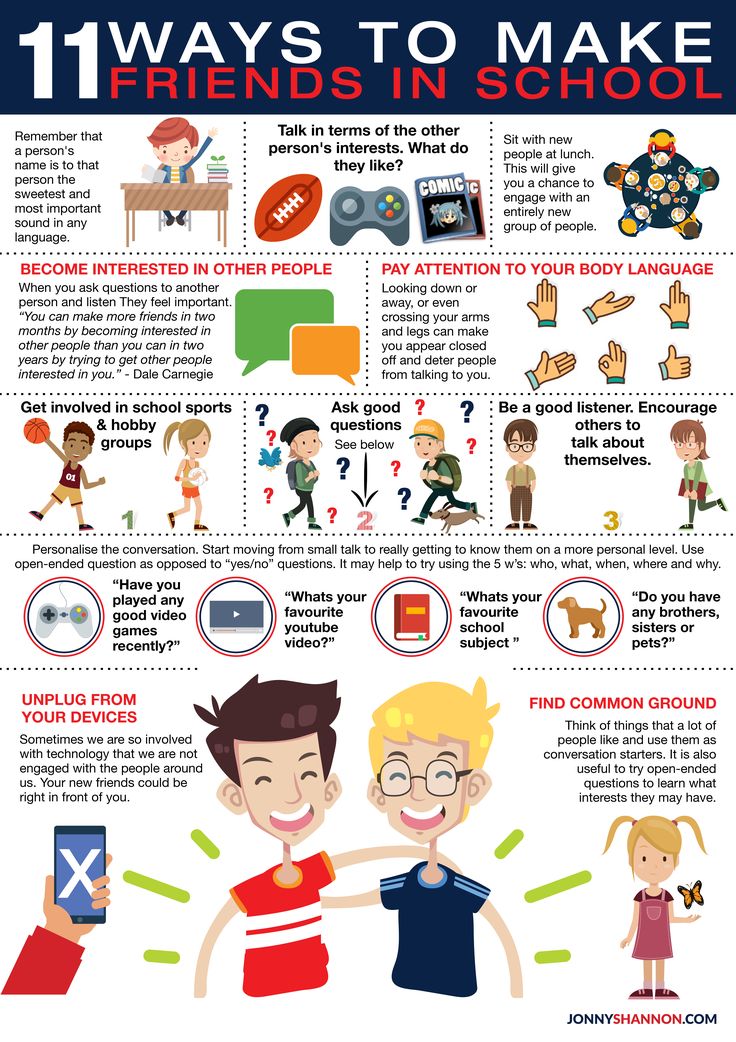
Down's syndrome is a relatively common congenital pathology caused by the presence of an extra chromosome in 21 pairs. Down syndrome is also called trisomy on chromosome 21. Of all the chromosomal abnormalities, this pathology is the most well-known for a wide range of people.
Down's syndrome is associated with many significant malformations. Such as, for example, congenital heart defects, duodenal atresia (a condition where part of the small intestine does not develop), an increased risk of developing acute leukemia (leukemia). About 50% of children with this condition are born with heart defects, the most common of which is a ventricular septal defect. Another disease that is often observed in such children is Hirschsprung's disease - an aganglionic area of the large intestine, leading to the development of intestinal obstruction.
Screening tests during pregnancy are used to assess the risk of having a baby with Down syndrome. These tests are painless and non-invasive (do not cause damage to the tissues of the pregnant woman and fetus), but do not accurately judge the presence or absence of congenital pathology. Nevertheless, their widespread use makes it possible to suspect risks and recommend invasive procedures. Invasive diagnostic methods include interventions performed under ultrasound guidance - amniocentesis, chordocentesis and chorionic villus sampling.
Fetal ultrasound is the main way to diagnose the likelihood of Down syndrome
Fetal ultrasound to detect developmental abnormalities is called ultrasound screening. Most often, ultrasound screening is combined with biochemical blood tests of a pregnant woman, and such a study is called combined screening. Depending on the choice of criteria, the probability of detecting Down syndrome in the second trimester is 60-91%. The use of color Doppler technology for ultrasound screening makes it possible to detect defects in the fetal heart and increases the accuracy of diagnosis.
Major ultrasound markers of Down syndrome
Ultrasound markers are ultrasound measurements that are most likely to be associated with a high chance of having Down syndrome in the fetus. None of these markers is specific, that is, inherent only in this pathology. The following indicators are taken into account: the thickness of the collar space (75% of sensitivity), the absence of the nasal bone (58%), heart defects, short femurs and humeri, hyperechoic intestines, choroid plexus cysts, echogenic foci in the heart, signs of duodenal atresia . Of course, none of these markers is an absolute sign of a chromosomal abnormality.
Measuring the thickness of the nuchal space
The nuchal space (cervical fold) is the area between the folds of fetal tissues, which is transparent on ultrasound, located below the occiput in the area of neck formation. In children with chromosomal abnormalities, such as Down's syndrome or trisomy 18, fluid accumulates in this place. The measurement of this space is carried out from 11 to 14 weeks during the ultrasound of pregnancy in the first trimester. Measurements obtained at this time are the most significant for predicting the individual risk of developing fetal chromosomal abnormalities. The thickness of the collar space more than 3 mm is taken as an indicator of an increased likelihood of genetic defects in the fetus. The increase in the collar space does not mean that the fetus necessarily has Down syndrome, but if we take into account the gestational age of the fetus on which measurements were taken, the age of the mother and biochemical screening indicators, the probability of a correct diagnosis becomes higher than 80%. Although nuchal thickness screening is one of the most important tests for detecting chromosomal abnormalities, there are biases. The error arises due to violations of the measurement technique. This applies not only to measurements of the collar space, but also to the nasal bone. There are errors due to the human factor. The correct measurement technique and the experience of the ultrasound specialist are very important.
Fetal Pelvis and Brain Measurements
In fetuses with trisomy 21, ultrasonography may show cerebellar hypoplasia (reduction in size) and a reduction in the frontal lobe. These facts are used to assess the likelihood of Down syndrome. The combination of a shortened transverse size of the cerebellum and a decrease in the fronto-thalamic distance is of greater importance for the diagnosis of this chromosomal anomaly than measuring each distance of intracerebral structures separately.
In a fetus with Down's syndrome during ultrasound, the length of the iliac bones is clearly reduced and the angle between these bones is increased. This can be measured most clearly at the level of the middle of the sacrum in cross section.
Signs of Down's syndrome during ultrasound in the second trimester of pregnancy
Ultrasound of the fetus in the second trimester of pregnancy can reveal such abnormalities as a violation of the formation of bones of the skeleton, expansion of the collar space by more than 5 mm, the presence of heart defects, expansion of the renal pelvis (pyeloectasia ), echogenicity of the intestine, cysts of the choroid plexus of the brain. Moreover, only heart defects, disorders of skeletal formation and expansion of the collar space are independent risk factors.
The use of only one of the measurements in assessing the probability of the presence of chromosomal abnormalities of the fetus, for example, determining the thickness of the collar space or measuring the iliac angle, is less accurate than taking into account the totality of measurements.
Another way to determine the risk of having a baby with Down syndrome
In addition to ultrasound signs, biochemical screening in the first and second trimester is used to determine the degree of risk. In this case, the blood of a pregnant woman is examined. The best results are obtained by combining ultrasonic and biochemical signs. If the combination of factors is threatening, a decision may be made to conduct invasive methods for diagnosing Down syndrome and other chromosomal abnormalities. Invasive techniques consist in obtaining particles of the skin or blood of the fetus, followed by genetic analysis. These methods are 100% reliable. At the same time, even complete well-being during screening in the first and second trimester using ultrasound and biochemical markers cannot guarantee the absence of chromosomal abnormalities in the fetus. The choice and determination of the sufficiency of diagnostic methods is carried out taking into account the age of the parents, family history and the presence of abnormalities during previous pregnancies.
We carry out all types of ultrasound diagnostics:
- 3D and 4D ultrasound during pregnancy
- Fetometry data at various times
- Ultrasound diagnostics of Down syndrome and other chromosomal abnormalities
- Evaluation of the correct development of the fetus by ultrasound
Ultrasound during pregnancy
- Hydrotubation (echohydrotubation): examination of the patency of the fallopian tubes (ultrasonic hysterosalpingoscopy)
- Transvaginal
- Ovaries
- Uterus
- Mammary glands
Female ultrasound
- Duplex Scan
- Cerebral vessels
- Neck vessels (duplex angioscanning of the main arteries of the head)
- Veins of the lower extremities
Vascular ultrasound
- Transrectal (trusion): prostate
- Scrotum (testicles)
- Vessels of the penis
Male ultrasound
- Appendicitis
- Abdomen
- Gallbladder
- Stomach
- Intestines
- Bladder
- Soft tissue
- Pancreas
- Liver
- Kidney
- Joints
- Thyroid
- Echocardiography (ultrasound of the heart)
Organ ultrasound
- Varicose veins: ultrasound diagnosis of varicose veins
- Hypertension: Ultrasound diagnosis of hypertension
- Thrombosis: ultrasound diagnosis of vein thrombosis
- Ultrasound diagnosis of chronic pancreatitis
- for kidney stones
- for cholecystitis
Ultrasound diagnostics of diseases
- Hip joints in newborns (if hip dysplasia is suspected)
Pediatric ultrasound
Screening for Down syndrome during pregnancy in Moscow, prices
Down syndrome - human trisomy on the 21st chromosome - the most common of this group of diseases, occurs in about 1 in 700-800 newborns. The probability of having a child with Down syndrome increases with the age of the expectant mother. However, taking into account the birth rate in different age groups, 80% of children with Down syndrome are born to mothers under the age of 30 years.
After the birth of one child with Down syndrome, the mother's risk of relapse for each subsequent child is approximately 1% higher than her age risk. This fact is especially important for young mothers, whose age-related risks are small.
Prenatal screening, recommended for all pregnant women, regardless of risk group, to detect chromosomal pathology, includes the study of serum markers (alpha-fetoprotein, free estriol, human chorionic gonadotropin) and ultrasound, performed routinely in the first trimester of pregnancy. The most characteristic ultrasound findings in Down's syndrome include heart defects, shortened long bones, underdeveloped fetal nasal bone, malformations of the small intestine, etc. If the results show an increased risk of the presence of chromosomal abnormalities, a diagnostic invasive intervention is performed to take fetal biomaterial for confirmatory research. Depending on the duration of pregnancy, this can be a chorionic villus biopsy (10-12 weeks) or amniocentesis (from the 15th week). According to the results of genetic diagnosis of the obtained fetal material, a prenatal diagnosis is made, and when the syndrome is confirmed in the fetus, the issue of maintaining pregnancy is decided.
Most patients with Down syndrome, unless diagnosed prenatally, are usually recognized at birth due to typical phenotypic features, but in some cases prematurity or ethnic differences may make clinical diagnosis less straightforward. In addition to the characteristic external features, malformations of many organs and systems are described in Down syndrome. The most common of these are congenital heart defects (observed in approximately 50% of cases), often requiring surgical intervention. Malformations of the gastrointestinal tract are also noted, especially duodenal atresia (2-5% of cases), Hirschsprung's disease, esophageal atresia and other serious disorders.
Ophthalmic diseases - strabismus, cataracts, myopia and glaucoma - are more common in children with Down syndrome. There may also be hearing loss of heterogeneous origin, spinal cord compression, myopathic symptoms associated with spinal cord compression (weakness, abnormal reflexes, urinary incontinence, etc.).
For all the problems associated with their mental development, Down syndrome patients show a wide range of developmental abilities, with high variability in personality and behavioral phenotypes. The most common causes of death in patients with Down syndrome are associated with congenital heart defects, infections (such as pneumonia), and malignant neoplasms of the blood system. Surgical correction of associated congenital malformations is usually successful, and long-term survival is good. However, less than half of patients survive to age 60, and less than 15% survive past age 68. Neurodegenerative diseases with features of Alzheimer's disease occur in most patients over 40 years of age.
The development of laboratory and ultrasound diagnostics has made it possible to develop and introduce into the practice of practical healthcare, including in our country, a number of comprehensive measures that solve many problems of monitoring the condition of the fetus and the course of pregnancy, including prenatal detection of Down syndrome in the fetus in the early stages pregnancy. These tasks can be solved by traditional screening of the first trimester, which combines the study of serum hormonal markers and ultrasound.
Non-invasive prenatal testing (NIPT) supplements the data of traditional screening, because, due to its higher sensitivity and specificity, it allows you to refine the results. The use of Prenetix NIPT as an expert-level screening reduces the number of invasive interventions that verify chromosomal diseases, and thereby avoids potential complications of invasion and reduces the risk of miscarriage as a result of diagnostic measures.
![]()