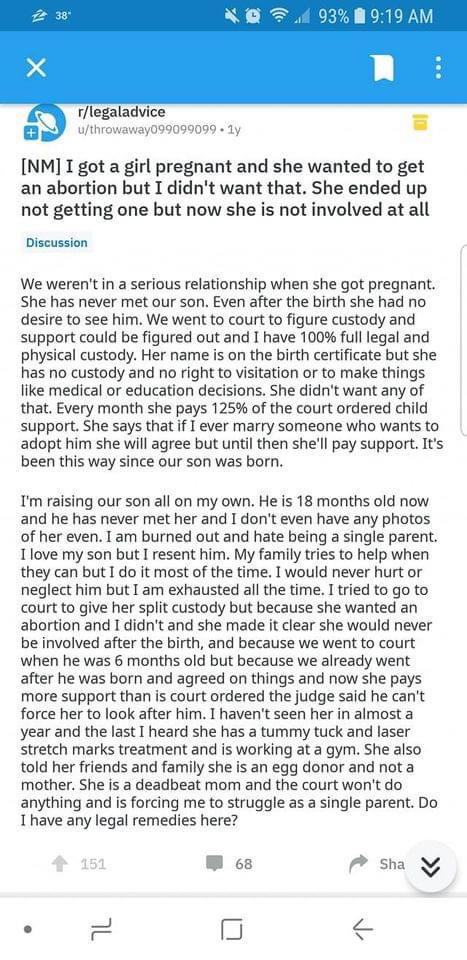
Is it ok to take valacyclovir while pregnant
Safety of antiviral medication for the treatment of herpes during pregnancy
Can Fam Physician. 2011 Apr; 57(4): 427–428.
Language: English | French
So-Hee Kang, RPh, Angela Chua-Gocheco, MD, Pina Bozzo, and Adrienne Einarson, RN
Copyright and License information Disclaimer
Question One of my patients is a pregnant woman in her first trimester with a history of recurrent genital herpes. She is concerned about whether use of her antiviral medication will adversely affect her baby. What should I tell her?
Answer Studies have shown that the use of acyclovir or valacyclovir is not associated with an increase in birth defects. Limited data exist for famciclovir and therefore it would not be considered a first-line choice for treatment of herpes during pregnancy.
Question L’une de mes patientes enceintes en est à son premier trimestre de grossesse et elle a des antécédents d’herpès génital récurrent. Elle se demande si l’utilisation de ses médicaments antiviraux pourrait nuire à son bébé. Que devrais-je lui répondre?
Réponse Des études ont démontré que l’utilisation de l’acyclovir ou du valacyclovir n’est pas associée à une augmentation des anomalies congénitales. Les données concernant le famciclovir sont limitées et ce médicament ne devrait donc pas être considéré comme choix de traitement de première intention pour l’herpès durant la grossesse.
Herpes simplex virus (HSV) infections are common viral infections, with almost 40% of infected patients encountering frequent recurrence within the first year of disease onset.1 In Ontario the seroprevalence for HSV type 1 (HSV-1) and type 2 was 51.1% and 9.1%, respectively.2 A Canadian study revealed HSV type 2 seropositivity in pregnant women to be 17.3%, which raises the concern of potential viral transmission from mother to infant.3 It is also important to note that genital herpes due to HSV-1 infections has increased in frequency and is responsible for up to 30% to 50% of new genital HSV infections. 3,4 Recent findings indicate that pregnant women who acquire HSV as a primary infection in the latter half of their pregnancies are at greatest risk of transmission to neonates.5
3,4 Recent findings indicate that pregnant women who acquire HSV as a primary infection in the latter half of their pregnancies are at greatest risk of transmission to neonates.5
Neonatal HSV infections are considered more serious compared with adult infections, having consequences that include the following: skin, eye, and mouth infections; central nervous system diseases; disseminated infections; and death. The Canadian neonatal HSV surveillance data indicate that there are 5.9 cases per 100 000 live births, stressing the importance of antiviral treatment during pregnancy to reduce such complications.6 Treatment with antivirals in adults has established their efficacy and safety, but evidence of the safety of acyclovir, famciclovir, and valacyclovir during pregnancy is relatively lacking. It is important to discuss the use of topical antiviral preparations owing to the need to prevent potential orolabial to genital transmission of HSV-1.
Valacyclovir is well absorbed after oral administration; absorption of a single dose of 1000 mg is 54% higher than that achieved after single 200- or 800-mg doses of oral acyclovir.7 Valacyclovir is rapidly metabolized to acyclovir, the triphosphorylated form of which selectively inhibits human HSV DNA polymerase, reducing viral DNA replication. Similarly, famciclovir is the oral prodrug of its active form penciclovir; it is more stable than acyclovir triphosphate, as evidenced by longer half-lives intracellularly, which might account for the prolonged in vitro antiviral activity.8 Acyclovir 5% and penciclovir 1% creams are used to treat orolabial herpes (HSV-1). Penciclovir was not detected in the plasma or urine of healthy volunteers after single or repeated application of the 1% cream.9 Systemic absorption of acyclovir following topical application is minimal or undetectable in adults.10
Although controlled studies evaluating the effectiveness of oral antivirals for recurrent HSV outbreaks in the mother and neonatal HSV infections at delivery do exist, they are limited by small sample sizes, the lack of fetal safety outcomes, and variation in timing of exposure. 11 However, data on the safety of antiviral drugs during pregnancy have been collected in pregnancy registries, usually established by the manufacturers. The oldest registry (ie, Acyclovir Pregnancy Registry) was open from 1984 to 1998, evaluating the use of oral or intravenous acyclovir in pregnant women. In total, 1234 pregnancies were noted with 1246 outcomes from 24 countries; 756 exposed pregnancies were studied in the first trimester. The risk of birth defects was 3.2% (95% confidence interval 2.0% to 5.0%), which is similar to the baseline risk of birth defects in the general population. No unusual defects or patterns of defects were seen, but the limitation of the results was the high loss to follow-up (27% of registrants).12 During the years 1995 to 1999, the manufacturer of valacyclovir maintained its pregnancy registry, and 110 exposures were reported with 111 known outcomes. Within the first trimester, 1 birth defect was reported out of 28 exposures; 2 of 31 and 1 of 51 exposures resulted in defects in the second and third trimesters, respectively.
11 However, data on the safety of antiviral drugs during pregnancy have been collected in pregnancy registries, usually established by the manufacturers. The oldest registry (ie, Acyclovir Pregnancy Registry) was open from 1984 to 1998, evaluating the use of oral or intravenous acyclovir in pregnant women. In total, 1234 pregnancies were noted with 1246 outcomes from 24 countries; 756 exposed pregnancies were studied in the first trimester. The risk of birth defects was 3.2% (95% confidence interval 2.0% to 5.0%), which is similar to the baseline risk of birth defects in the general population. No unusual defects or patterns of defects were seen, but the limitation of the results was the high loss to follow-up (27% of registrants).12 During the years 1995 to 1999, the manufacturer of valacyclovir maintained its pregnancy registry, and 110 exposures were reported with 111 known outcomes. Within the first trimester, 1 birth defect was reported out of 28 exposures; 2 of 31 and 1 of 51 exposures resulted in defects in the second and third trimesters, respectively. Prenatal exposures were too limited to provide pregnancy outcomes. According to personal written correspondence from GlaxoSmithKline to Motherisk (January 2010), this registry was limited by the number of registrants and the length of monitoring.
Prenatal exposures were too limited to provide pregnancy outcomes. According to personal written correspondence from GlaxoSmithKline to Motherisk (January 2010), this registry was limited by the number of registrants and the length of monitoring.
A recent Danish population-based retrospective cohort study used data from its nationwide registry to examine live-born infants born between 1996 and 2008 who were exposed to antivirals during pregnancy and the rate of major birth defects within the first year of life. Of the 837 795 enrolled infants, 1804 pregnancies were exposed to acyclovir, valacyclovir, or famciclovir in the first trimester; prevalence odds ratios were considered no different between exposed and unexposed cohorts. Similar results were found when evaluating second and third trimester data. There was also no significant difference in the prevalence of malformations between exposed and unexposed groups when examining exposure during the first trimester to individual antivirals. The rate of malformations was 2.0% for acyclovir (32 of 1561 infants) and 3.1% for valacyclovir (7 of 229 infants). For famciclovir, exposure was uncommon with 1 infant of 26 exposed having a birth defect. As a supplementary analysis, the association between the use of dermatologic acyclovir and penciclovir creams and major birth defects was evaluated. The rate of malformations in those exposed to acyclovir cream and penciclovir cream in the first trimester (2.3%, 65 of 2850 infants, and 4.2%, 5 of 118 infants, respectively) was not different from the unexposed cohort; similar results were found in the second and third trimesters of pregnancy.13
The rate of malformations was 2.0% for acyclovir (32 of 1561 infants) and 3.1% for valacyclovir (7 of 229 infants). For famciclovir, exposure was uncommon with 1 infant of 26 exposed having a birth defect. As a supplementary analysis, the association between the use of dermatologic acyclovir and penciclovir creams and major birth defects was evaluated. The rate of malformations in those exposed to acyclovir cream and penciclovir cream in the first trimester (2.3%, 65 of 2850 infants, and 4.2%, 5 of 118 infants, respectively) was not different from the unexposed cohort; similar results were found in the second and third trimesters of pregnancy.13
The accumulated evidence for the safety of oral acyclovir and valacyclovir, established from the manufacturer’s pregnancy registries and as a result of the Danish cohort study, does not demonstrate an increase in the rate of major birth defects when compared with the general population or an unexposed group. Data on the safety of famciclovir’s use during pregnancy is quite limited, and although it might not be expected to increase the risk of major malformations, it should not be the first choice of medication for treatment of HSV during pregnancy. In addition, topical antiviral preparations of acyclovir and penciclovir resulted in no increased rate of major birth defects during pregnancy. Limitations of the safety data on antivirals include a high lost-to-follow-up rate in the registries and the lack of prospective controlled studies. However, these data are reassuring, allowing physicians to offer pregnant patients either acyclovir or valacyclovir for treatment of primary or recurrent HSV infection, which not only treats the mother’s condition, but also reduces the likelihood of transmission to the neonate, without unduly compromising fetal safety.
In addition, topical antiviral preparations of acyclovir and penciclovir resulted in no increased rate of major birth defects during pregnancy. Limitations of the safety data on antivirals include a high lost-to-follow-up rate in the registries and the lack of prospective controlled studies. However, these data are reassuring, allowing physicians to offer pregnant patients either acyclovir or valacyclovir for treatment of primary or recurrent HSV infection, which not only treats the mother’s condition, but also reduces the likelihood of transmission to the neonate, without unduly compromising fetal safety.
Motherisk
Motherisk questions are prepared by the Motherisk Team at the Hospital for Sick Children in Toronto, Ont. Ms Kang is a doctoral candidate in the Faculty of Pharmacy at the University of Toronto. Dr Chua-Gocheco is a member of the Motherisk Program. At the time this paper was written, Ms Bozzo was a member and Ms Einarson was Assistant Director of the Motherisk Program. Ms Bozzo is now Assistant Director and Ms Einarson has retired but continues to be a member of the Motherisk Program.
Ms Bozzo is now Assistant Director and Ms Einarson has retired but continues to be a member of the Motherisk Program.
Do you have questions about the effects of drugs, chemicals, radiation, or infections in women who are pregnant or breastfeeding? We invite you to submit them to the Motherisk Program by fax at 416 813-7562; they will be addressed in future Motherisk Updates.
Published Motherisk Updates are available on the Canadian Family Physician website (www.cfp.ca) and also on the Motherisk website (www.motherisk.org).
Competing interests
None declared
1. Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121(11):847–54. [PubMed] [Google Scholar]
2. Howard M, Sellors JW, Jang D, Robinson NJ, Fearon M, Kaczorowski J, et al. Regional distribution of antibodies to herpes simplex virus type 1 (HSV-1) and HSV-2 in men and women in Ontario, Canada. J Clin Microbiol. 2003;41(1):84–9. [PMC free article] [PubMed] [Google Scholar]
J Clin Microbiol. 2003;41(1):84–9. [PMC free article] [PubMed] [Google Scholar]
3. Patrick DM, Dawar M, Cook DA, Krajden M, Ng HC, Rekart ML. Antenatal seroprevalence of herpes simplex virus type 2 (HSV-2) in Canadian women: HSV-2 prevalence increases throughout the reproductive years. Sex Transm Dis. 2001;28(7):424–8. [PubMed] [Google Scholar]
4. Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–73. [PubMed] [Google Scholar]
5. Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Barucca V, Chiarini F, et al. Herpes simplex virus infection in pregnancy and in neonate: status of art of epidemiology, diagnosis, therapy and prevention. Virol J. 2009;6:40. [PMC free article] [PubMed] [Google Scholar]
6. Kropp RY, Wong T, Cormier L, Ringrose A, Burton S, Embree JE, et al. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006;117(6):1955–62. [PubMed] [Google Scholar]
Pediatrics. 2006;117(6):1955–62. [PubMed] [Google Scholar]
7. Ormrod D, Scott LJ, Perry CM. Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs. 2000;59(4):839–63. [PubMed] [Google Scholar]
8. Simpson D, Lyseng-Williamson KA. Famciclovir: a review of its use in herpes zoster and genital and orolabial herpes. Drugs. 2006;66(18):2397–416. [PubMed] [Google Scholar]
9. Denavir cream 1% [package insert] Parsippany, NJ: Novartis Consumer Health, Inc; 2002. [Google Scholar]
10. Zovirax cream 5% [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2002. [Google Scholar]
11. Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database Syst Rev. 2008;(1):CD004946. [PubMed] [Google Scholar]
12. GlaxoSmithKline . Acyclovir pregnancy registry and valacyclovir pregnancy registry [interim report] Research Triangle Park, NC: Glaxo Wellcome; 1997. [Google Scholar]
[Google Scholar]
13. Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA. 2010;304(8):859–66. [PubMed] [Google Scholar]
Use of Acyclovir, Valacyclovir, and Famciclovir in the First Trimester of Pregnancy and the Risk of Birth Defects | Congenital Defects | JAMA
Abstract
Context Herpes simplex and herpes zoster infections are common and often treated with antiviral drugs including acyclovir, valacyclovir, and famciclovir. Safety of these antivirals when used in the first trimester of pregnancy is insufficiently documented.
Objective To investigate associations between exposure to acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and risk of major birth defects.
Design, Setting, and Participants Population-based historical cohort study of 837 795 live-born infants in Denmark from January 1, 1996, to September 30, 2008. Participants had no diagnoses of chromosomal aberrations, genetic syndromes, birth defect syndromes with known causes, or congenital viral infections. Nationwide registries were used to ascertain individual-level information on dispensed antiviral drugs, birth defect diagnoses (categorized according to a standardized classification scheme), and potential confounders.
Participants had no diagnoses of chromosomal aberrations, genetic syndromes, birth defect syndromes with known causes, or congenital viral infections. Nationwide registries were used to ascertain individual-level information on dispensed antiviral drugs, birth defect diagnoses (categorized according to a standardized classification scheme), and potential confounders.
Main Outcome Measure Prevalence odds ratios (PORs) of any major birth defect diagnosed within the first year of life by exposure to antiviral drugs.
Results Among 1804 pregnancies exposed to acyclovir, valacyclovir, or famciclovir in the first trimester, 40 infants (2.2%) were diagnosed with a major birth defect compared with 19 920 (2.4%) among the unexposed (adjusted POR, 0.89; 95% confidence interval [CI], 0.65-1.22). For individual antivirals, a major birth defect was diagnosed in 32 of 1561 infants (2.0%) with first-trimester exposure to acyclovir (adjusted POR, 0.82; 95% CI, 0. 57-1.17) and in 7 of 229 infants (3.1%) with first-trimester exposure to valacyclovir (adjusted POR, 1.21; 95% CI, 0.56-2.62). Famciclovir exposure was uncommon (n = 26), with 1 infant (3.8%) diagnosed with a birth defect. Exploratory analyses revealed no associations between antiviral drug exposure and 13 different subgroups of birth defects, but the number of exposed cases in each subgroup was small.
57-1.17) and in 7 of 229 infants (3.1%) with first-trimester exposure to valacyclovir (adjusted POR, 1.21; 95% CI, 0.56-2.62). Famciclovir exposure was uncommon (n = 26), with 1 infant (3.8%) diagnosed with a birth defect. Exploratory analyses revealed no associations between antiviral drug exposure and 13 different subgroups of birth defects, but the number of exposed cases in each subgroup was small.
Conclusion In this large nationwide cohort, exposure to acyclovir or valacyclovir in the first trimester of pregnancy was not associated with an increased risk of major birth defects.
Acyclovir, valacyclovir, and famciclovir are antiviral agents used in the treatment of herpes simplex and herpes zoster infections. For genital and labial herpes, these antivirals are used as short-course treatment in primary infections and as episodic or chronic suppressive therapy for frequently recurring disease.1,2 In herpes zoster, antivirals effectively reduce acute symptoms as well as the risk for postherpetic neuralgia. 3
3
The prevalence of herpes simplex is high4,5 and almost 40% of individuals with genital herpes experience at least 6 recurrences in the first year after disease onset,6 which is often the threshold for episodic or chronic suppressive treatment.1 More than 1% of susceptible women acquire herpes simplex during the first trimester of pregnancy7 and the incidence of herpes zoster is 1.5 to 2 per 1000 person-years in the third and fourth decades of life.8 Given this background, antiviral treatment will be indicated for a significant number of women in pregnancy.
Although the safety of acyclovir, valacyclovir, and famciclovir in general has been well established,9,10 data on the use of these antivirals in early pregnancy are limited. Animal studies, although of uncertain applicability to humans,11 did not demonstrate teratogenic effects initially, whereas later studies suggested that multiple defects may be induced with very high doses of acyclovir. 12,13 The US Food and Drug Administration has classified acyclovir, valacyclovir, and famciclovir as category B drugs in pregnancy.14-16 Information on the safety of acyclovir, with regard to teratogenicity in humans, is mainly based on data from a pregnancy registry managed by the drug manufacturer.17 This study reported that the rate of major birth defects in 596 pregnancies exposed in the first trimester was 3.2% and compared it to the rate expected (3.2%) in the general population. In addition to the lack of a valid control group, recruitment to this study relied on spontaneous reporting. Two other acyclovir studies were very limited in size.18,19 There are no published data on valacyclovir in early pregnancy and experience with famciclovir is limited to 4 pregnancies.19
12,13 The US Food and Drug Administration has classified acyclovir, valacyclovir, and famciclovir as category B drugs in pregnancy.14-16 Information on the safety of acyclovir, with regard to teratogenicity in humans, is mainly based on data from a pregnancy registry managed by the drug manufacturer.17 This study reported that the rate of major birth defects in 596 pregnancies exposed in the first trimester was 3.2% and compared it to the rate expected (3.2%) in the general population. In addition to the lack of a valid control group, recruitment to this study relied on spontaneous reporting. Two other acyclovir studies were very limited in size.18,19 There are no published data on valacyclovir in early pregnancy and experience with famciclovir is limited to 4 pregnancies.19
We conducted a nationwide registry-based cohort study to assess associations between acyclovir, valacyclovir, and famciclovir use in the first trimester of pregnancy and major birth defects. Our primary objective was to investigate the risk of any major birth defects. In secondary explorative analyses, we examined risks in subgroups of major birth defects by organ system.
Our primary objective was to investigate the risk of any major birth defects. In secondary explorative analyses, we examined risks in subgroups of major birth defects by organ system.
Methods
We used data from nationwide registries to conduct a historical cohort study including all infants born alive in Denmark from January 1, 1996, to September 30, 2008, and evaluated associations between exposure to oral acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and major birth defects diagnosed within the first year of life. In supplementary analyses, we investigated associations between use of dermatological acyclovir and penciclovir creams and the risk of birth defects. Individual-level data were linked between registries using the unique personal identification number assigned to all inhabitants in Denmark. The study was approved by the Danish Data Protection Agency. Ethics approval is not required for registry-based research in Denmark.
Study Cohort
The Medical Birth Register (MBR) contains individual-level information on all deliveries by women living in Denmark,20 including the personal identification numbers of the parents and the newborn, date of birth, multiple births, gestational age, and various physical characteristics of the newborn. On the basis of the MBR, we identified a cohort of all live births in Denmark from January 1, 1996, to September 30, 2008. The date of conception was calculated by subtracting gestational age from date of birth. For births in which gestational age was missing (0.9%), we imputed the cohort median of 280 days. The MBR records gestational age based on the last menstrual period and corrected by ultrasonographic measurements in most women.21 A validation study of gestational age registration in the MBR compared registered data with medical records and found that 87% of registrations were correct if agreement was defined as within 1 week. 22
Antiviral Drug Exposure
The Prescription Drug Register,23 established in 1995, contains individual-level information on all prescriptions filled at all Danish pharmacies. Each record contains the personal identification number of the patient, date of filling the prescription, anatomic therapeutic chemical (ATC) code, number of packages, package size, and number of daily defined doses in the prescription. We obtained information (ATC code) on oral acyclovir (J05AB01), valacyclovir (J05AB11), famciclovir (J05AB09), and dermatologic acyclovir (D06BB03) and penciclovir (D06BB06) prescriptions filled by cohort mothers from 4 weeks before conception until birth. Systemic acyclovir, valacyclovir, and famciclovir are prescription-only drugs in Denmark, while dermatological acyclovir and penciclovir creams have also been available over the counter since 1993 and 1999, respectively. We lacked information on inpatient antiviral drug treatment.
Birth Defects
Cases of birth defects were identified through the National Patient Register (NPR),24 which contains individual-level information on hospital visits (emergency department and outpatient) and admissions including diagnoses assigned by clinicians according to the International Classification of Disease (ICD). The NPR does not contain diagnostic data from the primary care setting, which limits the detection of diagnoses to those that have been diagnosed in hospitals. We accessed NPR data covering January 1, 1996, to March 31, 2009. For multiple births, any child was considered as a potential case. Major birth defects were defined according to the EUROCAT (a European network for surveillance of congenital anomalies) classification for subgroups of major congenital anomalies.25 We modified the EUROCAT protocol for the purpose of our study: infants with diagnoses of chromosomal aberrations, genetic disorders, and birth defect syndromes with known causes (n = 2944), and congenital viral infections possibly associated with birth defects (n = 259) were identified and excluded (ICD codes are available in eAppendix). Minor defects were excluded from evaluation according to the EUROCAT exclusion list.26
Minor defects were excluded from evaluation according to the EUROCAT exclusion list.26
Potential Confounders
From the MBR, the Central Person Register,27 and Statistics Denmark, we obtained information on birth year and the mother's parity, age at conception, smoking status during pregnancy, country or continent of origin, place of residence at the time of conception, and educational level and socioeconomic class in the year of conception. From the NPR and the Prescription Drug Register, we obtained information on maternal diseases and drug exposures that may be associated both with herpes simplex or herpes zoster and birth defects; infectious diseases in the first trimester; history of sexually transmitted infections, diabetes mellitus, and immunodeficiency; and filled prescriptions for antineoplastic and immunomodulating agents from 3 months before conception throughout the first trimester, oral glucocorticoids in the first trimester, and oral antibiotics in the first trimester (ICD and ATC codes in eAppendix). We also identified high-risk pregnancies and history of birth defects in siblings back to 1977. We did not have data on folic acid exposure.
We also identified high-risk pregnancies and history of birth defects in siblings back to 1977. We did not have data on folic acid exposure.
Statistical Analysis
We used logistic regression to estimate prevalence odds ratios (PORs) with 95% confidence intervals (CIs) comparing prevalence odds of major birth defects in infants from pregnancies exposed to antivirals and in infants from unexposed pregnancies (SAS software version 9.1, SAS Institute Inc, Cary, North Carolina). Results were considered statistically significant when the 95% CI did not overlap 1.0 in either direction. Potential confounders were included in regression models if they were significantly (P < .05; 2-sided) associated with major birth defects in univariate analyses. P values in univariate analyses were estimated with missing values excluded. In adjusted models, we applied multiple imputation for variables with missing values using the Markov Chain Monte Carlo method.
Children were observed for the first registered diagnosis of a major defect within 1 year after birth. For the primary outcome measure of all major birth defects, we combined all subgroups of malformations. In preplanned exploratory analyses we evaluated subgroups by organ system without correction for multiple comparisons. Any filling of an acyclovir, valacyclovir, or famciclovir prescription was considered as exposure. The main exposure time window comprised the first trimester (12 weeks). Analysis of exposure within 4 weeks before conception and in the second and third trimesters was performed for comparison. The timing of exposure was defined by the date of filling the prescription, and any pregnancy could contribute to any exposure time window. In case a pregnancy was exposed in more than 1 time window, the corresponding PORs (except for crude estimates) were adjusted for effects of each other. The study had 80% power to detect a 47% relative increase (POR, 1.47) in the risk of birth defects in those exposed to any antiviral (n = 1804; 5% 2-sided α level; EpiInfo, version 3. 5.1, http://www.cdc.gov/epiinfo).
5.1, http://www.cdc.gov/epiinfo).
Results
The cohort included 837 795 live births (34 787 multiple births), among whom 19 960 (2.4%) were diagnosed with a major birth defect during the first year of life. Table 1 and Table 2 present descriptive characteristics of cohort participants.
Among 1804 pregnancies exposed to acyclovir, valacyclovir, or famciclovir at any time in the first trimester, 40 infants (2.2%) had a diagnosis of a major birth defect, compared with 19 920 of 835 991 infants (2.4%) among the unexposed pregnancies (crude POR, 0.93; 95% CI, 0.68-1.27). Risk estimates for those potential confounders that were statistically significant risk factors for birth defects in univariate analyses are shown in eTable 1. Adjusting for these variables in a multivariate model, acyclovir, valacyclovir, or famciclovir exposure at any time in the first trimester was not associated with increased risk of major birth defects (POR, 0. 89; 95% CI, 0.65-1.22), as compared with unexposed pregnancies. Table 3 presents risk estimates for the association between use of antivirals and major birth defects for the 3 antivirals together and individually in the different exposure time windows. First-trimester use of acyclovir, the most commonly prescribed antiviral, was not associated with major birth defects (32 cases among 1561 exposed [2.0%] vs 2.4% in the unexposed; adjusted POR, 0.82; 95% CI, 0.57-1.17). Neither valacyclovir nor famciclovir were associated with major birth defects, although use of the latter was very uncommon.
89; 95% CI, 0.65-1.22), as compared with unexposed pregnancies. Table 3 presents risk estimates for the association between use of antivirals and major birth defects for the 3 antivirals together and individually in the different exposure time windows. First-trimester use of acyclovir, the most commonly prescribed antiviral, was not associated with major birth defects (32 cases among 1561 exposed [2.0%] vs 2.4% in the unexposed; adjusted POR, 0.82; 95% CI, 0.57-1.17). Neither valacyclovir nor famciclovir were associated with major birth defects, although use of the latter was very uncommon.
The Figure shows exploratory analyses of associations between use of antivirals and major birth defect subgroups by organ system. There was no significant increase in the prevalence of any major birth defect subgroup among mothers exposed to antivirals in the first trimester of pregnancy. Results were similar when restricted to acyclovir. However, these analyses were based on a small number of cases among the exposed and should be interpreted with caution. Exposure to any antiviral within 4 weeks before conception (eTable 2) was associated with increased risk of major heart defects (POR, 1.71; 95% CI, 1.05-2.79), while major eye defects (POR, 2.76; 95% CI, 0.98-7.76) and nervous system defects (POR, 2.37; 95% CI, 0.87-6.50) had relatively high PORs but were not statistically significant. There were no statistically significant associations between antiviral exposure and any other subgroup of major birth defects among those exposed within 4 weeks before conception or any subgroup of birth defects among those exposed in the second and third trimesters (eTable 2).
Exposure to any antiviral within 4 weeks before conception (eTable 2) was associated with increased risk of major heart defects (POR, 1.71; 95% CI, 1.05-2.79), while major eye defects (POR, 2.76; 95% CI, 0.98-7.76) and nervous system defects (POR, 2.37; 95% CI, 0.87-6.50) had relatively high PORs but were not statistically significant. There were no statistically significant associations between antiviral exposure and any other subgroup of major birth defects among those exposed within 4 weeks before conception or any subgroup of birth defects among those exposed in the second and third trimesters (eTable 2).
Supplementary analyses of acyclovir and penciclovir dermatological creams found no significant associations between exposure to these drugs and the risk of major birth defects (Table 4).
We conducted alternative analyses to test the robustness of our results. First, to account for the possibility that women who had started taking antivirals before conception may have continued use beyond conception, we performed analyses that accounted for the number of daily defined doses in individual antiviral drug prescriptions (daily drug intake and consumption of the entire drug package were assumed). Women who had filled antiviral prescriptions within 4 weeks before conception and received enough doses to have a theoretical chance of continued exposure beyond the day of conception were grouped with women who had filled prescriptions in the first trimester. The adjusted POR for having offspring with major birth defects in this group was almost identical to that of the main analysis (0.89; 95% CI, 0.65-1.21; 42 cases among 1896 exposed [2.2%] vs 2.4% unexposed). Mothers who had filled prescriptions within 4 weeks before conception but had not received enough doses to have a theoretical chance of continued exposure beyond conception had an adjusted POR similar to that of the main analysis (1.20; 95% CI, 0.87-1.67; 38 cases among 1339 exposed [2.8%] vs 2.4% unexposed). An analogous analysis of the heart defects subgroup, also accounting for daily doses in prescriptions, showed that an increased risk remained among those exposed within 4 weeks before conception (18 cases among 1339 exposed [1.
Women who had filled antiviral prescriptions within 4 weeks before conception and received enough doses to have a theoretical chance of continued exposure beyond the day of conception were grouped with women who had filled prescriptions in the first trimester. The adjusted POR for having offspring with major birth defects in this group was almost identical to that of the main analysis (0.89; 95% CI, 0.65-1.21; 42 cases among 1896 exposed [2.2%] vs 2.4% unexposed). Mothers who had filled prescriptions within 4 weeks before conception but had not received enough doses to have a theoretical chance of continued exposure beyond conception had an adjusted POR similar to that of the main analysis (1.20; 95% CI, 0.87-1.67; 38 cases among 1339 exposed [2.8%] vs 2.4% unexposed). An analogous analysis of the heart defects subgroup, also accounting for daily doses in prescriptions, showed that an increased risk remained among those exposed within 4 weeks before conception (18 cases among 1339 exposed [1. 3%] vs 0.8% in the unexposed; adjusted POR, 1.83 [95% CI, 1.14-2.95]), while exposure in the first trimester was not associated with heart defects (13 cases among 1896 exposed [0.7%] vs 0.8% in the unexposed; adjusted POR, 0.79 [95% CI, 0.45-1.39]).
3%] vs 0.8% in the unexposed; adjusted POR, 1.83 [95% CI, 1.14-2.95]), while exposure in the first trimester was not associated with heart defects (13 cases among 1896 exposed [0.7%] vs 0.8% in the unexposed; adjusted POR, 0.79 [95% CI, 0.45-1.39]).
Second, in analyses restricted to those who used antivirals exclusively in the first trimester (no prescriptions filled in the other 2 exposure time windows), antiviral exposure was not associated with major birth defects (31 cases among 1339 exposed [2.3%]; adjusted POR, 0.92 [95% CI, 0.65-1.32]).
Third, filling an antiviral prescription at any time in the period of maximal susceptibility to teratogenic agents, 2 to 8 weeks after conception,11 was not associated with increased risk of major birth defects (15 cases among 997 exposed [1.5%]; adjusted POR, 0.58; 95% CI, 0.35-0.98). Analyses restricted to acyclovir produced similar estimates (13 cases among 857 exposed [1.5%]; adjusted POR, 0.60; 95% CI, 0.34-1.04).
Fourth, as correlation among infants from the same pregnancy could affect risk estimates, we performed analyses excluding multiple births. Among 1738 singleton pregnancies exposed to any antiviral in the first trimester, 36 (2.1%) were diagnosed with a major birth defect (adjusted POR, 0.86; 95% CI, 0.62-1.21).
Among 1738 singleton pregnancies exposed to any antiviral in the first trimester, 36 (2.1%) were diagnosed with a major birth defect (adjusted POR, 0.86; 95% CI, 0.62-1.21).
Fifth, because prepregnancy antiviral use may be associated with undetected reuse in pregnancy, we conducted an analysis restricted to mothers without a history of antiviral use (prescription history available until 9 months before conception on average) and without a history of sexually transmitted infections including anogenital herpes. There were 16 cases among 717 exposed to any antiviral in the first trimester (2.2%), compared with 18 940 cases among 797 922 unexposed (2.4%; adjusted POR, 0.89; 95% CI, 0.54-1.47).
Finally, as multiple imputation was used for variables with missing values, we wanted to address the potential for bias introduced by missing data. We therefore performed analyses restricted to cohort participants without any missing values among covariates included in the multivariate model. Adjusted PORs were very similar to those of the main analysis: 0.86 for any antiviral (95% CI, 0.62-1.21; 36 cases among 1657 exposed [2.2%]) and 0.83 for acyclovir alone (95% CI, 0.57-1.19; 30 cases among 1441 exposed [2.1%]).
Adjusted PORs were very similar to those of the main analysis: 0.86 for any antiviral (95% CI, 0.62-1.21; 36 cases among 1657 exposed [2.2%]) and 0.83 for acyclovir alone (95% CI, 0.57-1.19; 30 cases among 1441 exposed [2.1%]).
Comment
This large nationwide historical cohort study found no association between exposure to acyclovir, valacyclovir, or famciclovir in the first trimester of pregnancy and the risk of any major birth defect. In analyses of individual antivirals, risk estimates were similar for acyclovir and valacyclovir. While analysis of valacyclovir was based on a limited number of exposed cases, results at least indicate that it is not a major human teratogen. Analyses of famciclovir were based on only 26 exposed pregnancies and should therefore not be viewed as evidence of safety of this drug. Exploratory analyses of subgroups of major birth defects by organ system showed no statistically significant associations with first-trimester exposure to antiviral drugs or acyclovir alone. Finally, supplementary analyses showed that exposure to dermatological acyclovir cream was not associated with major birth defects, whereas the number exposed to penciclovir cream was insufficient to draw firm conclusions.
Finally, supplementary analyses showed that exposure to dermatological acyclovir cream was not associated with major birth defects, whereas the number exposed to penciclovir cream was insufficient to draw firm conclusions.
Our results are in concert with previously published reports. A study using the acyclovir pregnancy registry found that the rate of major birth defects among 596 pregnancies exposed in the first trimester was 3.2%.17 This was compared with the expected rate in the general population (3.2%). However, the study lacked statistical testing and a proper control group. Further limitations included recruitment of exposed pregnancies by spontaneous reporting, which may have introduced selection bias, and a loss to follow-up of 27%. Surveillance for birth defects was restricted to the immediate postnatal period so that malformations diagnosed later would have been missed. A regional Danish study applied a methodology similar to ours, but was limited by a small sample size (n = 72) with respect to oral acyclovir. 18 This study also included dermatological acyclovir exposure in early pregnancy (n = 474) and found no association with birth defects.18 A study using prescription data and questionnaire information on pregnancy outcomes found no birth defects in 22 pregnancies exposed to acyclovir or famciclovir.19 There are no published data on valacyclovir. Thus, previous studies have reported on fewer than 700 pregnancies exposed in the first trimester or just 72 if only analytical studies are considered, as compared with 1804 in our study.
18 This study also included dermatological acyclovir exposure in early pregnancy (n = 474) and found no association with birth defects.18 A study using prescription data and questionnaire information on pregnancy outcomes found no birth defects in 22 pregnancies exposed to acyclovir or famciclovir.19 There are no published data on valacyclovir. Thus, previous studies have reported on fewer than 700 pregnancies exposed in the first trimester or just 72 if only analytical studies are considered, as compared with 1804 in our study.
Several strengths and limitations of our study deserve particular mention. The registry-based study design allowed nationwide coverage over 13 years, independent ascertainment of dispensed prescriptions and birth defect diagnoses, and a complete 1-year follow-up. Defects diagnosed after 1 year of age would have been missed. A series of sensitivity analyses demonstrated the robustness of our results for variable exposure definitions, including an analysis restricted to the period of maximal susceptibility to teratogenic agents 2 to 8 weeks after conception. 11 Diagnostic information in the NPR has high validity, ie, registrations were correct for 88% of birth defect diagnoses overall28 and 89% of cardiac malformations29 while the completeness of registration was 90%.28 Our study did not include abortions. If drug-induced birth defects were associated with increased risk of planned or spontaneous abortion, results of the study would have been biased toward the null.
11 Diagnostic information in the NPR has high validity, ie, registrations were correct for 88% of birth defect diagnoses overall28 and 89% of cardiac malformations29 while the completeness of registration was 90%.28 Our study did not include abortions. If drug-induced birth defects were associated with increased risk of planned or spontaneous abortion, results of the study would have been biased toward the null.
We included a wide range of potential confounders but evaluation of maternal comorbidity was incomplete because the NPR is restricted to the hospital setting. Furthermore, unmeasured confounding remains a possibility. Because of the size of the cohort, a factor masking a birth defect risk associated with antiviral use would have to be either common or the associations with both antiviral use and a reduced risk of birth defects would have to be very strong.
Use of filled prescriptions as a measure of drug exposure eliminates recall bias and increases the precision of the information on the type of drug used, as compared with interview data. A major limitation is, however, that nonadherence to the dispensed drugs would bias results toward no effect and obscure teratogenic effects, if present, among women who were adherent.
A major limitation is, however, that nonadherence to the dispensed drugs would bias results toward no effect and obscure teratogenic effects, if present, among women who were adherent.
Exposure within 4 weeks before conception was included mainly for comparison. The observations of increased risk of heart defects and elevated, but nonsignificant, PORs for eye and nervous system defects in those exposed within 4 weeks before conception lack a biological explanation. Maximal vulnerability to teratogenic agents is typically confined to the period of organogenesis (2-8 weeks postconception) and heart defects most often arise between 6.5 and 8 weeks of gestation.11 The observations are therefore probably chance findings partly attributable to multiple comparisons of 13 subgroups in the different time periods or the result of unmeasured confounding.
The exploratory analyses of subgroups of major defects involved few exposed cases in each subgroup and therefore cannot exclude teratogenic effects with certainty. Risks of birth defects by subgroup and of specific defects should be investigated further in larger studies.
Risks of birth defects by subgroup and of specific defects should be investigated further in larger studies.
Acyclovir and penciclovir creams are also available as over-the-counter drugs and nonprescription use in those classified as unexposed in our study would therefore bias results toward no effect. Such a bias might also be conferred by inpatient antiviral exposure, which was not detected in the registries. Given the size of the group classified as unexposed, however, effects of exposure misclassification would be minimal.
Our study, to our knowledge the largest of its kind, found no significant association between first-trimester exposure to antiherpetic antiviral drugs and major birth defects. Consequently, it has immediate clinical implications and may support informed decisions on safety when prescribing antivirals for herpes infections in early pregnancy. Acyclovir is the most extensively documented antiviral and should therefore be the drug of choice in early pregnancy, while data on valacyclovir and famciclovir are still insufficient. Future research on antiherpetic antivirals and mother-child health should include safety studies with regard to spontaneous abortion and preterm birth, and during breastfeeding.
Future research on antiherpetic antivirals and mother-child health should include safety studies with regard to spontaneous abortion and preterm birth, and during breastfeeding.
Back to top
Article Information
Corresponding Author: Björn Pasternak, MD, PhD, Department of Epidemiology Research, Statens Serum Institut, Artillerivej 5, 2300 Copenhagen S, Denmark ([email protected]).
Author Contributions: Dr Pasternak had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Pasternak, Hviid.
Acquisition of data: Hviid.
Analysis and interpretation of data: Pasternak, Hviid.
Drafting of the manuscript: Pasternak.
Critical revision of the manuscript for important intellectual content: Pasternak, Hviid.
Statistical analysis: Hviid.
Obtained funding: Hviid.
Study supervision: Hviid.
Financial Disclosures: None reported.
Funding/Support: This work was supported by grants from the Danish Medical Research Council and the Lundbeck Foundation.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of the manuscript.
References
1.
Sen P, Barton SE. Genital herpes and its management. BMJ. 2007;334(7602):1048-105217510153PubMedGoogle ScholarCrossref
2.
Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168(11):1137-114418541820PubMedGoogle ScholarCrossref
Arch Intern Med. 2008;168(11):1137-114418541820PubMedGoogle ScholarCrossref
3.
Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334(7605):1211-121517556477PubMedGoogle ScholarCrossref
4.
Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186:(suppl 1) S3-S2812353183PubMedGoogle ScholarCrossref
5.
Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964-97316926356PubMedGoogle ScholarCrossref
6.
Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first-episode infection. Ann Intern Med. 1994;121(11):847-8547978697PubMedGoogle ScholarCrossref
Ann Intern Med. 1994;121(11):847-8547978697PubMedGoogle ScholarCrossref
7.
Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337(8):509-5159262493PubMedGoogle ScholarCrossref
8.
Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341-134917976353PubMedGoogle ScholarCrossref
9.
Tyring SK, Baker D, Snowden W. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir. J Infect Dis. 2002;186:(suppl 1) S40-S4612353186PubMedGoogle ScholarCrossref
10.
Saltzman R, Jurewicz R, Boon R. Safety of famciclovir in patients with herpes zoster and genital herpes. Antimicrob Agents Chemother. 1994;38(10):2454-24577840587PubMedGoogle ScholarCrossref
11.
Buhimschi CS, Weiner CP. Medications in pregnancy and lactation: part 1 teratology. Obstet Gynecol. 2009;113(1):166-18819104374PubMedGoogle Scholar
12.
Moore HL Jr, Szczech GM, Rodwell DE, Kapp RW Jr, de Miranda P, Tucker WE Jr. Preclinical toxicology studies with acyclovir: teratologic, reproductive and neonatal tests. Fundam Appl Toxicol. 1983;3(6):560-5686662297PubMedGoogle ScholarCrossref
13.
Chahoud I, Stahlmann R, Bochert G, Dillmann I, Neubert D. Gross-structural defects in rats after acyclovir application on day 10 of gestation. Arch Toxicol. 1988;62(1):8-143190462PubMedGoogle ScholarCrossref
Arch Toxicol. 1988;62(1):8-143190462PubMedGoogle ScholarCrossref
14.
US Food and Drug Administration. Zovirax (acyclovir product information), GlaxoSmithKline, 2005. http://www.accessdata.fda.gov/drugsatfda_docs/label/2005/018828s030,020089s019,019909s020lbl.pdf. Accessed July 5, 2010
15.
US Food and Drug Administration. Valtrex (valacyclovir product information), GlaxoSmithKline, 2008. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020487s016lbl.pdf. Accessed July 5, 2010
16.
US Food and Drug Administration. Famvir (famciclovir product information), Novartis, 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020363s036lbl.pdf. Accessed July 5, 2010
17.
Stone KM, Reiff-Eldridge R, White AD,
et al. Pregnancy outcomes following systemic prenatal acyclovir exposure: conclusions from the international acyclovir pregnancy registry, 1984-1999. Birth Defects Res A Clin Mol Teratol. 2004;70(4):201-20715108247PubMedGoogle ScholarCrossref
Birth Defects Res A Clin Mol Teratol. 2004;70(4):201-20715108247PubMedGoogle ScholarCrossref
18.
Ratanajamit C, Vinther Skriver M, et al. Adverse pregnancy outcome in women exposed to acyclovir during pregnancy: a population-based observational study. Scand J Infect Dis. 2003;35(4):255-25912839155PubMedGoogle ScholarCrossref
19.
Wilton LV, Pearce GL, Martin RM, Mackay FJ, Mann RD. The outcomes of pregnancy in women exposed to newly marketed drugs in general practice in England. Br J Obstet Gynaecol. 1998;105(8):882-8899746382PubMedGoogle ScholarCrossref
20.
Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320-3239675544PubMedGoogle Scholar
21.
Jørgensen FS. Ultrasonography of pregnant women in Denmark 1999-2000: description of the development since 1980-1990. Ugeskr Laeger. 2003;165(46):4409-441514655565PubMedGoogle Scholar
22.
Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. Validation of the Danish Birth Registration. J Clin Epidemiol. 1996;49(8):893-8978699210PubMedGoogle ScholarCrossref
23.
Danmarks Statistik. Research databases: data sources available at Statistics Denmark [in Danish] (ISBN 8701-501-1301-1). http://www.dst.dk/upload/forskningsdatabaser.pdf. Accessed August 2, 2010
24.
Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register: a valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263-26810421985PubMedGoogle Scholar
1999;46(3):263-26810421985PubMedGoogle Scholar
25.
Eurocat Network. Chapter 3.3: coding of Eurocat subgroups of congenital anomalies, issued on January, 03, 2007. http://www.eurocat-network.eu/content/EUROCAT-Guide-1.3.pdf. Accessed March 3, 2010
26.
Eurocat Network. Chapter 3.2: minor anomalies for exclusion, issued on 31-08-2007. http://www.eurocat-network.eu/content/EUROCAT-Guide-1.3.pdf. Accessed March 3, 2010
27.
Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull. 2006;53(4):441-44917150149PubMedGoogle Scholar
28.
Larsen H, Nielsen GL, Bendsen J, Flint C, Olsen J, Sørensen HT. Predictive value and completeness of the registration of congenital abnormalities in three Danish population-based registries. Scand J Public Health. 2003;31(1):12-1612623519PubMedGoogle ScholarCrossref
Scand J Public Health. 2003;31(1):12-1612623519PubMedGoogle ScholarCrossref
29.
Jepsen B, Jepsen P, Johnsen SP, Espersen GT, Sørensen HT. Validity of diagnoses of cardiac malformations in a Danish population-based hospital-discharge registry. Int J Risk Saf Med. 2006;18(2):77-81Google Scholar
Herpes during pregnancy - consequences of lichen for pregnant women
Herpes viruses are many-sided and very dangerous for humans. Of particular concern is the infection in a woman expecting a baby.
Doctor's consultation
You can get the consultation of the necessary specialist online in the Doctis application
Laboratory
You can undergo a comprehensive examination of all major body systems
- Genital herpes in early pregnancy
- Genital herpes in late pregnancy
- Pregnancy herpes test
- Chicken pox and herpes zoster during pregnancy
- Prevention of infection of the newborn with chickenpox
- Treatment and prevention of exacerbations of herpes
There is an opinion that if a pregnant woman is infected with the herpes simplex virus, then the unborn danger! But everything is not so scary if the disease does not manifest itself (is in stable remission) or a pregnant woman with an active form of the disease is observed by an infectious disease specialist. Today approaches to maintaining patients with genital herpes changed
Today approaches to maintaining patients with genital herpes changed
Why is herpes dangerous during pregnancy
I am pregnant, short term, and I have genital herpes. Does this mean that I need to terminate the pregnancy?
No way! Genital herpes is not an indication for abortion. Virus crosses the placenta extremely rarely. But for a child, herpes is dangerous if it first appeared a month before birth. or repeated a few days before the birth, since there is a risk of infection of the baby at the time of his passage through the infected birth canal. If infected, the infant will develop severe the disease is neonatal herpes, often occurring with damage to the central nervous system.
Although during pregnancy the risk of transmission of herpes virus from mother to fetus minimal, tolerate the manifestations of genital herpes and not take antiviral drugs, being afraid the consequences of treatment for the unborn child are not worth it.
After 14 weeks of pregnancy, if genital herpes occurs, treatment with an antiviral drug is possible acyclovir. After 22 weeks, therapy with valaciclovir is possible.
In the vast majority of cases, the herpes simplex virus is transmitted from the mother child during childbirth, so the closer to the end of pregnancy there is a recurrence of genital herpes, the higher the risk of infection of the child and the more relevant the treatment of the disease.
If primary genital herpes or recurrence occurs at or after 36 weeks of gestation, clinicians do not limit treatment to 5-10 days, but continue the entire period until the moment of delivery. As in the case very frequent recurrences of genital herpes during pregnancy (one outbreak in 1-2 months) - at week 36, proactive treatment with acyclovir or its analogues begins and continues until the moment childbirth. The goal of proactive treatment is to prevent recurrence shortly before delivery and to reduce the likelihood of asymptomatic carriage.
It must be remembered that it is the asymptomatic shedding of the virus from the urogenital tract that can often be cause of infection of the child during childbirth.
Even when there seems to be no cause for concern, since the manifestations genital herpes are absent in the last months of pregnancy, still at 32-34 weeks pregnancy, it is necessary to conduct a smear (scraping) examination from the cervical canal for the presence of DNA herpes simplex virus types 1 and 2 by PCR.
This analysis is necessary for primary genital herpes or its recurrence in the 1st and / or 2nd trimester, relapses genital herpes before pregnancy, relapses of genital herpes in a sexual partner, lesions urogenital tract of unknown cause, antibodies to herpes simplex virus type 1 and type 2 IgM class, detected during a routine examination during pregnancy.
For rashes or virus shedding on Wednesday within 7 days before delivery, especially in the presence of genital herpes by the beginning of childbirth, a caesarean section is performed to reduce the risk of transmission virus from mother to child.
I'm just planning a pregnancy. And I want to get rid of herpes recurrences that have been haunting me for a long time me. How do you feel about the treatment of genital herpes with interferon inducers and immunomodulators?
Widespread use of immunomodulators and interferon preparations in Russian medical centers (viferon, polyoxidonium, isoprinosine, etc.) for the treatment of herpesvirus infections is completely unreasonable. The so-called "ozone therapy" will not help the patient either. You will not find these methods in international protocols, recommendations for the treatment of viral infections in children and adults, including pregnant women women. Neither in Russia nor abroad have studies been conducted proving the effectiveness of these drugs in accordance with all international regulations.
An infectious disease specialist, a professional in his field, will never turn to immunomodulators and inducers interferon, but will look for the cause of the disease and prescribe therapy that acts on the pathogen itself - drugs acyclovir, valaciclovir or famaciclovir.With primary genital herpes for 10 days, with relapses diseases - in appropriate doses for 5 days.
Therapy should be started as early as possible at the very first signs of an exacerbation. Application possible antiherpetic drugs as a preventive treatment - 2-3 days before the expected relapse, if the patient is aware of the factors that provoke it, and for the entire period of the risk factor. If genital herpes disturbs a person more than 6 times a year and / or relapses reduce the quality of life of the patient and bring him not only physical, but also serious psychological discomfort, should be discussed with the patient long-term (at least 12 months) daily suppressive antiviral therapy (for example, valaciclovir). The effectiveness of such treatment tactics for the prevention of recurrence of herpes infection has been proven. all international rules.
Sequelae of chickenpox during pregnancy
How dangerous are chicken pox and shingles for a pregnant woman?
These diseases are caused by the varicella-zoster virus (VZV), which also belongs to the herpesvirus family. infect women are most often children who easily tolerate the disease. At the same time, chickenpox in an adult can be severe and dangerous to his health.
infect women are most often children who easily tolerate the disease. At the same time, chickenpox in an adult can be severe and dangerous to his health.
Infection is transmitted by airborne droplets from person to person already 48 hours before the onset of the rash, during the entire period of the rash and for a week after appearance of the last bubbles.
Expectant mothers who catch chickenpox may develop severe herpes pneumonia. Therefore, when sick chickenpox during pregnancy should be observed by an infectious disease specialist and in most cases of antiviral therapy.
In maternal varicella at 8 to 20 weeks of gestation infection fetus with the varicella-zoster virus can lead to fetal chickenpox with the development of a "syndrome congenital chicken pox" with severe malformations - damage to the brain, eyes, skeletal defects. Therefore, if a woman falls ill with chicken pox in the 1-3rd month of pregnancy, the doctor the woman should be informed about all possible risks of the disease for the fetus and discussed with her the question of a possible termination of pregnancy.
Second and third trimesters infectious disease doctor monitors the state of the future mothers. If the infection is severe or the infection occurred in the last month of pregnancy, prescribe antiviral treatment with acyclovir or its analogues. If ultrasound does not detect fetal pathology, pregnancy is not interrupted.
Within 96 hours (preferably within the first 48 hours) after contact a pregnant woman with chickenpox in the absence of IgG class antibodies to VVZ it is possible to introduce a specific VVZ-immunoglobulin as a measure to prevent the development of the disease. Immunity persists for 3-4 weeks, may be re-introduced after 21 days.
If the mother-to-be becomes ill with chicken pox in the last month of pregnancy , then the child may be born with skin rashes. If a woman falls ill in the last few days of pregnancy or in the first days after childbirth in a newborn infected during childbirth, the symptoms of chickenpox appear in the first 11 days of life. Chickenpox is most severe in infants whose mothers fell ill 5 days before or 2-3 days after delivery. In case of chickenpox in mothers, newborn children are given a specific immunoglobulin to prevent the development of the disease. In case of its severe course - appoint acyclovir for intravenous administration.
Chickenpox is most severe in infants whose mothers fell ill 5 days before or 2-3 days after delivery. In case of chickenpox in mothers, newborn children are given a specific immunoglobulin to prevent the development of the disease. In case of its severe course - appoint acyclovir for intravenous administration.
Infection of a child with VVV a few days after birth may manifest as postnatal varicella in the period of 12-28 day of his life. This form of the disease is less severe, it is possible to introduce a specific immunoglobulin with the risk of the disease and the appointment of intravenous acyclovir for destructive skin lesions.
Vaccination against the varicella-zoster virus is not given to pregnant women because this is a live vaccine. Therefore, if a young woman does not have IgG antibodies to VVZ before planning pregnancy, it is advisable to vaccinate against chicken pox.
Herpes zoster ("secondary" VVZ infection) in a pregnant woman is not dangerous for a child. Contact seronegative pregnant woman with herpes zoster is not desirable, although the threat of her infection There is practically no VVZ.
Contact seronegative pregnant woman with herpes zoster is not desirable, although the threat of her infection There is practically no VVZ.
I'm just planning a pregnancy. And I want to get rid of herpes recurrences that have been haunting me for a long time me. How do you feel about the treatment of genital herpes with interferon inducers and immunomodulators?
Widespread use of immunomodulators and interferon preparations in Russian medical centers (viferon, polyoxidonium, isoprinosine, etc.) for the treatment of herpesvirus infections is completely unreasonable. The so-called "ozone therapy" will not help the patient either. You will not find these methods in international protocols, recommendations for the treatment of viral infections in children and adults, including pregnant women women. Neither in Russia nor abroad have studies been conducted proving the effectiveness of these drugs in accordance with all international regulations.
Infectionist, a professional in his field will never turn to immunomodulators and interferon inducers, but will look for the cause of the disease and prescribe an effective pathogen therapy - drugs acyclovir, valaciclovir or famaciclovir. With primary genital herpes for 10 days, with relapses of the disease - in appropriate doses for 5 days.
Therapy should be started as early as possible at the very first signs of an exacerbation. Application possible antiherpetic drugs as a preventive treatment - 2-3 days before the expected relapse, if the patient is aware of the factors that provoke it, and for the entire period of the risk factor. If genital herpes disturbs a person more than 6 times a year and / or relapses reduce the quality of life of the patient and bring him not only physical, but also serious psychological discomfort, should be discussed with the patient long-term (at least 12 months) daily suppressive antiviral therapy (for example, valaciclovir). The effectiveness of such treatment tactics for the prevention of recurrence of herpes infection has been proven. all international rules.
The effectiveness of such treatment tactics for the prevention of recurrence of herpes infection has been proven. all international rules.
If you have any questions, you can ask your doctor obstetrician-gynecologist or infectious disease specialist online at Doctis app.
The author of the article: Vasily Iosifovich Shakhgildyan
Valtrex pregnancy and lactation - Medum.ru
Pregnancy and lactationPlease note: the use of any drugs, including Valtrex drug, planning and during periods of pregnancy, lactation (breastfeeding) is necessary agree with the attending physician. Women of reproductive potential are advised to undergo pregnancy testing.
Use during pregnancy and lactation
Fertility
In animal studies, valaciclovir had no effect on fertility. However, the use of high doses of parenteral acyclovir caused testicular effects in rats and dogs.
Human fertility studies have not been conducted with valaciclovir. However, there were no changes in sperm count, motility and morphology in 20 patients after 6 months of daily use of valaciclovir at doses ranging from 400 mg to 1000 mg.
Pregnancy
There are limited data on the use of Valtrex ® during pregnancy. The drug should be used during pregnancy only if the potential benefit to the mother outweighs the potential risk to the fetus.
Pregnancy registries have documented pregnancy outcomes in women taking Valtrex ® or other drugs containing acyclovir (acyclovir is an active metabolite of Valtrex ® ), 111 and 1246 observations, respectively (of which 29 and 756 took drugs in the first trimester of pregnancy, were pregnancy outcomes), recorded prospectively. An analysis of the data presented in the registry of pregnant women exposed to acyclovir did not reveal an increase in the number of birth defects in their children compared with the general population, and no specificity or pattern was found for any of the malformations, indicating a common cause. Since a small number of women who took valaciclovir during pregnancy were included in the pregnancy registry, reliable and definite conclusions about the safety of the use of valaciclovir during pregnancy cannot be made.
Since a small number of women who took valaciclovir during pregnancy were included in the pregnancy registry, reliable and definite conclusions about the safety of the use of valaciclovir during pregnancy cannot be made.
Breastfeeding period
Aciclovir, the main metabolite of valaciclovir, passes into breast milk. After taking valacyclovir at a dose of 500 mg orally, C max in breast milk was 0.5-2.3 times (on average 1.4 times) higher than the corresponding concentrations of acyclovir in the mother's blood plasma. The ratio of AUC values of acyclovir in breast milk to AUC in maternal serum ranged from 1.4 to 2.6 (mean value 2.2). The mean concentration of acyclovir in breast milk was 2.24 µg/ml (9.95 µmol/l). When the mother takes valacyclovir at a dose of 500 mg 2 times a day, breast-fed children are exposed to the same effects of acyclovir as when taken orally at a dose of about 0.61 mg / kg / day. The half-life of acyclovir from breast milk is the same as from plasma.