Chromosome 21 test
Prenatal Testing for Down Syndrome | Patient Education
Down syndrome is a genetic condition caused by extra genes from the 21st chromosome. It results in certain characteristics, including some degree of cognitive disability and other developmental delays. Common physical traits include an upward slant of the eyes; flattened bridge of the nose; single, deep crease on the palm of the hand; and decreased muscle tone. A child with Down syndrome, however, may not have all these traits.
The incidence of Down syndrome in the United States is about 1 in 1,000 births. There is no association between Down syndrome and culture, ethnic group, socioeconomic status or geographic region.
Age-Related Risks
Generally, the chance of having a Down syndrome birth is related to the mother's age. Under age 25, the odds of having a child with Down syndrome are about 1 in 1,400. At age 35, the odds are about 1 in 350. At age 40, the odds are about 1 in 100.
Causes of Down Syndrome
There are three causes of Down syndrome:
Trisomy 21
An estimated 95 percent of people with Down syndrome have trisomy 21, meaning they have three number 21 chromosomes instead of two. We normally have 23 pairs of chromosomes, each made up of genes. During the formation of the egg and the sperm, a woman's or a man's pair of chromosomes normally split so that only one chromosome is in each egg or sperm. In trisomy 21, the 21st chromosome pair does not split and a double dose goes to the egg or sperm. An estimated 95 to 97 percent of the extra chromosome is of maternal origin.
Translocation
Translocation occurs in about 3 to 4 percent of people with Down syndrome. In this type, an extra part of the 21st chromosome gets stuck onto another chromosome. In about half of these situations, one parent carries the extra 21st chromosome material in a "balanced" or hidden form.
Mosaicism
In mosaicism, the person with Down syndrome has an extra 21st chromosome in some of the cells but not all of them. The other cells have the usual pair of 21st chromosomes. About 1 to 2 percent of people with Down syndrome have this type.
Prenatal Testing
Screening tests can identify women at increased risk of having a baby with Down syndrome. These tests have no risks of miscarriage, but can't determine with certainty whether a fetus is affected. Diagnostic tests, on the other hand, are extremely accurate at identifying certain abnormalities in the fetus, but carry a small — generally less than 1 percent — risk of miscarriage. We offer options for both screening and diagnostic testing.
These tests have no risks of miscarriage, but can't determine with certainty whether a fetus is affected. Diagnostic tests, on the other hand, are extremely accurate at identifying certain abnormalities in the fetus, but carry a small — generally less than 1 percent — risk of miscarriage. We offer options for both screening and diagnostic testing.
Continue reading
Screening Tests
Sequential Integrated Screening — Sequential integrated screening is offered to all pregnant women by the state of California. This non-invasive screening is performed in two steps.
In the first step, which is performed between 10 and 14 weeks of pregnancy, a blood sample is taken from the mother and a nuchal translucency ultrasound is performed to measure the amount of fluid at the back of the baby's neck. If the blood test is scheduled prior to the ultrasound, we can provide the results at the end of the ultrasound appointment. The results of the blood test, the nuchal translucency measurement and the mother's age are used to estimate the risk for Down syndrome and trisomy 18.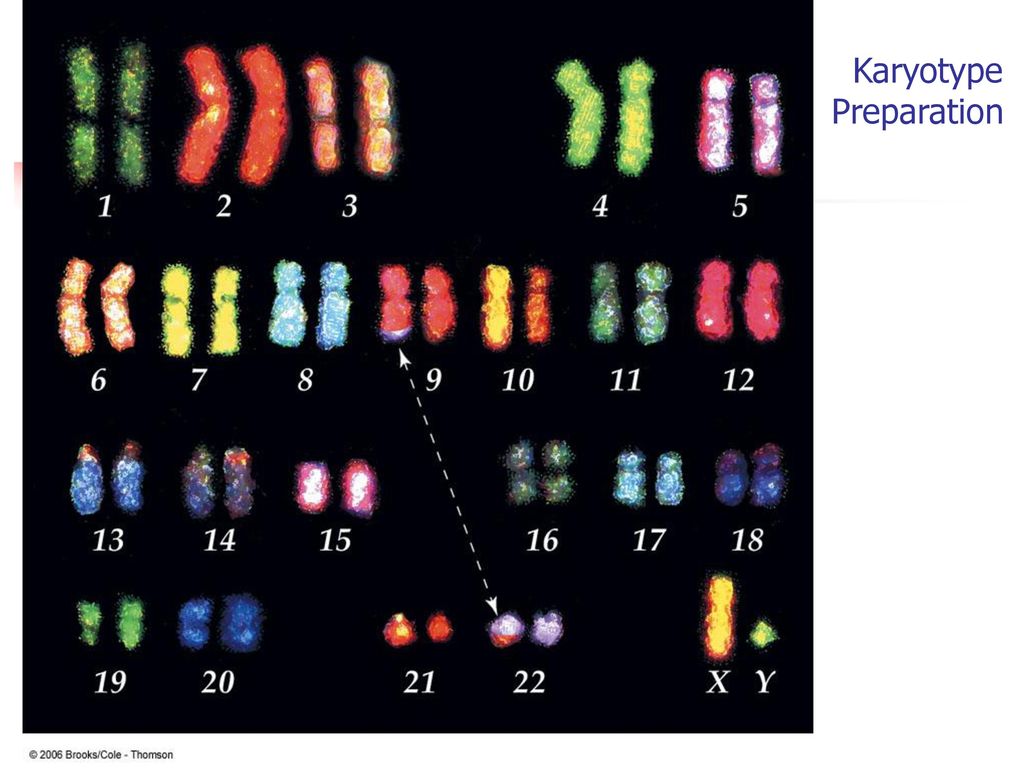
The second step is a maternal blood test between 15 to 20 weeks of pregnancy. When the results of this blood test are combined with the results from the first trimester blood test and nuchal translucency ultrasound, the detection rate for Down syndrome increases. This test also provides a personal risk assessment for having a fetus with trisomy 18, Smith-Lemli-Opitz syndrome, an open neural tube defect or an abdominal wall defect.
Diagnostic Tests
Amniocentesis, chorionic villus sampling (CVS) and ultrasound are the three primary procedures for diagnostic testing.
Amniocentesis — Amniocentesis is used most commonly to identify chromosomal problems such as Down syndrome. When the fetus is known to be at risk, it can detect other genetic diseases like cystic fibrosis, Tay-Sachs disease and sickle cell disease.
An amniocentesis procedure for genetic testing is typically performed between 15 and 20 weeks of pregnancy. Under ultrasound guidance, a needle is inserted through the abdomen to remove a small amount of amniotic fluid.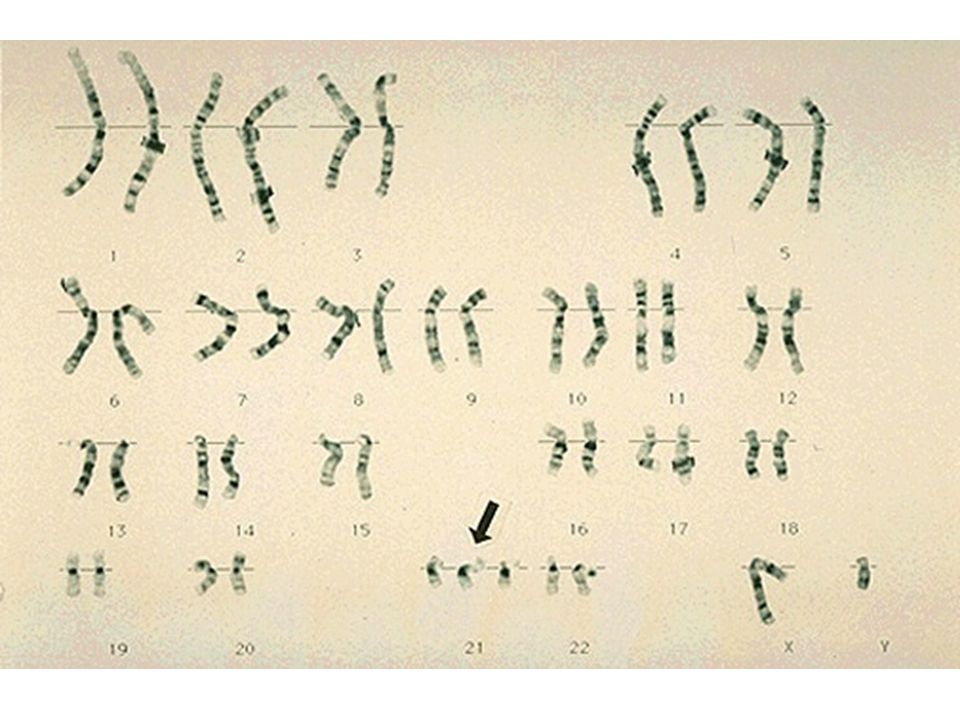 The cells from the fluid are then cultured and a karyotype analysis — an analysis of the chromosomal make-up of the cells — is performed. It takes about two weeks to receive the results of the test.
The cells from the fluid are then cultured and a karyotype analysis — an analysis of the chromosomal make-up of the cells — is performed. It takes about two weeks to receive the results of the test.
Amniocentesis detects most chromosomal disorders, such as Down syndrome, with a high degree of accuracy. Testing for other genetic diseases, such as Tay-Sachs disease, is not routinely performed but can be detected through specialized testing if your fetus is known to be at risk. Testing for neural tube defects, such as spina bifida, also can be performed.
There is a small risk of miscarriage as a result of amniocentesis — about 1 in 100 or less. Miscarriage rates for procedures performed at UCSF Medical Center are less than 1 in 350.
Chorionic Villus Sampling (CVS) — Like amniocentesis, chorionic villus sampling is used most commonly to identify chromosomal problems such as Down syndrome. It can detect other genetic diseases like cystic fibrosis, Tay-Sachs disease and sickle cell disease in at-risk fetuses. The main advantage of CVS over amniocentesis is that it is done much earlier in pregnancy, at 10 to 12 weeks rather than 15 to 20 weeks.
The main advantage of CVS over amniocentesis is that it is done much earlier in pregnancy, at 10 to 12 weeks rather than 15 to 20 weeks.
CVS involves removing a tiny piece of tissue from the placenta. Under ultrasound guidance, the tissue is obtained either with a needle inserted through the abdomen or a catheter inserted through the cervix. The tissue is then cultured and a karyotype analysis of the chromosomal make-up of the cells is performed. It takes about two weeks to receive the results.
The advantage of CVS over amniocentesis is that the test is performed much earlier in pregnancy, so results are typically available by the end of the third month. A disadvantage is that spinal cord defects cannot be detected. Expanded alpha fetoprotein (AFP) blood testing or ultrasound can be performed later in the pregnancy to screen for spinal cord defects.
There is a small risk of miscarriage as a result of CVS — 1 in 100 or less. Miscarriage rates for procedures performed at UCSF Medical Center are less than 1 in 350.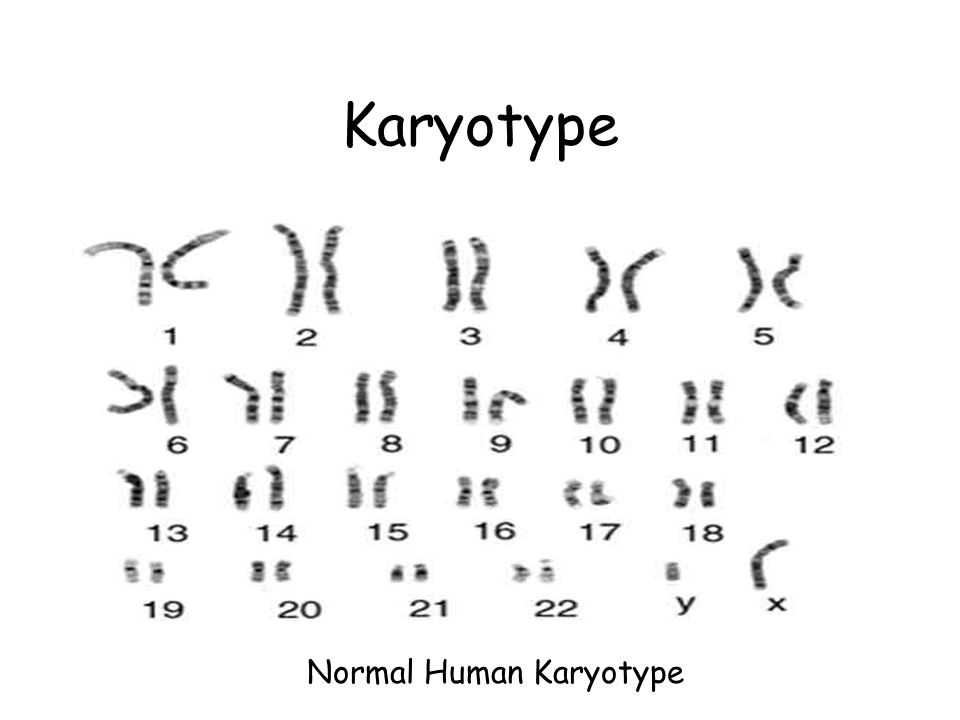
Ultrasound — The primary purpose of ultrasound is to determine the status of a pregnancy — the due date, size of the fetus and if the mother is carrying multiples. Ultrasound also can provide some information about possible birth defects in a fetus. All patients at UCSF Medical Center undergo a comprehensive ultrasound examination before any invasive tests are performed. Results of the ultrasound are explained at the time of the visit.
In some patients, an ultrasound raises concern of a possible abnormality in the fetus. We have extensive experience in performing and interpreting ultrasounds in pregnancy.
If You Receive a Positive Result
If you receive positive results on a screening test, we recommend that you discuss this with your doctor and a genetic counselor. Options for further diagnostic testing will be explained. The decision as to whether to have invasive genetic testing is up to you.
If a diagnostic test finds a genetic abnormality, the significance of such results should be discussed with experts familiar with the condition, including a medical geneticist and a genetic counselor, as well as your own doctor.
MaterniT 21 Plus | Labcorp
Find Locations
For hours, walk-ins and appointments.
Locate Me
Reason for your visit
- Select Service -Routine labworkPediatric collectionsEmployee wellness with body measurementH pylori breath testEmployment drug testingRadar (point of collection drug screen)Saliva alcohol testingHair collectionCOVID-19 Unexposed/Symptom Free
With a blood draw from you as early as nine weeks into your pregnancy, the MaterniT® 21 PLUS test can screen for certain chromosomal abnormalities that could affect your baby’s health and development, providing you with more information earlier in your pregnancy.
What it screens for—and why
Like most noninvasive prenatal tests (NIPT), MaterniT 21 PLUS screens for certain chromosomal abnormalities called trisomies, including trisomy 21 (Down syndrome), trisomy 18 (Edwards syndrome), and trisomy 13 (Patau syndrome).
But it digs deeper, screening for certain sex chromosome aneuploidies (SCAs, abnormal numbers of X or Y chromosomes) and select microdeletions (missing parts of chromosomes).
While rare, these chromosomal abnormalities can have profound consequences in the life and health of your child. Detecting this information early can help your doctor recommend specialized care for you and your baby, before and after delivery.
The MaterniT 21 PLUS test detects the following chromosomal abnormalities:
| TRISOMIES |
|---|
| Trisomy 21 (Down syndrome) |
| Trisomy 18 (Edwards syndrome) |
| Trisomy 13 (Patau syndrome) |
| Trisomy 16* |
| Trisomy 22* |
| SCAS* |
|---|
| 45,X (Turner syndrome) |
| 47,XXY (Klinefelter syndrome) |
| 47,XXX (Triple X syndrome) |
| 47,XYY (XYY syndrome) |
| MICRODELETIONS | |
|---|---|
| 22q (DiGeorge syndrome)* | 11q (Jacobsen syndrome)* |
| 5p (Cri-du-chat syndrome)* | 8q (Langer-Giedion syndrome)* |
| 1p36 deletion syndrome* | 4p (Wolf-Hirschhorn syndrome)* |
| 15q (Prader-Willi syndrome; Angelman syndrome)* | |
* Reported as an additional finding.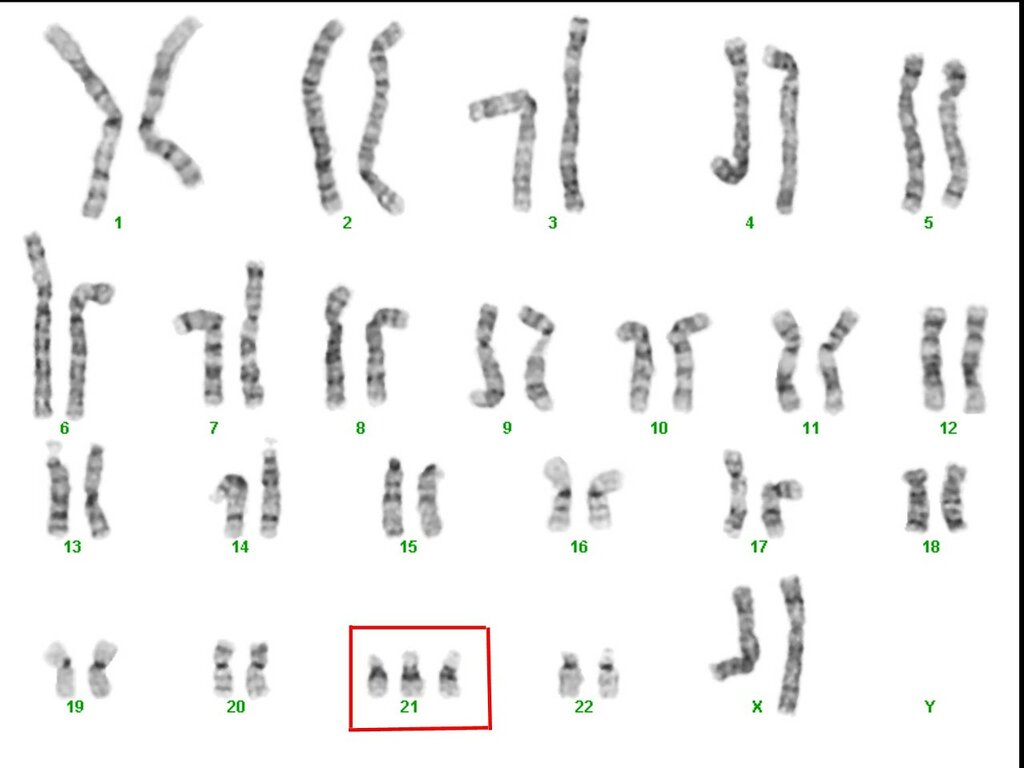 Talk to your doctor about your options.
Talk to your doctor about your options.
Why “noninvasive?”
There are many ways to get this information, including methods such as serum screens and diagnostic procedures such as amniocentesis.
As a noninvasive prenatal test, MaterniT 21 PLUS is different from both. It has higher detection rates than serum screening1 (determined to be 97.9% positive predictive value for trisomy 21 in a high-risk cohort2), and requires only a blood draw from the mother; amniocentesis requires withdrawing fluid from around the developing baby.
Most women who get the MaterniT 21 PLUS will screen negative for chromosomal abnormalities and may not require further testing.
However, any patient with a positive test result may be offered genetic counseling and/or diagnostic testing for confirmation of test results.
Clear results, delivered quickly
The test delivers clear positive or negative results for well known chromosomal abnormalities, such as trisomy 21 (Down syndrome), typically returned in about five days from the receipt of your blood draw at our lab in California.
Also, if you’re carrying twins, MaterniT 21 PLUS can detect common chromosomal abnormalities in multiple gestation pregnancies.
References
- Practice Bulletin No. 163 Summary. (2016). Obstetrics & Gynecology, 127(5), 979-981.
- Porreco RP, Garite TJ, Maurel K et al. Noninvasive prenatal screening for fetal trisomies 21, 18, 13 and the common sex chromosome aneuploidies from maternal blood using massively parallel genomic sequencing of DNA. Am J Obstet Gynecol 2014;211:365.e1–12.
- llumina. https://www.illumina.com/clinical/illumina_clinical_laboratory/verifi-prenatal-tests.html. Accessed August 15, 2018.
Trisomy test (13,18 and 21)
TRISOMY test is a quick, easy and safe method to learn more about the health of your unborn child.
The TRISOMY test is designed to rule out the most common genetic disorders (trisomy) of chromosomes 21, 18 and 13 that cause Down, Edwards and Patau syndromes.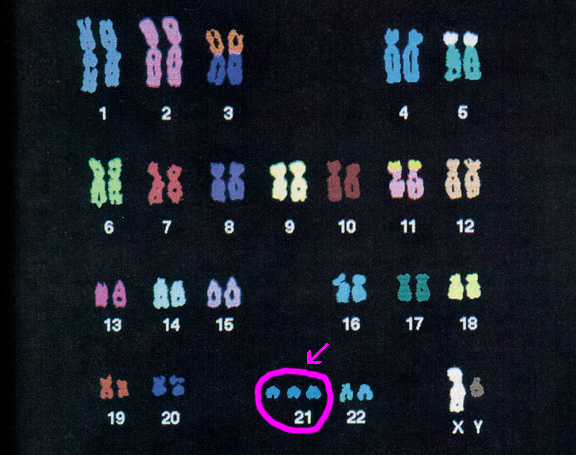 Before taking the test, you should consult a specialist in gynecology and obstetrics or a clinical geneticist.
Before taking the test, you should consult a specialist in gynecology and obstetrics or a clinical geneticist.
Non-Invasive Prenatal Testing (NIPT) offered by various laboratories detects specific chromosome 21, 18 and 13 abnormalities and sex chromosome abnormalities. The tests give results that do not reveal any information about genetic or morphological fetal or maternal disorders other than those for which they are intended. Positive test results obtained with NIPT require confirmation by an invasive method.
Trisomy 21 (Down syndrome)
NIPT screening can detect more than 99% of cases of trisomy 21. Overall, only 1 in 1650 trisomy 21 as part of NIPT results based on normal pregnancy (0.06%)* was identified as false positive. With respect to the TRISOMY test, only 1 out of 1,842 results based on normal pregnancy was found to be false positive (i.e., less than 0.05%). The results of the latest validation study, which included a set of samples obtained from pregnant women and a set of samples containing embryonic trisomy 21, showed that the TRISOMY test is very sensitive.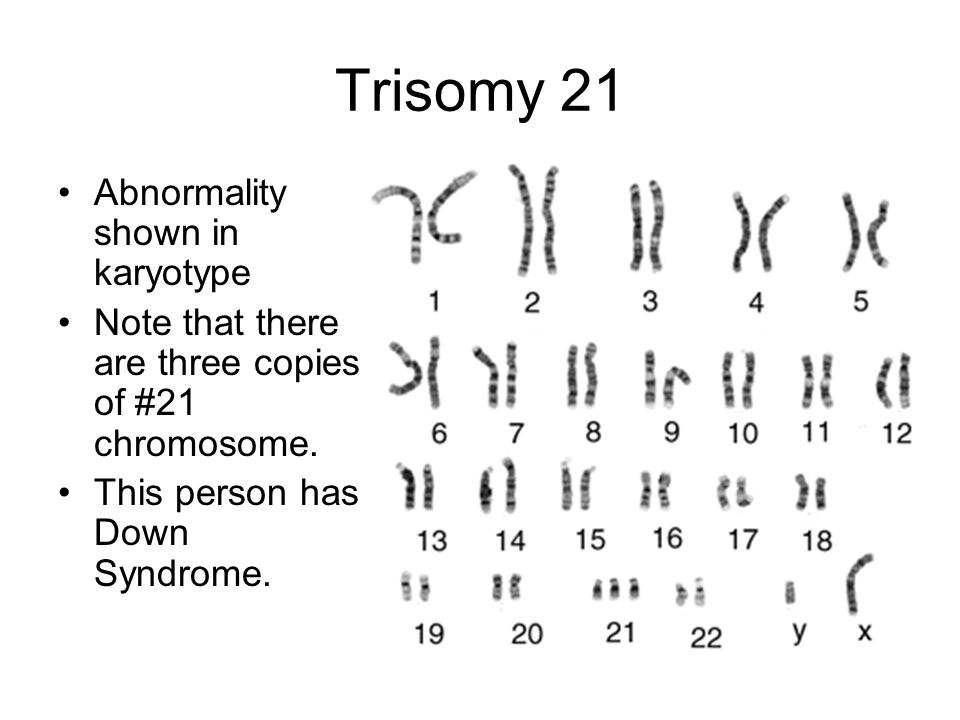
Trisomy 18 (Edwards syndrome)
The sensitivity of NIPT in detecting trisomy 18 is lower than the sensitivity in detecting trisomy 21, namely in the amount of 90% *. The false positive rate of the TRISOMY test is 0.01%*. The false negative rate of the TRISOMY test (1 in 9 cases*) is comparable to the global rate (1 in 10 cases*).
Trisomy 13 (Patau syndrome)
Cases of trisomy 13 are rare in prospective studies, making calculations of the sensitivity and specificity of NIPT for this type of syndrome quite complex. In a recent international study based on 11,185 samples, 2 out of 2 positive cases* were successfully identified. The false positive rate for this type of trisomy in this particular study reached 0.02%*. With regard to the TRISOMY test screening test, 3 out of 3 trisomies were correctly identified within 13 cases, with a false positive rate of 0.05%.
TRISOMY test is a non-invasive maternal blood test that, due to its high sensitivity and specificity, can exclude the presence of chromosomal abnormalities already after the 11th week of pregnancy. The test can also determine the sex of the unborn child if the mother-to-be wishes to know.
The test can also determine the sex of the unborn child if the mother-to-be wishes to know.
The TRISOMY test is suitable for any pregnant woman as early as the 11th week of pregnancy.
Compared to traditional screening methods, the TRISOMY test has higher sensitivity and specificity for the types of trisomy for which it is intended. As a result, it provides a significantly lower number of false positives.
The TRISOMY test is suitable for pregnant women who:
are anxious about possible health problems for the unborn child caused by the presence of one of the studied types of trisomy;
who are 35 or older at birth and have a negative biochemical screening test;
conceived as a result of IVF,
had a positive biochemical screening*,
received an ultrasound screening result indicating a higher risk of traceable trisomy types,*
have had a fetal chromosomal abnormality in the past,*
their parent(s) have been diagnosed with a Robertsonian translocation (increased risk of trisomy 13 or 21),*
suffer from recurrent miscarriage. *
*
*Medical genetic counseling should be done prior to the test.
TRISOMY test is also useful in case of gynecological and obstetrical contraindications that may complicate invasive prenatal testing (amniocentesis), for example:
- increased risk of miscarriage,
- current anticoagulant therapy (low blood clotting),
- immune risk due to Rh incompatibility (Rhesus negative),
- gestational age from 14 to 16 weeks of gestation (increased risk of complications due to amniocentesis),
- placenta previa,
- uterine fibroids.
Consult your gynecologist/obstetrician for any obstetric contraindications.
Consultation with an OB/GYN or clinical geneticist is required prior to non-invasive prenatal testing.
TRISOMY test results
Non-invasive prenatal testing results provide information on a selected number of the most common genetic chromosomal disorders (up to 85% of all fetal genetic disorders).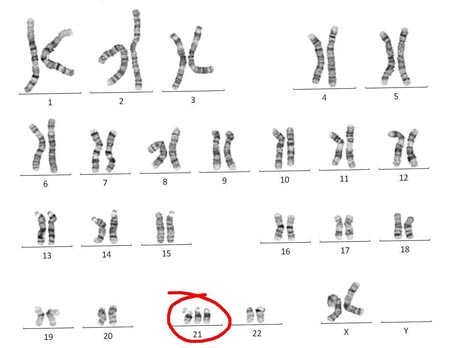 It should also be remembered that testing cannot detect all existing genetic or developmental disorders.
It should also be remembered that testing cannot detect all existing genetic or developmental disorders.
If your NIPT test is negative, it will help you avoid invasive diagnostic tests such as amniocentesis, which carry certain risks associated with taking amniotic fluid.
The sensitivity and specificity of the TRISOMY test are over 99%.
Attention: Despite the high sensitivity and specificity in the detection of fetal trisomy on the 21st, 18th and 13th chromosomes, the TRISOMY test is considered a type of screening and not a diagnostic method. Therefore, a positive result should always be confirmed by taking amniotic fluid or chorionic villi.
A negative TRISOMY test result does not need to be confirmed by diagnostic amniocentesis, which means that in most cases the mother-to-be can avoid invasive tests and their associated risks.
If the TRISOMY test is positive, you should consult with a geneticist who will refer you for the examination required in this situation.
Non-Invasive Prenatal Test - NIPT Prenetics
Non-Invasive Prenatal Test is a genetic screening test based on the blood of a pregnant woman to rule out the most common fetal chromosomal abnormalities such as Down syndrome (extra 21 chromosome), Edwards syndrome (extra 18 chromosome) and Patau syndrome (extra 13 chromosome), Turner and Kleinfelter syndrome (change in the number of sex chromosomes). Currently, the world medical communities recommend the use of NIPT as a screening test in pregnant women of any age and risk group, indicating that the results of non-invasive testing must be confirmed using diagnostic methods. A significant difference from traditional biochemical screening, which is carried out in the first trimester, is the analysis of not indirect markers (hormones), but directly the biomaterial (DNA) of the fetus. Unlike invasive procedures, where the material of the fetus, cells, is examined, NIPT is absolutely safe for both the mother and the fetus itself. The test is based on determining the percentage of the sequence of short fragments of fetal DNA circulating in the mother's bloodstream, starting from the 5th week of pregnancy. After delivery, the fetal DNA disappears from the mother's bloodstream within two hours. The proportion of fetal DNA in the mother's blood increases with increasing gestational age and decreases with increasing body weight. Every woman can take a blood test starting from 10 obstetric weeks, when the necessary concentration of fetal DNA for analysis is obtained. The sensitivity and specificity of NIPT can be as high as 99.9%, with a false positive rate of 0.1% (a high risk of the syndrome was identified, but it was not confirmed) in the detection of such a common disease as Down syndrome, despite the fact that the same indicators with combined screening have values of 85%, and the test may not be confirmed in about 15% of women.
The test is based on determining the percentage of the sequence of short fragments of fetal DNA circulating in the mother's bloodstream, starting from the 5th week of pregnancy. After delivery, the fetal DNA disappears from the mother's bloodstream within two hours. The proportion of fetal DNA in the mother's blood increases with increasing gestational age and decreases with increasing body weight. Every woman can take a blood test starting from 10 obstetric weeks, when the necessary concentration of fetal DNA for analysis is obtained. The sensitivity and specificity of NIPT can be as high as 99.9%, with a false positive rate of 0.1% (a high risk of the syndrome was identified, but it was not confirmed) in the detection of such a common disease as Down syndrome, despite the fact that the same indicators with combined screening have values of 85%, and the test may not be confirmed in about 15% of women.
In 2015, a large Noninvasive Examination of Trisomy study was conducted, which included a survey of almost 16,000 pregnant women of different ages from different risk groups from 35 medical centers in Europe and the USA.
As a result, non-invasive extracellular DNA testing was shown to be more sensitive and specific than standard screening for the detection of trisomy 21. False positive test results were almost 100 times lower than with standard screening, regardless of maternal age. It is especially noteworthy that in the group of patients examined at low risk according to the results of the study of freely circulating DNA, none of the children was born with trisomy 21, which indicates an extremely low number of false negative results, which are the most important indicator of screening methods.
Despite the fact that the non-invasive prenatal test has rather high parameters of sensitivity and specificity, a 100% reliable result could not be obtained due to a number of limitations:
-
Maternal cancers
-
Presence of balanced rearrangements of chromosomes in parents
-
Maternal aneuploidy (change in the number of chromosomes in pairs)
-
Vanishing Twin Syndrome
- Organ transplantation and blood transfusion
-
Bone marrow transplantation and stem cell treatment
-
Mosaicism (cells have a different chromosome set)
-
Pregnancy with more than two fetuses
NIPT Prenetix is recommended for pregnant women of all ages and all risk groups from week 10 of pregnancy.