Flu shot in first trimester
Flu shots and miscarriage: Let’s clear up misunderstandings | Your Pregnancy Matters
With flu season in full swing, September is possibly the worst time to release a confusing study about the safety of flu shots for pregnant woman. But that’s what happened, and now doctors across the country are running defense to protect pregnant women and their babies from the flu.
The study in question was funded by the Centers for Disease Control and Prevention (CDC) and published in Vaccine. The CDC promptly released a statement that the study does not quantify miscarriage risk and does not prove flu shots can cause miscarriage, even Vaccine Editor-in-Chief Gregory Poland, CRED, who is also director of vaccine research at the Mayo Clinic, was quoted in The New York Times saying he does “not at all” believe flu shots caused the miscarriages reported in the study.
Unfortunately, click-baiting media outlets and grassroots anti-vaccination advocates got wind of the study. Inflammatory headlines and misinformation added to the confusion about the study, leaving fearful pregnant women scrambling to decide whether to get vaccinated.
Let’s make one thing clear: UT Southwestern Ob/Gyns and infectious disease experts recommend that all pregnant women get the flu shot. The benefits for moms and babies clearly outweigh the risks, no matter what you might have read online. Let’s examine why we can confidently make this recommendation and what to do if you’re on the fence about getting vaccinated for the flu.
Holes in the ‘flu shots and miscarriage’ study
The most important thing I want women to understand is this: This study does not say that you are at increased risk for miscarriage if you are in the first trimester, have a healthy pregnancy, and get a flu shot.
The study looked at a population of women who were diagnosed with a miscarriage during pregnancy and compared them to women who have live births. They then studied whether and when the women received a flu shot during pregnancy. The study did not specify when the women who got flu shots were vaccinated, but it stated that many received a flu shot within 28 days of the miscarriage and also received a flu shot during the previous year’s flu season.
The definition of pregnancy in the study is very broad. The reported pregnancies could have been diagnoses from doctors, self-reported by the patients, or determined by a lab test only. There was no requirement that the pregnancies were proved to be viable, or have a chance to be successful, before women were included in the analysis.
The majority of miscarriages in the women in the study occurred in the first trimester, with the greatest number occurring between five and seven weeks. This isn’t surprising because miscarriage is so common in the general population. In fact, 80 percent of spontaneous miscarriages occur in the first trimester. There’s no way to know whether these pregnancies were going to be successful regardless of whether the women received flu shots.
Getting a flu shot is still best for mom and baby
The CDC, the American College of Obstetricians and Gynecologists (ACOG), and the American Academy of Pediatrics continue to recommend the flu shot for pregnant women at any stage of pregnancy, not only for the benefit of the mom but also because it provides the baby with antibodies that can help protect the baby after birth.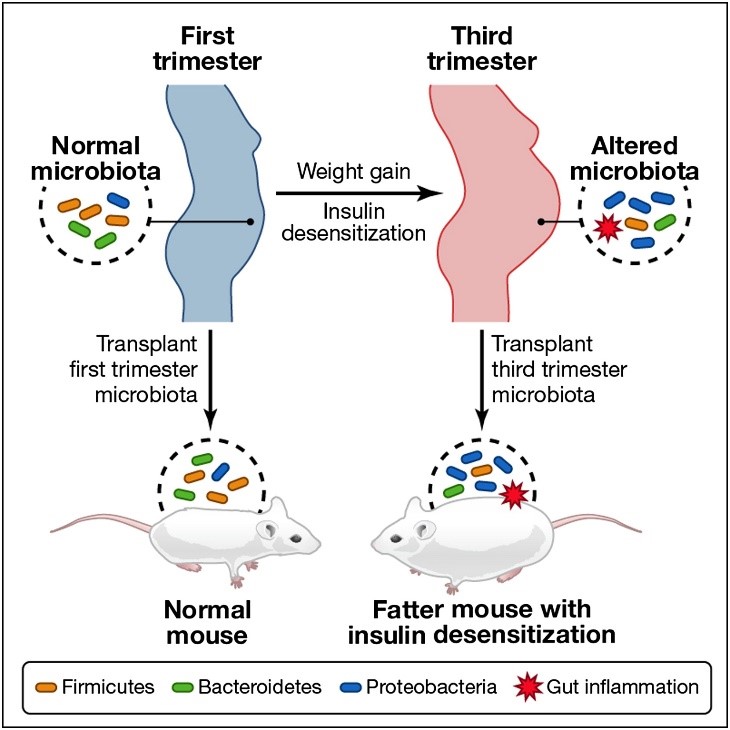
Infants cannot get their own flu vaccine until 6 months of age, so the antibodies you pass to your child are vital. As of September 2, 2017, more than 100 pediatric flu deaths already have been reported for the 2016/2017 flu season. The peak flu season traditionally is September through March, but there’s no way to predict with certainty how early the flu season will start or how long it will last. Early indications from countries in the southern hemisphere where flu season has already started suggest this year will see a lot of influenza activity.
Some women worry that the flu shot will make them sick. This is untrue. Flu shots are made from dead viruses that cannot give you the flu – but you may notice some minor side effects such as soreness at the injection site, low-grade fever, or muscle aches, which are annoying but not worrisome.
The nasal spray flu vaccine is not recommended for pregnant women. The spray is made from a live attenuated virus, which means the virus is weakened but still alive and could potentially infect you with the flu. But this year, the CDC is not recommending the spray vaccine for anyone, including children, because it’s likely to be ineffective against the flu strains that are expected to be strongest this flu season.
But this year, the CDC is not recommending the spray vaccine for anyone, including children, because it’s likely to be ineffective against the flu strains that are expected to be strongest this flu season.
What to do if you’re on the fence about getting vaccinated
Getting the flu shot during any trimester of pregnancy is reasonable and safe, and being vaccinated against the flu in the first trimester will not put your baby at risk. But if you’re nervous about getting the flu shot during the first trimester, don’t refuse to be vaccinated. Just hold off until after you reach 20 weeks of pregnancy. The Vaccine study reported there was no increased risk after 20 weeks of pregnancy. You might not be as fully protected from the flu, which can lead to serious complications including hospitalization and even death in pregnant women, but your baby will still get the antibody benefit.
The key takeaway for women is that scientific research studies have never proven a cause/effect relationship between the flu vaccine and miscarriage. In fact, January 2013 study showed no link between miscarriage rates and maternal flu vaccination using clinical data, and an August 2017 study found no increased risk between maternal flu vaccination and birth defects in babies. Even the study published in Vaccine states that the data retrieved demonstrate an association, not a cause/effect relationship, between flu shots and miscarriage.
In fact, January 2013 study showed no link between miscarriage rates and maternal flu vaccination using clinical data, and an August 2017 study found no increased risk between maternal flu vaccination and birth defects in babies. Even the study published in Vaccine states that the data retrieved demonstrate an association, not a cause/effect relationship, between flu shots and miscarriage.
I appreciate that researchers continue to question what is and isn’t safe for pregnant women and their babies. However, as medical professionals, we have to be careful about what we say and how we say it. This inconclusive study is a prime example of how associative data, particularly when it is distributed without proper context, can do more harm than good for public health.
Influenza Vaccination During Pregnancy | ACOG
Number 732 (Replaces Committee Opinion Number 608, September 2014. Reaffirmed 2021)
Committee on Obstetric Practice
This Committee Opinion was developed by the American College of Obstetricians and Gynecologists’ Immunization and Emerging Infections Expert Work Group and the Committee on Obstetric Practice in collaboration with Neil S. Silverman, MD, and Richard Beigi, MD.
Silverman, MD, and Richard Beigi, MD.
ABSTRACT: Influenza vaccination is an essential element of prepregnancy, prenatal, and postpartum care because influenza can result in serious illness, including a higher chance of progressing to pneumonia, when it occurs during the antepartum or postpartum period. In addition to hospitalization, pregnant women with influenza are at increased risk of intensive care unit admission and adverse perinatal and neonatal outcomes. The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices and the American College of Obstetricians and Gynecologists recommend that all adults receive an annual influenza vaccine and that women who are or will be pregnant during influenza season receive an inactivated influenza vaccine as soon as it is available. In the United States, the influenza season typically occurs from October to May. Ideally, an influenza vaccination should be given before the end of October, but vaccination throughout the influenza season is encouraged to ensure protection during the period of circulation. Any of the licensed, recommended, age-appropriate, inactivated influenza vaccines can be given safely during any trimester. Therefore, it is critically important that obstetrician–gynecologists and other obstetric care providers recommend and advocate for the influenza vaccine. Obstetrician–gynecologists are encouraged to stock and administer the influenza vaccine to their pregnant patients in their offices, and should get the influenza vaccine themselves every season. If the influenza vaccine cannot be offered in a practice, obstetrician–gynecologists and obstetric care providers should refer patients to another health care provider, pharmacy, or community vaccination center. This updated Committee Opinion includes more recent data on the safety and efficacy of influenza vaccination during pregnancy and recommendations for treatment and postexposure chemoprophylaxis.
Any of the licensed, recommended, age-appropriate, inactivated influenza vaccines can be given safely during any trimester. Therefore, it is critically important that obstetrician–gynecologists and other obstetric care providers recommend and advocate for the influenza vaccine. Obstetrician–gynecologists are encouraged to stock and administer the influenza vaccine to their pregnant patients in their offices, and should get the influenza vaccine themselves every season. If the influenza vaccine cannot be offered in a practice, obstetrician–gynecologists and obstetric care providers should refer patients to another health care provider, pharmacy, or community vaccination center. This updated Committee Opinion includes more recent data on the safety and efficacy of influenza vaccination during pregnancy and recommendations for treatment and postexposure chemoprophylaxis.
Recommendations
The American College of Obstetricians and Gynecologists (ACOG) makes the following recommendations:
The Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices and ACOG recommend that all adults receive an annual influenza vaccine and that women who are or will be pregnant during influenza (flu) season receive an inactivated influenza vaccine as soon as it is available.
 Any of the licensed, recommended, age-appropriate, inactivated influenza vaccines can be given safely during any trimester.
Any of the licensed, recommended, age-appropriate, inactivated influenza vaccines can be given safely during any trimester.Maternal influenza immunization is an essential component of prenatal care for women and their newborns. Obstetrician–gynecologists and other health care providers should counsel pregnant women about the safety and benefits of influenza immunization for themselves and their fetuses and advocate for the benefits of passive immunity from maternal immunization for their newborns.
Obstetrician–gynecologists are encouraged to stock and administer the influenza vaccine to their pregnant patients in their offices, and should get the influenza vaccine themselves every season.
If the influenza vaccine cannot be offered in a practice, obstetrician–gynecologists and obstetric care providers should refer patients to another health care provider, pharmacy, or community vaccination center.
Obstetrician–gynecologists should strongly encourage their office staff to be vaccinated against influenza every season.

Individuals with a history of egg allergy who have experienced only hives after exposure to egg can receive any licensed and recommended influenza vaccine that is otherwise appropriate for their age and health status.
In the case of allergic symptoms more serious than hives, the vaccine should be administered in an inpatient or outpatient medical setting (including, but not necessarily limited to hospitals, clinics, health departments, and physician offices).
Patients with flu-like illness should be treated with antiviral medications presumptively regardless of vaccination status. Health care providers should not rely on test results to initiate treatment and should treat patients presumptively based on clinical evaluation.
Because of the high potential for morbidity, the CDC and ACOG recommend that postexposure antiviral chemoprophylaxis (75 mg of oseltamivir once daily for 10 days) be considered for pregnant women and women who are up to 2 weeks postpartum (including pregnancy loss) who have had close contact with someone likely to have been infected with influenza.
 If oseltamivir is unavailable, zanamiver can be substituted, two inhalations once daily for 10 days.
If oseltamivir is unavailable, zanamiver can be substituted, two inhalations once daily for 10 days.
Introduction
Published data continue to demonstrate the need for influenza vaccination during pregnancy as well as the importance of recommending and providing vaccination in the office 1 2 3 4. During the 2016–2017 influenza season, 53.6% of women reported receiving the influenza vaccine before or during pregnancy 5. Although these numbers reflect significant progress, much room remains for improvement to meet the U.S. Health and Human Services’ Healthy People 2020 goal of vaccinating 80% of pregnant women against influenza 6. The American College of Obstetricians and Gynecologists’ Immunization and Emerging Infections Expert Work Group and the Committee on Obstetric Practice recommend that all women who are pregnant during influenza season receive an inactivated influenza vaccine in accordance with recommendations from the CDC’s Advisory Committee on Immunization Practices 5. This updated Committee Opinion includes more recent data on the safety and efficacy of influenza vaccination during pregnancy and recommendations for treatment and postexposure chemoprophylaxis.
This updated Committee Opinion includes more recent data on the safety and efficacy of influenza vaccination during pregnancy and recommendations for treatment and postexposure chemoprophylaxis.
Background
Influenza vaccination is an essential element of prepregnancy, prenatal, and postpartum care because influenza can result in serious illness, including a higher chance of progressing to pneumonia, when it occurs during the antepartum or postpartum period. For example, a retrospective cohort study in Nova Scotia found that women hospitalized for respiratory illness during pregnancy (especially during the third trimester) were more likely to have an increased number of medical visits or an increased length of stay when compared with the number of visits the year before their pregnancy 7. In this study, the association between pregnancy status and hospital admission was particularly striking for women with comorbidities 7. However, it is important to note that many studies, including the aforementioned study, were not able to confirm the influenza diagnosis with laboratory results, and more studies using confirmatory laboratory results are needed in pregnant women. In addition to hospitalization, pregnant women with influenza are at an increased risk of intensive care unit admission and adverse perinatal and neonatal outcomes 8 9 10. Finally, morbidity and mortality among pregnant women increases during influenza pandemics, including the 2009 h2N1 influenza pandemic 10 11 12 13 14 15 16 17 18. Taken together, these data emphasize the importance of influenza vaccination as a vital intervention that all obstetrician–gynecologists and other obstetric care providers should recommend and administer.
In addition to hospitalization, pregnant women with influenza are at an increased risk of intensive care unit admission and adverse perinatal and neonatal outcomes 8 9 10. Finally, morbidity and mortality among pregnant women increases during influenza pandemics, including the 2009 h2N1 influenza pandemic 10 11 12 13 14 15 16 17 18. Taken together, these data emphasize the importance of influenza vaccination as a vital intervention that all obstetrician–gynecologists and other obstetric care providers should recommend and administer.
In the United States, the influenza season typically occurs from October to May. The CDC’s Advisory Committee on Immunization Practices and ACOG recommend that all adults receive an annual influenza vaccine and that women who are or will be pregnant during influenza season receive an inactivated influenza vaccine as soon as it is available. Ideally, an influenza vaccination should be given by the end of October, but vaccination throughout the influenza season is encouraged to ensure protection during the period of circulation. The inactivated influenza vaccine can be given to all pregnant women during any trimester 5. Because influenza vaccines are recommended annually for all adults, pregnant women should be vaccinated even if they received an influenza vaccine during a previous pregnancy. Vaccination in the postpartum period is an alternative only when vaccination during pregnancy cannot be completed.
The inactivated influenza vaccine can be given to all pregnant women during any trimester 5. Because influenza vaccines are recommended annually for all adults, pregnant women should be vaccinated even if they received an influenza vaccine during a previous pregnancy. Vaccination in the postpartum period is an alternative only when vaccination during pregnancy cannot be completed.
Safety
Numerous studies, including clinical trials and observational studies, and data from safety reporting systems have demonstrated consistently the safety of influenza vaccination during pregnancy 19 20 21 22 23. To date, only one small retrospective case–control study has suggested a possible association between receipt of an influenza vaccine containing A/h2N1pdm early in the first trimester and spontaneous abortion in women who also received an influenza vaccine containing A/h2N1pdm in the previous influenza season 24. This association has not been observed during other seasons or other versions of the influenza vaccine. Because of the lack of evidence of biological plausibility, several notable flaws in this study, and the preponderance of other data showing no association, the recommendation for influenza vaccine given in any trimester has not changed 24 25. Although some researchers have raised concerns that thimerosal, a mercury-containing preservative used in multidose vials of the influenza vaccine, may be unsafe, there is no scientific evidence that thimerosal-containing vaccines cause health or developmental problems in children born to women who received vaccines with thimerosal during pregnancy 26 27 28. Therefore, although thimerosal-free formulations of the influenza vaccine are available, the CDC’s Advisory Committee on Immunization Practices does not indicate a preference for thimerosal-containing or thimerosal-free vaccines for any group, including pregnant women 19.
Because of the lack of evidence of biological plausibility, several notable flaws in this study, and the preponderance of other data showing no association, the recommendation for influenza vaccine given in any trimester has not changed 24 25. Although some researchers have raised concerns that thimerosal, a mercury-containing preservative used in multidose vials of the influenza vaccine, may be unsafe, there is no scientific evidence that thimerosal-containing vaccines cause health or developmental problems in children born to women who received vaccines with thimerosal during pregnancy 26 27 28. Therefore, although thimerosal-free formulations of the influenza vaccine are available, the CDC’s Advisory Committee on Immunization Practices does not indicate a preference for thimerosal-containing or thimerosal-free vaccines for any group, including pregnant women 19.
Individuals with a history of egg allergy who have experienced only hives after exposure to egg can receive any licensed and recommended influenza vaccine that is otherwise appropriate for their age and health status. A recent study found the rate of anaphylaxis after all vaccines to be 1.31 per one million vaccine doses given 29.Individuals who report having had reactions to egg involving symptoms other than hives (such as angioedema, respiratory distress, lightheadedness, or recurrent emesis) or those who have required epinephrine or another emergency medical intervention, also may receive any licensed and recommended influenza vaccine. However, in the case of allergic symptoms more serious than hives, the vaccine should be administered in an inpatient or outpatient medical setting (including, but not necessarily limited to hospitals, clinics, health departments, and physician offices).
A recent study found the rate of anaphylaxis after all vaccines to be 1.31 per one million vaccine doses given 29.Individuals who report having had reactions to egg involving symptoms other than hives (such as angioedema, respiratory distress, lightheadedness, or recurrent emesis) or those who have required epinephrine or another emergency medical intervention, also may receive any licensed and recommended influenza vaccine. However, in the case of allergic symptoms more serious than hives, the vaccine should be administered in an inpatient or outpatient medical setting (including, but not necessarily limited to hospitals, clinics, health departments, and physician offices).
Vaccine administration should be supervised by a health care provider who is able to recognize and manage severe allergic conditions. A previous severe allergic reaction to influenza vaccine, not to eggs, regardless of the component suspected of being responsible for the reaction, is the only current contraindication to future receipt of the influenza vaccine 5.
Currently, pregnant women should receive any licensed, recommended, age-appropriate, inactivated influenza vaccine during any trimester 5. If the timing of the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine and the influenza vaccine align, it is safe and effective to administer both vaccines during the same visit. It is also safe for breastfeeding women to receive the influenza vaccine if they did not receive it during pregnancy.
Efficacy and Benefits
The efficacy of seasonal influenza vaccination in pregnant women is similar to its efficacy among the general adult population 30. Although the effectiveness of the influenza vaccine can be lower than that of other adult vaccines, vaccination still offers significant protection against influenza. It can mitigate the severity of the effect of influenza when infection does occur and is the primary preventive intervention for pregnant women. A study during the 2012–2013 influenza season demonstrated that pregnant women who were vaccinated had significantly fewer hospitalizations than those who were not 31.
Influenza vaccination during pregnancy also can benefit the newborns of women who received the vaccine. Four large-scale, randomized controlled trials and numerous observational studies have demonstrated neonatal protection from maternal influenza vaccination 32 33 33 34 35. Studies also have demonstrated a reduction in hospitalization related to influenza infection among infants born to women who received the vaccine during pregnancy 36 37. Therefore, because the influenza vaccine is not effective in infants younger than 6 months, passive immunization of fetuses through transplacentally transmitted antibodies is currently the best prevention strategy for newborns 32. Thus, maternal influenza immunization is an essential component of prenatal care for women and their newborns. Obstetrician–gynecologists and other health care providers should counsel pregnant women about the benefits of influenza immunization for themselves and their fetuses and advocate for the benefits of passive immunity from maternal immunization for their newborns.
Treatment and Postexposure Chemoprophylaxis in Pregnant Women
Pregnant women are at high risk of serious complications of influenza infection such as intensive care unit admission, preterm delivery, and maternal death. Patients with flu-like illness should be treated with antiviral medications presumptively regardless of vaccination status. Treatment with oseltamivir (75 mg twice daily for 5 days) is preferred; however, if oseltamivir is unavailable, zanamivir (two inhalations [10 mg] twice daily for 5 days) may be substituted. Health care providers should not rely on test results to initiate treatment and should treat patients presumptively based on clinical evaluation 38.
Because of the high potential for morbidity, the CDC and ACOG recommend that postexposure antiviral chemoprophylaxis (75 mg of oseltamivir once daily for 10 days) be considered for pregnant women and women who are up to 2 weeks postpartum (including pregnancy loss) who have had close contact with someone likely to have been infected with influenza. If oseltamivir is unavailable, zanamivir can be substituted, two inhalations once daily for 10 days. All women who are pregnant or are in the first 2 weeks postpartum should be counseled to call for evaluation immediately if the early signs and symptoms of influenza infection (eg, a fever greater than 100°F coupled with shortness of breath, syncope, or chest pain) develop 38. For more information about treatment and dosage see ACOG and the Society for Maternal–Fetal Medicine’s Seasonal Influenza Assessment and Treatment of Pregnant Women with Influenza-like Illness algorithm at www.acog.org/More-Info/FluVaccine.
The Obstetrician–Gynecologist’s Role
Discussion with patients regarding the effects of influenza and the potential benefits of vaccination during pregnancy is particularly important because a lack of knowledge about the benefits of the influenza vaccine has been shown to be a barrier to vaccine acceptance 39 40 41. Educational tools with simple chart prompts increase the frequency of discussion between physicians and pregnant women regarding influenza vaccination 42. Moreover, studies consistently suggest that when recommendations for influenza vaccination during pregnancy come directly from a woman’s obstetrician–gynecologist or other obstetric care provider and the vaccine is available in the physician’s office, the odds of vaccine acceptance and receipt are 5-fold to 50-fold higher 1 2. Therefore, it is critically important that all obstetrician–gynecologists and other obstetric care providers recommend and advocate for the influenza vaccine. Obstetrician–gynecologists are encouraged to stock and administer the influenza vaccine to their pregnant patients in their offices, and should get the influenza vaccine themselves every season. Depending on the size of a practice and services provided, there may not be the means to stock and offer the influenza vaccine in the office. If the influenza vaccine cannot be offered in a practice, obstetrician–gynecologists and obstetric care providers should refer patients to another health care provider, pharmacy, or community vaccination center.
Moreover, studies consistently suggest that when recommendations for influenza vaccination during pregnancy come directly from a woman’s obstetrician–gynecologist or other obstetric care provider and the vaccine is available in the physician’s office, the odds of vaccine acceptance and receipt are 5-fold to 50-fold higher 1 2. Therefore, it is critically important that all obstetrician–gynecologists and other obstetric care providers recommend and advocate for the influenza vaccine. Obstetrician–gynecologists are encouraged to stock and administer the influenza vaccine to their pregnant patients in their offices, and should get the influenza vaccine themselves every season. Depending on the size of a practice and services provided, there may not be the means to stock and offer the influenza vaccine in the office. If the influenza vaccine cannot be offered in a practice, obstetrician–gynecologists and obstetric care providers should refer patients to another health care provider, pharmacy, or community vaccination center.
If a patient receives the influenza vaccine outside of the obstetrician–gynecologist’s office, it is important for the site that provided the vaccination to provide proper vaccine documentation if the site does not work directly with a centralized vaccine registration program, so that the patient’s immunization record can be updated appropriately. These combined efforts send a powerful message to pregnant women that vaccination is very important for their protection and for their newborns.
Conclusion
Pregnant women are particularly vulnerable to influenza infection and its resulting morbidities; therefore, influenza vaccination is an integral element of prepregnancy, prenatal, and postpartum care. It is imperative that obstetrician–gynecologists, other health care providers, health care organizations, and public health officials continue efforts to improve the rate of influenza vaccination among pregnant women. Doing so will benefit women and their newborns.
For More Information
The American College of Obstetricians and Gynecologists has identified additional resources on topics related to this document that may be helpful for obstetrician–gynecologists, other health care providers, and patients. You may view these resources at: www.acog.org/More-Info/FluVaccine.
You may view these resources at: www.acog.org/More-Info/FluVaccine.
These resources are for information only and are not meant to be comprehensive. Referral to these resources does not imply the American College of Obstetricians and Gynecologists’ endorsement of the organization, the organization’s website, or the content of the resource. The resources may change without notice.
Copyright April 2018 by the American College of Obstetricians and Gynecologists. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, posted on the Internet, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without prior written permission from the publisher.
Requests for authorization to make photocopies should be directed to Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400.
American College of Obstetricians and Gynecologists 409 12th Street, SW, PO Box 96920, Washington, DC 20090-6920
Influenza vaccination during pregnancy. ACOG Committee Opinion No. 732. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;131:e109–14.
ACOG Committee Opinion No. 732. American College of Obstetricians and Gynecologists. Obstet Gynecol 2018;131:e109–14.
This information is designed as an educational resource to aid clinicians in providing obstetric and gynecologic care, and use of this information is voluntary. This information should not be considered as inclusive of all proper treatments or methods of care or as a statement of the standard of care. It is not intended to substitute for the independent professional judgment of the treating clinician. Variations in practice may be warranted when, in the reasonable judgment of the treating clinician, such course of action is indicated by the condition of the patient, limitations of available resources, or advances in knowledge or technology. The American College of Obstetricians and Gynecologists reviews its publications regularly; however, its publications may not reflect the most recent evidence. Any updates to this document can be found on www.acog.org or by calling the ACOG Resource Center.
While ACOG makes every effort to present accurate and reliable information, this publication is provided “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. ACOG does not guarantee, warrant, or endorse the products or services of any firm, organization, or person. Neither ACOG nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with this publication or reliance on the information presented.
Flu shot for pregnant women | Surgut City Clinical Polyclinic No. 4
Is it possible to get a flu shot during pregnancy? In most cases, experts say: it is not only possible, but also necessary: during the gestation period, a woman belongs to the most vulnerable group of the population, along with small children, the elderly and people with chronic diseases.
Is influenza dangerous during pregnancy?
Influenza is an infectious disease, depending on the strain and the body's immune response, it can proceed quite easily, or it can cause severe complications and consequences. Pregnancy is a physiological state in which the body's defenses are naturally reduced, the mother's immune system is suppressed to reduce the likelihood of fetal rejection, which leads to a reduced ability to resist various infectious agents, and the influenza virus is no exception. nine0003
Pregnant women are much more likely than in the same age period outside of gestation to have influenza complications: viral, bacterial pneumonia and pneumonia of mixed etiology, sinusitis, bronchitis, otitis media, pyelonephritis, etc.
which is also noted in pregnant women much more often, complications of the cardiovascular system (myocarditis, heart failure) may develop. Also, against the background of influenza, diseases of the endocrine system often become aggravated or debut ( diabetes mellitus ), urinary system (nephritis, cystitis), there are episodes of bronchial asthma.
Bacterial diseases that are not directly related to exposure to the influenza virus, such as candidiasis, can also worsen or develop after a viral infection due to a decrease in general immunity. In addition to a more severe course of the disease and complications, influenza in pregnant women can lead to the threat of premature birth, spontaneous abortion. In the early stages, the influenza virus and some medications can adversely affect the fetal organs and systems, and in the later stages of gestation, the virus that has penetrated the placental barrier can cause influenza in the child. nine0003
Can pregnant women get the flu shot?
The World Health Organization has a program to vaccinate pregnant women against influenza. In the absence of contraindications, experts recommend that all pregnant women be vaccinated against influenza, although it is not included in the mandatory vaccination list and the choice remains with the expectant mother.
What are the reasons for getting a flu shot during pregnancy?
- A weakened immune system in a pregnant woman puts her at a higher risk of contracting the influenza virus during and outside of seasonal outbreaks.
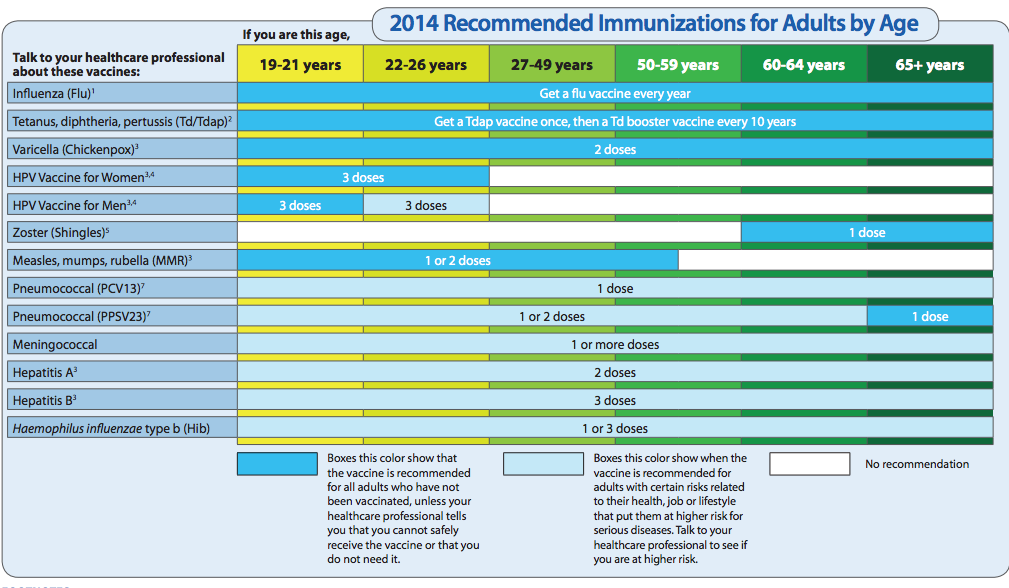 nine0029
nine0029 - When infected, the course of the disease can be much more severe than the average characteristic for a given strain; when new mutated influenza strains appear, pregnant women are one of the groups with the highest mortality.
- Pregnant women are much more likely to develop influenza complications.
- Influenza can affect not only the mother's body, but also cause spontaneous abortion or abnormal development of the fetus.
- Vaccination produces antibodies against influenza, which are passed on to the child and protect against infection during the first 6 months of life. nine0029
Are there any contraindications for influenza vaccination during pregnancy?
An absolute contraindication is the presence of an allergic reaction to chicken protein, the basic substance for creating a vaccine, as well as individual intolerance to vaccine components.
Relative contraindications, which must be assessed for each woman separately, include allergies to various groups of antibiotics, an allergic reaction to a previous vaccination. It is highly recommended not to get a flu shot in the early stages (in the first trimester before the formation of the placenta). Annual preventive vaccination before conception helps to avoid the risk of disease during the season of high viral load. nine0003
It is highly recommended not to get a flu shot in the early stages (in the first trimester before the formation of the placenta). Annual preventive vaccination before conception helps to avoid the risk of disease during the season of high viral load. nine0003
Temporary contraindications, in which it is necessary to postpone the vaccination period until the period of full health, include respiratory diseases, exacerbation of somatic diseases, allergies, preeclampsia of the second and third trimester.
When to get vaccinated during pregnancy and when planning to conceive?
Pregnant women are recommended to get influenza vaccination at the beginning of the second trimester, especially if the pregnancy occurs during the seasonal period of epidemics. Vaccination is preferred 1 month before the start of the increase in the infectious activity of the virus, most often in September-early October. nine0003
The process of developing an immune response takes 2 to 4 weeks, so 1 month before the onset of the average activity of the virus, it is necessary to vaccinate.
When planning pregnancy, vaccination is carried out 1 month before attempting to conceive. It must be remembered that the vaccination is valid for about 12 months, so regular annual revaccination is necessary for long-term protection.
Flu vaccination preparation
In extremely rare cases, allergic reactions of a pronounced nature (up to Quincke's edema) occur on the components of the vaccine, which were not expected during the previous examination of the woman. To reduce the likelihood of an allergy of any severity, it is necessary to follow the rules for preparing for vaccination:
- Vaccination is carried out against the background of full health and not earlier than 2 weeks after a viral or bacterial disease;
- malaise on the day of vaccination - a reason for examination by a doctor and postponement of the date of vaccination; nine0029
- 2-3 days before vaccination, on the day of vaccination and a week after, avoid unusual foods, drinks, potential allergens in food, cosmetics, etc.
Choosing a vaccine for pregnant women
All vaccines offered to develop immunity against the most common strains of influenza are inactivated, that is, the live virus does not enter the body, which eliminates both the negative impact of the infectious agent on the expectant mother and teratogenic effects to the fruit. However, vaccines may differ in the presence and amount of a preservative, the focus of action on one or two virus strains predicted in the next epidemic, etc.
The most commonly chosen preparations for vaccination of pregnant women include:
- Sovigripp;
- Vaxigripp;
- Grippol;
- Begrivak;
- Grippo Plus and others.
The most common and affordable vaccines in Russia are Grippol and Grippol Plus. The second option is considered the most suitable for women during the gestation period, since there are no preservatives in the vaccine.
Despite the fact that the effect of the vaccine is not an absolute protection against infection with the virus, in the event of an illness, the clinical picture in patients, including pregnant women, is characterized by less severe symptoms, mild forms of the disease, and rare complications. Timely flu vaccination helps protect not only the mother, but also the baby, and is recommended for almost all pregnant women and women planning a child. nine0003
Timely flu vaccination helps protect not only the mother, but also the baby, and is recommended for almost all pregnant women and women planning a child. nine0003
Recommendations for influenza vaccination of pregnant women
Pregnant women and women after childbirth have a high risk of developing a severe form of influenza with the subsequent development of complications, which is associated with factors such as:
-
General immunosuppression due to the products and metabolism of certain hormones: chorionic gonadotropin, progesterone, alpha-fetoprotein, placental proteins Syncytin-1 and Syncytin-2 and other factors. Immunosuppression is directed mainly at T-cell immunity and NK cells. nine0003
-
High oxygen demand of the mother and fetus. Oxygen uptake progressively increases in the second and third trimesters of pregnancy. The growing fetus puts pressure on the diaphragm, which makes breathing difficult, especially during exercise and movement.
 Any decrease in lung function greatly affects the general condition of pregnant women.
Any decrease in lung function greatly affects the general condition of pregnant women. -
High sensitivity to hypoxia. In the case of hypoxia, a high level of production of destructive reactive oxygen species is observed. Free oxygen radicals reduce the ability of the lungs and placenta to transport oxygen, and with the development of influenza, they cause a generalized pro-inflammatory reaction. nine0003
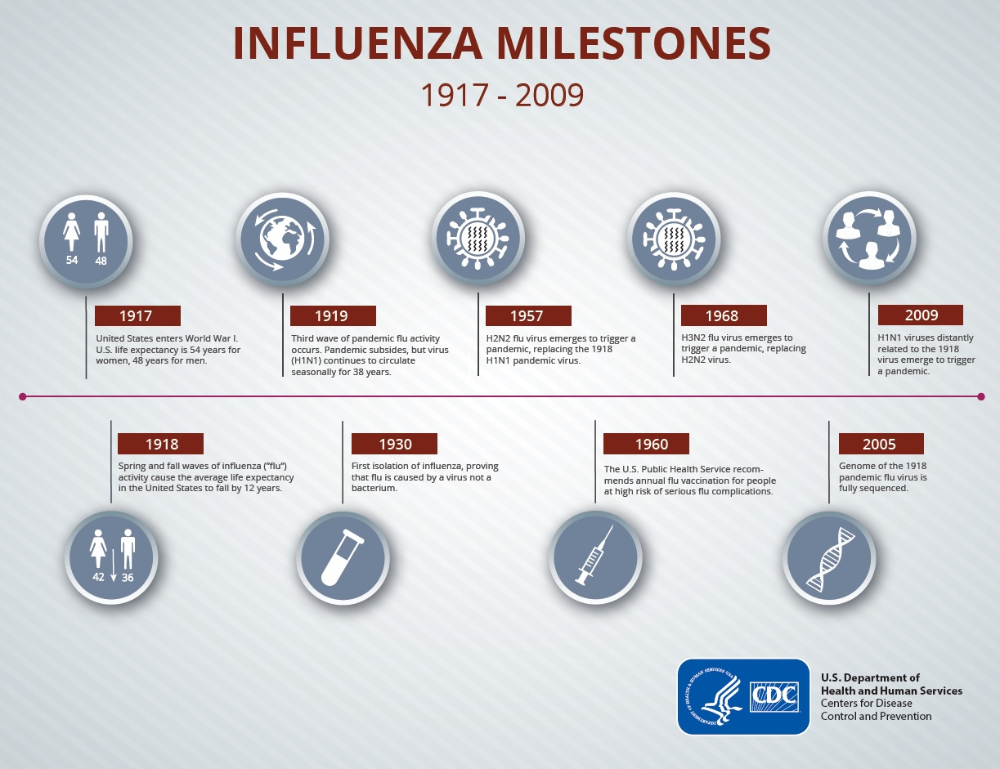
The 1957 influenza A/h3N2/pandemic revealed that 50% of women of childbearing age who died were pregnant, accounting for up to 10% of all influenza deaths. During the 2009 pandemic, maternal mortality in the Russian Federation from influenza and its complications was 83 cases (15.8% of the total).
The need for pregnant women with influenza for medical care in the intensive care unit is 10 times higher than for patients diagnosed with influenza from other categories of the population. After analyzing the most critical period of pregnancy for a woman, experts concluded that the majority of deaths occur in the third trimester of gestation. nine0003
nine0003
The 2009 influenza A/h2N1/v pandemic allowed the WHO Advisory Committee on Immunization Practices to place pregnant women in the highest priority group for influenza immunization.
Vaccination of pregnant women against influenza with subunit and split vaccines has been planned for more than 20 years in a number of European and American countries, while its immunological efficiency reaches 70-85%.
Special studies conducted in the USA, which involved more than 2,000 women, have shown that immunization of pregnant women with modern inactivated vaccines does not affect the normal development of the fetus and does not cause undesirable post-vaccination effects. There is evidence that among infants born to mothers vaccinated against influenza during pregnancy, it reduces the incidence of influenza in children in the first 6 months of life by 50-63% due to the passive transfer of antibodies against influenza from mother to fetus. nine0003
The studies conducted in recent years by domestic scientists substantiate and expand the indications for vaccination of pregnant women with modern polymer-subunit preparations. It has been proven that pregnancy is not a factor limiting the formation of full-fledged post-vaccination immunity.
It has been proven that pregnancy is not a factor limiting the formation of full-fledged post-vaccination immunity.
It has been shown that vaccination of women in the second trimester of pregnancy with immunoadjuvant drugs in terms of immunogenicity and the duration of post-vaccination seroprotection in women themselves and their infants is preferable to vaccination in the third trimester of gestation. At the same time, non-adjuvanted subunit influenza vaccine forms a more pronounced humoral immunity to influenza when administered at late gestation. nine0003
Thus, influenza vaccination is strongly recommended for pregnant women and women planning pregnancy in the current epidemic season with inactivated influenza vaccines. Vaccination of pregnant women can be carried out at all stages of pregnancy. The best results are achieved in early pregnancy and in the 3rd trimester of pregnancy.
The decision to vaccinate pregnant and breastfeeding women should be made by the doctor individually, taking into account the particular health of the pregnant woman.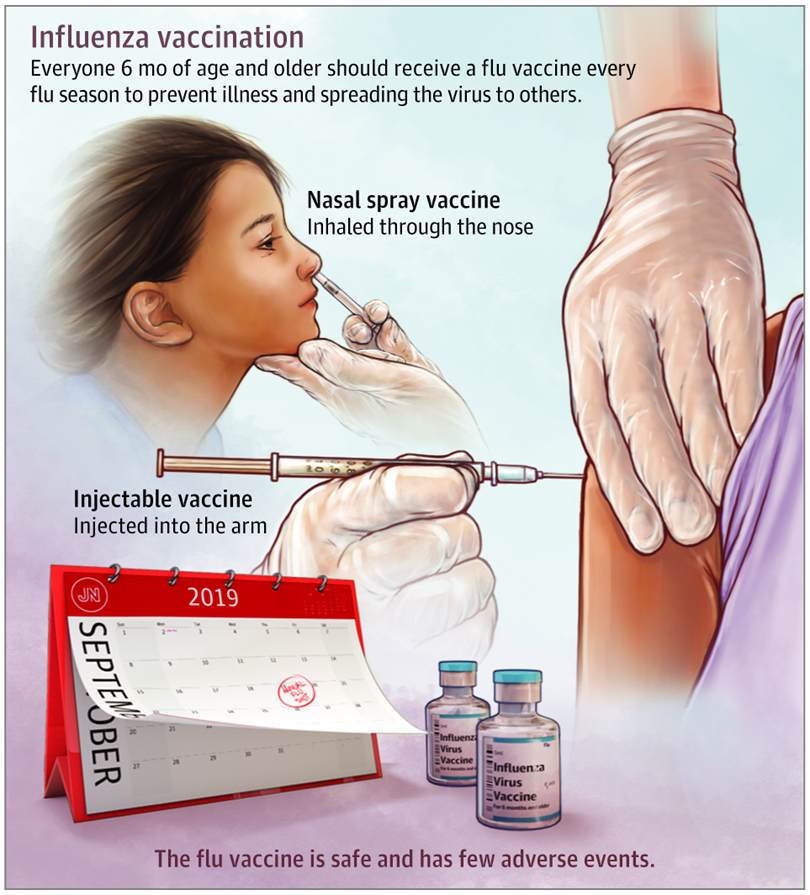 nine0003
nine0003
Vaccination is recommended during the second (preferably with immunoadjuvanted vaccines) and third (vaccines without adjuvants) trimester of pregnancy.
For vaccination, only inactivated vaccines that do not contain preservatives and are allowed in Russia should be used.
Live influenza vaccine is not indicated for vaccination in pregnant women.
According to the WHO and the Atlanta Center for Disease Control (CDC, Atlanta, US): nine0003
-
Vaccination of millions of pregnant women at various stages of pregnancy has shown no side effects for either pregnant women or their children.”
-
Vaccination is indicated at all stages of pregnancy.
-
Vaccination of pregnant women is carried out only by injectable vaccines by us. Live influenza nasal spray is not suitable for vaccination during pregnancy.
- Postpartum women may be vaccinated with any type of vaccine during any breastfeeding period.