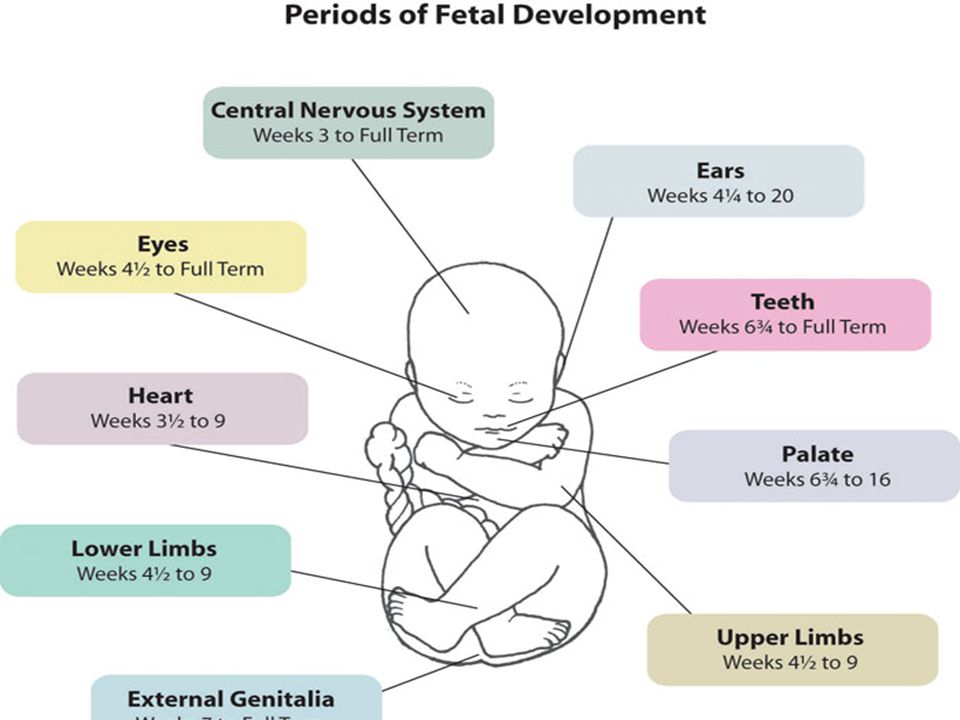
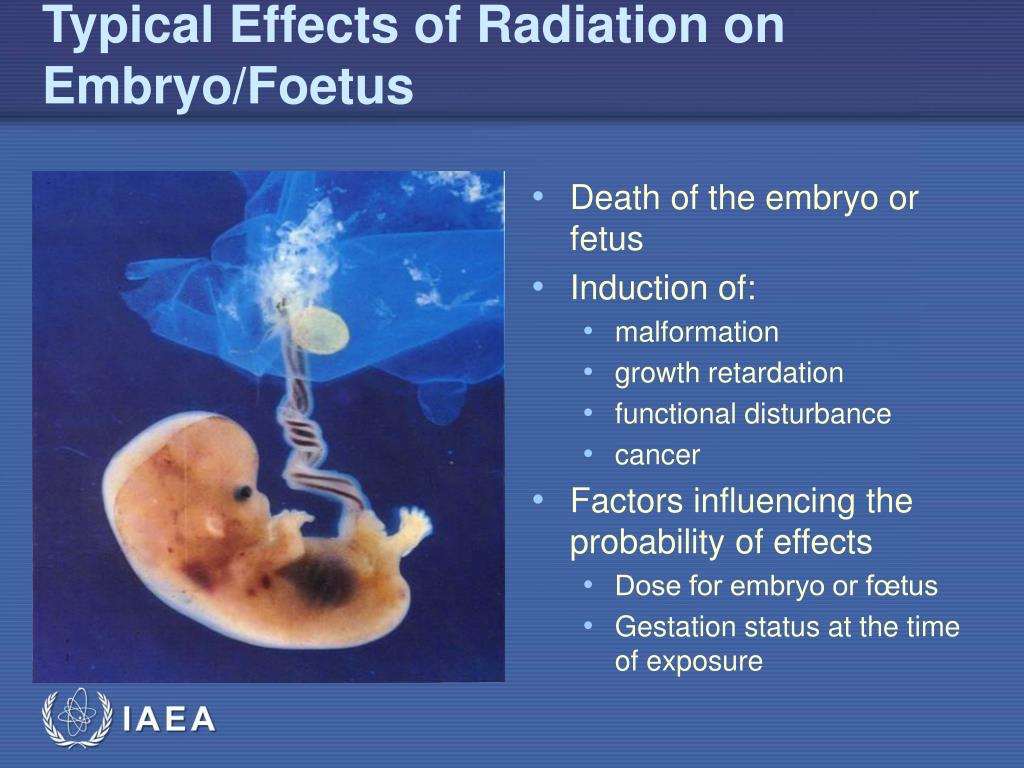
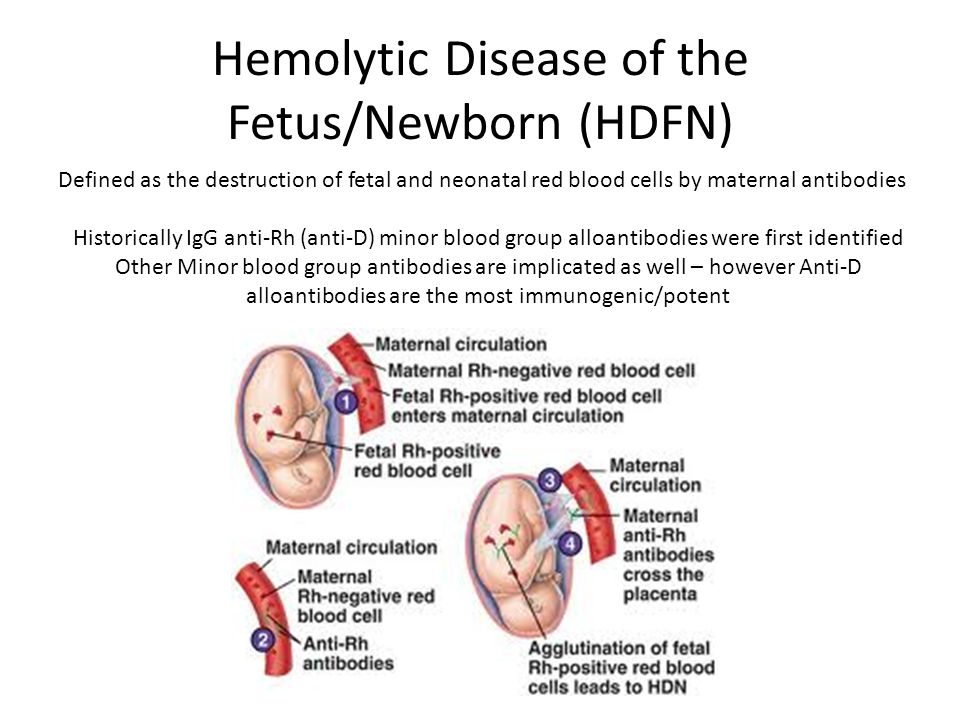
Cmv effects on fetus
Babies Born with Congenital CMV
Español (Spanish) | Print
When a baby is born with cytomegalovirus (CMV) infection, it is called congenital CMV. Most babies with congenital CMV never show signs or have health problems. However, some babies have health problems at birth or that develop later.
Some babies with congenital CMV infection have signs at birth, such as:
- Rash
- Jaundice (yellowing of the skin or whites of the eyes)
- Microcephaly (small head)
- Low birth weight
- Hepatosplenomegaly (enlarged liver and spleen)
- Seizures
- Retinitis (damaged eye retina)
Some babies with signs of congenital CMV infection at birth can have long-term health problems, such as:
- Hearing loss
- Developmental and motor delay
- Vision loss
- Microcephaly (small head)
- Seizures
Some babies can have hearing loss at birth or can develop it later, even babies who passed the newborn hearing test or didn’t have any other sings at birth.
CMV is the most common infectious cause of birth defects in the United States. About 1 out of 200 babies is born with congenital CMV.
One out of 5 babies with congenital CMV will have symptoms or long-term health problems, such as hearing loss.
In the most severe cases, CMV can cause pregnancy loss.
Most people with CMV infection have no symptoms and aren’t aware that they have been infected. If you are pregnant and get infected with CMV, you can pass the virus to your baby during pregnancy. This can happen when you are infected with CMV for the first time or again during pregnancy.
Young children are a common source of CMV.
By the age of 5 years, one in three children has been infected with CMV, but usually does not have symptoms. The virus can stay in a child’s body fluids like saliva and urine for months after the infection. People who are around young children a lot are at greater risk of CMV infection.
Parents and childcare providers can lower their risk of getting CMV by reducing contact with saliva (spit) and urine from babies and young children.
- Do not share food, utensils, or cups with a child.
- Wash your hands with soap and water after changing diapers or helping a child to use the toilet.
Congenital CMV infection can be diagnosed by testing a newborn baby’s urine (preferred specimen), saliva, or blood. These specimens must be collected for testing within 2 to 3 weeks after the baby is born to confirm a diagnosis of congenital CMV infection.
For babies with signs of congenital CMV infection at birth, antiviral medications (primarily valganciclovir) might improve hearing and developmental outcomes. Valganciclovir can have serious side effects and has only been studied in babies with signs of congenital CMV infection. There is limited information on the effectiveness of valganciclovir to treat infants with hearing loss alone.
Last Reviewed: May 27, 2022
Source: National Center for Immunization and Respiratory Diseases, Division of Viral Diseases
- Handwashing: Clean Hands Save Lives
- Basics about Hearing Loss in Children
- CMV Facts for Pregnant Women and Parents
CMV Fact Sheet for Pregnant Women and Parents
Español (Spanish)
Most people have been infected with cytomegalovirus (CMV), but do not have symptoms. If a pregnant woman is infected with CMV, she can pass it to her developing baby. This is called congenital CMV, and it can cause birth defects and other health problems.
If a pregnant woman is infected with CMV, she can pass it to her developing baby. This is called congenital CMV, and it can cause birth defects and other health problems.
For pregnant women
You can pass CMV to your baby
If you are pregnant and have CMV, the virus in your blood can cross through your placenta and infect your developing baby. This is more likely to happen if you have a frst-time CMV infection while pregnant but can also happen if you have a subsequent infection during pregnancy.
You are not likely to be tested for CMV
It is not recommended that doctors routinely test pregnant women for CMV infection. This is because laboratory tests cannot predict which developing babies will become infected with CMV or have long-term health problems.
You may be able to reduce your risk
You may be able to lessen your risk of getting CMV by reducing contact with saliva and urine from babies and young children.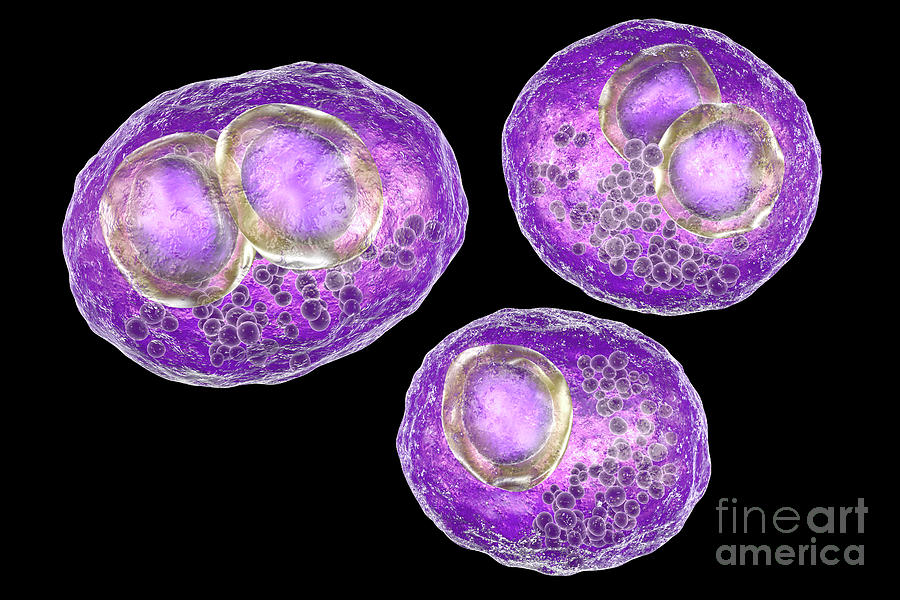 The saliva and urine of children with CMV have high amounts of the virus. You can avoid getting a child’s saliva in your mouth by, for example, not sharing food, utensils, or cups with a child. Also, you should wash your hands after changing diapers. These cannot eliminate your risk of getting CMV, but may lessen the chances of getting it.
The saliva and urine of children with CMV have high amounts of the virus. You can avoid getting a child’s saliva in your mouth by, for example, not sharing food, utensils, or cups with a child. Also, you should wash your hands after changing diapers. These cannot eliminate your risk of getting CMV, but may lessen the chances of getting it.
For parents
About 1 out of every 200 babies is born with congenital CMV. About 1 out of 5 of these babies will have birth defects or other long-term health problems.
Babies with congenital CMV may show signs at birth
Some signs that a baby might have congenital CMV infection when they are born are:
- Small head size
- Seizures
- Rash
- Liver, spleen, and lung problems
Tests on a baby’s saliva, urine, or blood done within two to three weeks after birth can confirm if the baby has congenital CMV.
Early treatment may help
Babies who show signs of congenital CMV at birth may be treated with medicines called antivirals. Antivirals may decrease the severity of health problems and hearing loss but should be used with caution due to side effects.
Antivirals may decrease the severity of health problems and hearing loss but should be used with caution due to side effects.
Long-term health problems may occur
Babies with signs of congenital CMV at birth are more likely to have long-term health problems, such as:
- hearing loss
- intellectual disability
- vision loss
- seizures
- lack of coordination or weakness
Some babies with congenital CMV but without signs of disease at birth may still have or develop hearing loss. Hearing loss may be present at birth or may develop later in babies who passed their newborn hearing test. Sometimes, hearing loss worsens with age.
Hearing checks and therapies are recommended
Children with congenital CMV should have regular hearing checks. Children with hearing loss should receive services such as speech or occupational therapy. These services help ensure they develop language, social, and communication skills.
The earlier your child can get hearing checks and therapies, the more he or she can benefit from them.
Download the print version and share pdf icon[1 page]
Diagnosis and treatment of cytomegalovirus during pregnancy H-Clinic blog CMV treatment
Diagnosis and treatment of cytomegalovirus during pregnancy H-Clinic blog CMV treatment
04/15/2020
Cytomegalovirus (CMV) is one of the members of the herpes virus family (it also includes the causative agents of herpes simplex, chickenpox, Epstein-Barr virus). The first contact with the virus most often occurs in childhood, and most adults have antibodies to it. Like many viruses of this family, CMV has the ability to persist for life in the body's cellular reservoirs, usually without causing any symptoms, since the immune system does not allow the virus to multiply uncontrollably. However, cytomegalovirus during pregnancy can be reactivated from reservoir cells against the background of a physiological decrease in immune function in a pregnant woman. This can lead to serious fetal developmental disorders such as: nine0003
This can lead to serious fetal developmental disorders such as: nine0003
• lack of hearing;
• decreased vision or blindness;
• hepatitis and jaundice;
• microcephaly;
• decrease in the level of platelets;
• learning difficulties;
• epilepsy.
Cytomegalovirus in pregnant women is especially dangerous for the fetus if the woman has not previously been in contact with the virus, does not have immunity to it, and became infected with CMV during the current pregnancy or shortly before its onset. nine0003
This can be checked by testing for antibodies to the virus. During pregnancy, monitoring is carried out for the so-called TORCH complex (this is an analysis for infections that can be transmitted from mother to fetus during pregnancy and cause developmental disorders in the child: toxoplasma, rubella, cytomegalovirus and herpes). It is optimal to pass this analysis even before the onset of the planned pregnancy to assess possible risks.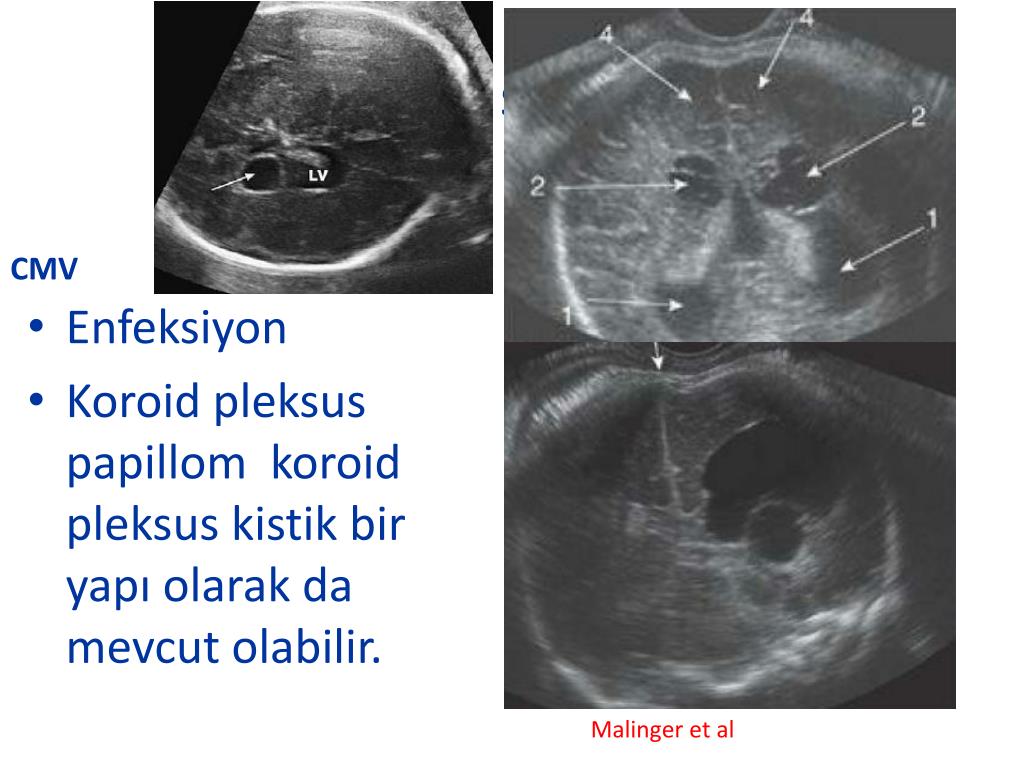
Upon contact of the body with any pathogen (be it a bacterium, virus or helminth), at least two types of antibodies can be produced in the blood: antibodies of the IgM class and antibodies of the IgG class. The former are an "immediate" reaction of the human immune system to the entry of a foreign organism and usually indicate a recent infection. The second type of antibodies is aimed at the final suppression of the disease and the maintenance of immunity for a long time (sometimes for life). nine0003
Thus, if IgG antibodies are detected before pregnancy, and IgM antibodies give a negative result, this indicates that your body has previously encountered the virus, has a certain level of immune protection, and the risk of re-infection is very small. If the test for IgG is negative, then additional precautions are necessary. In the practice of monitoring a pregnant woman, PCR tests of blood and urine for CMV infection are also widely used. All women are shown mandatory screening for cytomegalovirus during pregnancy. The timing and order of the examination are determined by the doctor. nine0003
The timing and order of the examination are determined by the doctor. nine0003
How is CMV transmitted?
CMV is usually transmitted through close contact with a person who already has the virus in their body and sheds it into the environment. The virus can spread through saliva, blood, urine, seminal fluid, breastfeeding. CMV can only be transmitted from a person whose virus is in an "active" state and is excreted in bodily fluids. This happens, for example, if:
• a person contracted the virus for the first time: children often transmit the virus to each other through close contact in kindergarten;
• the virus "reactivated": began to multiply uncontrollably after a certain time after the initial infection. This occurs as a result of a significant weakening of the immune system;
• if reinfection (re-infection) has occurred: the person has become infected with another subtype of CMV.
Thus, the source of infection can most often be children, in whom, after the first contact with the infection, the virus actively multiplies in the body for some time, sometimes causing symptoms like acute respiratory infections, and sometimes completely asymptomatic. In the "risk zone" are pregnant women who work with children or already have children. nine0003
In the "risk zone" are pregnant women who work with children or already have children. nine0003
How to prevent infection?
It is impossible to completely prevent infection: a CMV vaccine has not yet been developed. But during pregnancy, you can reduce your risk by doing the following:
• Wash your hands regularly with warm water and soap, especially if you've changed your baby's diapers or work in day care;
• do not kiss small children on the lips or cheek: it is better to kiss them on the top of their head or just hug them; nine0003
• do not finish eating after the child, do not use the same utensils with him;
• Do not lick baby's pacifiers.
How to cure an infection?
In the event that a planned screening examination nevertheless revealed signs of infection or reactivation of an infection in a pregnant woman, further tactics of treatment and management of pregnancy are determined by the doctor together with the woman, taking into account the risks of infection of the fetus. Sometimes invasive diagnostic interventions (such as amniocentesis) may be required. nine0003
Sometimes invasive diagnostic interventions (such as amniocentesis) may be required. nine0003
The following drugs are registered in Russia for the treatment of cytomegalovirus infection: ganciclovir and valganciclovir. Foscarnet and cidofovir are also used abroad. All of these drugs have a direct antiviral effect. However, possible effects on the fetus limit the use of some of them during pregnancy. There are studies showing the efficacy and safety of hyperimmune globulin G (that is, a drug containing antibodies to the virus) in preventing transmission to the fetus. The choice of treatment tactics, therapy regimens is determined by the attending physician, based on a number of data: laboratory test results, gestational age, etc. As for drugs that indirectly affect the immune system (panavir, isoprinosine, viferon), their effectiveness in the prevention of congenital CMV infection has not been proven. nine0003
Diagnosis and treatment of cytomegalovirus during pregnancy is a complex task, which usually requires the simultaneous participation of an experienced obstetrician-gynecologist and an infectious disease specialist.
Author: infectious disease specialist at the H-Clinic Degtyareva Svetlana Yurievna .
Medical editor: Head of the University Clinic, Candidate of Medical Sciences, infectious disease specialist Konnov Danila Sergeevich . nine0037
You can also read about cytomegalovirus in our article CYTOMEGALOVIRUS TREATMENT .
Return to the list
Cytomegalovirus infection and pregnancy (pregravid preparation and therapy) uMEDp
The article presents the results of studies aimed at studying cytomegalovirus infection in pregnant women, its pathogenesis, clinic, diagnosis, therapy. Recommendations are given for preconception preparation of women with cytomegalovirus infection. nine0007
Table. Drugs used to treat active forms of CMVI in pregnant women
Cytomegalovirus infection and the immune system
The problem of cytomegalovirus infection (CMVI) remains relevant at the present time. The need to study CMVI is due to its wide distribution and the fact that CMV can cause various disorders in newborns and children whose mothers had CMVI during pregnancy. nine0003
The need to study CMVI is due to its wide distribution and the fact that CMV can cause various disorders in newborns and children whose mothers had CMVI during pregnancy. nine0003
The causative agent of CMVI is an opportunistic agent, a typical anthroponosis, belonging to the family Herpesviridae , subfamily Betaherpesvirinae - Human herpesvirus 5 (official name). The common name is Cytomegalovirus . CMV is different:
- the ability to infect almost all cells of the human body, which causes a variety of clinical manifestations;
- low tissue selectivity; nine0123
- slow replication;
- relatively low virulence;
- a high degree of dependence on the state of immunity and the ability to suppress cellular immunity;
- lifelong persistence in the host organism;
- periodic reactivation;
- the uncertainty of the moment and ways of infection.
Cytomegaly is coded according to ICD-10 as:
- B25.
 Cytomegalovirus disease; nine0123
Cytomegalovirus disease; nine0123 - B25.0. cytomegalovirus pneumonia;
- B25.1. Cytomegalovirus hepatitis;
- B25.2. Cytomegalovirus pancreatitis;
- B25.8. Other cytomegalovirus diseases;
- Q25.9. unspecified cytomegalovirus disease;
- O35.3. Damage to the fetus (suspected) as a result of a viral illness of the mother, requiring the provision of medical care to the mother.
The immune system plays an important role in the pathogenesis of CMVI. The body's first antiviral defense line is innate immunity. However, unlike acquired immunity, it does not provide long-term and reliable protection to the host. During the primary interaction, viruses are opposed by protective barriers (skin epithelium and mucous membranes). An important innate way of protecting the body against viruses is RNA interference [1, 2]. The acquired immune system, when confronted with a virus, produces specific antibodies that attach to the virus and often make it harmless.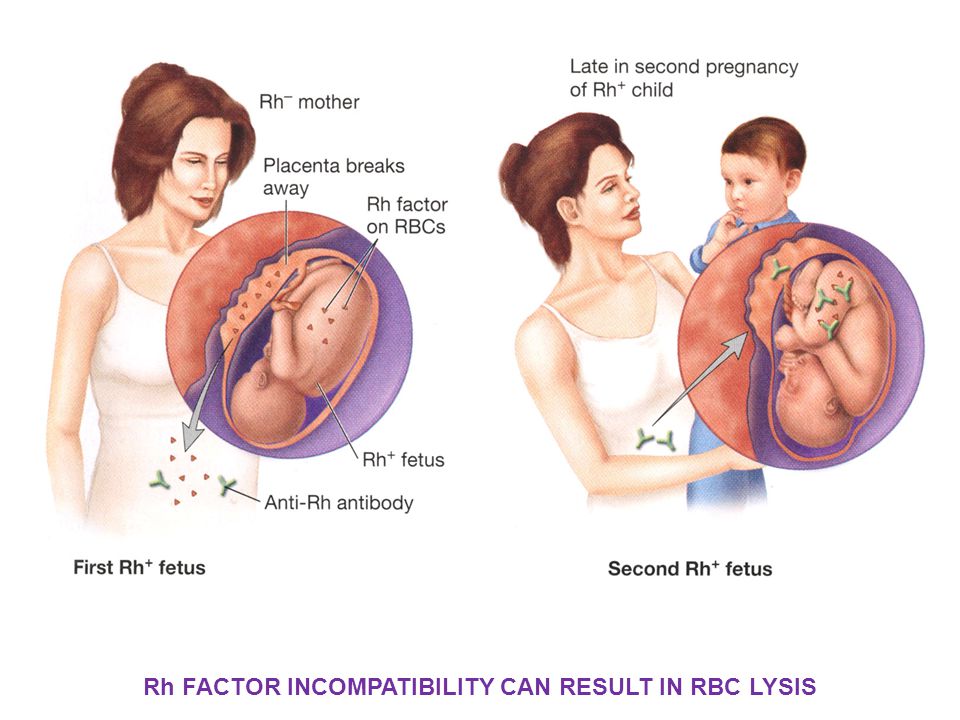 The most important are two types of antibodies. The first is class M immunoglobulin (Ig), which is highly effective in neutralizing viruses, but is produced by the cells of the immune system only for a few weeks. Synthesis of the second - IgG continues indefinitely. The presence of IgM in the blood indicates an acute infection, IgG indicates an infection suffered in the past [3–6]. nine0003
The most important are two types of antibodies. The first is class M immunoglobulin (Ig), which is highly effective in neutralizing viruses, but is produced by the cells of the immune system only for a few weeks. Synthesis of the second - IgG continues indefinitely. The presence of IgM in the blood indicates an acute infection, IgG indicates an infection suffered in the past [3–6]. nine0003
The second protective antiviral mechanism is cellular immunity, which includes immune cells - T-lymphocytes. An important protective reaction is also the production of interferon, which is formed in the body in response to the presence of the virus. Interferons suppress the intracellular stages of viral reproduction in infected cells, provide immunity to viruses of surrounding healthy cells, prevent dissemination of viruses in the body, and stop the formation of new viruses by affected cells, killing them [2, 7, 8]. nine0003
Epidemiology
The incidence of CMVI is endemic and is not subject to seasonal fluctuations.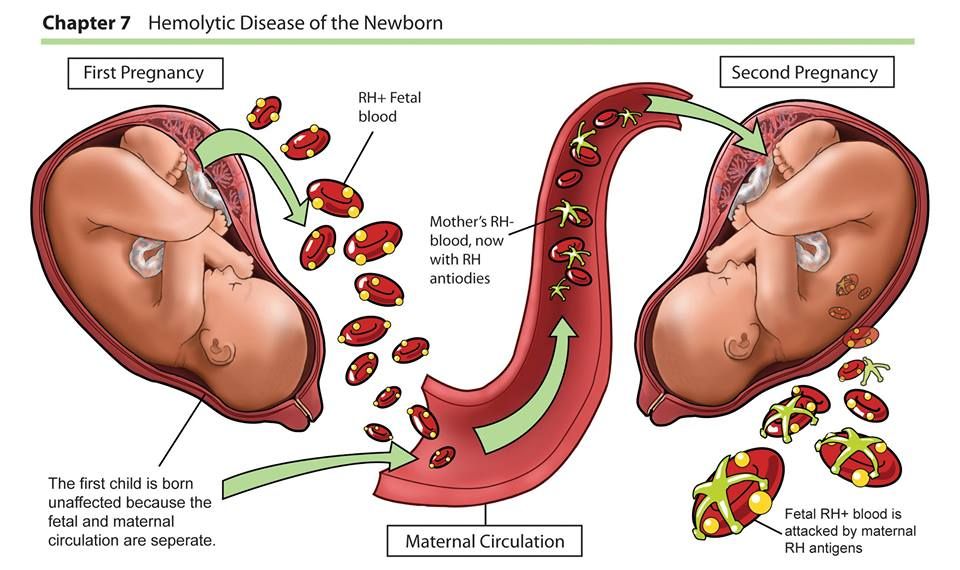 In Europe, CMVI occupies a leading position among congenital viral infections. The prevalence of antibodies to CMV among women of childbearing age varies in regions of the world from 40 to 100% [3, 9–11]. In the Russian Federation, according to various authors, the frequency of detection of CMVI markers in women reaches 90%. Among women over 30, 98% are infected. The seropositive response rate among pregnant women in Japan is 95%, in China - 92%, in Israel - 84%, in Australia - 71%, in France - 50% [3, 4, 7, 11].
In Europe, CMVI occupies a leading position among congenital viral infections. The prevalence of antibodies to CMV among women of childbearing age varies in regions of the world from 40 to 100% [3, 9–11]. In the Russian Federation, according to various authors, the frequency of detection of CMVI markers in women reaches 90%. Among women over 30, 98% are infected. The seropositive response rate among pregnant women in Japan is 95%, in China - 92%, in Israel - 84%, in Australia - 71%, in France - 50% [3, 4, 7, 11].
In Western Europe, there are three to five cases of congenital CMVI per 1000 births [1, 12]. The frequency of transplacental infection of newborns in different countries, according to the literature, ranges from 0.2 to 14%. It should be especially noted that 0.2–2.2% of newborns are diagnosed with intrauterine CMVI infection with the development of severe psychomotor and somatic disorders in subsequent years [3, 13]. In case of primary infection during pregnancy, infection of the fetus occurs in 30-40% of cases, and according to some data, it can reach 75% [2, 10, 13]. nine0003
nine0003
The frequency of CMVI is higher in developing countries among the population with a low socioeconomic level [1, 4, 7, 9].
High-risk groups for CMV infection include:
- pregnant;
- premature babies;
- newborns;
- young children;
- blood and organ recipients;
- cancer patients;
- hematological patients; nine0123
- AIDS patients and HIV-infected;
- patients with immunodeficiencies of various etiologies;
- homosexuals;
- medical workers.
Pathogenesis
The pathogenetic mechanisms of the development of CMVI depend on the dose of the virus, the routes of infection, the age of the patient, and genetic characteristics.
CMV is present in the blood of most people. The source of the virus can be urine, nasopharyngeal secretions, cervical mucus and vaginal secretions, semen, breast milk, tears, saliva, blood. In other words, CMV can be transmitted by feeding, transfusion of blood and its preparations, contact with secrets and excretions deposited on toys and household items, through all biological fluids and body secretions (saliva, urine, etc. ), coughing (contact-household route of infection), a sexually transmitted route of infection is also possible [12, 14]. nine0003
), coughing (contact-household route of infection), a sexually transmitted route of infection is also possible [12, 14]. nine0003
A distinctive feature of CMV is that, once it enters the body of a person with normal immunity, it invades cells that are sensitive to it and is preserved there for a long time. CMV can persist in blood leukocytes, vascular endothelium, salivary glands, kidneys, and other organs. The virus remains in an inactive state, since the normal state of the immune system is an insurmountable obstacle for it [2, 7, 12]. With a significant decrease in immunity, CMV is activated, destroys the nuclei of the cells in which it "hid" and other intracellular structures. As a result, the cell attracts liquid to itself, swells and acquires a characteristic appearance, which is why it was called "owl's eye" [1, 3, 9, 15, 16].
Clinical picture
As a rule, with CMVI there are no clinical manifestations or there is scanty nonspecific symptoms. The incubation period is 30-40 days, the minimum incubation period is two weeks, the maximum is three months. With clinically pronounced CMVI, a temperature reaction develops (38–40 ° C), which can last two to three weeks, hepatomegaly, splenomegaly, and adenopathy. Much less frequently, patients develop interstitial pneumonia, myocarditis, pericarditis, polyradiculoneuritis, myelitis, meningoencephalitis, hemolytic anemia, and thrombocytopenia. nine0003
The incubation period is 30-40 days, the minimum incubation period is two weeks, the maximum is three months. With clinically pronounced CMVI, a temperature reaction develops (38–40 ° C), which can last two to three weeks, hepatomegaly, splenomegaly, and adenopathy. Much less frequently, patients develop interstitial pneumonia, myocarditis, pericarditis, polyradiculoneuritis, myelitis, meningoencephalitis, hemolytic anemia, and thrombocytopenia. nine0003
In 1989, the staff of the Institute of Virology. DI. Ivanovsky developed a classification of CMVI manifestations depending on the route of infection, the most suitable for practical work.
1. Manifestations in perinatal infection:
- miscarriage, stillbirth;
- malformations;
- congenital CMVI.
2. Manifestations during intra- and postnatal infection: nine0003
- acute infectious disease;
- latent carriage, inapparent, subclinical forms of chronic infection;
- reactivation of infection.
3. Manifestations of infection through blood, saliva, urine, sexual contact:
- acute infectious disease;
- latent carriage, inapparent, subclinical forms;
- reactivation of infection. nine0138
Cytomegalovirus infection and pregnancy. Consequences of transplacental infection
The physiological course of pregnancy is accompanied by a suppressive restructuring of the immune system, the purpose of which is the formation and maintenance of immunological tolerance to fetal alloantigens. Viral infections cause the development of inflammatory processes, affect intercellular interactions and lead to changes in the synthesis of regulatory proteins by cells of the immune system [1, 3, 9, eleven]. Due to the peculiarities of immune protection during pregnancy, the state of immunity at the time of infection, the nature of the interaction between the host's immune response and viral replication, as well as the state of the pregnant woman's cellular immunity system are of great importance [1, 3, 7, 15].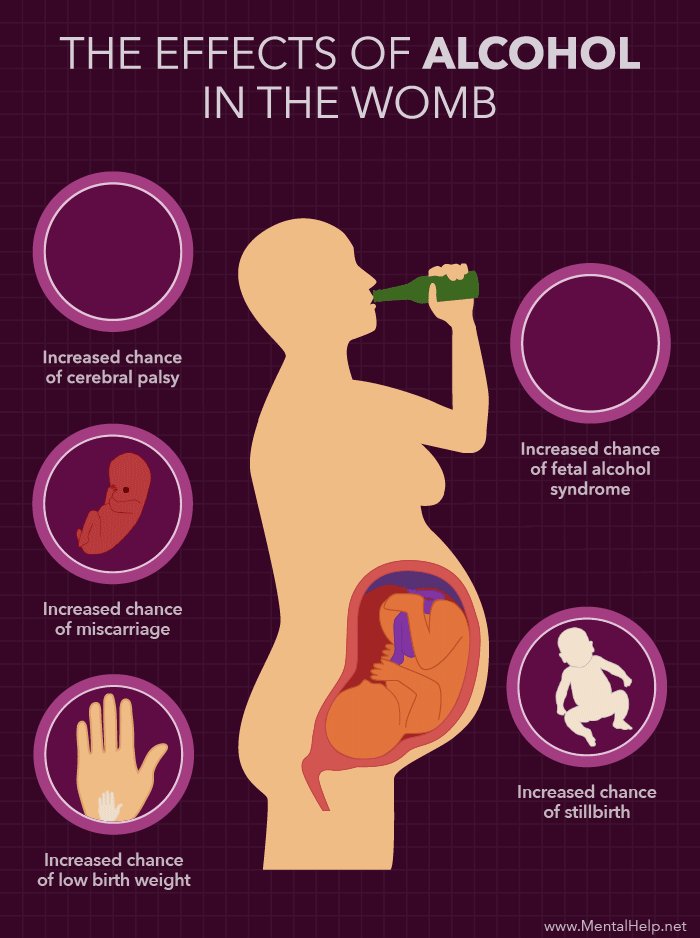
Women with recurrent miscarriage are significantly more likely than other pregnant women to be diagnosed with a persistent form of CMVI (41.9%, p
The frequency of transplacental infection in different countries, according to the literature, ranges from 0.2 to 14%. Risk factors for intrauterine infection of the fetus include the presence of active CMVI in the mother, a high level of viremia, an immunodeficient state of the mother, increased permeability of the fetoplacental barrier [1, 2, 3, 19].
In the presence of CMVI, an asymptomatic infection may develop without consequences for the health of the child. However, in some cases, the presence of CMVI increases the risk of impaired embryogenesis, intrauterine damage to the fetus, spontaneous abortion, placental insufficiency, congenital pathology of the fetus and newborn, and the formation of immunodeficiencies in the postnatal period. The risk of adverse effects is high in both severe and subclinical variants of cytomegaly. An analysis of the condition of newborns showed that in pregnant women with a burdened obstetric and gynecological history, the risk of giving birth to children in serious condition is 4.9times higher when CMVI is detected in the third trimester of pregnancy [9, 16].
An analysis of the condition of newborns showed that in pregnant women with a burdened obstetric and gynecological history, the risk of giving birth to children in serious condition is 4.9times higher when CMVI is detected in the third trimester of pregnancy [9, 16].
Congenital CMVI in the second - fifth year of a child's life can manifest itself as blindness, deafness, speech inhibition, lag in psychomotor and mental development. The risk group for the development of congenital CMVI includes children of patients who do not have basic immunity to CMV (absence of immunoglobulins to CMV in serum) [11, 14, 17].
Diagnostics
CMVI must be differentiated from acquired immunodeficiency syndrome, infectious mononucleosis, acute leukemia, lymphogranulomatosis, viral hepatitis, and sepsis. The congenital form of CMVI should be distinguished from such infectious embryopathies and fetopathy as rubella, listeriosis, toxoplasmosis, herpes infection, syphilis. Differential diagnosis is also carried out between CMVI and hemolytic disease of the newborn, birth injuries, and hereditary syndromes. At the same time, the anamnestic data of the woman and the results of instrumental examination methods are of great importance. An important role in establishing the causes of the disease is played by the pathomorphological examination of the placenta, umbilical cord and fetal membranes. In case of antenatal or intranatal death of the fetus, it is necessary to conduct a thorough histological, bacteriological and virological examination of the tissues of the deceased fetus and newborn [4, 15]. nine0003
Differential diagnosis is also carried out between CMVI and hemolytic disease of the newborn, birth injuries, and hereditary syndromes. At the same time, the anamnestic data of the woman and the results of instrumental examination methods are of great importance. An important role in establishing the causes of the disease is played by the pathomorphological examination of the placenta, umbilical cord and fetal membranes. In case of antenatal or intranatal death of the fetus, it is necessary to conduct a thorough histological, bacteriological and virological examination of the tissues of the deceased fetus and newborn [4, 15]. nine0003
Laboratory diagnosis of CMVI includes verification of the etiological agent (identification of the pathogen, its genome, antigen), detection of serological markers (serological antibodies), determination of the severity of the infectious process - the activity of virus replication (separate determination of anticytomegalovirus antibodies of the IgM and IgG classes) [4, 11].
Laboratory criteria for CMVI activity include detection of a virus, CMV antigen (pp65, pp72) or CMV DNA, anti-CMV IgM class antibodies in the blood, multiple (4 times or more) increase in titers of anti-CMV IgG class antibodies in "paired sera" or the appearance of IgG class antibodies in previously seronegative women, the detection of IgG class antibodies in serum with a low avidity index. nine0003
To clarify the nature of the infection (primary or chronic), it is necessary to take into account the avidity index (the degree of strength of binding of an antibody molecule to an antigen molecule). The detection of IgG antibodies with an avidity index below 30-35% in the test serum indicates a fresh primary infection. An IgG antibody level with an avidity index of ≥ 40% indicates past infection. The presence of IgG antibodies with an avidity index of 31–39% indicates a late stage of primary infection or a recent infection when high concentrations of IgG antibodies are detected [2–4]. nine0003
nine0003
Optimal diagnosis of CMVI includes a combination of virological, serological and molecular biological methods, which allows not only to diagnose the infection, but also to determine its activity.
During pregnancy, the active form of CMVI is detected in 40–50% of seropositive women, while the infection can become active at any stage of pregnancy, which requires examination of pregnant women for CMVI markers. Recommended timing for virological monitoring: 8–12 weeks, 23–25 weeks, 33–35 weeks of gestation [2, 4, 9, 20].
Treatment
The objectives of the treatment of women with an active form of CMVI are the termination of viral excretion and the elimination of IgM CMV from the blood, the transformation of reactivated and persistent forms of infection into a latent one.
In the treatment of severe generalized forms of CMVI, antiviral agents are used: acyclovir, ganciclovir, valaciclovir, famciclovir, foscarnet, cidofovir, fomivirsen.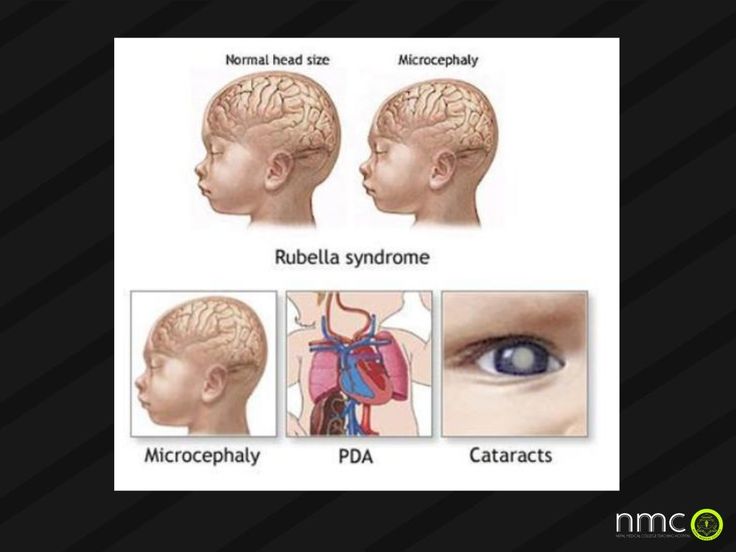 However, they are highly toxic, which greatly limits their use in pregnant, lactating women, and newborns. They are also not recommended for patients planning pregnancy [11, 16]. The only safe alternative to etiotropic drugs is Neocytotect. nine0003
However, they are highly toxic, which greatly limits their use in pregnant, lactating women, and newborns. They are also not recommended for patients planning pregnancy [11, 16]. The only safe alternative to etiotropic drugs is Neocytotect. nine0003
The table shows the drugs used to treat active forms of CMVI in pregnant women. The active form or reactivation of CMVI in pregnant women may serve as an indication for the appointment of hyperimmune anticytomegalovirus immunoglobulin (Neocytotect, Biotest Pharma, Germany). Activation of CMVI occurs at a low titer of specific IgG. Neocytotec contains ten times more IgG antibodies to CMV than standard immunoglobulins, blocks CMV and limits its dissemination in the body. The introduction of Neocytotect to pregnant women reduces the risk of intrauterine infection of the fetus and the development of long-term consequences of congenital CMVI. According to many researchers, the use of Viferon in the complex therapy of CMVI during pregnancy makes it possible to reduce the concentration of interferon gamma and interleukin 8 in the blood serum by two to three times [9, 15, 16, 21].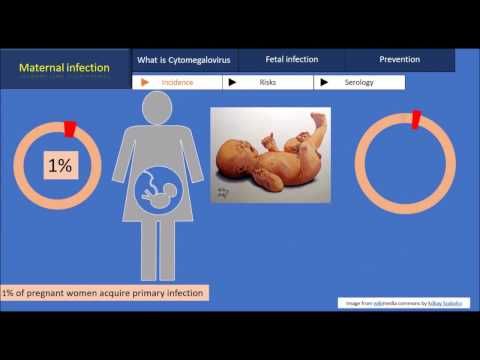 The composition of the drug Viferon, produced by Feron LLC (Moscow), in addition to recombinant human interferon-alpha-2b, includes antioxidants.
The composition of the drug Viferon, produced by Feron LLC (Moscow), in addition to recombinant human interferon-alpha-2b, includes antioxidants.
Women with an active form of CMVI are at risk of possible intrauterine transmission of the infection. Such women outside of pregnancy are recommended to carry out immunocorrective therapy using mainly Taktivin, Cycloferon, Panavir, Viferon. Pregnancy planning is allowed when the active infection is eliminated and the latent form of CMVI is established. Treatment of women with an active form of CMVI before pregnancy planning prevents the activation of CMVI in the most dangerous first trimester of pregnancy in 75% of cases. nine0003
Conclusion
CMVI is an important problem in obstetrics, gynecology and perinatology due to the negative consequences of the infection in relation to reproductive health. To date, CMVI therapy remains insufficiently effective, which necessitates the development of standardized methods for the prevention and treatment of this disease.