Accuracy of genetic testing during pregnancy
Genetic Non-Invasive Prenatal Screening Tests May Have False Results: FDA Safety Communication
Discuss the results of genetic prenatal screening tests with a genetic counselor or other health care provider.Date Issued: April 19, 2022
Español
The U.S. Food and Drug Administration (FDA) is warning patients and health care providers about the risks of false results with genetic non-invasive prenatal screening (NIPS) tests, sometimes called noninvasive prenatal testing or tests (NIPT). Results from NIPS tests can provide information about the possibility of a fetus having certain genetic abnormalities that could result in a child being born with a serious health condition.
While health care providers widely use NIPS tests, none have yet been authorized, cleared, or approved by the FDA. The accuracy and performance of NIPS tests have not been evaluated by the FDA and these tests can give false results, such as reporting a genetic abnormality when the fetus does not actually have one. NIPS tests are screening tests, which means the NIPS test may only tell you the risk of the fetus having certain genetic abnormalities. They are not diagnostic tests, which are generally used to more definitively confirm or rule out a suspected genetic abnormality.
The FDA is aware of reports that patients and health care providers have made critical health care decisions based on results from these screening tests alone and without additional confirmatory testing. Specifically, pregnant people have ended pregnancies based only on the results of NIPS tests. Without confirming the results with a diagnostic test, there is no way to know whether the fetus actually had the genetic abnormality reported by the screening test. The FDA is aware of cases where a screening test reported a genetic abnormality and a confirmatory diagnostic test later found that the fetus was healthy.
Given the increased use of these tests and concerns raised in recent media reports, the FDA is providing this information to educate patients and health care providers and to help reduce the inappropriate use of NIPS tests. The FDA recommends that patients discuss the benefits and risks of NIPS tests with a genetic counselor or other health care provider before deciding to get these tests. Patients should also discuss the results of NIPS tests with a genetic counselor or other health care provider before making any decisions about their pregnancy. Health care providers should be aware of the risks and limitations of using these screening tests and should not use the results from these tests alone to diagnose chromosomal (genetic) abnormalities or disorders.
The FDA recommends that patients discuss the benefits and risks of NIPS tests with a genetic counselor or other health care provider before deciding to get these tests. Patients should also discuss the results of NIPS tests with a genetic counselor or other health care provider before making any decisions about their pregnancy. Health care providers should be aware of the risks and limitations of using these screening tests and should not use the results from these tests alone to diagnose chromosomal (genetic) abnormalities or disorders.
Recommendations for Patients
- Talk with a genetic counselor or other health care provider before deciding to have prenatal testing and to discuss which tests to use, including genetic screening tests such as NIPS tests. Genetic counselors and other health care providers can help you understand the benefits and risks of these tests.
- Do not use the results of screening tests such as NIPS tests alone to make decisions about your pregnancy because the results of these tests may not accurately reflect whether your fetus has a genetic abnormality.
 Additional testing may require invasive procedures to obtain a sample, such as amniocentesis or chorionic villous sampling (CVS), which carry a small risk of miscarriage. The diagnostic confirmatory tests performed on these samples may not have been reviewed by the FDA.
Additional testing may require invasive procedures to obtain a sample, such as amniocentesis or chorionic villous sampling (CVS), which carry a small risk of miscarriage. The diagnostic confirmatory tests performed on these samples may not have been reviewed by the FDA. - Discuss the results of genetic prenatal screening tests and what the results may mean with a genetic counselor or other health care provider. They can help you decide whether to get additional testing to confirm results from a screening test.
- A positive screening test result means that the fetus has a higher risk of having a genetic abnormality compared with the average risk. It does not mean that the fetus definitively has a genetic abnormality, or a condition caused by a genetic abnormality.
- A negative screening test result means that the fetus has a lower risk of having a genetic abnormality compared with the average risk. It does not rule out the possibility that the fetus has a genetic abnormality, or a condition caused by a genetic abnormality.
- The ability of a NIPS test to correctly tell whether a fetus is at risk for a genetic abnormality depends on how common or rare the genetic abnormality is and on underlying risk factors. Disorders caused by a microdeletion (small missing piece of a chromosome) are rare. Because these conditions are so rare, a positive result may be more likely to be from a healthy fetus than one that actually has the reported genetic abnormality.
Recommendations for Health Care Providers
- Review the Recommendations for Patients with your pregnant patients.
- In addition to technical issues, multiple biological factors can influence NIPS results. For example, in some cases, a positive NIPS test result may accurately detect a chromosomal abnormality, but that abnormality is in the placenta and not in the fetus. In these cases, the fetus may be healthy. Additional confirmatory diagnostic tests should be performed to determine whether or not the fetus is affected.

- Discuss with your patients the benefits and risks of prenatal tests, including genetic screening tests such as NIPS tests.
- Do not use the results of screening tests such as NIPS tests alone to diagnose chromosomal abnormalities or disorders.
- Ensure your patients receive the appropriate follow-up testing and care, including genetic counseling, as needed.
Test Description and Background
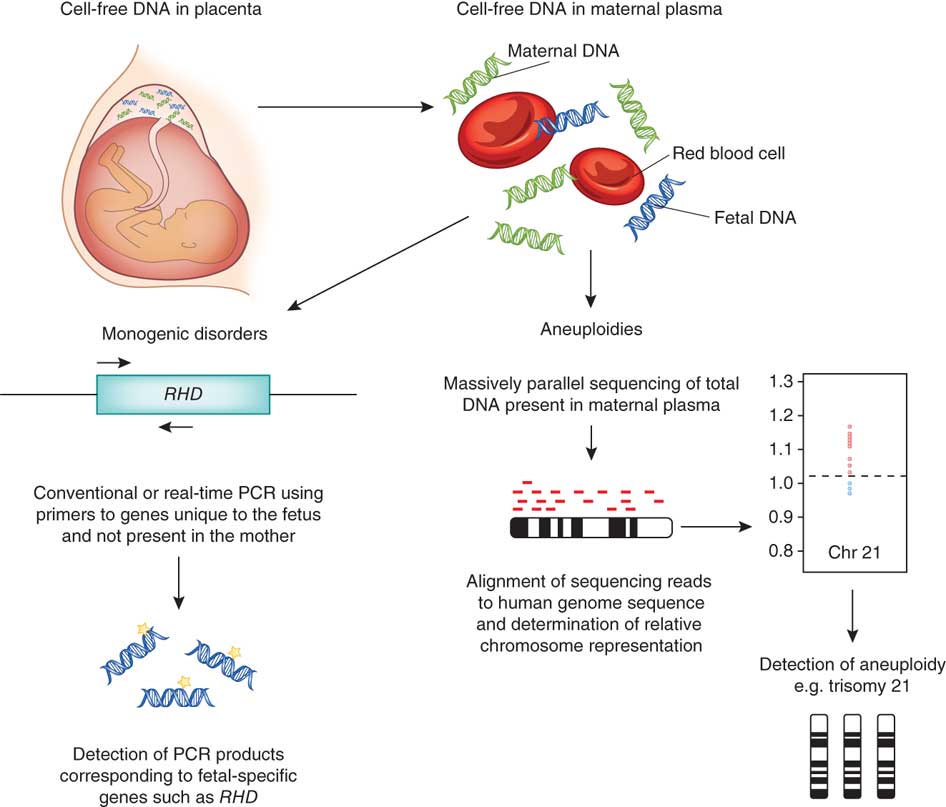
Noninvasive prenatal screening (NIPS) tests analyze small fragments of fetal DNA, called cell-free DNA, that are circulating in a pregnant person's blood with the goal of determining the risk that the fetus has certain genetic abnormalities. When used appropriately, these tests offer a non-invasive approach for prenatal screening and may provide useful information to assess the risk that a fetus has (or does not have) a genetic abnormality. It is important for patients and health care providers to be aware that these are screening tests, not diagnostic tests, and to understand the benefits, risks, and limitations of these tests.
Many laboratories that offer these tests claim the tests are "reliable" and "highly accurate," offering "peace of mind" for patients. The FDA is concerned that these claims may not be supported with sound scientific evidence. False claims may cause patients as well as health care providers to believe the test results are reliable and can be used alone to make decisions about the pregnancy. In addition, because some of the genetic abnormalities and disorders are so rare, in cases such as detection of a microdeletion, there may be a high chance that a positive result is actually from a fetus that does not have the genetic abnormality reported by the test.
The NIPS tests currently being offered are marketed as laboratory developed tests (LDTs). Most LDTs, including NIPS tests, are offered without FDA review. While LDTs are medical devices under the Federal Food, Drug, and Cosmetic Act, the FDA has had a general policy of enforcement discretion for most LDTs since the Medical Device Amendments were enacted in 1976. That means that FDA does not generally enforce applicable regulatory requirements for most LDTs. The FDA is continuing to work with Congress on legislation to establish a modern regulatory framework for all tests, including LDTs.
That means that FDA does not generally enforce applicable regulatory requirements for most LDTs. The FDA is continuing to work with Congress on legislation to establish a modern regulatory framework for all tests, including LDTs.
Additional Information for Health Care Providers
The FDA recommends that health care providers also be aware of the positions of relevant professional societies, including the American College of Obstetricians and Gynecologists (ACOG), the Society for Maternal-Fetal Medicine (SMFM), and the American College of Medical Genetics and Genomics (ACMG):
- These medical professional societies recommend that prenatal genetic screening should be discussed and offered to all patients regardless of their age or risk for a chromosomal abnormality.
- Patient education is emphasized in order to support informed decision making about whether to accept or decline screening. Emphasis is placed on education surrounding the positive predictive value of NIPS tests and the appropriate use of cell-free DNA tests as screening and not diagnostic tests.
- ACMG specifically recommends against testing for aneuploidies (missing or extra chromosomes) other than those involving chromosomes related to Down syndrome (21), Edwards syndrome (18) and Patau syndrome (13).
- ACOG does not recommend the use of NIPS tests to detect microdeletions.
Published studies also strongly support the importance of performing confirmatory diagnostic testing to determine whether or not the fetus truly has a chromosomal abnormality following a positive screening test result. The scientific literature related to the use of NIPS tests from laboratories, including 25 peer-reviewed publications covering 13 studies evaluating more than 10,000 individuals undergoing NIPS, indicates that the NIPS tests evaluated generally perform well for ruling out disorders caused by chromosomal abnormalities. The scientific literature generally report high negative predictive values, greater than 99.9% when calculated, for the NIPS tests studied. This means that the fetus is very likely not to have a chromosomal abnormality if the test returns a negative result.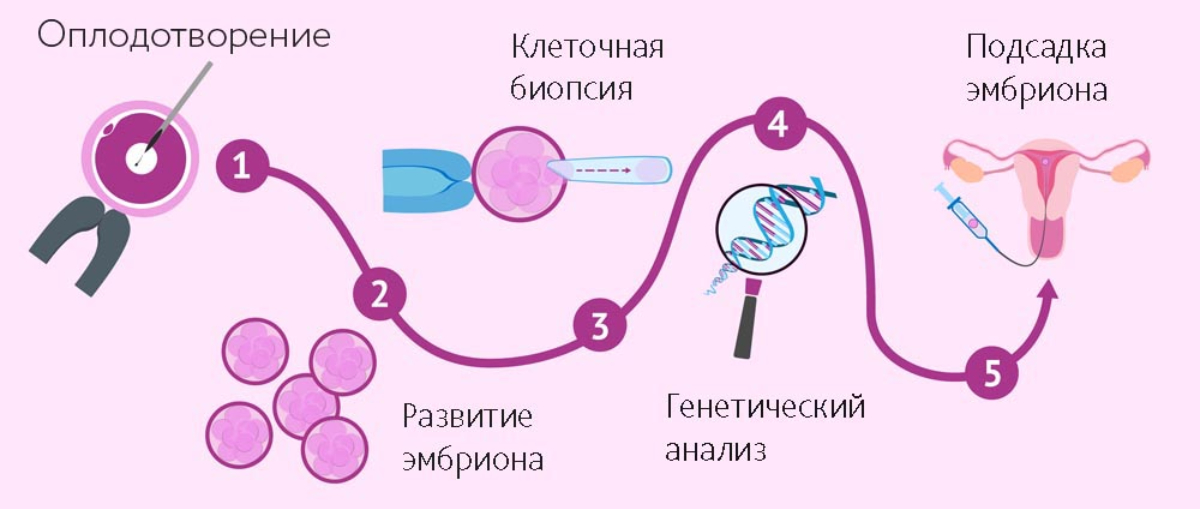 However, the literature confirms that the reliability of positive screening results is limited, particularly for microdeletions. Reliability of positive screening results in these studies was best for Down syndrome, with a positive predictive value of about 90%, meaning that one in 10 positive results are not confirmed as Down syndrome. However, reliability of positive screening results was far lower for microdeletions, with the positive predictive value ranging from about 2% to 30%, depending on the condition. For example, Di George syndrome, which is caused by a microdeletion on chromosome 22, showed a positive predictive value of about 30%. This means that, out of 10 patients receiving a positive result for Di George syndrome on a screening test, it is not confirmed in 7 of those patients when diagnostic testing is performed with CVS or amniocentesis.
However, the literature confirms that the reliability of positive screening results is limited, particularly for microdeletions. Reliability of positive screening results in these studies was best for Down syndrome, with a positive predictive value of about 90%, meaning that one in 10 positive results are not confirmed as Down syndrome. However, reliability of positive screening results was far lower for microdeletions, with the positive predictive value ranging from about 2% to 30%, depending on the condition. For example, Di George syndrome, which is caused by a microdeletion on chromosome 22, showed a positive predictive value of about 30%. This means that, out of 10 patients receiving a positive result for Di George syndrome on a screening test, it is not confirmed in 7 of those patients when diagnostic testing is performed with CVS or amniocentesis.
FDA Actions
The FDA is informing the public of the risks related to the use of genetic prenatal screening and the potential harm if NIPS test results are not used and interpreted appropriately.
The FDA encourages test developers to provide accurate, clear, and complete information about the performance of their tests, how they should be used, and what the results may or may not mean. The FDA also encourages test developers to work with the FDA toward authorization, clearance, or approval of their tests.
The FDA will continue to closely monitor safety issues around the use of NIPS tests and is committed to protecting public health. The FDA will keep the public informed if significant new information becomes available.
Reporting Problems with Your Device
If you think you had a problem with a non-invasive prenatal screening (NIPS) test, the FDA encourages you to report the problem through the MedWatch Voluntary Reporting Form.
Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.
Questions?
If you have questions, email the Division of Industry and Consumer Education (DICE) at DICE@FDA. HHS.GOV or call 800-638-2041 or 301-796-7100.
HHS.GOV or call 800-638-2041 or 301-796-7100.
Prenatal Genetic Testing for Birth Defects: Pros and Cons
Birth defects affect 1 in every 33 babies born in the US, according to the CDC. While that may not seem like a lot in the grand scheme, consider that every 4.5 minutes a baby is born in the U.S. with a birth defect. That translates to about 120,000 babies each year [*]. Worldwide, the percentage of babies born with birth defects is closer to 6% [*].
What causes birth defects is largely a mystery, though known risk factors include smoking or drinking alcohol, taking certain drugs, the presence of maternal infection such as Zika virus, and high fever. But for the bulk of birth defects, scientists point to a complex and unknown interaction between the parents’ genes, the mother’s age, health issues like diabetes, behavior, and environmental factors.
The good news is, there are a wealth of tests that can help parents make informed decisions on the road to parenthood — even before the baby is conceived.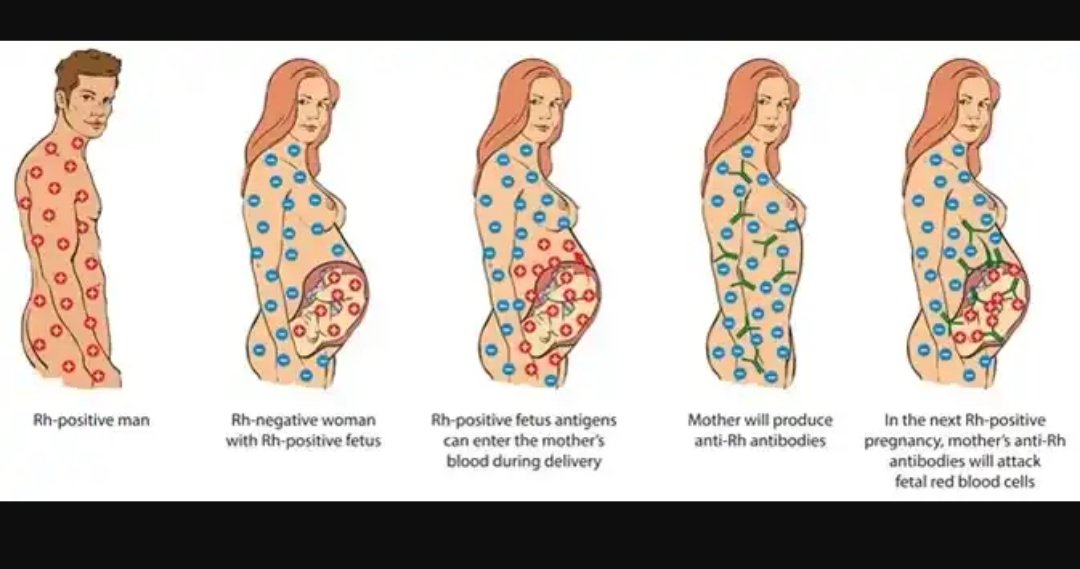
Here, we look at the types of tests and examine their pros and cons.
What Is Prenatal Testing?
Prenatal tests provide you and your doctor a view into your baby’s development while also screening for certain genetic issues. While no testing can guarantee a healthy baby, prenatal testing can help identify pregnancy complications and birth defects at the earliest possible moment. In some cases, these prenatal tests can be lifesaving to mother and baby.
What Are the Types of Prenatal Tests?
Prenatal testing divides into two categories: screening and diagnostic. The main difference is that screening tests are designed to quantify your risk of having a child with a birth defect while diagnostic testing can offer more definitive answers and test for more abnormalities. Screening tests rely on bloodwork and ultrasound imaging while diagnostic tests require tissue or cells.
Prenatal Screening Tests
Screening tests are performed via blood tests, urinalysis, and ultrasound. During pregnancy, these tests monitor the baby’s progress and detect abnormalities characteristic of potential disorders or infections.
During pregnancy, these tests monitor the baby’s progress and detect abnormalities characteristic of potential disorders or infections.
The amount of genetic information doctors can get from a simple blood draw is expanding rapidly, thanks to advancements in testing [*].
For instance, prior to conception, parental carrier screening can help couples determine what genetic anomalies they carry and calculate the likelihood of them passing them on to their offspring. This information could help them determine whether to go with IVF to mitigate such risks through pre-implantation genetic diagnosis.
For parents who conceive via the traditional route, screening results coupled with other risk factors — the mother’s age, the parents’ ethnic background, the family history of genetic disorders — are used to calculate the odds that the fetus might be born with certain genetic disorders, such as Down syndrome, cystic fibrosis, Tay-Sachs disease, or sickle cell anemia. There are no risks to the fetus from these genetic screening tests.
There are no risks to the fetus from these genetic screening tests.
- A blood test performed in the first trimester checks for certain markers — proteins or hormones — indicative of Down syndrome. Bloodwork is also performed early on to test for Rh factor and diseases like rubella, hepatitis B and C, HIV, and tuberculosis.
- A nuchal translucency ultrasound is offered in the first trimester. This test measures increased fluid or thickness at the back of the baby’s neck indicative of Down syndrome. Together, the blood and ultrasound tests are remarkably effective at detecting Down syndrome and aneuploidy (chromosome abnormality) [*]. Abnormal nuchal translucency can also be indicative of risk of other birth defects such as Turner syndrome or congenital heart disease.
- A blood test in the second trimester screens for three or four markers indicative of aneuploidy. Tests done later in pregnancy may also include a glucose screening and GBS (Group B streptococcus) screening.
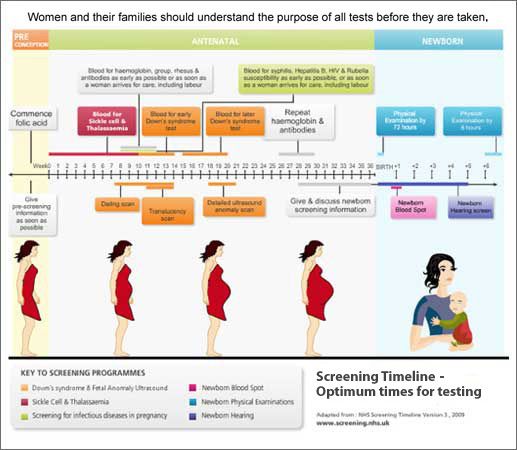
- A combined test (sometimes called an integrated test) uses the blood test and ultrasound from the first trimester together with the second trimester blood test to calculate a risk rating.
Screening Test Risk & Accuracy
These standard tests now reach a detection rate of up to 88–96% for Down syndrome and up to 85–95% for trisomy 18, depending on whether screening is performed in the first or second trimester of pregnancy, or both [*].
There is the potential for a false positive of around 5%. If the mother is pregnant with multiples, a blood test will not be as reliable because the markers from a Down syndrome fetus may be harder to detect.
There are no risks to the fetus, or the mother associated with screening tests.
Non-Invasive Prenatal Testing for Genetic Screening
Non-invasive prenatal testing (NIPT), also known as cell-free fetal DNA screening (CFDNA or CFFDNA), is the most recent addition to the genetic screening toolkit. Available since 2011, NIPT is the most accurate screen for chromosome abnormalities, and in certain countries like the Netherlands, it has become the standard of care for all pregnant women.
Available since 2011, NIPT is the most accurate screen for chromosome abnormalities, and in certain countries like the Netherlands, it has become the standard of care for all pregnant women.
Unlike the other screening tests that look for hormone levels or proteins, this test relies on bits of DNA fragments from the placenta circulating in the mother’s blood (assuming that the placenta DNA matches that of the baby). It can be done any time after 10 weeks’ gestation and is usually done before 21 weeks.
It is used to detect Down syndrome, Trisomy 13 and Trisomy 18, sex chromosome abnormalities, and the fetus’s Rh factor. It has a very high detection rate for common aneuploidies (98-99%) and low false positives (.1-.2%). It does not detect other abnormalities and insurance coverage for it may be conditional, but if your screening reveals an issue you want to formally diagnose, it makes sense to do it since it’s noninvasive and highly accurate.
Out-of-pocket costs for NIPT range from $800-$2,000 in the U. S [*]. Contact your insurer to determine your coverage or review The American College of Obstetrics and Gynecology NIPT insurance coverage overview here.
S [*]. Contact your insurer to determine your coverage or review The American College of Obstetrics and Gynecology NIPT insurance coverage overview here.
Prenatal Diagnostic Tests
Prenatal diagnostic tests tell parents if their baby is affected with a genetic disorder, as opposed to screening tests which calculate their risk of having a baby affected.
For patients at increased risk due to maternal age, abnormal screening results, genetic markers, or family history, diagnostic testing is indicated. These tests require tissue or cells to be collected and are invasive.
While some women choose to do this after a genetic screening indicates a high risk, others may bypass the screening step if they want a definite answer earlier in their pregnancy. Diagnostic tests also can detect other types of chromosomal disorders that genetic screening tests cannot.
There are three diagnostic tests:
- Chorionic villus sampling (CVS): CVS is performed in the first trimester, between 10-13 weeks of pregnancy.
 It tests placenta tissue, which is taken either transcervically or transabdominally, i.e., via the cervix akin to a Pap smear or by using a needle inserted through the mother’s abdomen into the uterus. CVS cannot detect neural tube defects like spina bifida, so if that is a concern, an ultrasound or genetic amniocentesis might be a better choice.
It tests placenta tissue, which is taken either transcervically or transabdominally, i.e., via the cervix akin to a Pap smear or by using a needle inserted through the mother’s abdomen into the uterus. CVS cannot detect neural tube defects like spina bifida, so if that is a concern, an ultrasound or genetic amniocentesis might be a better choice. - Amniocentesis (Amnio): Amnio is a second trimester test performed at 15 weeks or later in which a needle is inserted through the mother’s abdomen to extract fluid surrounding the baby. The amniotic fluid contains fetal cells used for testing.
- Percutaneous umbilical blood sampling (PUBS): PUBS, also known as cordocentesis, is the most accurate diagnostic method but is less often used. It involves taking a sample of fetal blood from the umbilical cord via the uterus. A needle is guided by ultrasound and then introduced into a blood vessel (usually the vein) of the umbilical cord. The blood can be tested for genetic disorders, blood conditions (PUBS is most often used to test for anemia) and infections.
 It can also be used to confirm the results of CVS or amnio but it cannot be performed until 18-22 weeks’ gestation [*]. PUBS may also be used to treat a critically ill fetus by delivering blood or medication through the umbilical cord [*].
It can also be used to confirm the results of CVS or amnio but it cannot be performed until 18-22 weeks’ gestation [*]. PUBS may also be used to treat a critically ill fetus by delivering blood or medication through the umbilical cord [*].
Diagnostic Test Risk & Accuracy
Both CVS and Amnio carry minimal risk of complications like infection, premature rupture of membranes, or even miscarriage. CVS has a .5-1% risk of miscarriage, according to the Mayo Clinic. Amnio risk of miscarriage is 1 in 400, or .25% [*]. PUBS carries higher risk of complications or miscarriage, 1-2%, so it is usually only done when a diagnosis cannot be made via amnio or CVS. According to the Mayo Clinic, the risk numbers for PUBS may be skewed by the fact that many fetuses are already quite ill when the test is done at 18-22 weeks. [*]
For women of any age, at least half of first trimester miscarriages occur due to a chromosome abnormality in the baby [*].
How Common Are Birth Defects From Genetics?
To date, more than 7,000 different birth defects of genetic or partially genetic origin have been identified.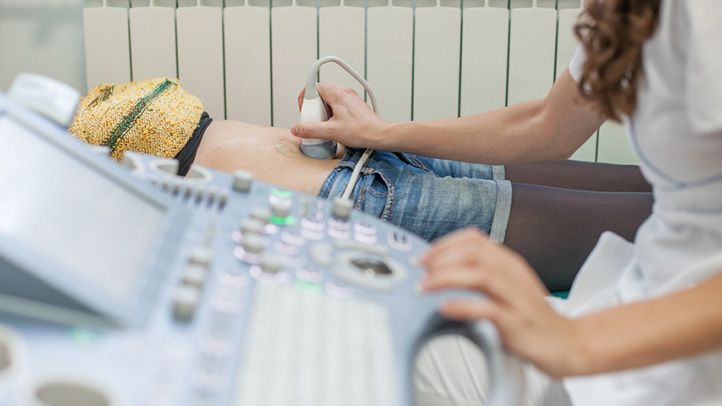
The prevalence of chromosomal abnormalities in clinically recognized miscarriage is greater than 50%. Fetuses with aneuploidy account for 6-11% of all stillbirths and neonatal deaths [*].
According to the March of Dimes, experience from high-income countries shows that nearly 70% of birth defects can either be prevented or the affected children can be offered care that could save their lives or reduce the severity of disability. These interventions include appropriate treatment, particularly surgery, and prevention, especially before conception or in very early pregnancy.
Do Doctors Recommend Prenatal Genetic Testing?
Doctors invariably look at patient outcomes when they make recommendations, and the facts here speak for themselves: The United States reported a remarkable 46% decline in infant mortality rates from birth defects over the period 1980 to 2001, and much of this reduction can be attributed to improvements in diagnosis, care, and prevention. Other high-income countries have reported similar declines.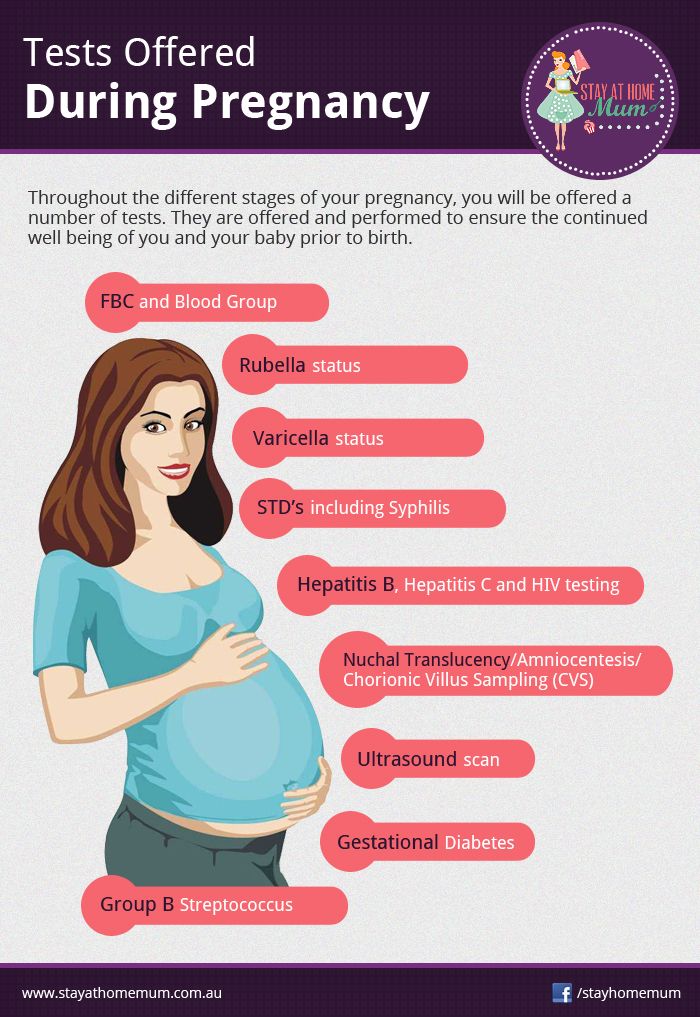 And that was 20 years ago, before NIPT and other advancements in prenatal testing [*].
And that was 20 years ago, before NIPT and other advancements in prenatal testing [*].
The objective of prenatal genetic testing is to detect health problems that could affect the woman, fetus, or newborn and provide the patient and her obstetric care provider with the information they need to make fully informed decisions about their pregnancy.
The American College of Obstetricians and Gynecologists urges doctors to make the aims and limitations of prenatal testing clear to patients and ensure the tests are aligned with the patient’s risks, reproductive goals, and preferences [*].
The Pros and Cons of Genetic Testing
To know or not to know… this is the question. Here are the pros and cons:
Pros of Genetic Testing
- Reassure patients when results are normal.
- Empower parents to make fully informed decisions to manage their pregnancy, even before conception.
- Empower doctors to identify and treat (where feasible) fetal conditions in utero, significantly improving the chance of survival and reducing the need for major surgery after birth [*].

- Optimize neonatal outcomes by ensuring an appropriate delivery location and personnel to care for an affected infant or manage a potentially complicated birth.
- Ensure parents are prepared, emotionally and medically, to care for an infant with special needs.
- Allow for the opportunity to terminate a pregnancy.
Cons of Genetic Testing
- Minor risk of complications or even miscarriage associated with invasive diagnostic testing.
- The emotional toll of pregnancy management when your fetus is diagnosed with a birth defect.
- The possibility of a false positive in a screening test (usually resolved with diagnostic testing).
- Cost of certain tests where insurance coverage is unavailable or limited.
- Lack of availability to underserved communities.
What to Do if Your Child Is Predisposed
If a parent is known to carry a heritable genetic disease or carrier genetic testing reveals this, pre-implantation genetic diagnosis and IVF can ensure that the disease is not passed on to the offspring, but IVF is expensive, and not all insurance providers cover it.
If you know your baby may carry a genetic disease or suspect it, consider private cord blood banking for that child and especially, its siblings.
Cord blood and cord tissue banking have proven clinical benefits in more than 80 FDA-approved stem cell treatments.
MiracleCord has been awarded Best U.S. Cord Blood Bank from Global Health and Pharma in 2021 and 2022 for our cutting-edge technology, service, and value. To learn more about cord blood and cord tissue banking, request MiracleCord’s Free Info Kit.
Final Thoughts
Screening or testing for birth defects is a personal choice, not a requirement. There are times when, for emotional or religious reasons, parents prefer not to know if they are at risk of having a child with a birth defect or whether their child will have one.
Conversely, there are women who would very much welcome the chance to better manage their pregnancy risks but cannot afford it or simply don’t have access.
Meanwhile, parents who opt for in vitro fertilization often opt for some genetic screening and pre-implantation genetic diagnosis to avoid costly multiple cycles and failed pregnancies.
Whatever the emotions surrounding it, prenatal genetic testing is a miracle that improves lives. Though not all birth defects can be detected or avoided, prenatal genetic screening and diagnostic tests can help parents determine whether to continue the pregnancy, prepare for a child with special needs, or perhaps avoid the defect or mitigate its severity while the fetus is still in the womb. And that’s remarkable.
what does genetic analysis show during pregnancy and where to do it in Moscow?
Every expectant mother wants to be 100% sure that her baby will be born healthy. But until recently, it was possible to verify this only with the help of risky research methods, which were prescribed only for vital indications. Now there is a safe way to detect genetic abnormalities in a fetus - a non-invasive DNA test. What does it show and how is it carried out? We understand the topic and answer questions.
During pregnancy, most genetic defects in the fetus can be diagnosed with a DNA test, and are often performed for this purpose.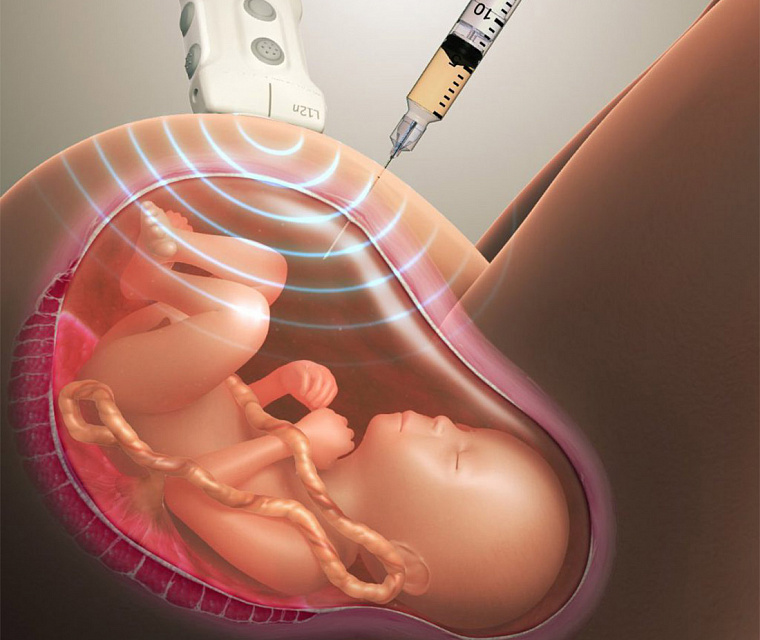
Here are the main purposes of a DNA test for pregnant women:
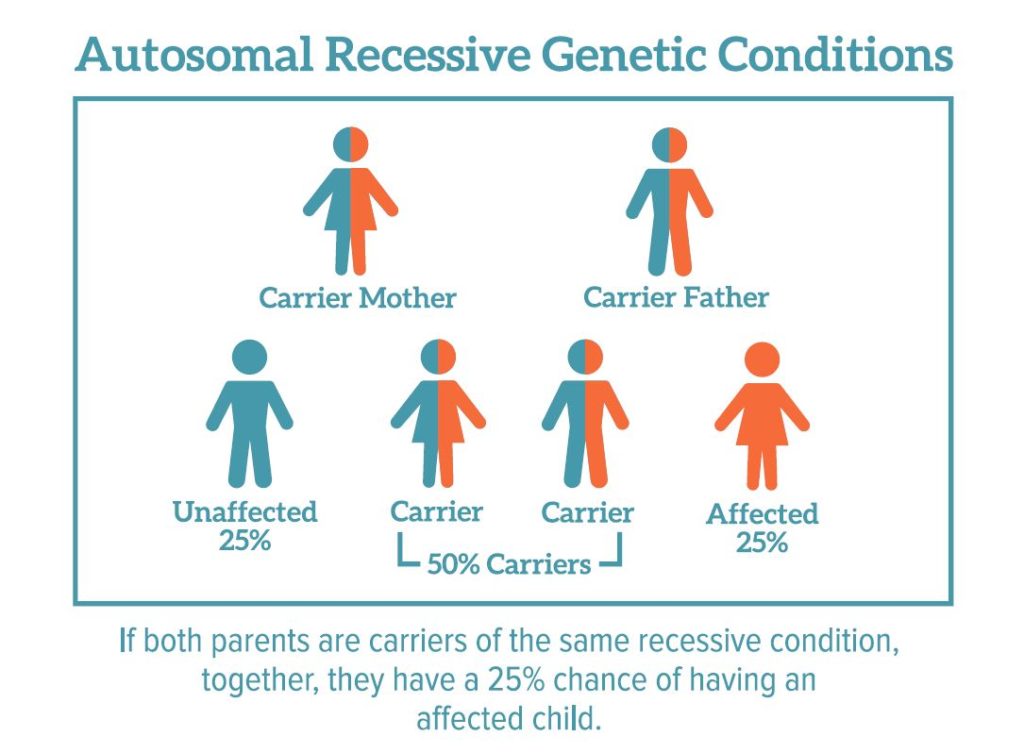
Diagnosis of chromosomal abnormalities:
- Down syndrome (additional chromosome in the twenty-first pair) occurs in about one in a thousand newborns [1] . With the increase in the age of the expectant mother, the risk of giving birth to a baby with an anomaly increases. Children with Down syndrome are often born with heart defects, epilepsy, their physical development lags behind the norm, and all have more or less severe mental retardation.
- Edwards syndrome occurs due to the presence of an extra chromosome in the eighteenth pair. This is a rare anomaly: it occurs in about one in 7000 cases [2] . Newborns with Edwards syndrome have numerous malformations: 90-95% of them die in the first months.
- Patau syndrome is trisomy of the thirteenth pair. This anomaly is even rarer, affecting approximately one in 14,000 newborns [3] . Like Edwards syndrome, this type of hereditary pathology is manifested by multiple gross malformations; life expectancy of patients rarely exceeds a year.
Important
Ultrasound screening is performed for early diagnosis of Down, Edwards and Patau syndromes. Some features of the development of the fetus allow us to suggest chromosomal abnormalities: an increased width of the collar zone, a shortened nasal bone, and others. But in the first trimester, ultrasound signs cannot be considered as reliable evidence of disorders, and later, when the screening results become more convincing, time may be lost. Meanwhile, research among 18 955 women showed that the most accurate test for the presence of chromosomal abnormalities is DNA analysis during pregnancy (in the first trimester) [4] .
- Anomalies in the number of sex chromosomes . These include Klinefelter syndrome, Shereshevsky-Turner syndrome and many other abnormalities associated with a decrease or increase in the normal number of X and Y chromosomes. Such genetic defects are often accompanied by underdevelopment of the gonads (sex glands), infertility, various changes in appearance, often mental retardation.

Diagnosis of congenital pathologies of the nervous system . For example, a DNA test early in pregnancy can detect Rett syndrome (a severe neuropsychiatric disorder that does not become apparent until several months after birth), infantile epileptic encephalopathy, and other CNS disorders.
Identification of hereditary forms of craniosynostosis (premature fusion of the bones of the skull) - Pfeiffer, Aper, Cruzon, Müncke syndromes.
Many other genetic diseases poorly diagnosed by traditional screening can be detected by DNA testing during pregnancy.
Determination of the sex of the unborn baby . Yes, you can see it on the screen of the ultrasound machine already during the second screening. But sometimes you need to get a 100% accurate result as early as possible. This is necessary primarily for the timely diagnosis of hereditary anomalies associated with sex. For example, hemophilia and Klinefelter's syndrome occur only in boys, while Turner's syndrome affects only girls. The reliability of sex determination by prenatal DNA testing at seven to nine weeks of gestation is 95%, from 12 weeks onwards it reaches 99% [5] .
The reliability of sex determination by prenatal DNA testing at seven to nine weeks of gestation is 95%, from 12 weeks onwards it reaches 99% [5] .
Establishment of paternity . The need for this study arises for various reasons. For example, it may be required if the future father refuses to admit his involvement in the conception. In controversial cases, the results of the examination can serve as evidence in court.
Indications and contraindications for testing
It is natural for any expectant mother to worry about the health of her baby, so the mere desire of a pregnant woman is enough to perform a non-invasive genetic test. This cannot be said about invasive research methods that carry a risk to the fetus and therefore are not carried out without strict indications. But there are situations when genetic analysis is simply necessary for pregnant women. Such a study is highly desirable if:
- Routine screening showed that there is a high risk of giving birth to a child with anomalies.
 According to the results of ultrasound, it is impossible to draw a conclusion about the presence of Down syndrome and other hereditary pathologies in the fetus, one can only suspect this for a number of reasons. To clarify the diagnosis, the pregnant woman is sent for DNA analysis. Previously, it was possible to confirm or refute malformations only through traumatic and risky methods - amniocentesis, cordocentesis and others. Not so long ago, a safe alternative appeared - a non-invasive prenatal genetic test.
According to the results of ultrasound, it is impossible to draw a conclusion about the presence of Down syndrome and other hereditary pathologies in the fetus, one can only suspect this for a number of reasons. To clarify the diagnosis, the pregnant woman is sent for DNA analysis. Previously, it was possible to confirm or refute malformations only through traumatic and risky methods - amniocentesis, cordocentesis and others. Not so long ago, a safe alternative appeared - a non-invasive prenatal genetic test. - Expectant mother over 35 years old. With age, the likelihood of having a child with Down syndrome increases. Other chromosomal abnormalities are less dependent on this indicator, but some association can also be traced.
- The woman has a history of miscarriages or miscarriages. A common cause of miscarriage lies precisely in the genetic defects of the fetus. If the last pregnancy ended unsuccessfully, it makes sense to make sure that everything is in order this time.
- In the family of the future mother or father, there were cases of the appearance of children with hereditary pathologies. Until the results of the study are available, a recurrence of such a situation cannot be completely ruled out.
- One of the future parents suffered from alcohol or drug addiction. Even if the problem is in the past, the risk to the child's health still exists.
- In the first weeks of pregnancy, a woman suffered from an acute infectious disease (the most dangerous of them is rubella) or experienced the effects of other teratogenic factors (which can lead to abnormalities in the development of the fetus). The latter include radiation exposure, taking certain medications (for example, tetracycline antibiotics), drinking alcohol, poisoning with salts of heavy metals.
There are cases when it is not possible to carry out genetic testing of pregnant women. The main contraindication to invasive tests is the threat of miscarriage. Also, studies are not performed for acute infections, inflammatory gynecological diseases, myoma. A non-invasive test for genetic abnormalities is not done for multiple pregnancies (triplets or more), since in this case it is difficult to determine the DNA of each fetus and the data may be inaccurate. The analysis is not performed in surrogate mothers and women who conceived by fertilization with a donor egg (with the exception of certain types of testing). It is impossible to obtain reliable test data if a blood transfusion or bone marrow transplantation was performed shortly before pregnancy.
Also, studies are not performed for acute infections, inflammatory gynecological diseases, myoma. A non-invasive test for genetic abnormalities is not done for multiple pregnancies (triplets or more), since in this case it is difficult to determine the DNA of each fetus and the data may be inaccurate. The analysis is not performed in surrogate mothers and women who conceived by fertilization with a donor egg (with the exception of certain types of testing). It is impossible to obtain reliable test data if a blood transfusion or bone marrow transplantation was performed shortly before pregnancy.
How testing is done
There are many types of genetic tests performed during pregnancy, but all of them can be divided into two groups depending on the method of sampling. From this point of view, invasive and non-invasive methods are distinguished. The former are associated with the penetration of the future mother and fetus into the body, the latter do not imply such an intervention.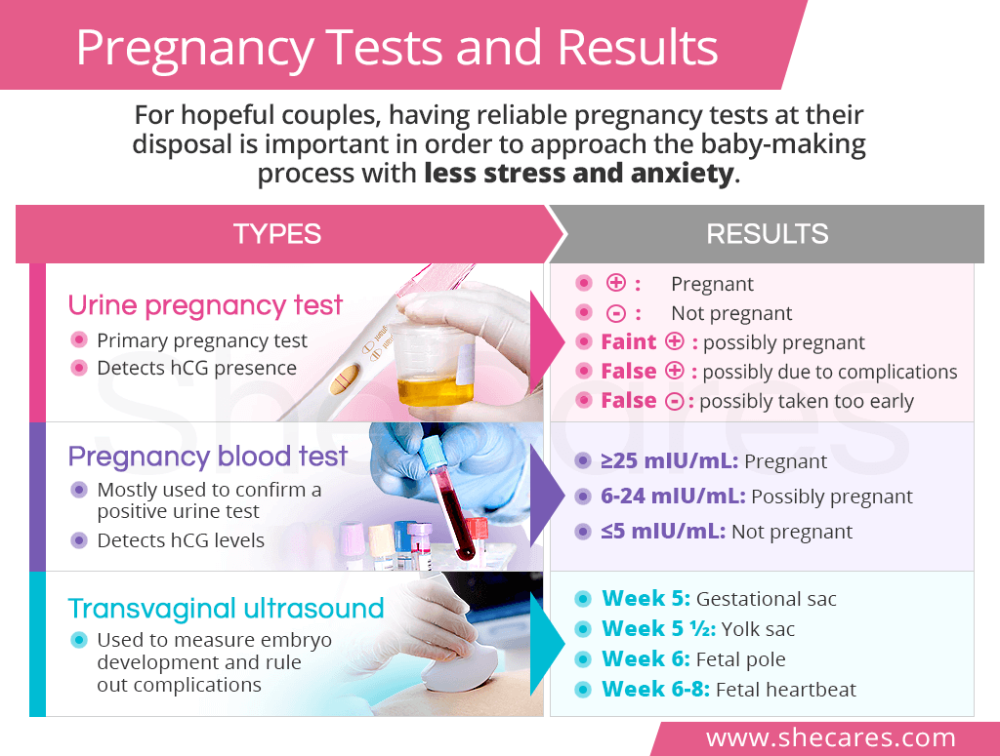
Invasive tests
All studies in this group carry a certain risk of spontaneous abortion, so they are resorted to only in extreme cases - if the likelihood of genetic abnormalities in the fetus is high enough.
Chorionic villus biopsy is the earliest invasive test and is performed at 10-14 weeks. The material for analysis is the tissue cells of the chorion - the future placenta. The fence is carried out by puncturing the abdominal wall and uterus with a long needle. The procedure is performed under ultrasound guidance. The accuracy of diagnosing genetic abnormalities by this method is 99% [6] . The same study performed at a later date is called placentocentesis.
Amniocentesis - analysis of fetal DNA contained in amniotic fluid cells. It is used in the second trimester of pregnancy. Using a syringe with a needle, the fetal bladder is punctured through the abdominal wall and a small amount of amniotic fluid (about 30 ml) is taken. The results of the study need to wait two to three weeks. The accuracy of the method also reaches 99%.
The results of the study need to wait two to three weeks. The accuracy of the method also reaches 99%.
Cordocentesis is an analysis of cord blood, which is also taken through the abdominal wall. The procedure is also performed in the II trimester. With the help of cordocentesis, genetic diseases, infections and other intrauterine pathologies can be determined.
Non-invasive genetic test
The technique is relatively new for Russia, and such testing is not yet carried out in all medical centers. This is due to the insufficient equipment of the laboratories of state institutions, and the rather high cost of the study (because it cannot be used everywhere, like screening).
In contrast to all the methods described above, non-invasive testing for fetal genetic abnormalities can be said to be safe. To take a biomaterial, you do not need to resort to a puncture and other traumatic interventions that can lead to complications. For analysis, only the blood of a pregnant woman is needed, which is taken from a vein in the usual way. No special preparation is required for the study.
For analysis, only the blood of a pregnant woman is needed, which is taken from a vein in the usual way. No special preparation is required for the study.
The test is carried out using high-tech medical equipment. The blood taken from a woman in a centrifuge is divided into erythrocyte mass, a layer of leukocytes and plasma. DNA from the last two fractions is "deciphered" by sequencing, separating the genomes of the mother and fetus. The resulting material is analyzed for the risk of chromosomal and other pathologies. The method of analysis depends on the type of testing. The entire research process takes an average of two weeks.
The informativeness of tests differs depending on their type. Almost all such analyzes are able to determine the presence or absence of Down syndrome, Edwards and Patau, sex chromosome anomalies, the sex of the unborn child. Some methods are suitable for studying the genetic risks of pregnancy after IVF (including with a donor egg). The reliability of the results of non-invasive testing reaches 99%.
In addition to safety and high accuracy, the study has another weight advantage - the ability to perform DNA analysis in early pregnancy. It is done starting at nine to ten weeks, when fetal DNA begins to be detected in the mother's blood. No screening method or invasive test is performed at such times. Early diagnosis of pathologies is crucial in maintaining pregnancy, and the confidence that the unborn child is healthy allows you to expect his birth with joy and without fear.
Thanks to the latest technology in medicine, it has become possible to determine the risk of hereditary diseases in the fetus without resorting to invasive research methods. Genetic analysis of the blood of pregnant women is accurate and safe, and besides, it is the earliest of all possible ways to detect intrauterine pathologies. This testing has two drawbacks: firstly, it is paid, and secondly, it is carried out so far only in a few clinics.
Frequently asked questions about biochemical screening of pregnant women
Which tests are performed on pregnant women?
Currently, two main types of tests are recommended for pregnant women in St.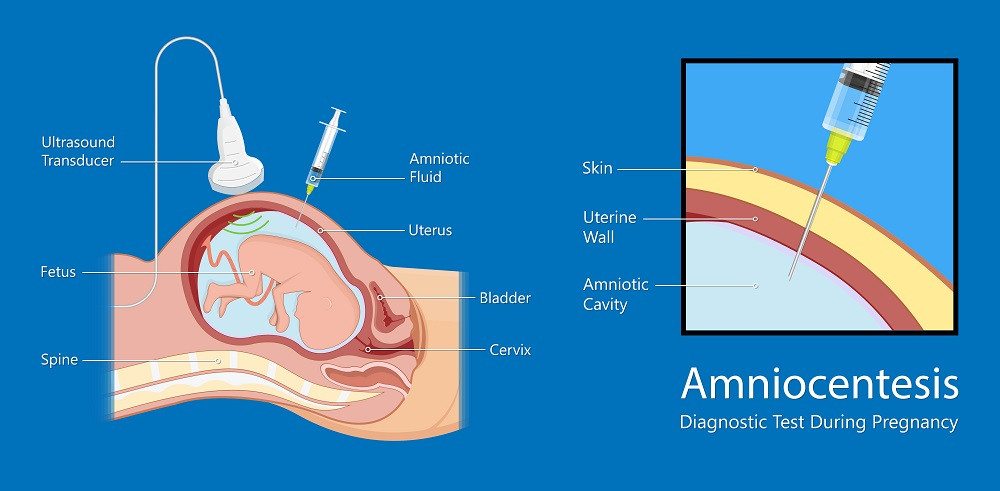 Petersburg:
Petersburg:
- analysis for PAPP-A and beta-hCG in the period of 9-13 weeks
- analysis for AFP and hCG.
Should I do a “triple test”?
Some private laboratories use the so-called "triple test", using kits that, in addition to AFP and hCG, have added the determination of the concentration of another hormone - unconjugated estriol. According to modern data, its assessment for clarifying the risks of fetal chromosomal pathology has too little weight and is highly dependent on many other factors of the woman's condition, which is not significant for calculating risks for fetal chromosomal pathology. If the results of the “double” test reveal an increased risk for you, it is better to contact a geneticist and understand the situation, taking into account a professional clinical assessment of the test results.
How important is it to accurately indicate the weight of a pregnant woman when taking blood for “fetal proteins”?
Each woman's weight must be recorded on the examination referral. In the absence of such information, the risk can be calculated "according to the average" weight of pregnant women in this period - 60 kg.
In the absence of such information, the risk can be calculated "according to the average" weight of pregnant women in this period - 60 kg.
All women are different - there are pregnant women weighing 45 and 145 kg. For a more accurate assessment of the results, an amendment is introduced in accordance with the "weight category". But absolute accuracy is not required here - individual grams will not change the calculations. An individual approach is important. Therefore, we always measure a woman's weight before taking tests.
I took a blood test for fetal proteins, as the doctor prescribed them immediately after taking them, now I'm worried, so I had a hearty breakfast in the morning. Will this affect the outcome of the study? Can I retake blood on an empty stomach?
Don't worry! Unlike most "adult" tests that are sensitive to food intake, the measurement of the amount of any proteins entering the mother's blood from the fetus is not dependent on the time of the meal. The most important thing here is to know exactly the gestational age established by ultrasound. It makes no sense to retake the analysis. And it is very important for pregnant women to eat breakfast and continue to eat more often during the day than usual.
The most important thing here is to know exactly the gestational age established by ultrasound. It makes no sense to retake the analysis. And it is very important for pregnant women to eat breakfast and continue to eat more often during the day than usual.
In clinics where an individual approach to the examination of pregnant women is possible, and of course, in our Center for Fetal Medicine, blood for screening tests can be donated throughout the working day.
Which test is better - PAPP-A and beta-hCG or AFP and hCG?
At present, the first one has the absolute advantage. It has been proven that it is more specific for assessing risks for chromosomal pathology, including Down syndrome. Its important advantage is that it is carried out in the first trimester, you can donate blood from the age of 9weeks of pregnancy (determined by the size of the fetus on ultrasound). The most optimal terms for this analysis are 9-12 weeks. A study period of up to almost 14 (13 weeks 6 days) is allowed, but the reliability of the risk assessment will be lower.
If you have completed a full first trimester test, performed an ultrasound and received a conclusion from a geneticist that the fetus is at low risk for chromosomal abnormalities, testing for AFP and hCG is not worth it.
In special cases, after the first screening, a test for AFP and hCG is prescribed as an additional test on the recommendation of a geneticist.
If you missed the first screening test, then, of course, you need to donate blood at least for the second one within 15-18 weeks.
I would like to emphasize that, on the recommendation of international experts in prenatal diagnosis, analysis for PAPP-A and beta-hCG in a period of 9-12 weeks is recommended for all pregnant women at any age.
I went through the IVF procedure and used a donor cell, since my cells do not mature at 46 years old. Now 12 weeks. How do I correctly pass a biochemical test?
You urgently need to donate blood for PAPP-A and beta-hCG and perform an ultrasound. From your age, the IVF procedure itself does not change the amount of proteins. And the risk "by proteins" will be assessed depending on certain concentrations.
From your age, the IVF procedure itself does not change the amount of proteins. And the risk "by proteins" will be assessed depending on certain concentrations.
But the computer program calculates the "combined" risks - by proteins, by ultrasound and by the age of the woman, more precisely, by the "age of the egg." Accordingly, for this analysis, the age of the egg donor must be indicated in the calculation referral. If you do not know it exactly, you can calculate by low age risk, since all donors have age restrictions for participating in the IVF program. If you have already been calculated according to your age, don't worry, we can reassess the risks taking into account real data and issue you a Medical Genetic Conclusion based on the results of prenatal studies.
Doctors of the Center for Fetal Medicine are one of the leading specialists in prenatal diagnostics, candidates of medical sciences, doctors of the highest categories with a narrow specialization and extensive experience in prenatal medicine.