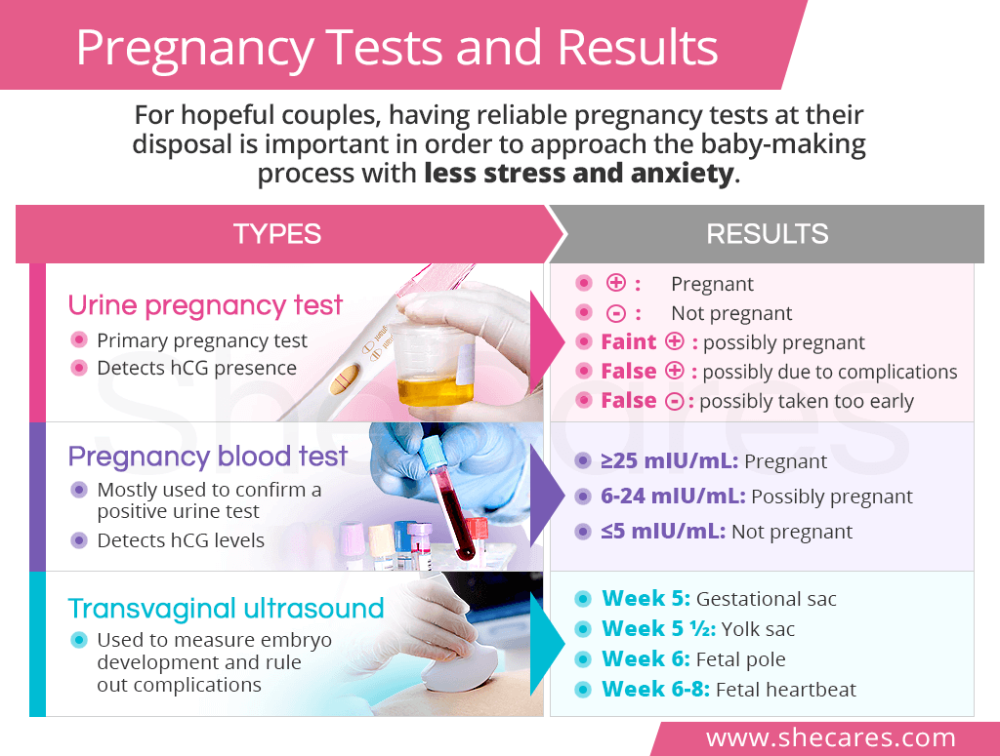
What is human chorionic gonadotropin hcg
Human Chorionic Gonadotropin - StatPearls
Danielle Betz; Kathleen Fane.
Author Information
Last Update: August 8, 2022.
Introduction
Human chorionic gonadotropin (hCG) is a chemical created by trophoblast tissue, tissue typically found in early embryos and which will eventually be part of the placenta. Measuring hCG levels can be helpful in identifying a normal pregnancy, pathologic pregnancy, and can also be useful following an aborted pregnancy. There is also a benefit in measuring hCG in a variety of cancers including choriocarcinoma and extra-uterine malignancies.
Etiology and Epidemiology
Human chorionic gonadotropin is a hormone produced primarily by syncytiotrophoblastic cells of the placenta during pregnancy. The hormone stimulates the corpus luteum to produce progesterone to maintain the pregnancy. Smaller amounts of hCG are also produced in the pituitary gland, the liver, and the colon.[1] As previously mentioned, certain malignancies can also produce either hCG or hCG-related hormone. Trophoblastic cancers (hydatidiform mole, choriocarcinoma, and germ cell tumors) are associated with high serum levels of hCG-related molecules.
The hormone itself is a glycoprotein composed of two subunits, the alpha and beta subunits.[1] There are multiple forms found in the serum and urine during pregnancy including the intact hormone and each of the free subunits. HCG is primarily catabolized by the liver, although about 20% is excreted in the urine. The beta subunit is degraded in the kidney to make a core fragment which is measured by urine hCG tests.
Specimen Requirements and Procedure
Urine Testing
Urine should not be collected after the patient has been drinking a large amount of fluid, as a dilute specimen may result in a falsely negative test.[2]
Blood in the urine may cause a false positive test result.
Serum Testing
Diagnostic Tests
Serum tests for hCG are immunometric assays. This means that they use 2two antibodies that bind to the hCG molecule, a fixed antibody and a radiolabeled antibody which adhere to different sites on the molecule, sandwiching and immobilize the molecule to make it detectable.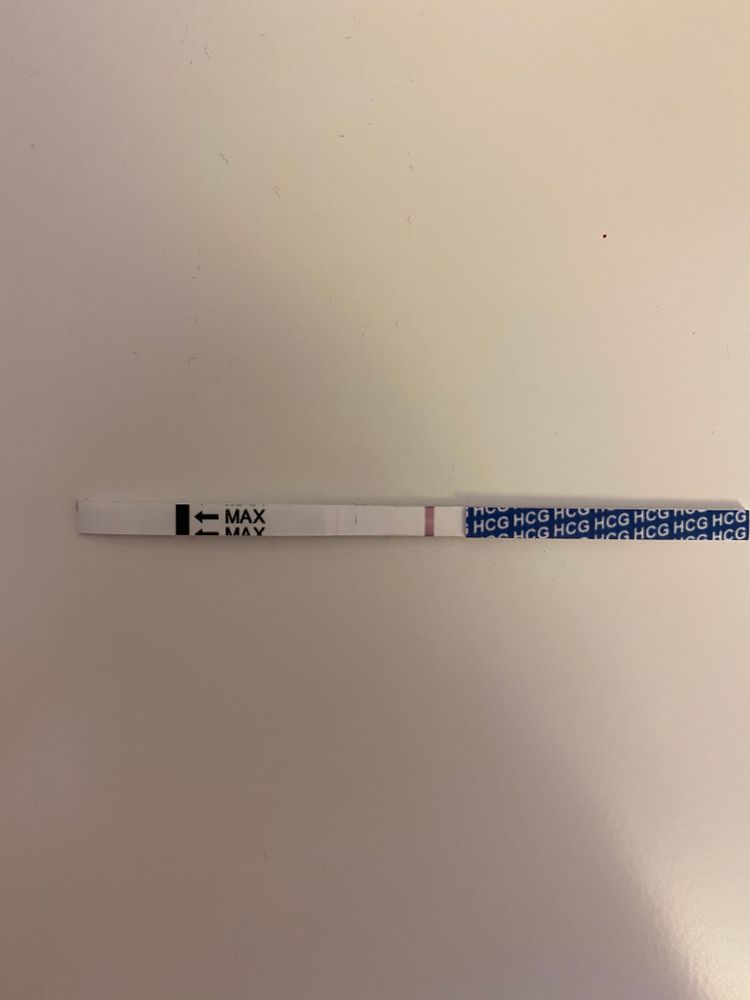 [3] Assays involve washing away the excess serum components and measuring the amount of remaining labeled hCG to give a quantitative result. There are more than 100 different assays commercially available which results in significant variability in reported values.
[3] Assays involve washing away the excess serum components and measuring the amount of remaining labeled hCG to give a quantitative result. There are more than 100 different assays commercially available which results in significant variability in reported values.
Urine assays are similar, although many detect total hCG levels greater than 20 mIU/mL.[4] Many over-the-counter urine pregnancy tests do not detect hyperglycosylated hCG, which accounts for most of the hCG in early pregnancy, resulting in a wide range of sensitivities of these tests.
Serum testing is much more sensitive and specific than urine testing. Urine testing, however, is more convenient, affordable, comfortable for patients, has a fast turnaround (5 to 10 minutes), and does not require a medical prescription.
Testing Procedures
Urine Testing
Urine is placed in or on a designated receptacle (most commercially available and medical point of care tests)
An indicator (typically a colored line or symbol), along with a control, will appear if the test is positive
An isolated control line/symbol will be evident if the test is negative
Serum Testing
Serum hCG testing is performed in a laboratory equipped with the proper machinery and uses a peripheral blood sample
If a hook effect/gestational trophoblastic disease is suspected, the lab should perform a dilution prior to testing
Interfering Factors
There are multiple reasons why an hCG test (serum or urine) may have a false report. While uncommon, false positive hCG tests can result in unnecessary medical care and/or irreversible surgical procedures. False negatives may be equally concerning and result in a delay in care or diagnostic evaluation. Potential causes of false results are listed and briefly discussed.
While uncommon, false positive hCG tests can result in unnecessary medical care and/or irreversible surgical procedures. False negatives may be equally concerning and result in a delay in care or diagnostic evaluation. Potential causes of false results are listed and briefly discussed.
Serum False Positives (1/1000 to 1/10,000) [5]
Ectopic production of hCG (hydatidiform mole, choriocarcinoma, and germ cell tumors,[6], in addition to multiple myeloma, stomach, liver, lung, bladder, pancreatic, breast, colon, cervical, and endometrial cancers)[7][8][9][10][11]
Heterophile antibodies (autoantibodies and antibodies formed after exposure to animal products that interact with the assay antibodies)[12][13]
Rheumatoid factors (can bind the antibodies in the assay as well)
IgA deficiency[14]
Chronic renal failure or ESRD on hemodialysis (rare)[15]
Red blood cell or plasma transfusion of blood with hCG in it have been reported
Exogenous hCG preparations for weight loss, assisted reproduction, doping[16]
Serum False Negatives
Early measurement after conception
"Hook effect" can occur when hCG levels are about 500,000 mIU/mL.
 [17] This is because there are so many hCG molecules that they saturate both the tracer and the antibodies separately, which doesn't allow for the sandwiching of the tracer-hCG-antibody required for the measurement. This means that all of the complexes are washed away, giving a false-negative result. If gestational trophoblastic disease is suspected, the lab should perform a dilution prior to testing.
[17] This is because there are so many hCG molecules that they saturate both the tracer and the antibodies separately, which doesn't allow for the sandwiching of the tracer-hCG-antibody required for the measurement. This means that all of the complexes are washed away, giving a false-negative result. If gestational trophoblastic disease is suspected, the lab should perform a dilution prior to testing.
Urine False Positives
Blood or protein in the urine
Human error in result interpretation
Ectopic production of hCG
Exogenous hCG
Drugs (aspirin, carbamazepine, methadone, high urinary pH and seminal fluid)[18]
Urine False Negatives
Early measurement after conception
Dilute urine specimen[2]
"Hook effect" as discussed above
Results, Reporting, Critical Findings
HCG levels are reported in milli-international units of hCG hormone per milliliter of blood, or mIU/mL.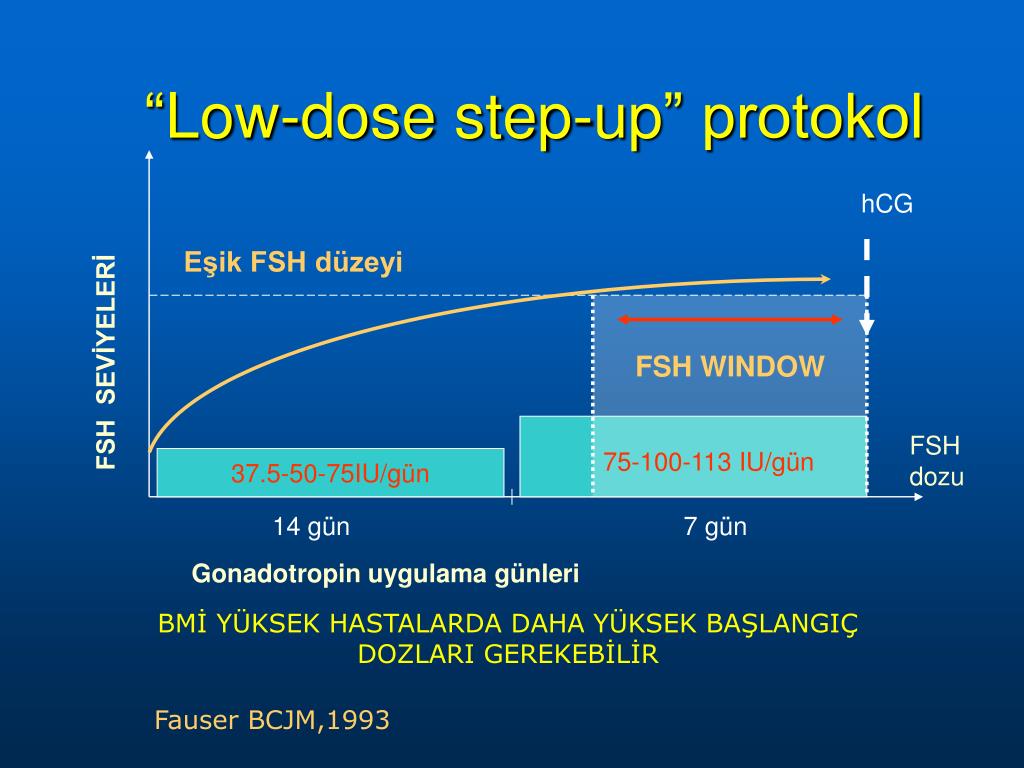 International unit per liter (IU/L) may also be used.
International unit per liter (IU/L) may also be used.
Urine hCG testing is qualitative, reporting a positive or negative result. The assays detect hCG levels typically starting at 20 to 50 (reportedly as low as 6.3 to 12.5)[19] mIU/mL, corresponding to levels at approximately 4 weeks post-conception.
Serum assays can measure beta-hCG as low as 1 to 2 mIU/mL.
Clinical Significance
Pregnancy
HCG is an important hormone in pregnancy, and its clinical utility is primarily centered around its detection in early pregnancy, along with serial measurement during pregnancy and pregnancy-related complications.
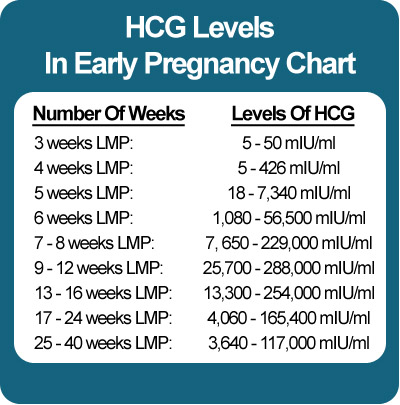
Levels of hCG can vary widely between women with normal pregnancies. Typically, serum and urine concentrations of hCG rise exponentially in the first trimester of pregnancy, doubling about every 24 hours during the first 8 weeks. The peak is usually around 10 weeks of gestation and then levels decrease until about the 16th week of gestation where they remain fairly constant until term. [3]
[3]
Patients who have hCG levels that plateau prior to 8 weeks or that fail to double commonly have a nonviable pregnancy, whether intra-uterine or extra-uterine. Extra-uterine (ectopic) pregnancies usually have a rate-of-rise that is low without the typical doubling. However, given the large range of normal hCG levels and inconsistent rates-of-rise of this hormone, checking serum levels is typically paired with ultrasound evaluation to improve sensitivity and specificity.[20]
Return of hCG to zero following delivery or termination of pregnancy ranges from 7 to 60 days.[21] Trending the fall of hCG levels can be important in termination of molar pregnancies and also following the termination of normal or ectopic pregnancies to be assured that the therapy has been successful.
It notable that there are many different combinations of antibodies used in commercial assays. This results in heterogeneous results with as much as a 50-fold difference in immunoassay results.[3] This is clinically relevant, particularly when comparing results from different laboratories in different facilities/hospitals when examining low values following pregnancy termination or trophoblastic disease.
Gestational Trophoblastic Disease
Detection of hCG is also useful in the evaluation of trophoblastic disease, including complete and partial hydatidiform mole, postmolar tumor, gestational choriocarcinoma, testicular choriocarcinoma, and placental site trophoblastic disease. All of these entities produce hCG, varying levels of which are reported on commercial assays. A total hCG level of greater than 100,000 mIU/mL in early pregnancy, for example, is highly suggestive of a complete hydatidiform mole,[22] although many normal pregnancies may reach this level at their peak around weeks 8 to 11 of gestation. Precise hCG measurements are important to assess the tumor mass, the successful treatment of malignancy, and to test for recurrence or persistence of disease.[6]
Non-Pregnant Patients
HCG in the serum increases with age in nonpregnant women. A cut off of 14 mIU/mL has been suggested for use in interpreting results in women over the age of 55. In all nonpregnant patients, testicular cancer, ovarian cancer, bladder cancer, or other malignancy should be evaluated as a source of persistently positive hCG testing.
Enhancing Healthcare Team Outcomes
Knowing the utility and variability of different hCG assays is clinically relevant to a wide range of medical providers. False positive and false negative testing has a large impact on patient care. All providers in a patient care team should be aware of common limitations in testing, for example, urine assay false positives with hematuria, false negatives with dilute urine, along with more obscure but still very relevant causes of inaccurate testing. Interpreting results that may be false should be undergone with care to help prevent unnecessary testing and treatment.[23] (Level V) Collaboration, shared decision making, and communication are critical elements in good patient care.
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
References
- 1.
Montagnana M, Trenti T, Aloe R, Cervellin G, Lippi G. Human chorionic gonadotropin in pregnancy diagnostics.
 Clin Chim Acta. 2011 Aug 17;412(17-18):1515-20. [PubMed: 21635878]
Clin Chim Acta. 2011 Aug 17;412(17-18):1515-20. [PubMed: 21635878]- 2.
Ong S, Beebeejaun H. The effect of physiological urine dilution on pregnancy test results in complicated early pregnancies. Br J Obstet Gynaecol. 1999 Jan;106(1):87-8. [PubMed: 10426268]
- 3.
Cole LA. Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem. 1997 Dec;43(12):2233-43. [PubMed: 9439438]
- 4.
Greene DN, Schmidt RL, Kamer SM, Grenache DG, Hoke C, Lorey TS. Limitations in qualitative point of care hCG tests for detecting early pregnancy. Clin Chim Acta. 2013 Jan 16;415:317-21. [PubMed: 23159297]
- 5.
Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002 Jul;187(1):217-24. [PubMed: 12114913]
- 6.
Cole LA, Shahabi S, Butler SA, Mitchell H, Newlands ES, Behrman HR, Verrill HL. Utility of commonly used commercial human chorionic gonadotropin immunoassays in the diagnosis and management of trophoblastic diseases.
 Clin Chem. 2001 Feb;47(2):308-15. [PubMed: 11159780]
Clin Chem. 2001 Feb;47(2):308-15. [PubMed: 11159780]- 7.
Marcillac I, Troalen F, Bidart JM, Ghillani P, Ribrag V, Escudier B, Malassagne B, Droz JP, Lhommé C, Rougier P. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992 Jul 15;52(14):3901-7. [PubMed: 1377600]
- 8.
Alfthan H, Haglund C, Roberts P, Stenman UH. Elevation of free beta subunit of human choriogonadotropin and core beta fragment of human choriogonadotropin in the serum and urine of patients with malignant pancreatic and biliary disease. Cancer Res. 1992 Sep 01;52(17):4628-33. [PubMed: 1324787]
- 9.
Sheaff MT, Martin JE, Badenoch DF, Baithun SI. beta hCG as a prognostic marker in adenocarcinoma of the prostate. J Clin Pathol. 1996 Apr;49(4):329-32. [PMC free article: PMC500461] [PubMed: 8655711]
- 10.
Lundin M, Nordling S, Carpelan-Holmstrom M, Louhimo J, Alfthan H, Stenman UH, Haglund C. A comparison of serum and tissue hCG beta as prognostic markers in colorectal cancer.
 Anticancer Res. 2000 Nov-Dec;20(6D):4949-51. [PubMed: 11326644]
Anticancer Res. 2000 Nov-Dec;20(6D):4949-51. [PubMed: 11326644]- 11.
Reisenbichler ES, Krontiras H, Hameed O. Beta-human chorionic gonadotropin production associated with phyllodes tumor of the breast: an unusual paraneoplastic phenomenon. Breast J. 2009 Sep-Oct;15(5):527-30. [PubMed: 19624411]
- 12.
Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999 Jul;45(7):942-56. [PubMed: 10388468]
- 13.
Check JH, Nowroozi K, Chase JS, Lauer C, Elkins B, Wu CH. False-positive human chorionic gonadotropin levels caused by a heterophile antibody with the immunoradiometric assay. Am J Obstet Gynecol. 1988 Jan;158(1):99-100. [PubMed: 2447778]
- 14.
Knight AK, Bingemann T, Cole L, Cunningham-Rundles C. Frequent false positive beta human chorionic gonadotropin tests in immunoglobulin A deficiency. Clin Exp Immunol. 2005 Aug;141(2):333-7. [PMC free article: PMC1809437] [PubMed: 15996198]
- 15.

Fahy BG, Gouzd VA, Atallah JN. Pregnancy tests with end-stage renal disease. J Clin Anesth. 2008 Dec;20(8):609-13. [PubMed: 19100935]
- 16.
Delbeke FT, Van Eenoo P, De Backer P. Detection of human chorionic gonadotrophin misuse in sports. Int J Sports Med. 1998 May;19(4):287-90. [PubMed: 9657371]
- 17.
Griffey RT, Trent CJ, Bavolek RA, Keeperman JB, Sampson C, Poirier RF. "Hook-like effect" causes false-negative point-of-care urine pregnancy testing in emergency patients. J Emerg Med. 2013 Jan;44(1):155-60. [PubMed: 21835572]
- 18.
Chard T. Pregnancy tests: a review. Hum Reprod. 1992 May;7(5):701-10. [PubMed: 1639991]
- 19.
Cervinski MA, Lockwood CM, Ferguson AM, Odem RR, Stenman UH, Alfthan H, Grenache DG, Gronowski AM. Qualitative point-of-care and over-the-counter urine hCG devices differentially detect the hCG variants of early pregnancy. Clin Chim Acta. 2009 Aug;406(1-2):81-5. [PubMed: 19477170]
- 20.
Davies S, Byrn F, Cole LA. Human chorionic gonadotropin testing for early pregnancy viability and complications. Clin Lab Med. 2003 Jun;23(2):257-64, vii. [PubMed: 12848444]
- 21.
Butts SF, Guo W, Cary MS, Chung K, Takacs P, Sammel MD, Barnhart KT. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013 Aug;122(2 Pt 1):337-343. [PMC free article: PMC3752097] [PubMed: 23969803]
- 22.
Menczer J, Modan M, Serr DM. Prospective follow-up of patients with hydatidiform mole. Obstet Gynecol. 1980 Mar;55(3):346-9. [PubMed: 7360433]
- 23.
Cole LA. Phantom hCG and phantom choriocarcinoma. Gynecol Oncol. 1998 Nov;71(2):325-9. [PubMed: 9826481]
Human Chorionic Gonadotropin - StatPearls
Danielle Betz; Kathleen Fane.
Author Information
Last Update: August 8, 2022.
Introduction
Human chorionic gonadotropin (hCG) is a chemical created by trophoblast tissue, tissue typically found in early embryos and which will eventually be part of the placenta. Measuring hCG levels can be helpful in identifying a normal pregnancy, pathologic pregnancy, and can also be useful following an aborted pregnancy. There is also a benefit in measuring hCG in a variety of cancers including choriocarcinoma and extra-uterine malignancies.
Measuring hCG levels can be helpful in identifying a normal pregnancy, pathologic pregnancy, and can also be useful following an aborted pregnancy. There is also a benefit in measuring hCG in a variety of cancers including choriocarcinoma and extra-uterine malignancies.
Etiology and Epidemiology
Human chorionic gonadotropin is a hormone produced primarily by syncytiotrophoblastic cells of the placenta during pregnancy. The hormone stimulates the corpus luteum to produce progesterone to maintain the pregnancy. Smaller amounts of hCG are also produced in the pituitary gland, the liver, and the colon.[1] As previously mentioned, certain malignancies can also produce either hCG or hCG-related hormone. Trophoblastic cancers (hydatidiform mole, choriocarcinoma, and germ cell tumors) are associated with high serum levels of hCG-related molecules.
The hormone itself is a glycoprotein composed of two subunits, the alpha and beta subunits.[1] There are multiple forms found in the serum and urine during pregnancy including the intact hormone and each of the free subunits. HCG is primarily catabolized by the liver, although about 20% is excreted in the urine. The beta subunit is degraded in the kidney to make a core fragment which is measured by urine hCG tests.
HCG is primarily catabolized by the liver, although about 20% is excreted in the urine. The beta subunit is degraded in the kidney to make a core fragment which is measured by urine hCG tests.
Specimen Requirements and Procedure
Urine Testing
Urine should not be collected after the patient has been drinking a large amount of fluid, as a dilute specimen may result in a falsely negative test.[2]
Blood in the urine may cause a false positive test result.
Serum Testing
Diagnostic Tests
Serum tests for hCG are immunometric assays. This means that they use 2two antibodies that bind to the hCG molecule, a fixed antibody and a radiolabeled antibody which adhere to different sites on the molecule, sandwiching and immobilize the molecule to make it detectable.[3] Assays involve washing away the excess serum components and measuring the amount of remaining labeled hCG to give a quantitative result. There are more than 100 different assays commercially available which results in significant variability in reported values.
Urine assays are similar, although many detect total hCG levels greater than 20 mIU/mL.[4] Many over-the-counter urine pregnancy tests do not detect hyperglycosylated hCG, which accounts for most of the hCG in early pregnancy, resulting in a wide range of sensitivities of these tests.
Serum testing is much more sensitive and specific than urine testing. Urine testing, however, is more convenient, affordable, comfortable for patients, has a fast turnaround (5 to 10 minutes), and does not require a medical prescription.
Testing Procedures
Urine Testing
Urine is placed in or on a designated receptacle (most commercially available and medical point of care tests)
An indicator (typically a colored line or symbol), along with a control, will appear if the test is positive
An isolated control line/symbol will be evident if the test is negative
Serum Testing
Serum hCG testing is performed in a laboratory equipped with the proper machinery and uses a peripheral blood sample
If a hook effect/gestational trophoblastic disease is suspected, the lab should perform a dilution prior to testing
Interfering Factors
There are multiple reasons why an hCG test (serum or urine) may have a false report.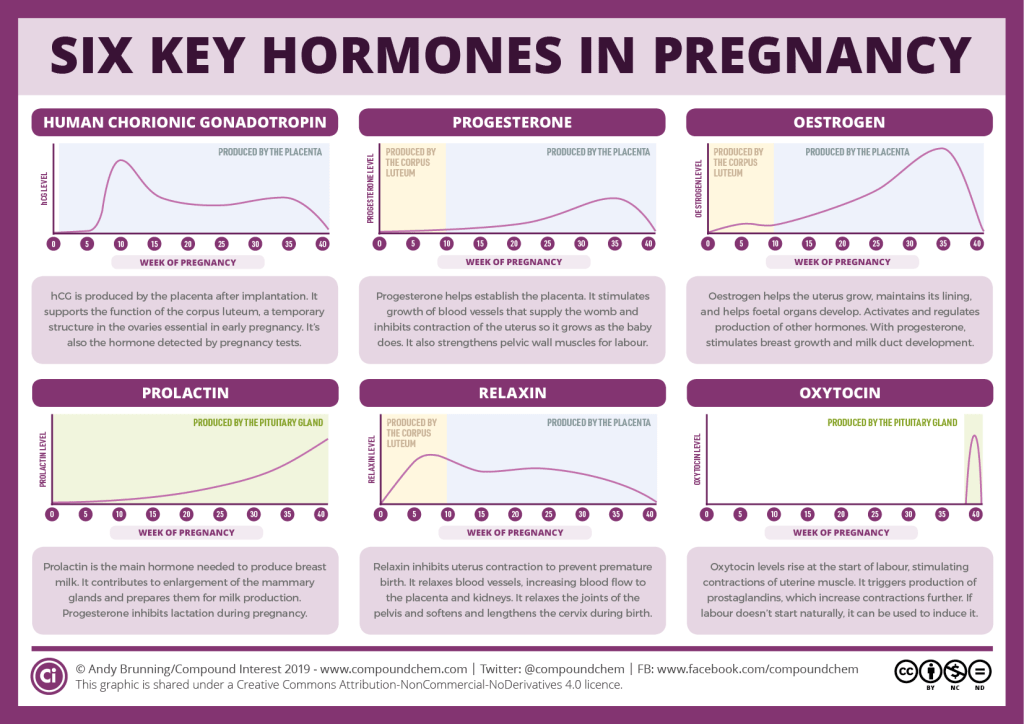 While uncommon, false positive hCG tests can result in unnecessary medical care and/or irreversible surgical procedures. False negatives may be equally concerning and result in a delay in care or diagnostic evaluation. Potential causes of false results are listed and briefly discussed.
While uncommon, false positive hCG tests can result in unnecessary medical care and/or irreversible surgical procedures. False negatives may be equally concerning and result in a delay in care or diagnostic evaluation. Potential causes of false results are listed and briefly discussed.
Serum False Positives (1/1000 to 1/10,000) [5]
Ectopic production of hCG (hydatidiform mole, choriocarcinoma, and germ cell tumors,[6], in addition to multiple myeloma, stomach, liver, lung, bladder, pancreatic, breast, colon, cervical, and endometrial cancers)[7][8][9][10][11]
Heterophile antibodies (autoantibodies and antibodies formed after exposure to animal products that interact with the assay antibodies)[12][13]
Rheumatoid factors (can bind the antibodies in the assay as well)
IgA deficiency[14]
Chronic renal failure or ESRD on hemodialysis (rare)[15]
Red blood cell or plasma transfusion of blood with hCG in it have been reported
Exogenous hCG preparations for weight loss, assisted reproduction, doping[16]
Serum False Negatives
Early measurement after conception
"Hook effect" can occur when hCG levels are about 500,000 mIU/mL.
 [17] This is because there are so many hCG molecules that they saturate both the tracer and the antibodies separately, which doesn't allow for the sandwiching of the tracer-hCG-antibody required for the measurement. This means that all of the complexes are washed away, giving a false-negative result. If gestational trophoblastic disease is suspected, the lab should perform a dilution prior to testing.
[17] This is because there are so many hCG molecules that they saturate both the tracer and the antibodies separately, which doesn't allow for the sandwiching of the tracer-hCG-antibody required for the measurement. This means that all of the complexes are washed away, giving a false-negative result. If gestational trophoblastic disease is suspected, the lab should perform a dilution prior to testing.
Urine False Positives
Blood or protein in the urine
Human error in result interpretation
Ectopic production of hCG
Exogenous hCG
Drugs (aspirin, carbamazepine, methadone, high urinary pH and seminal fluid)[18]
Urine False Negatives
Early measurement after conception
Dilute urine specimen[2]
"Hook effect" as discussed above
Results, Reporting, Critical Findings
HCG levels are reported in milli-international units of hCG hormone per milliliter of blood, or mIU/mL. International unit per liter (IU/L) may also be used.
International unit per liter (IU/L) may also be used.
Urine hCG testing is qualitative, reporting a positive or negative result. The assays detect hCG levels typically starting at 20 to 50 (reportedly as low as 6.3 to 12.5)[19] mIU/mL, corresponding to levels at approximately 4 weeks post-conception.
Serum assays can measure beta-hCG as low as 1 to 2 mIU/mL.
Clinical Significance
Pregnancy
HCG is an important hormone in pregnancy, and its clinical utility is primarily centered around its detection in early pregnancy, along with serial measurement during pregnancy and pregnancy-related complications.
Levels of hCG can vary widely between women with normal pregnancies. Typically, serum and urine concentrations of hCG rise exponentially in the first trimester of pregnancy, doubling about every 24 hours during the first 8 weeks. The peak is usually around 10 weeks of gestation and then levels decrease until about the 16th week of gestation where they remain fairly constant until term. [3]
[3]
Patients who have hCG levels that plateau prior to 8 weeks or that fail to double commonly have a nonviable pregnancy, whether intra-uterine or extra-uterine. Extra-uterine (ectopic) pregnancies usually have a rate-of-rise that is low without the typical doubling. However, given the large range of normal hCG levels and inconsistent rates-of-rise of this hormone, checking serum levels is typically paired with ultrasound evaluation to improve sensitivity and specificity.[20]
Return of hCG to zero following delivery or termination of pregnancy ranges from 7 to 60 days.[21] Trending the fall of hCG levels can be important in termination of molar pregnancies and also following the termination of normal or ectopic pregnancies to be assured that the therapy has been successful.
It notable that there are many different combinations of antibodies used in commercial assays. This results in heterogeneous results with as much as a 50-fold difference in immunoassay results.[3] This is clinically relevant, particularly when comparing results from different laboratories in different facilities/hospitals when examining low values following pregnancy termination or trophoblastic disease.
Gestational Trophoblastic Disease
Detection of hCG is also useful in the evaluation of trophoblastic disease, including complete and partial hydatidiform mole, postmolar tumor, gestational choriocarcinoma, testicular choriocarcinoma, and placental site trophoblastic disease. All of these entities produce hCG, varying levels of which are reported on commercial assays. A total hCG level of greater than 100,000 mIU/mL in early pregnancy, for example, is highly suggestive of a complete hydatidiform mole,[22] although many normal pregnancies may reach this level at their peak around weeks 8 to 11 of gestation. Precise hCG measurements are important to assess the tumor mass, the successful treatment of malignancy, and to test for recurrence or persistence of disease.[6]
Non-Pregnant Patients
HCG in the serum increases with age in nonpregnant women. A cut off of 14 mIU/mL has been suggested for use in interpreting results in women over the age of 55. In all nonpregnant patients, testicular cancer, ovarian cancer, bladder cancer, or other malignancy should be evaluated as a source of persistently positive hCG testing.
Enhancing Healthcare Team Outcomes
Knowing the utility and variability of different hCG assays is clinically relevant to a wide range of medical providers. False positive and false negative testing has a large impact on patient care. All providers in a patient care team should be aware of common limitations in testing, for example, urine assay false positives with hematuria, false negatives with dilute urine, along with more obscure but still very relevant causes of inaccurate testing. Interpreting results that may be false should be undergone with care to help prevent unnecessary testing and treatment.[23] (Level V) Collaboration, shared decision making, and communication are critical elements in good patient care.
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
References
- 1.
Montagnana M, Trenti T, Aloe R, Cervellin G, Lippi G. Human chorionic gonadotropin in pregnancy diagnostics.
 Clin Chim Acta. 2011 Aug 17;412(17-18):1515-20. [PubMed: 21635878]
Clin Chim Acta. 2011 Aug 17;412(17-18):1515-20. [PubMed: 21635878]- 2.
Ong S, Beebeejaun H. The effect of physiological urine dilution on pregnancy test results in complicated early pregnancies. Br J Obstet Gynaecol. 1999 Jan;106(1):87-8. [PubMed: 10426268]
- 3.
Cole LA. Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem. 1997 Dec;43(12):2233-43. [PubMed: 9439438]
- 4.
Greene DN, Schmidt RL, Kamer SM, Grenache DG, Hoke C, Lorey TS. Limitations in qualitative point of care hCG tests for detecting early pregnancy. Clin Chim Acta. 2013 Jan 16;415:317-21. [PubMed: 23159297]
- 5.
Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol. 2002 Jul;187(1):217-24. [PubMed: 12114913]
- 6.
Cole LA, Shahabi S, Butler SA, Mitchell H, Newlands ES, Behrman HR, Verrill HL. Utility of commonly used commercial human chorionic gonadotropin immunoassays in the diagnosis and management of trophoblastic diseases.
 Clin Chem. 2001 Feb;47(2):308-15. [PubMed: 11159780]
Clin Chem. 2001 Feb;47(2):308-15. [PubMed: 11159780]- 7.
Marcillac I, Troalen F, Bidart JM, Ghillani P, Ribrag V, Escudier B, Malassagne B, Droz JP, Lhommé C, Rougier P. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res. 1992 Jul 15;52(14):3901-7. [PubMed: 1377600]
- 8.
Alfthan H, Haglund C, Roberts P, Stenman UH. Elevation of free beta subunit of human choriogonadotropin and core beta fragment of human choriogonadotropin in the serum and urine of patients with malignant pancreatic and biliary disease. Cancer Res. 1992 Sep 01;52(17):4628-33. [PubMed: 1324787]
- 9.
Sheaff MT, Martin JE, Badenoch DF, Baithun SI. beta hCG as a prognostic marker in adenocarcinoma of the prostate. J Clin Pathol. 1996 Apr;49(4):329-32. [PMC free article: PMC500461] [PubMed: 8655711]
- 10.
Lundin M, Nordling S, Carpelan-Holmstrom M, Louhimo J, Alfthan H, Stenman UH, Haglund C. A comparison of serum and tissue hCG beta as prognostic markers in colorectal cancer.
 Anticancer Res. 2000 Nov-Dec;20(6D):4949-51. [PubMed: 11326644]
Anticancer Res. 2000 Nov-Dec;20(6D):4949-51. [PubMed: 11326644]- 11.
Reisenbichler ES, Krontiras H, Hameed O. Beta-human chorionic gonadotropin production associated with phyllodes tumor of the breast: an unusual paraneoplastic phenomenon. Breast J. 2009 Sep-Oct;15(5):527-30. [PubMed: 19624411]
- 12.
Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999 Jul;45(7):942-56. [PubMed: 10388468]
- 13.
Check JH, Nowroozi K, Chase JS, Lauer C, Elkins B, Wu CH. False-positive human chorionic gonadotropin levels caused by a heterophile antibody with the immunoradiometric assay. Am J Obstet Gynecol. 1988 Jan;158(1):99-100. [PubMed: 2447778]
- 14.
Knight AK, Bingemann T, Cole L, Cunningham-Rundles C. Frequent false positive beta human chorionic gonadotropin tests in immunoglobulin A deficiency. Clin Exp Immunol. 2005 Aug;141(2):333-7. [PMC free article: PMC1809437] [PubMed: 15996198]
- 15.
Fahy BG, Gouzd VA, Atallah JN. Pregnancy tests with end-stage renal disease. J Clin Anesth. 2008 Dec;20(8):609-13. [PubMed: 19100935]
- 16.
Delbeke FT, Van Eenoo P, De Backer P. Detection of human chorionic gonadotrophin misuse in sports. Int J Sports Med. 1998 May;19(4):287-90. [PubMed: 9657371]
- 17.
Griffey RT, Trent CJ, Bavolek RA, Keeperman JB, Sampson C, Poirier RF. "Hook-like effect" causes false-negative point-of-care urine pregnancy testing in emergency patients. J Emerg Med. 2013 Jan;44(1):155-60. [PubMed: 21835572]
- 18.
Chard T. Pregnancy tests: a review. Hum Reprod. 1992 May;7(5):701-10. [PubMed: 1639991]
- 19.
Cervinski MA, Lockwood CM, Ferguson AM, Odem RR, Stenman UH, Alfthan H, Grenache DG, Gronowski AM. Qualitative point-of-care and over-the-counter urine hCG devices differentially detect the hCG variants of early pregnancy. Clin Chim Acta. 2009 Aug;406(1-2):81-5. [PubMed: 19477170]
- 20.
Davies S, Byrn F, Cole LA. Human chorionic gonadotropin testing for early pregnancy viability and complications. Clin Lab Med. 2003 Jun;23(2):257-64, vii. [PubMed: 12848444]
- 21.
Butts SF, Guo W, Cary MS, Chung K, Takacs P, Sammel MD, Barnhart KT. Predicting the decline in human chorionic gonadotropin in a resolving pregnancy of unknown location. Obstet Gynecol. 2013 Aug;122(2 Pt 1):337-343. [PMC free article: PMC3752097] [PubMed: 23969803]
- 22.
Menczer J, Modan M, Serr DM. Prospective follow-up of patients with hydatidiform mole. Obstet Gynecol. 1980 Mar;55(3):346-9. [PubMed: 7360433]
- 23.
Cole LA. Phantom hCG and phantom choriocarcinoma. Gynecol Oncol. 1998 Nov;71(2):325-9. [PubMed: 9826481]
Human chorionic gonadotropin (hCG, beta-hCG, b-hCG, Human Chorionic)
Test material Blood serum
Method of determination Linked immunosorbent assay.
Specific pregnancy hormone.
Glycoprotein is a dimer with a molecular weight of about 46 kDa, synthesized in the placental syncytiotrophoblast. HCG is made up of two subunits: alpha and beta. The alpha subunit is identical to the alpha subunits of the pituitary hormones TSH, FSH, and LH. The beta subunit (β-hCG) used for the immunometric determination of the hormone is unique.
The level of beta-hCG in the blood already on the 6th - 8th day after conception makes it possible to diagnose pregnancy (the concentration of β-hCG in the urine reaches the diagnostic level 1-2 days later than in the blood serum).
In the first trimester of pregnancy, hCG provides the synthesis of progesterone and estrogen necessary to maintain pregnancy by the corpus luteum of the ovary. HCG acts on the corpus luteum like a luteinizing hormone, that is, it supports its existence. This happens until the "fetus-placenta" complex acquires the ability to independently form the necessary hormonal background.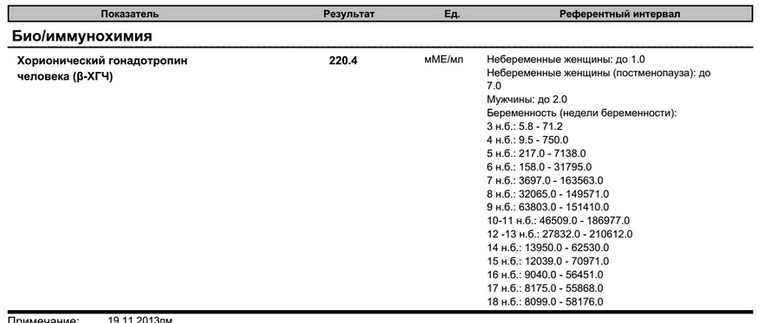 In the male fetus, hCG stimulates the Leydig cells that synthesize testosterone, which is necessary for the formation of male reproductive organs.
In the male fetus, hCG stimulates the Leydig cells that synthesize testosterone, which is necessary for the formation of male reproductive organs.
HCG is synthesized by trophoblast cells after embryo implantation and continues throughout pregnancy. In the normal course of pregnancy, between 2 - 5 weeks of pregnancy, the content of β-hCG doubles every 1.5 days. The peak concentration of hCG falls on the 10th - 11th week of pregnancy, then its concentration begins to slowly decrease. With multiple pregnancies, the content of hCG increases in proportion to the number of fetuses.
Low levels of hCG may indicate an ectopic pregnancy or threatened abortion. Determining the content of hCG in combination with other tests (alpha-fetoprotein and free estriol at 15-20 weeks of pregnancy, the so-called "triple test") is used in prenatal diagnosis to identify the risk of fetal abnormalities.
In addition to pregnancy, hCG is used in laboratory diagnostics as a tumor marker for tumors of trophoblastic tissue and germ cells of the ovaries and testes that secrete chorionic gonadotropin.
Early detection of pregnancy: determination of the level of hCG
What is hCG?
HCG (human chorionic gonadotropin) is a special pregnancy hormone, which is an important indicator of the development of pregnancy and its deviations. Chorionic gonadotropin is produced by cells of the chorion (shell of the embryo) immediately after its attachment to the wall of the uterus. Based on a blood test for chorionic gonadotropin, the doctor determines the presence of chorionic tissue in the body, and hence the onset of pregnancy in a woman.
When can an hCG test be done?
Determining the level of human chorionic gonadotropin in the blood is the most reliable method for determining pregnancy in the early stages. Chorionic gonadotropin appears in a woman's body from 5 to 6 days after fertilization. A common rapid pregnancy test that every woman can use at home is also based on the determination of human chorionic gonadotropin in the urine, but the necessary level of this hormone in the urine to diagnose pregnancy is reached a few days later.
In the absence of any pathology, in the first weeks of pregnancy, the level of the hormone doubles every 2 days, and its maximum concentration is reached by 10-11 weeks of pregnancy. After 11 weeks, the level of the hormone gradually goes down.
An increase in human chorionic gonadotropin during pregnancy can occur with:
-
multiple pregnancies;
-
toxicosis, gestosis;
-
maternal diabetes;
-
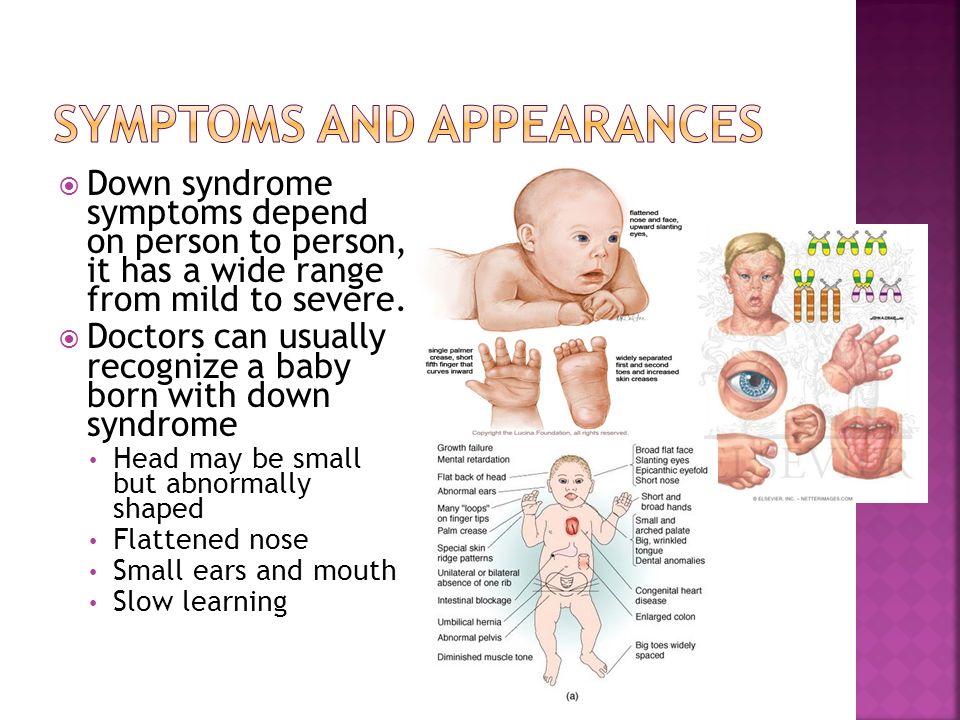
fetal pathologies, Down syndrome, multiple malformations;
-
incorrectly determined gestational age;
-
taking synthetic gestagens, etc.
Elevated values can also be seen within a week when taking a test after an abortion procedure. A high level of the hormone after a mini-abortion indicates a progressive pregnancy.
Low levels of human chorionic gonadotropin during pregnancy may indicate an incorrect gestational age or be a sign of serious disorders, such as:
-
ectopic pregnancy;
-
non-progressive pregnancy;
-
fetal growth retardation;
-
threatened miscarriage;
-
chronic placental insufficiency;
-
fetal death (in the II-III trimester of pregnancy).

Chorionic gonadotropin is included in the triple test, the results of which can be used to judge the presence of some anomalies in the development of the fetus, but an accurate diagnosis cannot be made. The study only allows you to identify women at risk. In this case, women will need to make a serious additional examination.
What is the role of the hCG hormone in the human body?
In addition to establishing the fact of pregnancy, by quantitative determination of the level of this hormone, one can judge the nature of the course of pregnancy, the presence of multiple pregnancy.
The most important task of human chorionic gonadotropin is to support the pregnancy itself. Under its control, the synthesis of the main hormones of pregnancy: estrogen and progesterone. In the first trimester, until the placenta is fully formed (up to 16 weeks), human chorionic gonadotropin maintains the normal functional activity of the corpus luteum, namely, the production of progesterone.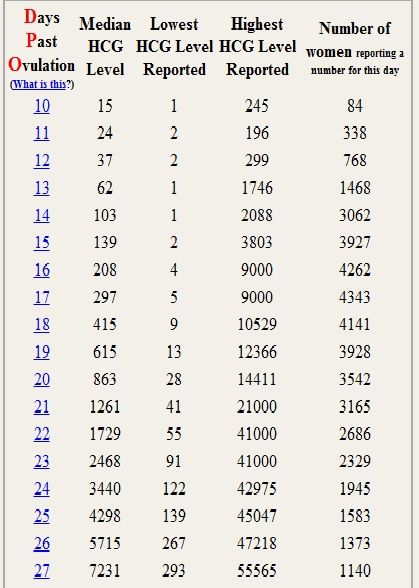
Another important function of human chorionic gonadotropin is to stimulate ovulation and maintain the vitality of the corpus luteum.
When does the doctor prescribe an hCG test?
In addition to diagnosing early pregnancy, chorionic gonadotropin is determined:
in women -
-
to detect amenorrhea;
-
exclude the possibility of ectopic pregnancy;
-
to assess the completeness of induced abortion;
-
for dynamic monitoring of pregnancy;
-
in case of threatened miscarriage and suspected non-developing pregnancy;
-
for the diagnosis of tumors - chorionepithelioma, hydatidiform mole;
-
for prenatal diagnosis of fetal malformations;
in men -
How to take a blood test for hCG hormone?
The independent laboratory INVITRO offers to undergo a laboratory test to determine the level of human chorionic gonadotropin.
The test is given by taking blood from a vein, preferably in the morning and on an empty stomach. A laboratory test is recommended to be carried out no earlier than 4-5 days of delayed menstruation, and can also be repeated after 2-3 days to clarify the results. To identify fetal pathology in pregnant women, it is recommended to take an analysis from the 14th to the 18th week of pregnancy.
In the complex diagnosis of fetal malformations, it is also recommended to take tests to determine the following markers: AFP (alpha-fetoprotein), E3 (free estriol), and also to do an ultrasound scan.
Limits of determination: 1.2 mU / ml-1125000 mU / ml
Beta subunit of human chorionic gonadotropin (beta-hCG) human embryo. The analysis is carried out for the purpose of early diagnosis of pregnancy, detection of its complications and diagnosis of diseases associated with impaired hCG secretion.
Synonyms Russian
Beta subunit of HCG.
Synonyms English
Human Chorionic Gonadotropin, hCG, b-HCG, Quantitative hCG; Beta hCG, Total hCG, Total beta hCG.
Test method
Electrochemiluminescent immunoassay (ECLIA).
Detection range: 0.1 - 1,000,000 IU/L.
Units
IU/L (international unit per litre).
What biomaterial can be used for research?
Venous blood.
How to properly prepare for an examination?
- Do not smoke for 30 minutes before the test.
General information about the study
Human chorionic gonadotropin (hCG) is a hormone produced in the fetal membrane of the human embryo. It is an important indicator of the development of pregnancy and its deviations. It is produced by the cells of the chorion (the shell of the embryo) immediately after it is attached to the wall of the uterus (this happens only a few days after fertilization). The embryo at this stage of pregnancy is a microscopic vesicle filled with fluid, the walls of which are made up of rapidly multiplying cells. From one part of these cells, the unborn child (embryoblast) develops, while from the cells outside the embryo, a trophoblast is formed - that part of the fetal egg, with which it is attached to the wall of the uterus. Later, the chorion is formed from the trophoblast.
The embryo at this stage of pregnancy is a microscopic vesicle filled with fluid, the walls of which are made up of rapidly multiplying cells. From one part of these cells, the unborn child (embryoblast) develops, while from the cells outside the embryo, a trophoblast is formed - that part of the fetal egg, with which it is attached to the wall of the uterus. Later, the chorion is formed from the trophoblast.
Chorion performs the function of nourishing the embryo, being an intermediary between the body of the mother and the child. In addition, it produces chorionic gonadotropin, which, on the one hand, affects the formation of the child, on the other hand, it specifically affects the mother's body, ensuring a successful pregnancy. The appearance of this hormone in the body of a future mother at the initial stage of pregnancy explains the importance of the test for early diagnosis of pregnancy.
Chorionic gonadotropin stimulates the secretory function of the corpus luteum of the ovaries, which should produce the hormone progesterone, which maintains the normal state of the inner lining of the uterine wall - the endometrium. The endometrium provides reliable attachment of the fetal egg to the mother's body and its nutrition with all the necessary substances.
The endometrium provides reliable attachment of the fetal egg to the mother's body and its nutrition with all the necessary substances.
Due to a sufficient amount of human chorionic gonadotropin, the corpus luteum, which normally exists for only about 2 weeks during each menstrual cycle, does not undergo resorption after successful conception and remains functionally active throughout the entire period of pregnancy. Moreover, it is in pregnant women under the influence of chorionic gonadotropin that it produces very large amounts of progesterone. In addition, CG stimulates the production of estrogens and weak androgens by ovarian cells and promotes the development of the functional activity of the chorion itself, and later the placenta, which is formed as a result of the maturation and growth of the chorionic tissue, improving its own nutrition and increasing the number of chorionic villi.
Thus, the role of human chorionic gonadotropin lies in the specific and multifaceted effect on the body of a woman and the fetus in order to ensure a successful pregnancy. Based on the analysis for chorionic gonadotropin, the presence of chorionic tissue in the woman's body is determined, and hence pregnancy.
Based on the analysis for chorionic gonadotropin, the presence of chorionic tissue in the woman's body is determined, and hence pregnancy.
According to its chemical structure, chorionic gonadotropin is a combination of protein and complex carbohydrates, consisting of two parts (subunits): alpha and beta. The alpha subunit of chorionic gonadotropin is completely identical to the alpha subunits of the luteinizing, follicle-stimulating and thyroid-stimulating hormones of the pituitary gland, which perform functions that are largely similar to the function of chorionic gonadotropin, but not during pregnancy. The beta subunit of chorionic gonadotropin is unique, which, on the one hand, determines the specificity of its action, and, on the other hand, allows it to be identified in biological media. In this regard, this test is called "beta-subunit of human chorionic gonadotropin (beta-hCG)".
Knowing the level of beta-hCG in the blood, it is possible to diagnose pregnancy already on the 6-8th day after conception (in the urine, the concentration of beta-hCG reaches the diagnostic level 1-2 days later). Normally, during pregnancy between the 2nd and 5th weeks, the amount of beta-hCG doubles every 1.5 days. With multiple pregnancy, it increases in proportion to the number of fetuses. The maximum level of hCG reaches the 10-11th week, and then gradually decreases. This is due to the fact that from the beginning of the 2nd third of pregnancy, the placenta is able to independently produce enough estrogens and progesterone, with the participation of which the endometrium functions normally, regardless of the secretion of hormones in the ovarian corpus luteum. At the same time, the concentration of CG in the blood of a pregnant woman gradually decreases, and the corpus luteum can function without the effects of CG. During this period, the role of the hormone is to stimulate the production of testosterone in the fetus, which is necessary for the normal development of the external genital organs of the embryo.
Normally, during pregnancy between the 2nd and 5th weeks, the amount of beta-hCG doubles every 1.5 days. With multiple pregnancy, it increases in proportion to the number of fetuses. The maximum level of hCG reaches the 10-11th week, and then gradually decreases. This is due to the fact that from the beginning of the 2nd third of pregnancy, the placenta is able to independently produce enough estrogens and progesterone, with the participation of which the endometrium functions normally, regardless of the secretion of hormones in the ovarian corpus luteum. At the same time, the concentration of CG in the blood of a pregnant woman gradually decreases, and the corpus luteum can function without the effects of CG. During this period, the role of the hormone is to stimulate the production of testosterone in the fetus, which is necessary for the normal development of the external genital organs of the embryo.
Thus, during pregnancy, the level of beta-hCG in the blood first increases and then decreases. According to this indicator, one can judge the successful course of pregnancy and identify violations of the development of the fetus. The test for hCG in the blood is the most reliable method for determining pregnancy in the early stages. HCG appears in the body of a woman from 6-8 days after fertilization. A common rapid pregnancy test that every woman can use at home is also based on measuring hCG in the urine.
According to this indicator, one can judge the successful course of pregnancy and identify violations of the development of the fetus. The test for hCG in the blood is the most reliable method for determining pregnancy in the early stages. HCG appears in the body of a woman from 6-8 days after fertilization. A common rapid pregnancy test that every woman can use at home is also based on measuring hCG in the urine.
Below normal hormone levels at various stages of fetal development suggest ectopic pregnancy, fetal growth retardation, threatened miscarriage, non-progressive pregnancy, or placental insufficiency. The reason for the increased content of beta-hCG may be toxicosis, diabetes mellitus, or an incorrectly set gestational age. A high level of the hormone after a mini-abortion indicates a progressing pregnancy.
Determining the level of hCG is included in the triple test study, the results of which can be used to judge some anomalies in the development of the fetus, but an accurate diagnosis cannot be made. The study only allows you to classify a woman as a risk group for this pathology. In this case, further investigation is necessary. In non-pregnant women, CG is normally absent, but it can be secreted by some abnormal chorion-derived tissues (hydatidiform mole, chorionepithelioma) and some other tumors.
The study only allows you to classify a woman as a risk group for this pathology. In this case, further investigation is necessary. In non-pregnant women, CG is normally absent, but it can be secreted by some abnormal chorion-derived tissues (hydatidiform mole, chorionepithelioma) and some other tumors.
What is research used for?
- For the diagnosis of pregnancy, including multiple, ectopic and non-developing.
- To monitor the progress of pregnancy.
- To detect fetal growth retardation, threatened miscarriage, placental insufficiency.
- For the diagnosis of amenorrhea.
- To monitor the effectiveness of induced abortion.
- As part of a comprehensive examination to identify fetal malformations.
- For the diagnosis of CG-producing tumors.
When is the test scheduled?
- If pregnancy is suspected, in particular multiple pregnancy.
- When monitoring the progress of pregnancy.
- When there is an assumption about a complication during pregnancy: fetal growth retardation, the threat of spontaneous abortion, non-developing or ectopic pregnancy, chronic placental insufficiency.
- Confirm successful induced abortion if necessary.
- With a comprehensive examination to identify fetal malformations.
- When finding out the reason for the absence of menstruation (amenorrhea).
- When is the diagnosis of tumors producing hCG.
What do the results mean?
Reference values
| Floor | Week of pregnancy (from conception) | Reference values |
|
Female | Not pregnant | Less than 5 IU/L |
| 3 weeks | 5. | |
| 4 weeks | 9.5 - 750 IU/L | |
| 5 weeks | 217 - 7138 IU/L | |
| 6 weeks | 158 - 31795 IU/l | |
| 7 weeks | 3697 - 163563 IU/L | |
| 8 weeks | 32065 - 149571 IU/l | |
| 9 weeks | 63803 - 151410 IU/L | |
| 10 weeks | 46509 - 186977 IU/L | |
| 11-12 weeks | 27832 - 210612 IU/L | |
| 13-14 weeks | 13950 - 62530 IU/L | |
| 15 weeks | 12039 - 70971 IU/L | |
| 16 weeks | 9040 - 56451 IU/L | |
| 17 weeks | 8175 - 55868 IU/L | |
| 18 weeks | 8099 - 58176 IU/L | |
| Male |
| Less than 5 IU/L |
Causes of high beta-hCG levels
In the absence of pregnancy, the result of the beta-hCG test should be negative. The detection of beta-hCG suggests that at least 5-6 days have passed since fertilization. Between the 2nd and 5th weeks of pregnancy, during its normal course, the level of beta-hCG doubles every 1.5 days and soon reaches its maximum. Then it starts to slowly decline. The results of the analysis are compared with the normal values for each gestational age. For a correct interpretation of the results, it is necessary to know exactly when the conception occurred.
The detection of beta-hCG suggests that at least 5-6 days have passed since fertilization. Between the 2nd and 5th weeks of pregnancy, during its normal course, the level of beta-hCG doubles every 1.5 days and soon reaches its maximum. Then it starts to slowly decline. The results of the analysis are compared with the normal values for each gestational age. For a correct interpretation of the results, it is necessary to know exactly when the conception occurred.
In pregnant women:
- multiple pregnancy (increases in proportion to the number of fetuses),
- toxicosis,
- prolonged pregnancy,
- maternal diabetes,
- fetal malformations,
- taking synthetic hormones.
Not pregnant:
- hCG-producing tumors,
- surgical abortion (first 4-5 days after the procedure),
- taking hCG preparations.
Causes of low beta-hCG levels (during pregnancy):
- ectopic or non-developing pregnancy,
- fetal growth retardation,
- threatened miscarriage,
- chronic placental insufficiency,
- fetal death (in the 2nd-3rd trimester of pregnancy).
What can influence the result?
- When diagnosing pregnancy, taking the test too early - when less than 5 days have passed since conception - can lead to a false negative result.
Also recommended
- Alpha-fetoprotein (alpha-FP)
- Antiphospholipid antibodies IgG
- Antiphospholipid antibodies IgM
- Placental lactogen
- Free estriol
- Pregnancy - Prenatal screening for trisomies of the first trimester of pregnancy (Down syndrome), PRISCA
- Plasma pregnancy-associated protein A (PAPP-A)
- Pregnancy - Prenatal screening for trisomies of the second trimester of pregnancy, PRISCA
- Antibodies to steroid-producing cells of the placenta
Who orders the examination?
General practitioner, obstetrician-gynecologist, oncologist.
Literature
- Handbook of Clinical Laboratory Testing During Pregnancy, edited by Ann M.
 Gronowski. Springer-Verlag LLC, New York, 2004
Gronowski. Springer-Verlag LLC, New York, 2004 - Prenatal Diagnosis in Obstetric Practice. M. J. J. Whittle, Blackwell Publishers 1995
- Endocrinology of Pregnancy. Fuller W. Bazer, Humana Press 1998
- Blood Biochemistry. N J Russell, G M Powell, J G Jones, P J, Winterburn and J M Basford, Croom Helm, London and Canberra, 1982
- Blood Chemistry and CBC analysis-Clinical Laboratory Testing from a Functional Perspective. Rychard Weatherby N.D and Scott Fergusson, N.D., Bear Mounting Publishing, 2002.
- Tietz Clinical Guide to Laboratory Tests. Alan H. B. Wu, Saunders/Elsevier, 2006
- Laboratory and Diagnostic Tests. Joyce LeFever Kee - Pearson, Prentice Hall, 8th Edition 2010
- District Laboratory Practice in Tropical Countries. Monica Cheesbrough, Cambridge University Press, second edition, 2005.
- Clinical Chemistry. A Laboratory Perspective. Wendy L. Arneson, Jean M. Brickell, F.A. Davis Company, 2007
- Clinical Chemistry.
Learn more
 8 - 71.2 IU/L
8 - 71.2 IU/L