Cystic fibrosis at birth
Newborn Screening for CF | Cystic Fibrosis Foundation
Diagnosing CF Early Is Important
Newborn screening (NBS) for cystic fibrosis is done in the first few days after birth. By diagnosing CF early, CF health care providers can start medicines for CF as early as possible and help you learn ways to keep your child as healthy as possible. This can help delay or prevent serious, lifelong health problems related to CF.
Research shows that children who receive CF care early in life have better nutrition and are healthier than those who are diagnosed later. Cystic fibrosis can affect people of every race and ethnicity, and all children should undergo newborn screening as well as follow-up sweat testing at a CF Foundation-accredited care center after a positive newborn screen. Early diagnosis and treatment can:
- Improve growth.
- Help keep lungs healthy.
- Add years to life.
How Is Newborn Screening Done?
Newborn screening is done during the first few days of your baby's life — usually by a health care provider in the hospital. A few drops of blood from a heel prick are placed on a special card, called a Guthrie card.
This card with your baby's information is mailed to a special state laboratory that will test the blood sample for certain health conditions, including CF. In some states, newborn screening involves two blood samples, one at birth and one a few weeks later.
CF Newborn Screening Can Differ by State
All 50 states and the District of Columbia screen newborns for CF, but the method for screening may differ from state to state.
Every state's CF newborn screening program begins with a blood test from the baby to check the levels of a chemical made by the pancreas called immunoreactive trypsinogen (IRT).
IRT is normally found in small levels in the body. In people who have CF, IRT levels tend to be high but IRT levels can also be high if a baby is premature, had a stressful delivery, or other reasons.
IRT is normally found in small levels in the body, but when the pancreas is stressed before a baby is born, more IRT is released into the baby's blood. In people who have CF, IRT levels tend to be high but IRT levels can also be high if a baby is premature, had a stressful delivery, or for other reasons.
In people who have CF, IRT levels tend to be high but IRT levels can also be high if a baby is premature, had a stressful delivery, or for other reasons.
States use two different methods for newborn screening.
- Some states test IRT levels twice before conducting a DNA test. These states are called IRT-IRT-DNA states.
- Some states test IRT levels once before conducting a DNA test. These are called IRT-DNA states.
To learn more about the newborn screening method used in the state where your baby was born, please contact [email protected].
Understanding Results From Newborn Screening
Results from newborn screening for CF can take longer than one week after a blood sample is collected. Ask your baby's primary health care provider when you can expect results.
When high IRT levels are detected in the blood, the results of the newborn screening are said to be positive. A positive newborn screening result tells you that your baby might have CF.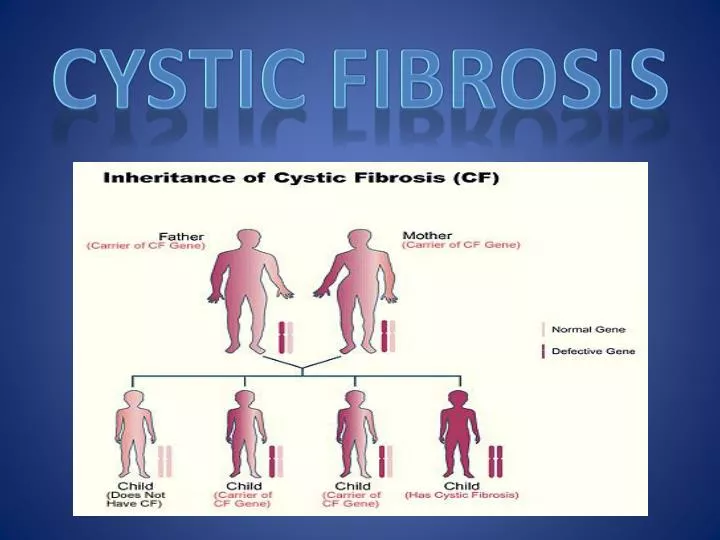
What to Do When Newborn Screening Results Are Positive for CF
If your baby had a positive NBS or you received a positive prenatal genetic test, it’s important to schedule a sweat test once your newborn is 48 hours old. At the latest, babies with a positive NBS or prenatal genetic test should have a sweat test performed by the age of 4 weeks to ensure that any health issues or changes can be found early and treated quickly.
The sweat test will measure how much salt is in your baby's sweat. This test is the only way to diagnose CF, although other forms of testing can help confirm or inform the diagnosis if the sweat test results are inconclusive.
The sweat test should be done at a CF Foundation-accredited care center. The staff will work to schedule the sweat test as soon as possible.
For more information about scheduling a sweat test appointment, please contact the CF care center closest to you.
Hear from other parents who received positive Newborn Screening results in this short video from Nemours.Cystic fibrosis and your baby
Cystic fibrosis (also called CF) is a condition that causes thick mucus to build up in the body. This causes problems with breathing and digestion.
CF is passed from parents to children through genes. A baby has to inherit a CF gene from both parents to have CF.
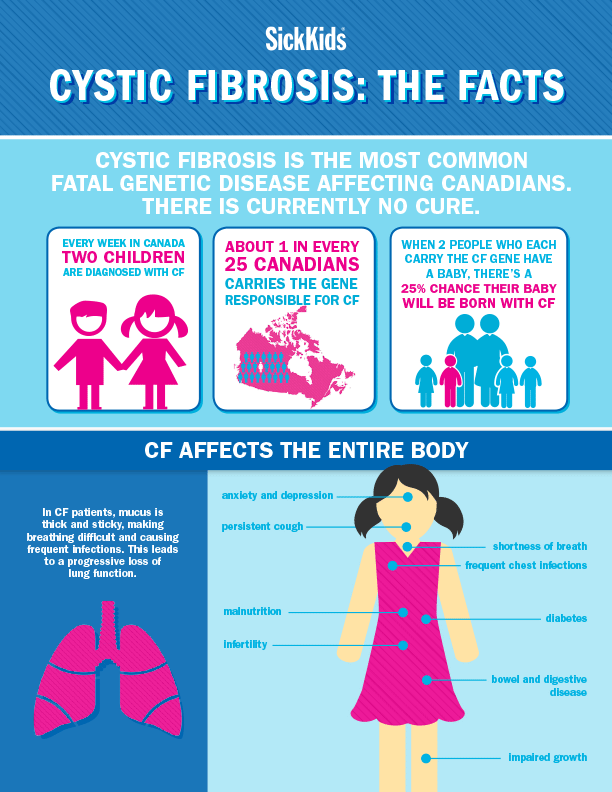
All babies have a newborn screening test for CF so it can be found and treated early.
Treatment can include medicines and chest therapy to help with your baby’s breathing and digestion.
Cystic fibrosis (CF) is a condition that affects breathing and digestion. It’s caused by very thick mucus that builds up in the body.
Mucus is a fluid that normally coats and protects parts of the body. It’s usually slippery and slightly thicker than water. But in CF, the mucus is thicker and sticky. It builds up in the lungs and digestive system and can cause problems with how you breathe and digest food.
CF affects about 30,000 children and adults in the United States. It is one of the most common genetic conditions in this country. CF is more common in white babies (about 1 in 3,500) than in Hispanic, Native American or Alaskan Native babies (about 1 in 10,000), in Black babies (about 1 in 15,000 black) and in Asian babies (about 1 in 30,000).
What causes CF?
CF is inherited.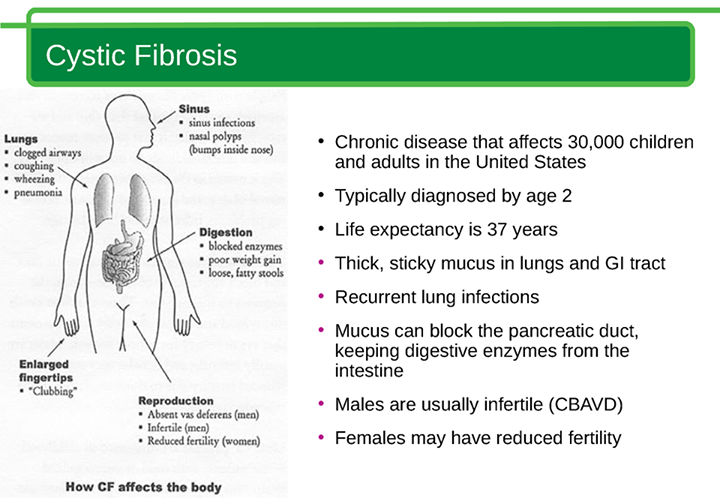 This means it’s passed from parent to child through genes. A gene is a part of your body’s cells that stores instructions for the way your body grows and works. Genes come in pairs—you get one of each pair from each parent.
This means it’s passed from parent to child through genes. A gene is a part of your body’s cells that stores instructions for the way your body grows and works. Genes come in pairs—you get one of each pair from each parent.
Sometimes the instructions in genes change. This is called a gene change or a mutation. Parents can pass gene changes to their children. Sometimes a gene change can cause a gene to not work correctly. Sometimes it can cause birth defects or other health conditions. A birth defect is a health condition that is present in a baby at birth.
Your baby has to inherit a gene change for CF from both parents to have CF. If they inherit the gene change from just one parent, they have the gene change for CF, but they doesn’t have the condition. When this happens, your baby is called a CF carrier.
What problems does CF cause?
Babies who have CF have very thick and sticky mucus that builds up in the body. When this mucus builds up in the lungs, it blocks airways and causes breathing problems and infections. Airways are tubes that carry air in and out of the lungs. As a baby with CF gets older, lung infections can get worse. This can lead to serious, and sometimes deadly, lung damage.
Airways are tubes that carry air in and out of the lungs. As a baby with CF gets older, lung infections can get worse. This can lead to serious, and sometimes deadly, lung damage.
When mucus builds up in the digestive system, it blocks tubes in the pancreas, an organ in the belly. This can make it hard for the body’s digestives system to break down food. When this happens, your baby may not get the nutrients they need to grow and stay healthy.
Some cases of CF are more serious than others. Babies who have CF are often sick with infections and need a lot of special medical care.
How do you know if your baby has CF?
All babies have newborn screening tests for CF. With newborn screening tests, CF can be found and treated early.
Before your baby leaves the hospital, their health care provider takes a few drops of blood from their heel to test for CF and other conditions. The blood is collected and dried on a special paper and sent to a lab for testing.
If newborn screening results aren’t normal, it simply means your baby needs more testing. Your baby’s provider can recommend another kind of test, called a diagnostic test. This test can check to see if your baby has CF or if there is some other cause for abnormal test results.
Your baby’s provider can recommend another kind of test, called a diagnostic test. This test can check to see if your baby has CF or if there is some other cause for abnormal test results.
Your provider may recommend that your baby have a sweat test to see if they have CF. This is a simple, painless test that checks the amount of salt in your baby’s sweat. Babies with CF have more salt in their sweat than healthy babies. Your baby’s provider also may recommend a genetic test for your baby.
If your baby does have CF, they may have these signs and symptoms that can be mild or serious:
- Coughing or wheezing
- Having lots of mucus in the lungs
- Many lung infections, such as pneumonia and bronchitis
- Shortness of breath
- Salty skin
- Slow growth, even with a big appetite
- Meconium ileus, when meconium gets stuck in a newborn’s intestine. Meconium is a baby's first bowel movement. It can be green, brown or black in color.
- Bowel movements that are frequent, loose, large or look greasy
- Stomach pain or bloating
If your baby has CF, how are lung and breathing problems treated?
Many lung infections in babies who have CF are caused by bacteria that don’t usually cause problems for healthy babies.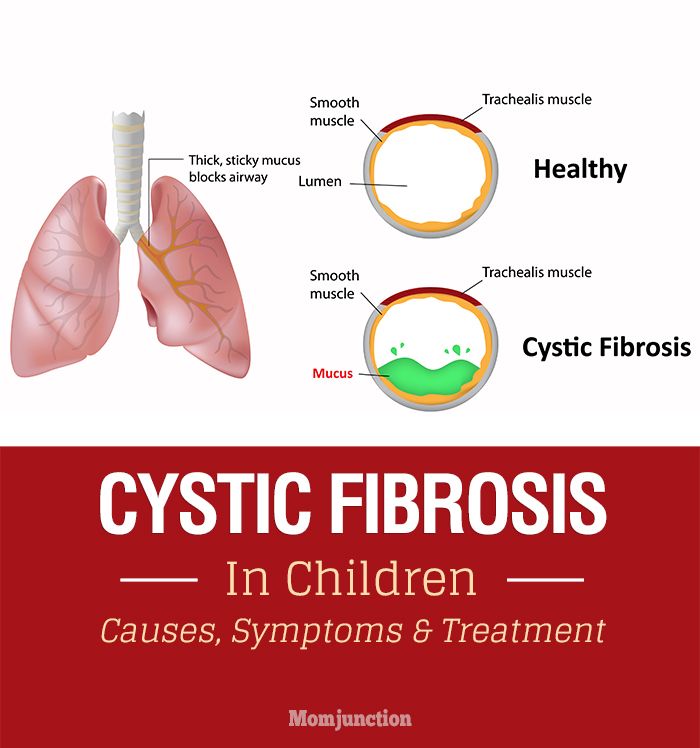 If your baby has CF, medicines like antibiotics often cannot get rid of all the bacteria in their lungs. These infections can lead to lung damage.
If your baby has CF, medicines like antibiotics often cannot get rid of all the bacteria in their lungs. These infections can lead to lung damage.
Your child’s treatment depends on the kind of symptoms they have and how severe the symptoms are. Certain medicines can help children with CF breathe better and prevent infections. Some come as a mist that your child breathes into the lungs. Medicines used for CF include:
- Mucus-thinners. Medicines like dornase alfa (Pulmozyme®) help thin mucus, making it easier to cough out.
- Bronchodilators. These medicines help open the airways to clear mucus from the lungs. Albuterol (Proventil® and Ventolin®) is an example.
- Antibiotics. These are medicines that kill infections caused by bacteria. Tobramycin ( Tobi®) is a common inhaled antibiotic, and azithromycin is a common antibiotic taken by mouth.
- Ibuprofen. This medicine can help reduce lung redness and swelling that make breathing difficult.
- Hypertonic saline. Inhaling this salt-water mist helps draw more water into the airways. This helps thin the mucus.
Your child’s provider may recommend that they get lots of physical activity or that you use other therapies to vibrate (shake) the chest to help loosen mucus in her lungs. This can make it easier for your child to cough mucus up and out of the lungs.
If your child’s CF becomes life-threatening, a lung transplant may be an option. This is a major operation that is becoming more successful in treating CF.
If your baby has CF, how are growth and digestion problems treated?
Some children with CF gain weight and grow normally. But many grow more slowly than other children.
Most children with CF need to take special medicines that help their bodies get nutrients from food. This helps with weight gain and digestion.
To help them grow, children with CF need healthy, high-calorie meals. They need extra vitamins, especially vitamins A, D, E, and K. A dietitian with experience in treating children who have CF can help you create your child’s meal plan for a healthy weight gain. A dietician is a person who has special training in helping people eat healthy.
A dietitian with experience in treating children who have CF can help you create your child’s meal plan for a healthy weight gain. A dietician is a person who has special training in helping people eat healthy.
Some teens or young adults with CF may get CF-related diabetes. This is usually treated by getting shots of insulin at mealtimes. It’s important to keep diabetes under control so that it doesn’t cause more lung problems.
More information
- Cystic Fibrosis Foundation
See also: Genetic counseling
Last updated: May, 2021
Mass screening of newborns for hereditary diseases. Cystic fibrosis - Krasnoyarsk Regional Medical Genetic Center
Read and take note, especially if you are young people. This examination is carried out in many countries and is called newborn screening or neonatal screening.
The purpose of newborn screening is, of course, not to identify newborns with hereditary diseases that have not yet manifested, but to treat them in order to prevent the appearance of clinical symptoms, in many cases very severe, or even fatal. As a result of early and carefully conducted treatment, instead of seriously ill children, and then adolescents and adults, healthy people are obtained, full-fledged members of society, often being the pride of the family.
As a result of early and carefully conducted treatment, instead of seriously ill children, and then adolescents and adults, healthy people are obtained, full-fledged members of society, often being the pride of the family.
Newborn screening in Russia is carried out for 5 hereditary and congenital diseases: phenylketonuria, hypothyroidism, galactosemia, adrenogenital syndrome and cystic fibrosis.
WHAT IS CYSTIC FIBRISIS?
Cystic fibrosis is a hereditary disease caused by a change (mutation) in the gene that is responsible for the synthesis of a protein that functions as a channel for chloride ions in cells. Due to dysfunction of this channel, mucus and other secretions become very thick and viscous in the lungs, pancreas and other organs. This leads to the development of a chronic infection in the lungs that damages the lung tissue; violation of food digestion, since pancreatic enzymes cannot enter the intestine, and other clinical manifestations.
HOW IS CYSTIC FIBRISIS INHERITED?
Cystic fibrosis is inherited in an autosomal recessive manner, when patients in the family appear in only one generation.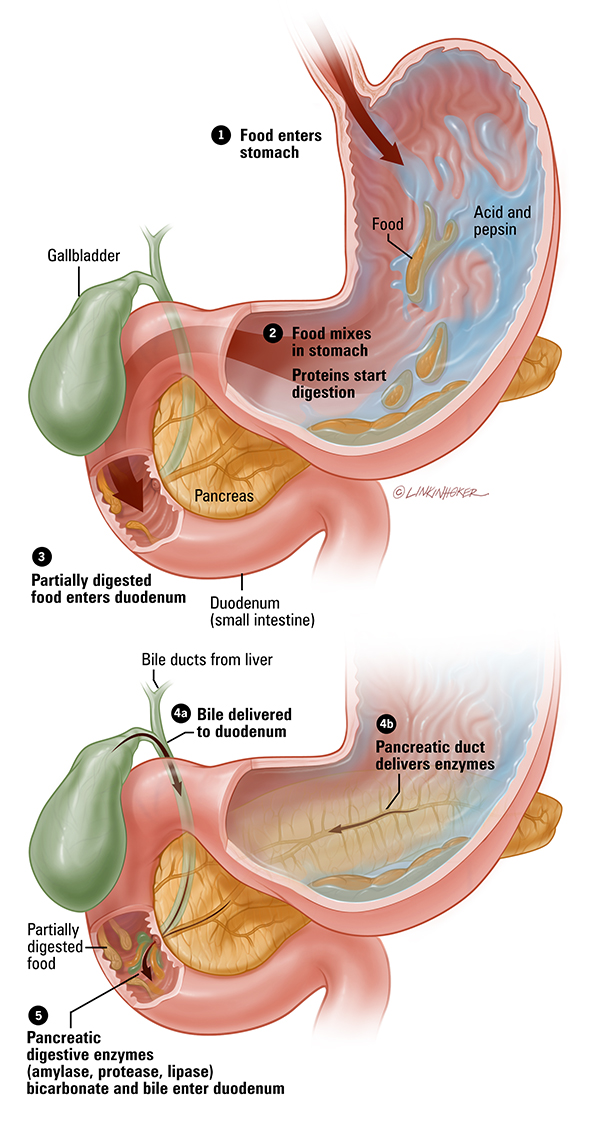 The scheme of such inheritance is shown in the figure, which shows a fragment of the pedigree of the family in which a child with cystic fibrosis was born. On the pedigree, males are indicated by a square, and females by a circle. Inside these squares and circles, only one chromosome (out of 23 pairs that a person has) is drawn, carrying a normal or mutant cystic fibrosis gene, which is marked with a black dot.
The scheme of such inheritance is shown in the figure, which shows a fragment of the pedigree of the family in which a child with cystic fibrosis was born. On the pedigree, males are indicated by a square, and females by a circle. Inside these squares and circles, only one chromosome (out of 23 pairs that a person has) is drawn, carrying a normal or mutant cystic fibrosis gene, which is marked with a black dot.
For simplicity, only the chromosome containing the gene that causes mutations in cystic fibrosis is shown in the figure. The child has a mutant gene on both chromosomes and is therefore ill. Each of the parents has the mutant gene only on one chromosome, while the second chromosome is normal and therefore they are healthy. Such people who have one normal and one defective gene are called carriers of the mutated gene. The maternal grandmother also has the mutant gene on only one chromosome, as does the paternal grandfather. They, like the parents of the child, are healthy, but they passed on the chromosomes containing the mutant gene to their children.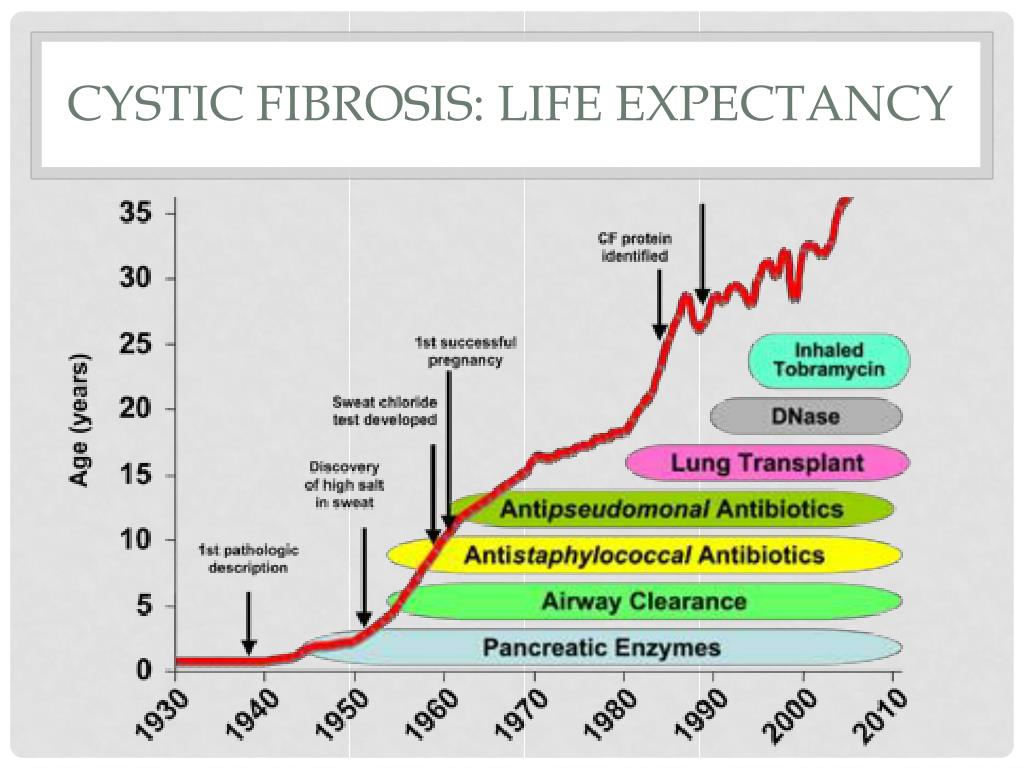 In the second grandparents, both chromosomes contain only the normal gene. Thus, in recessive inheritance, only the family member who received from his parents both chromosomes carrying the mutant gene is sick. All other family members are healthy, including those who are carriers of the mutant gene. The pedigree diagram shows that the parents of a sick child may still have sick children. The probability of the appearance of a sick child in families in which parents are carriers of the mutant gene is 1/4 or 25%. This probability does not change with the number of sick or healthy children in the family: for each subsequent child, the risk that he will be sick is 25%. The probability of having a healthy child, both chromosomes of which contain only a normal gene, is also 25%. And 50% of children will have one normal and one mutant gene, just like their parents. This means that with each pregnancy, carrier parents have a 3 in 4 (75%) chance of delivering a healthy baby. Many parents of children with cystic fibrosis and their relatives, having met a geneticist for the first time, insistently repeat that their child does not have a hereditary disease, since none of their relatives has ever had such a disease in their family.
In the second grandparents, both chromosomes contain only the normal gene. Thus, in recessive inheritance, only the family member who received from his parents both chromosomes carrying the mutant gene is sick. All other family members are healthy, including those who are carriers of the mutant gene. The pedigree diagram shows that the parents of a sick child may still have sick children. The probability of the appearance of a sick child in families in which parents are carriers of the mutant gene is 1/4 or 25%. This probability does not change with the number of sick or healthy children in the family: for each subsequent child, the risk that he will be sick is 25%. The probability of having a healthy child, both chromosomes of which contain only a normal gene, is also 25%. And 50% of children will have one normal and one mutant gene, just like their parents. This means that with each pregnancy, carrier parents have a 3 in 4 (75%) chance of delivering a healthy baby. Many parents of children with cystic fibrosis and their relatives, having met a geneticist for the first time, insistently repeat that their child does not have a hereditary disease, since none of their relatives has ever had such a disease in their family.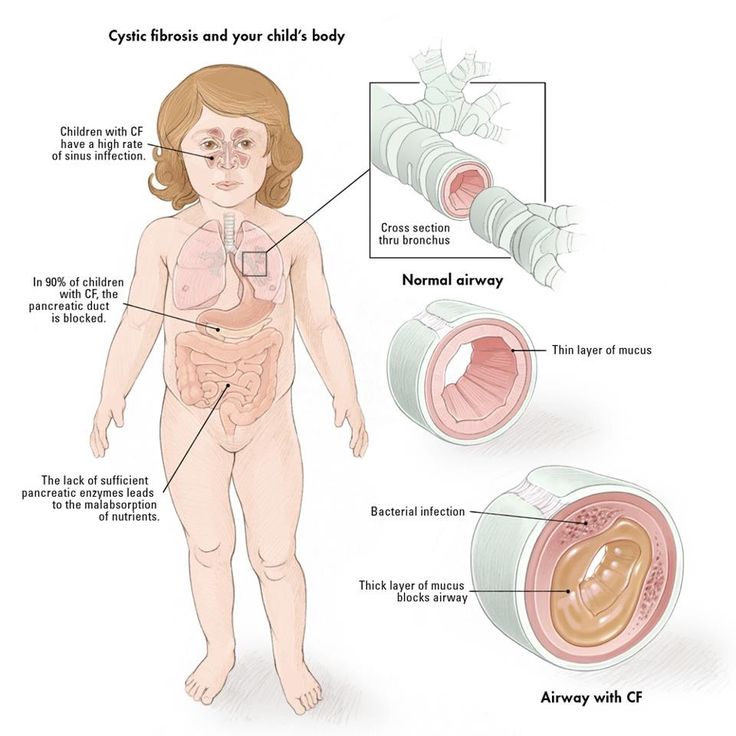 Only the explanation that the rules of inheritance are different, and it is not uncommon for a patient with a hereditary disease to be the only one in the family, allows them to understand what situation they are facing.
Only the explanation that the rules of inheritance are different, and it is not uncommon for a patient with a hereditary disease to be the only one in the family, allows them to understand what situation they are facing.
WHAT DEFECTS IN THE BODY DOES CYSTIC FISSIDOSIS CAUSE?
The disease usually begins at an early age. Clinicians distinguish three main forms of cystic fibrosis: pulmonary, intestinal, and mixed. The most common of these is the mixed form. It occurs in approximately 80% of patients with cystic fibrosis. The pulmonary form of cystic fibrosis is manifested by a chronic obstructive bronchopulmonary process. Due to the fact that the sputum of patients is thick and viscous, it is not expectorated. A large amount of protein in sputum makes it a good environment for the development of various microbes, including staphylococci and Pseudomonas aeruginosa. A chronic inflammatory process develops, leading to the destruction of lung tissue. The blood of patients is poorly saturated with oxygen, because of which the heart, liver and others begin to suffer. organs. Treatment of patients with pulmonary cystic fibrosis requires the use of powerful antibiotics in large doses. In the intestinal form of cystic fibrosis, the process of digestion of food is disrupted, since pancreatic enzymes that break down proteins and fats do not enter the intestine due to blockage of the ducts of the gland. Patients lag behind their relatives in height and weight. The main treatment for the intestinal form is to take pancreatic enzymes. The effectiveness of this treatment is easily monitored by the amount of fat in the child's stool. Pancreatic preparations should be given in such quantity that there is no fat in the stool. In the mixed form of cystic fibrosis, the intestinal manifestations of cystic fibrosis exacerbate lung damage. Treatment of the mixed form is the most difficult. In patients with cystic fibrosis who do not receive the necessary treatment, life expectancy is usually relatively short.
organs. Treatment of patients with pulmonary cystic fibrosis requires the use of powerful antibiotics in large doses. In the intestinal form of cystic fibrosis, the process of digestion of food is disrupted, since pancreatic enzymes that break down proteins and fats do not enter the intestine due to blockage of the ducts of the gland. Patients lag behind their relatives in height and weight. The main treatment for the intestinal form is to take pancreatic enzymes. The effectiveness of this treatment is easily monitored by the amount of fat in the child's stool. Pancreatic preparations should be given in such quantity that there is no fat in the stool. In the mixed form of cystic fibrosis, the intestinal manifestations of cystic fibrosis exacerbate lung damage. Treatment of the mixed form is the most difficult. In patients with cystic fibrosis who do not receive the necessary treatment, life expectancy is usually relatively short.
ARE THERE TESTS FOR EARLY DETECTION OF CYSTIC FIBRIOSIS?
Patients with cystic fibrosis can be detected by newborn screening.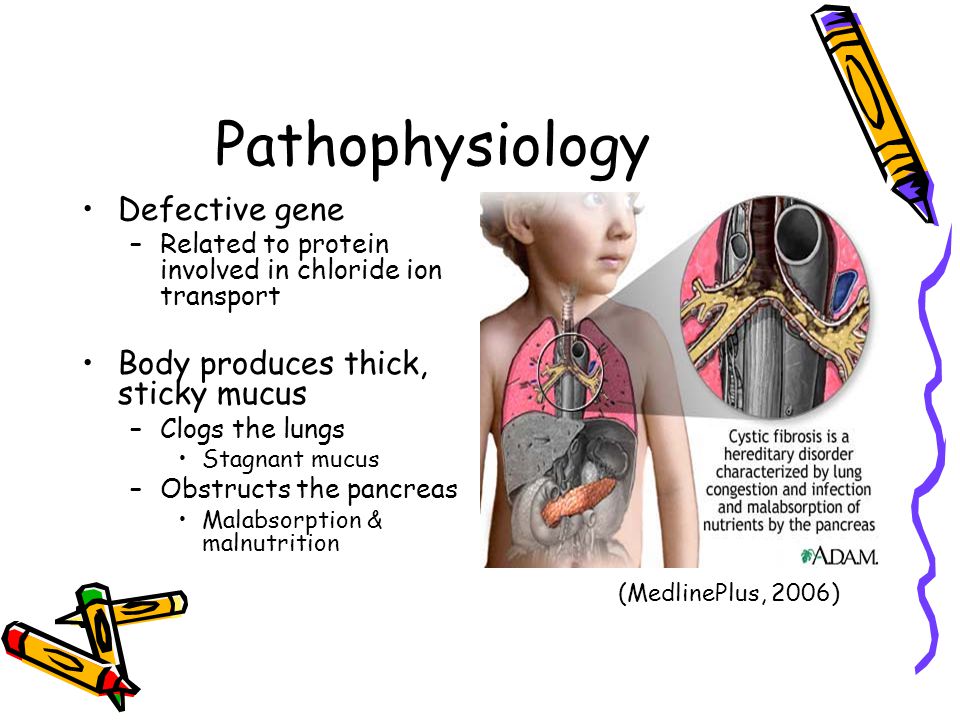 Unlike other hereditary screening diseases, in which early detection of the disease allows it to be treated so effectively that any clinical manifestations of the disease can be avoided altogether, newborn screening for cystic fibrosis has a slightly different goal. Doctors believe that if cystic fibrosis is detected in a newborn, and it is treated immediately, then the child develops normally physically and mentally. His lungs and other organs will be affected to a lesser extent, his life expectancy will increase, which at present, thanks to adequate treatment, is more than 35 years in developed countries. Screening for cystic fibrosis begins with the fact that a few drops of blood are taken from the heel of a newborn in the maternity hospital before discharge, which is applied to filter paper specially used for this purpose. The blood is dried, and such a form, which contains the name of the newborn and a number of other information necessary for his identification, is forwarded to the laboratory of the regional medical genetic consultation.
Unlike other hereditary screening diseases, in which early detection of the disease allows it to be treated so effectively that any clinical manifestations of the disease can be avoided altogether, newborn screening for cystic fibrosis has a slightly different goal. Doctors believe that if cystic fibrosis is detected in a newborn, and it is treated immediately, then the child develops normally physically and mentally. His lungs and other organs will be affected to a lesser extent, his life expectancy will increase, which at present, thanks to adequate treatment, is more than 35 years in developed countries. Screening for cystic fibrosis begins with the fact that a few drops of blood are taken from the heel of a newborn in the maternity hospital before discharge, which is applied to filter paper specially used for this purpose. The blood is dried, and such a form, which contains the name of the newborn and a number of other information necessary for his identification, is forwarded to the laboratory of the regional medical genetic consultation.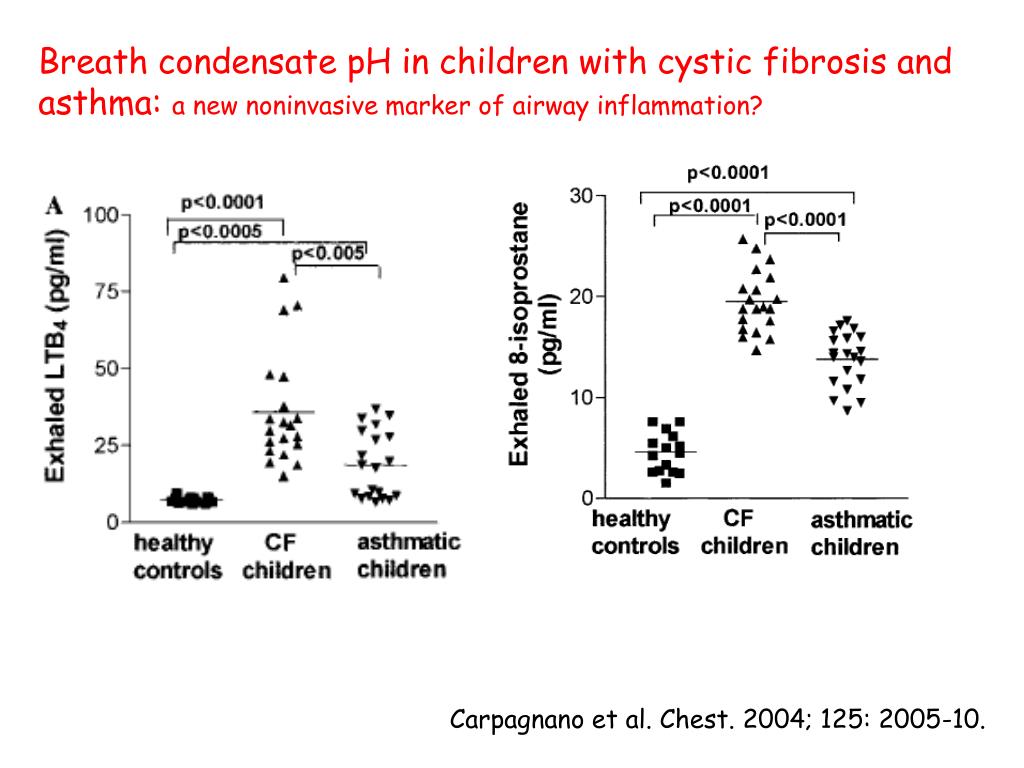 The laboratory conducts a special study that allows you to identify newborns who are suspected of having cystic fibrosis. In this case, the child is called to the laboratory for re-testing through the pediatrician. The pediatrician informs the parents that the first test for cystic fibrosis in their child was abnormal. They have cause for concern. Therefore, retesting the infant's blood sample, which is very important, should be done as soon as possible. In most cases, the test for cystic fibrosis is normal on re-examination. This means that the result of the first study was incorrect (it is called a false positive). The reasons for this may be different and related both to the condition of the infant and to some kind of laboratory error. This result, indicating that the child does not have cystic fibrosis, is immediately reported to the parents in order to relieve their sense of fear.
The laboratory conducts a special study that allows you to identify newborns who are suspected of having cystic fibrosis. In this case, the child is called to the laboratory for re-testing through the pediatrician. The pediatrician informs the parents that the first test for cystic fibrosis in their child was abnormal. They have cause for concern. Therefore, retesting the infant's blood sample, which is very important, should be done as soon as possible. In most cases, the test for cystic fibrosis is normal on re-examination. This means that the result of the first study was incorrect (it is called a false positive). The reasons for this may be different and related both to the condition of the infant and to some kind of laboratory error. This result, indicating that the child does not have cystic fibrosis, is immediately reported to the parents in order to relieve their sense of fear.
WHAT SHOULD I DO IF THE REPEATED LABORATORY TEST FOR CYSIC FISCIDIOSIS IS POSITIVE?
If the second laboratory test for cystic fibrosis is positive, then, unlike other screened hereditary diseases, this does not mean that the child has cystic fibrosis, although the probability of such a diagnosis is high. A family in which a positive test for cystic fibrosis has been re-confirmed in a child is invited to an appointment with a geneticist in a medical genetic consultation. Here, the family is explained what cystic fibrosis is and arranges for appointments with clinicians who are specialists in the diagnosis and treatment of cystic fibrosis. Now a group of clinicians takes over the observation of the child. To confirm the diagnosis of cystic fibrosis, an infant is given the so-called lot test. This completely harmless and painless test can be performed on an infant from the age of 3-4 weeks of life. If the lot test is negative, then the child is considered healthy, although the clinicians will still monitor him for some time. If the sweat test was positive, then the diagnosis of cystic fibrosis is considered established, even before the appearance of any clinical manifestations of the disease. In this case, doctors will prescribe a treatment for the child, which will have to be strictly observed.
A family in which a positive test for cystic fibrosis has been re-confirmed in a child is invited to an appointment with a geneticist in a medical genetic consultation. Here, the family is explained what cystic fibrosis is and arranges for appointments with clinicians who are specialists in the diagnosis and treatment of cystic fibrosis. Now a group of clinicians takes over the observation of the child. To confirm the diagnosis of cystic fibrosis, an infant is given the so-called lot test. This completely harmless and painless test can be performed on an infant from the age of 3-4 weeks of life. If the lot test is negative, then the child is considered healthy, although the clinicians will still monitor him for some time. If the sweat test was positive, then the diagnosis of cystic fibrosis is considered established, even before the appearance of any clinical manifestations of the disease. In this case, doctors will prescribe a treatment for the child, which will have to be strictly observed.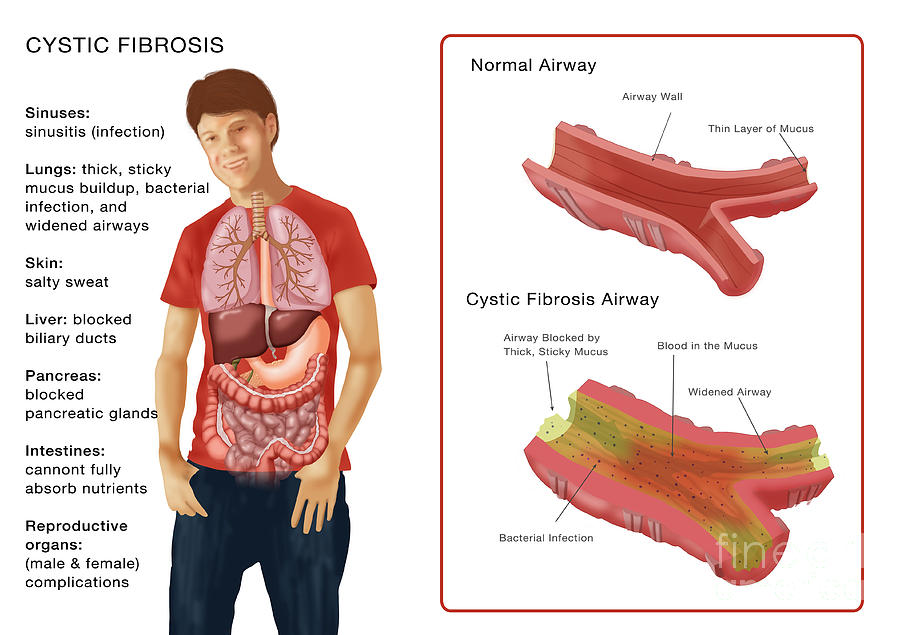 The child will be periodically examined by a group of specialists to constantly monitor his health.
The child will be periodically examined by a group of specialists to constantly monitor his health.
IS IT POSSIBLE TO HELP A FAMILY WITH A PATIENT WITH CYSTIC FIBRIOSIS TO HAVE HEALTHY CHILDREN?
Yes, and quite successfully. For cystic fibrosis, prenatal diagnosis is possible. The first step in this direction is to contact the medical genetic consultation, where the geneticist determines the indications and possible methodological approaches to prenatal diagnosis in each specific case. The procedure itself consists in the fact that during pregnancy at a period of 9-11 weeks or 16-18 weeks, an obstetrician-gynecologist collects a very small number of fetal cells and sends this material to a special laboratory for prenatal diagnosis. In this laboratory, laboratory geneticists perform molecular diagnostics, i.e. determine the presence or absence of a mutation in the gene responsible for cystic fibrosis. In the case of a positive result, the family decides whether to terminate the pregnancy with a sick fetus or adjusts to the appearance of another sick child.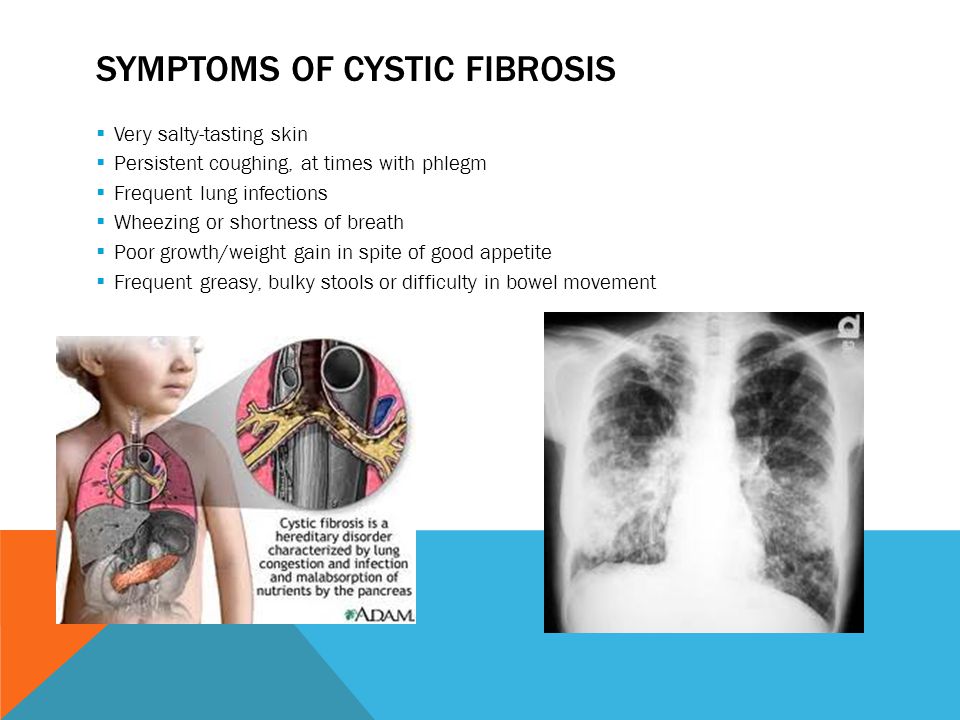 This choice is left to the family.
This choice is left to the family.
what it is, risk factors, symptoms, treatment
Cystic fibrosis or cystic fibrosis is a genetic disease that provokes damage to the external secretion glands, which leads to disruption of the respiratory and digestive organs. Men with cystic fibrosis are often infertile.
Contents
- Probability of disease and statistics
- Diagnosis of cystic fibrosis
- How cystic fibrosis manifests itself
- Treatment
- Cystic fibrosis today
- Note
Cystic fibrosis is usually diagnosed in children under two years of age, most often in the first weeks of life.
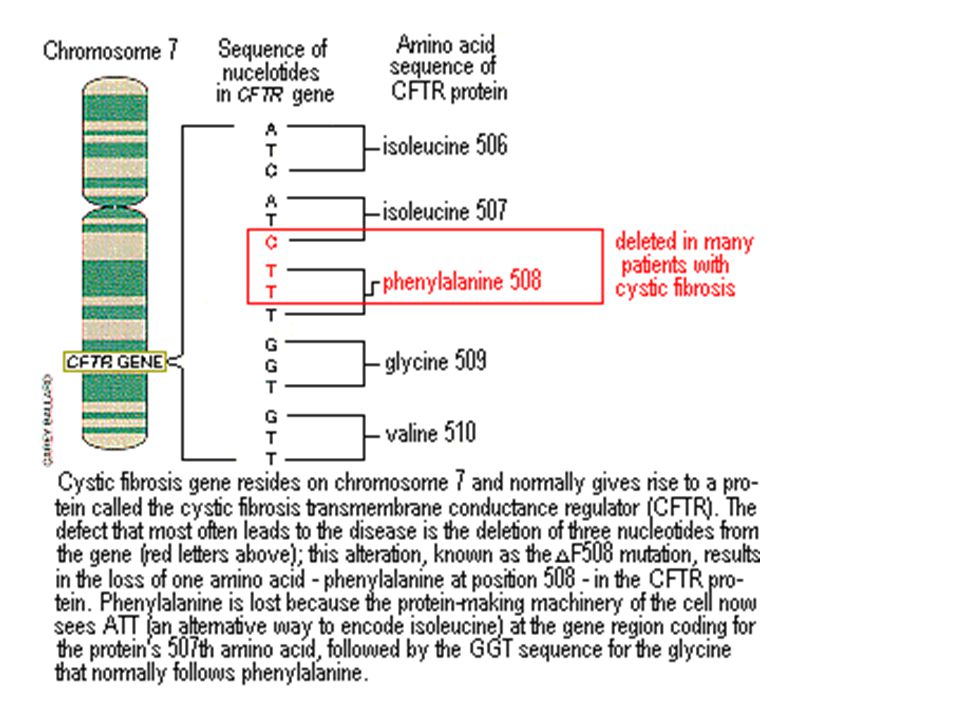
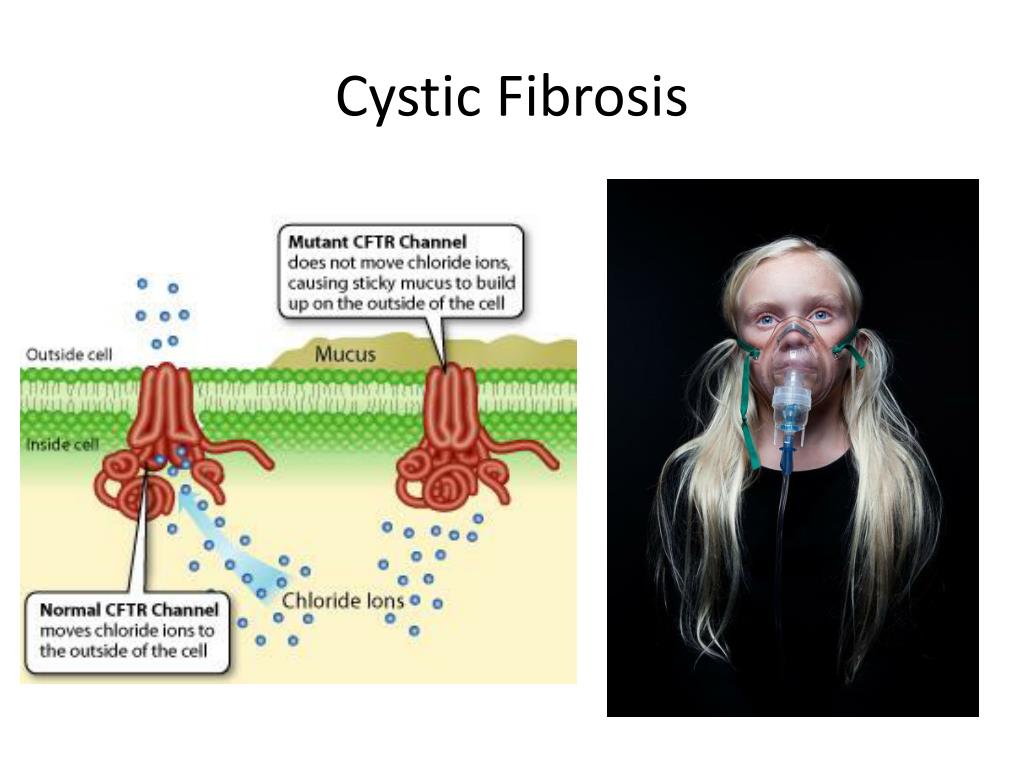
The disease is caused by mutations in the CFTR (cystic fibrosis transmembrane regulator) gene.
1000
new cases of cystic fibrosis are diagnosed worldwide every year
CFTR is a protein that transports chloride ions across the membrane of cells that produce mucus, sweat, saliva, tears and digestive enzymes.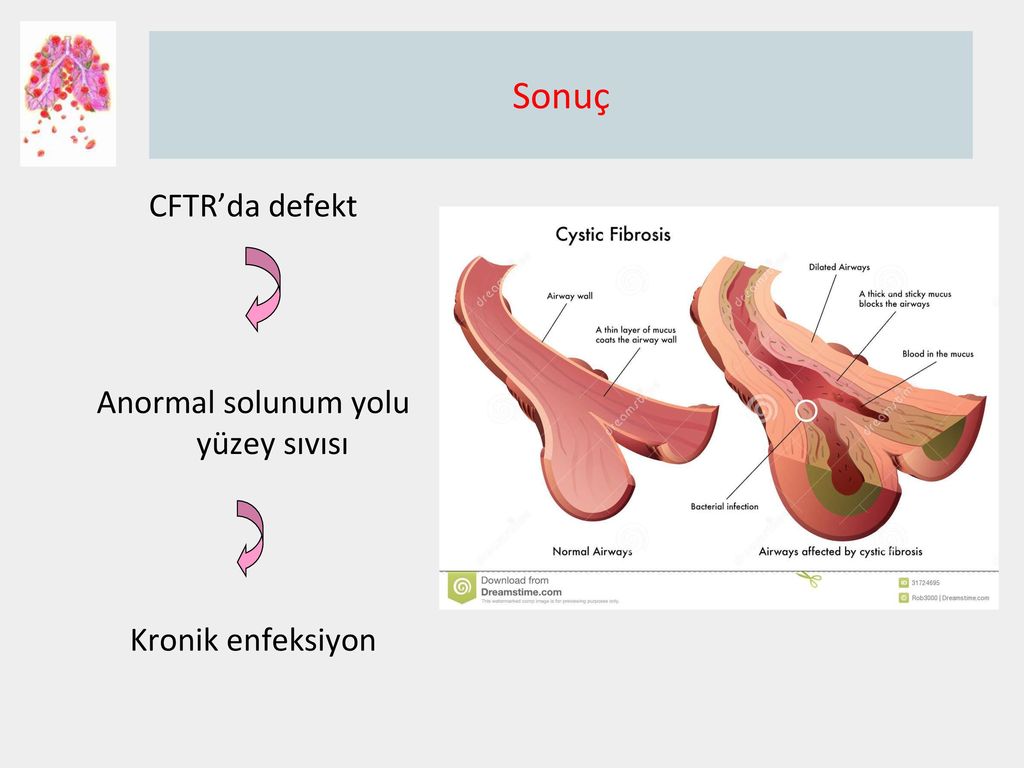 These ions are needed to control the movement of water, which liquefies the secretions of the glands. Due to mutations, the CFTR protein is not assembled correctly and cannot fully perform its function.
These ions are needed to control the movement of water, which liquefies the secretions of the glands. Due to mutations, the CFTR protein is not assembled correctly and cannot fully perform its function.
As a result, the transport of chlorine ions is disrupted, and consequently, the movement of water into and out of cells is impaired. The cells produce a thick and sticky secretion. And this can lead to the formation of cysts inside the organs.
Disease probability and statistics
Both parents must be asymptomatic carriers of the disease in order for a child to receive two copies of the gene that produces the defective protein. If only one of the parents is such a carrier, then the child will not have cystic fibrosis, but it may also be a carrier. Most often, the disease occurs in people of European descent.
More than 70,000 people worldwide are now living with cystic fibrosis, and more than 1,000 new cases are diagnosed each year. According to the registry of patients with cystic fibrosis in Russia in 2017, there were about 3,400 people with this diagnosis.
Probability of having a child with cystic fibrosis in carriers of mutated CFTR:
1. Both parents are carriers:
- 25% - children will have cystic fibrosis;
- 50% - children will be carriers;
- 25% - Children will neither be carriers nor become sick.
2. One parent is a carrier, the other has cystic fibrosis:
- 50% - children will be carriers;
- 50% - children will have cystic fibrosis.
One parent is healthy, the other one has cystic fibrosis:
- 100% - children will be carriers of cystic fibrosis.
One parent is healthy, the second is a carrier:
- 50% - children will be healthy;
- 50% - children will be carriers.
To find out the likelihood of illness in their future children, both parents should take a test for hereditary diseases.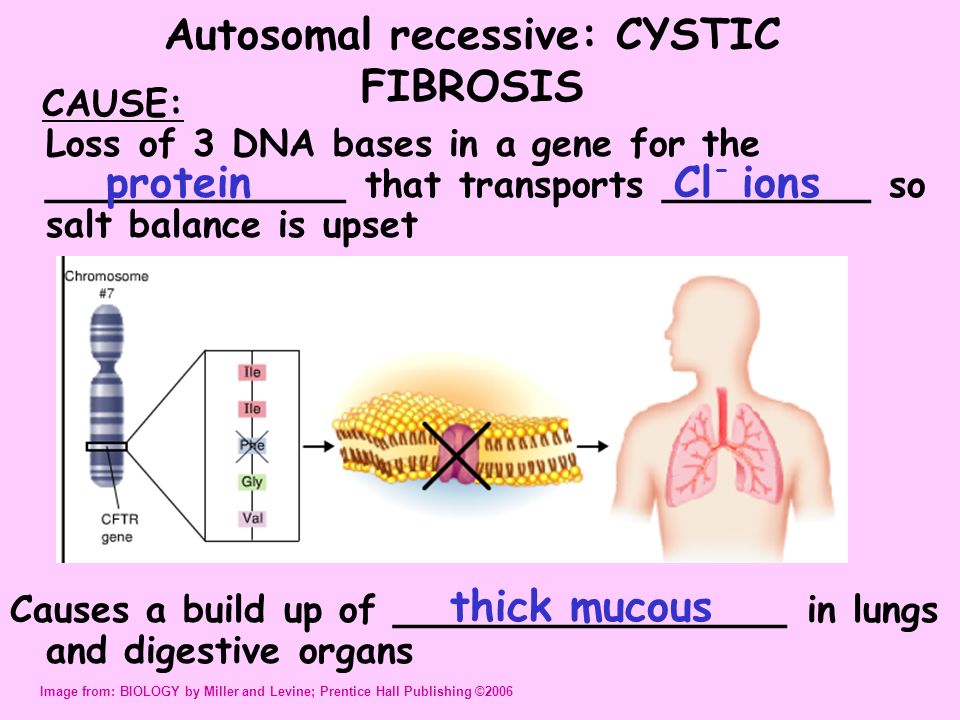
Diagnosis of cystic fibrosis
In some countries where cystic fibrosis is common, newborn screening is done at the hospital. This helps to start treatment in a timely manner.
At the first stage, the content of immunoreactive trypsinogen (IRT) is evaluated. It is a precursor of a pancreatic enzyme that enters the child's bloodstream due to damage to the pancreas in cystic fibrosis. If the result is positive, the test is repeated on the 21st-28th day of life.
Cystic fibrosis is not the only reason RTIs can be raised. This can happen, for example, if the baby is premature or due to meconium contamination of the sample (most likely). Therefore, for accurate diagnosis, a “sweat test” is prescribed - sweat samples are taken from the child to check the concentration of chlorides in it (with cystic fibrosis, their level is increased). With a borderline result of a sweat test, additional examination methods are prescribed.
How cystic fibrosis manifests itself
Main symptoms:
- persistent cough, sometimes with phlegm;
- frequent lung infections, including pneumonia and bronchitis;
- wheezing and shortness of breath;
- changes on the radiograph of the lungs;
- difficulty gaining height and weight with good appetite;
- Frequent loose, foul-smelling stools or difficult bowel movements.

In newborns
Meconium (original stool) becomes thick and sticky, so it accumulates in the intestines and is not excreted, requiring urgent surgical intervention.
In older children
In early childhood, the most obvious manifestation of the disease is pancreatic insufficiency. The secret clogs the ducts, so the intestines do not receive digestive enzymes in the right amount. The absorption of proteins, fats and fat-soluble vitamins is impaired, which is why the child does not gain weight well with a good appetite.
Excessive enzymes destroy cells leading to pancreatitis with development of cysts and fibrosis. Destruction of pancreatic tissue can disrupt its endocrine function and cause diabetes mellitus.
Pulmonary manifestations usually occur a little later. Due to the disease, the secret of the bronchi becomes viscous, sputum stagnates and often becomes infected, which leads to the development of pulmonary diseases.
Treatment
If a child is diagnosed with cystic fibrosis, the first thing parents can do is to work closely with the doctor as soon as the test results are available.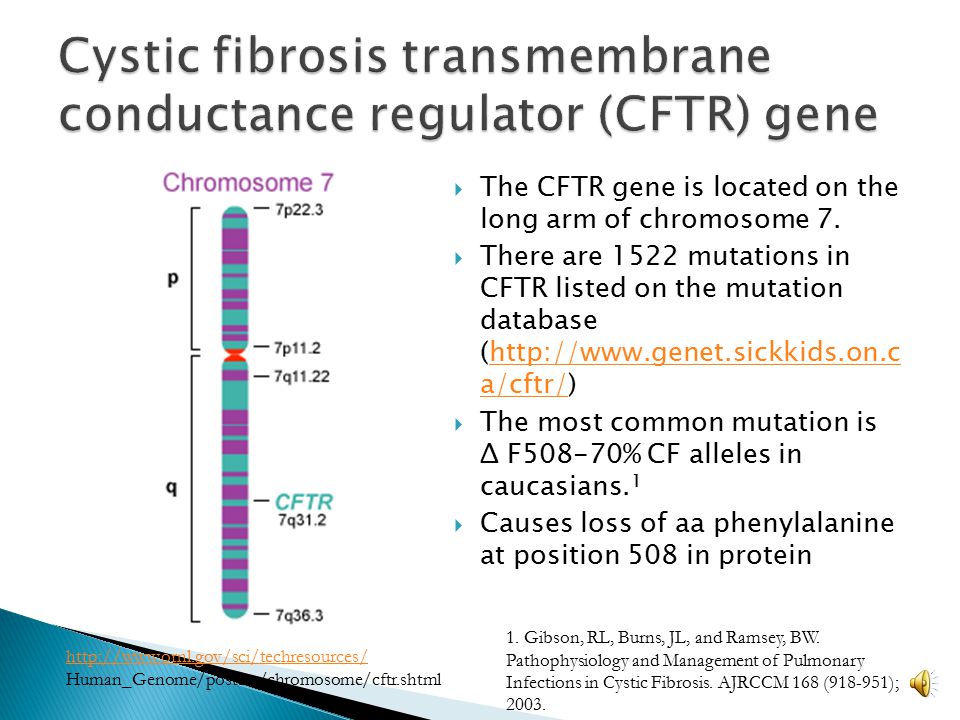
50 years
estimated median survival age for people with cystic fibrosis
It is important to understand that a lifelong treatment regimen is required - adherence to procedures literally every day.
Main recommendations:
- nutrition normalization;
- replacement therapy for pancreatic insufficiency;
- clearing the lungs of mucus;
- physiotherapy exercises;
- prescription of antibiotics in case of infection.
Since 2019, a number of pharmaceutical companies have reduced the supply of important antibiotics to fight cystic fibrosis in Russia. Instead, doctors use domestically produced generics, the active substance of which is less effective. Because of this, courses of antibiotics have to be carried out more often, which exacerbates side effects - for example, allergies. It is possible to achieve individual purchases of foreign-made drugs if the medical commission recognizes side effects from the use of generics.
For some genetic mutations, the drug Ivacoftor is indicated.
The Whole Genome test can be used to assess the individual characteristics of the use of Ivacoftor.
Cystic Fibrosis Today
In 1955, the Cystic Fibrosis Foundation was founded, which sees as its goal a detailed study of the disease and alleviation of the lives of thousands of people.
The activities of this and other scientific foundations have borne fruit - in the past, mortality was mainly among children and adolescents. More than half of the patients died in infancy.
Currently, in developed countries, the number of adult patients with cystic fibrosis exceeds the number of children, and the estimated average age of survival is approaching 50 years.
Note
- Cystic fibrosis is a genetic disease that causes damage to the external secretion glands, which leads to disruption of the respiratory and digestive organs.
- The disease is usually diagnosed before 2 years of age, more often in the first weeks of life through screening.