Big for gestational age
Large for Gestational Age and Obesity-Related Comorbidities
1. Bocca-Tjeertes IF, Kerstjens JM, Reijneveld SA, Veldman K, Bos AF, de Winte AF. Growth patterns of large for gestational age children up to age 4 years. Pediatrics. 2014;133:e643–9. doi: 10.1542/peds.2013-0985. [PubMed] [CrossRef] [Google Scholar]
2. Chiavaroli V, Derraik JG, Hofman PL, Cutfield WS. Born large for gestational age: bigger is not always better. J Pediatr. 2016;170:307–11. doi: 10.1016/j.jpeds.2015.11.043. [PubMed] [CrossRef] [Google Scholar]
3. Koyanagi A, Zhang J, Dagvadorj A, Hirayama F, Shibuya K, Souza JP, et al. Macrosomia in 23 developing countries: an analysis of a multicountry, facility-based, cross-sectional survey. Lancet. 2013;381:476–83. doi: 10.1016/S0140-6736(12)61605-5. [PubMed] [CrossRef] [Google Scholar]
4. Palatianou ME, Simos YV, Andronikou SK, Kiortsis DN. Long-term metabolic effects of high birth weight: a critical review of the literature. Horm Metab Res. 2014;46:911–20. doi: 10.1055/s-0034-1395561. [PubMed] [CrossRef] [Google Scholar]
5. Hong YH, Chung S. Small for gestational age and obesity related comorbidities. Ann Pediatr Endocrinol Metab. 2018;23:4–8. doi: 10.6065/apem.2018.23.1.4. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
6. Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non- insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–7. doi: 10.1007/BF00399095. [PubMed] [CrossRef] [Google Scholar]
7. Moldéus K, Cheng YW, Wikström AK, Stephansson O. Induction of labor versus expectant management of large-for-gestational-age infants in nulliparous women. PLoS One. 2017;12:e0180748. doi: 10.1371/journal.pone.0180748. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
8. Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
1991;303:1019–22. doi: 10.1136/bmj.303.6809.1019. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
9. Johnsson IW, Haglund B, Ahlsson F, Gustafsson J. A high birth weight is associated with increased risk of type 2 diabetes and obesity. Pediatr Obes. 2015;10:77–83. doi: 10.1111/ijpo.230. [PubMed] [CrossRef] [Google Scholar]
10. Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev. 2011;12:525–42. doi: 10.1111/j.1467-789X.2011.00867.x. [PubMed] [CrossRef] [Google Scholar]
11. Tian JY, Cheng Q, Song XM, Li G, Jiang GX, Gu YY, et al. Birth weight and risk of type 2 diabetes, abdominal obesity and hypertension among Chinese adults. Eur J Endocrinol. 2006;155:601–7. doi: 10.1530/eje.1.02265. [PubMed] [CrossRef] [Google Scholar]
12. Kim SY, Sharma AJ, Sappenfield W, Wilson HG, Salihu HM. Association of maternal body mass index, excessive weight gain, and gestational diabetes mellitus with large-for-gestational-age births. Obstet Gynecol. 2014;123:737–44. doi: 10.1097/AOG.0000000000000177. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Obstet Gynecol. 2014;123:737–44. doi: 10.1097/AOG.0000000000000177. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
13. Persson M, Pasupathy D, Hanson U, Norman M. Birth size distribution in 3,705 infants born to mothers with type 1 diabetes: a population-based study. Diabetes Care. 2011;34:1145–9. doi: 10.2337/dc10-2406. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
14. Persson M, Fadl H, Hanson U, Pasupathy D. Disproportionate body composition and neonatal outcome in offspring of mothers with and without gestational diabetes mellitus. Diabetes Care. 2013;36:3543–8. doi: 10.2337/dc13-0899. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
15. Kong L, Nilsson IAK, Gissler M, Lavebratt C. Associations of maternal diabetes and body mass index with offspring birth weight and prematurity. JAMA Pediatr. 2019;173:371–8. doi: 10.1001/jamapediatrics.2018.5541. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
16. Johnson JW, Longmate JA, Frentzen B.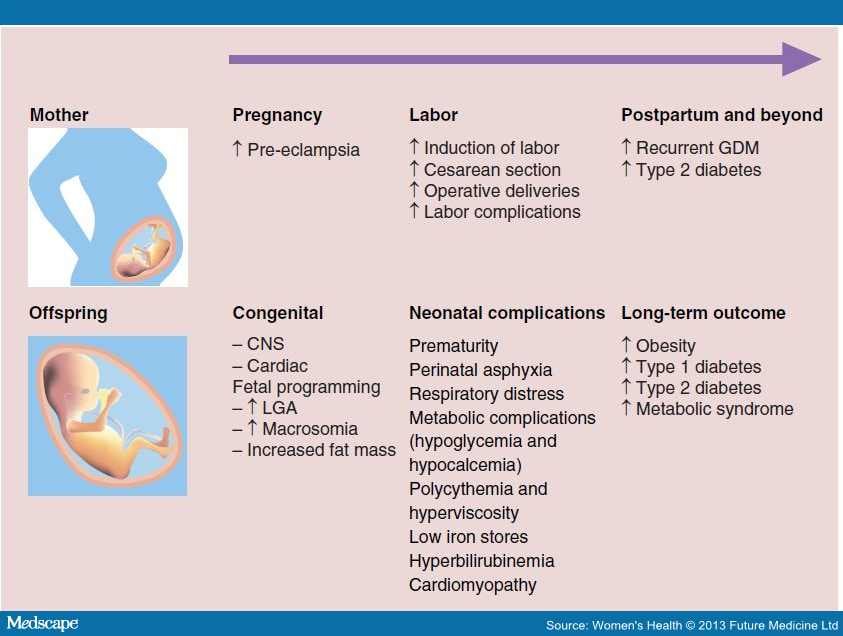 Excessive maternal weight and pregnancy outcome. Am J Obstet Gynecol. 1992;167:353–70. doi: 10.1016/S0002-9378(11)91414-8. [PubMed] [CrossRef] [Google Scholar]
Excessive maternal weight and pregnancy outcome. Am J Obstet Gynecol. 1992;167:353–70. doi: 10.1016/S0002-9378(11)91414-8. [PubMed] [CrossRef] [Google Scholar]
17. Frentzen BH, Dimperio DL, Cruz AC. Maternal weight gain: effect on infant birth weight among overweight and average-weight low-income women. Am J Obstet Gynecol. 1988;159:1114–7. doi: 10.1016/0002-9378(88)90424-3. [PubMed] [CrossRef] [Google Scholar]
18. Reynolds RM, Allan KM, Raja EA, Bhattacharya S, McNeill G, Hannaford PC, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ. 2013;347:f4539. doi: 10.1136/bmj.f4539. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
19. O'Reilly JR, Reynolds RM. The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf) 2013;78:9–16. doi: 10.1111/cen.12055. [PubMed] [CrossRef] [Google Scholar]
20. Alavi N, Haley S, Chow K, McDonald SD. Comparison of national gestational weight gain guidelines and energy intake recommendations. Obes Rev. 2013;14:68–85. doi: 10.1111/j.1467-789X.2012.01059.x. [PubMed] [CrossRef] [Google Scholar]
Comparison of national gestational weight gain guidelines and energy intake recommendations. Obes Rev. 2013;14:68–85. doi: 10.1111/j.1467-789X.2012.01059.x. [PubMed] [CrossRef] [Google Scholar]
21. Ekelund U, Ong K, Linné Y, Neovius M, Brage S, Dunger DB, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–30. doi: 10.1093/ajcn/83.2.324. [PubMed] [CrossRef] [Google Scholar]
22. Cnattingius S, Villamor E, Lagerros YT, Wikström AK, Granath F. High birth weight and obesity: a vicious circle across generations. Int J Obes (Lond) 2012;36:1320–4. doi: 10.1038/ijo.2011.248. [PubMed] [CrossRef] [Google Scholar]
23. Eriksen W, Sundet JM, Tambs K. Birth weight and the risk of overweight in young men born at term. Am J Hum Biol. 2015;27:564–9. doi: 10.1002/ajhb.22689. [PubMed] [CrossRef] [Google Scholar]
24. Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D.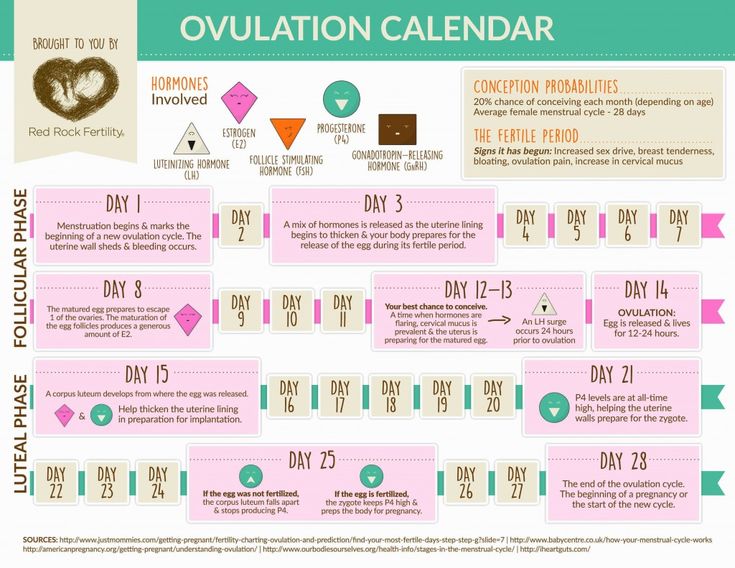 Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25:735–40. doi: 10.1038/sj.ijo.0801602. [PubMed] [CrossRef] [Google Scholar]
Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25:735–40. doi: 10.1038/sj.ijo.0801602. [PubMed] [CrossRef] [Google Scholar]
25. Gunnarsdottir I, Birgisdottir BE, Benediktsson R, Gudnason V, Thorsdottir I. Association between size at birth, truncal fat and obesity in adult life and its contribution to blood pressure and coronary heart disease; study in a high birth weight population. Eur J Clin Nutr. 2004;58:812–8. doi: 10.1038/sj.ejcn.1601881. [PubMed] [CrossRef] [Google Scholar]
26. Johannsson E, Arngrimsson SA, Thorsdottir I, Sveinsson T. Tracking of overweight from early childhood to adolescence in cohorts born 1988 and 1994: overweight in a high birth weight population. Int J Obes (Lond) 2006;30:1265–71. doi: 10.1038/sj.ijo.0803253. [PubMed] [CrossRef] [Google Scholar]
27. Gu S, An X, Fang L, Zhang X, Zhang C, Wang J, et al. Risk factors and long-term health consequences of macrosomia: a prospective study in Jiangsu Province, China.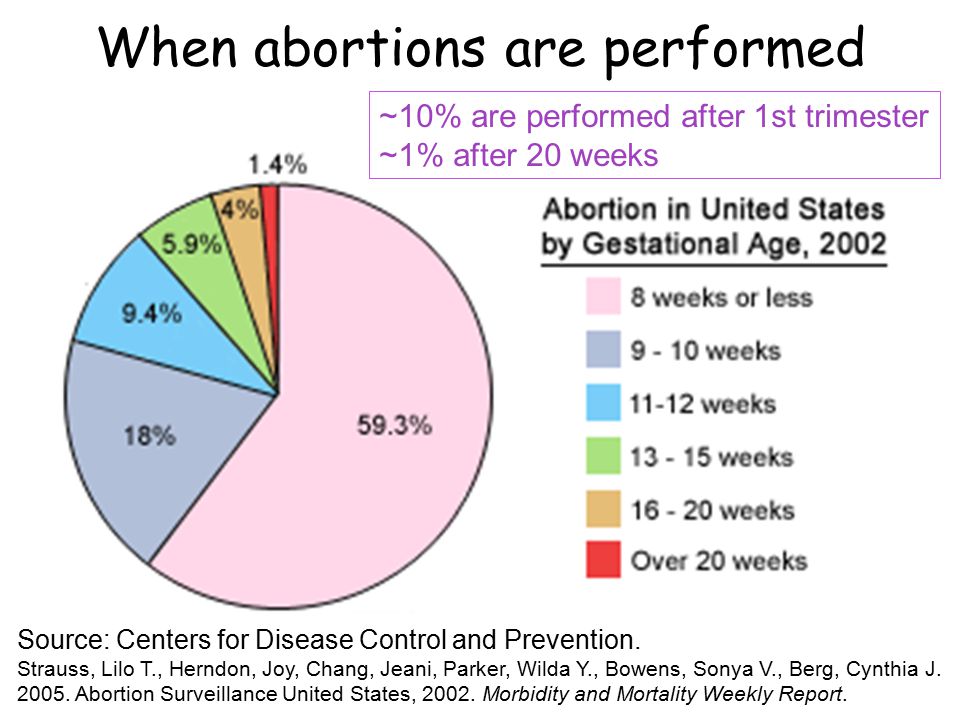 J Biomed Res. 2012;26:235–40. doi: 10.7555/JBR.26.20120037. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
J Biomed Res. 2012;26:235–40. doi: 10.7555/JBR.26.20120037. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
28. Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long-term overweight risk: systematic review and a meta-analysis including 643,902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7:e47776. doi: 10.1371/journal.pone.0047776. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
29. Kaul P, Bowker SL, Savu A, Yeung RO, Donovan LE, Ryan EA. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia. 2019;62:249–58. doi: 10.1007/s00125-018-4758-0. [PubMed] [CrossRef] [Google Scholar]
30. Derraik JG, Maessen SE, Gibbins JD, Cutfield WS, Lundgren M, Ahlsson F. Large-for-gestational-age phenotypes and obesity risk in adulthood: a study of 195,936 women. Sci Rep. 2020;10:2157. doi: 10.1038/s41598-020-58827-5. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
31. Cottrell EC, Ozanne SE. Early life programming of obesity and metabolic disease. Physiol Behav. 2008;94:17–28. doi: 10.1016/j.physbeh.2007.11.017. [PubMed] [CrossRef] [Google Scholar]
Cottrell EC, Ozanne SE. Early life programming of obesity and metabolic disease. Physiol Behav. 2008;94:17–28. doi: 10.1016/j.physbeh.2007.11.017. [PubMed] [CrossRef] [Google Scholar]
32. Xie C, Wang Y, Li X, Wen X. Childhood growth trajectories of etiological subgroups of large for gestational age newborns. J Pediatr. 2016;170:60–6.e1. doi: 10.1016/j.jpeds.2015.11.031. [PubMed] [CrossRef] [Google Scholar]
33. Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–94. doi: 10.2337/db06-0436. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
34. Lin XH, Wu DD, Gao L, Zhang JY, Pan HT, Wang H, et al. Altered DNA methylation in neonates born large-for-gestational-age is associated with cardiometabolic risk in children. Oncotarget. 2016;7:86511–21. doi: 10.18632/oncotarget.13442. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
35. Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101:E5. doi: 10.1542/peds.101.3.e5. [PubMed] [CrossRef] [Google Scholar]
Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101:E5. doi: 10.1542/peds.101.3.e5. [PubMed] [CrossRef] [Google Scholar]
36. Wang X, Liang L, Junfen FU, Lizhong DU. Metabolic syndrome in obese children born large for gestational age. Indian J Pediatr. 2007;74:561–5. doi: 10.1007/s12098-007-0108-9. [PubMed] [CrossRef] [Google Scholar]
37. Kahn HS, Narayan KM, Williamson DF, Valdez R. Relation of birth weight to lean and fat thigh tissue in young men. Int J Obes Relat Metab Disord. 2000;24:667–72. doi: 10.1038/sj.ijo.0801211. [PubMed] [CrossRef] [Google Scholar]
38. Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–30. doi: 10.1093/ajcn/77.3.726. [PubMed] [CrossRef] [Google Scholar]
39. Barker M, Robinson S, Osmond C, Barker DJ. Birth weight and body fat distribution in adolescent girls. Arch Dis Child. 1997;77:381–3. doi: 10.1136/adc.77.5.381. [PubMed] [CrossRef] [Google Scholar]
Arch Dis Child. 1997;77:381–3. doi: 10.1136/adc.77.5.381. [PubMed] [CrossRef] [Google Scholar]
40. Byberg L, McKeigue PM, Zethelius B, Lithell HO. Birth weight and the insulin resistance syndrome: association of low birth weight with truncal obesity and raised plasminogen activator inhibitor-1 but not with abdominal obesity or plasma lipid disturbances. Diabetologia. 2000;43:54–60. doi: 10.1007/s001250050007. [PubMed] [CrossRef] [Google Scholar]
41. Perng W, Hajj H, Belfort MB, Rifas-Shiman SL, Kramer MS, Gillman MW, et al. Birth size, early life weight gain, and midchildhood cardiometabolic health. J Pediatr. 2016;173:122–30. doi: 10.1016/j.jpeds.2016.02.053. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
42. Liu J, Au Yeung SL, He B, Kwok MK, Leung GM, Schooling CM. The effect of birth weight on body composition: evidence from a birth cohort and a Mendelian randomization study. PLoS One. 2019;14:e0222141. doi: 10.1371/journal.pone.0222141. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
43. Kang M, Yoo JE, Kim K, Choi S, Park SM. Associations between birth weight, obesity, fat mass and lean mass in Korean adolescents: the Fifth Korea National Health and Nutrition Examination Survey. BMJ Open. 2018;8:e018039. doi: 10.1136/bmjopen-2017-018039. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Kang M, Yoo JE, Kim K, Choi S, Park SM. Associations between birth weight, obesity, fat mass and lean mass in Korean adolescents: the Fifth Korea National Health and Nutrition Examination Survey. BMJ Open. 2018;8:e018039. doi: 10.1136/bmjopen-2017-018039. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
44. de Zegher F, Pérez-Cruz M, Sebastiani G, Díaz M, López-Bermejo A, Ibáñez L. Large for gestational age newborns from mothers without diabetes mellitus tend to become tall and lean toddlers. J Pediatr. 2016;178:278–80. doi: 10.1016/j.jpeds.2016.08.042. [PubMed] [CrossRef] [Google Scholar]
45. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6. doi: 10.1542/peds.2004-1808. [PubMed] [CrossRef] [Google Scholar]
46. Kelishadi R, Gouya MM, Adeli K, Ardalan G, Gheiratmand R, Majdzadeh R, et al. Factors associated with the metabolic syndrome in a national sample of youths: CASPIAN Study. Nutr Metab Cardiovasc Dis. 2008;18:461–70. doi: 10.1016/j.numecd.2007.02.014. [PubMed] [CrossRef] [Google Scholar]
Nutr Metab Cardiovasc Dis. 2008;18:461–70. doi: 10.1016/j.numecd.2007.02.014. [PubMed] [CrossRef] [Google Scholar]
47. Guerrero-Romero F, Aradillas-García C, Simental-Mendia LE, Monreal-Escalante E, de la Cruz Mendoza E, Rodríguez-Moran M. Birth weight, family history of diabetes, and metabolic syndrome in children and adolescents. J Pediatr. 2010;156:719–23. doi: 10.1016/j.jpeds.2009.11.043. [PubMed] [CrossRef] [Google Scholar]
48. Eyzaguirre F, Bancalari R, Román R, Silva R, Youlton R, Urquidi C, et al. Prevalence of components of the metabolic syndrome according to birthweight among overweight and obese children and adolescents. J Pediatr Endocrinol Metab. 2012;25:51–6. doi: 10.1515/jpem.2011.446. [PubMed] [CrossRef] [Google Scholar]
49. Harville EW, Srinivasan S, Chen W, Berenson GS. Is the metabolic syndrome a "small baby" syndrome? : the bogalusa heart study. Metab Syndr Relat Disord. 2012;10:413–21. doi: 10.1089/met.2012.0031. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
50. González-Jiménez E, Montero-Alonso MA, Schmidt-RioValle J, García-García CJ, Padez C. Metabolic syndrome in Spanish adolescents and its association with birth weight, breastfeeding duration, maternal smoking, and maternal obesity: a cross-sectional study. Eur J Nutr. 2015;54:589–97. doi: 10.1007/s00394-014-0740-x. [PubMed] [CrossRef] [Google Scholar]
González-Jiménez E, Montero-Alonso MA, Schmidt-RioValle J, García-García CJ, Padez C. Metabolic syndrome in Spanish adolescents and its association with birth weight, breastfeeding duration, maternal smoking, and maternal obesity: a cross-sectional study. Eur J Nutr. 2015;54:589–97. doi: 10.1007/s00394-014-0740-x. [PubMed] [CrossRef] [Google Scholar]
51. Romero-Velarde E, Aguirre-Salas LM, Álvarez-Román YA, Vásquez-Garibay EM, Casillas-Toral E, Fonseca-Reyes S. Prevalence of metabolic syndrome and associated factors in children and adolescents with obesity. Rev Med Inst Mex Seguro Soc. 2016;54:568–75. [PubMed] [Google Scholar]
52. Murtaugh MA, Jacobs DR, Jr, Moran A, Steinberger J, Sinaiko AR. Relation of birth weight to fasting insulin, insulin resistance, and body size in adolescence. Diabetes Care. 2003;26:187–92. doi: 10.2337/diacare.26.1.187. [PubMed] [CrossRef] [Google Scholar]
53. Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944–50. doi: 10.2337/diacare.22.6.944. [PubMed] [CrossRef] [Google Scholar]
Diabetes Care. 1999;22:944–50. doi: 10.2337/diacare.22.6.944. [PubMed] [CrossRef] [Google Scholar]
54. Chiavaroli V, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, Mohn A. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics. 2009;124:695–702. doi: 10.1542/peds.2008-3056. [PubMed] [CrossRef] [Google Scholar]
55. Spiegel E, Shoham-Vardi I, Sergienko R, Landau D, Sheiner E. The association between birth weight at term and long-term endocrine morbidity of the offspring. J Matern Fetal Neonatal Med. 2019;32:2657–61. doi: 10.1080/14767058.2018.1443440. [PubMed] [CrossRef] [Google Scholar]
56. Wei JN, Sung FC, Li CY, Chang CH, Lin RS, Lin CC, et al. Low birth weight and high birth weight infants are both at an increased risk to have type 2 diabetes among schoolchildren in Taiwan. Diabetes Care. 2003;26:343–8. doi: 10.2337/diacare.26.2.343. [PubMed] [CrossRef] [Google Scholar]
57. Zhang Y, Li H, Liu SJ, Fu GJ, Zhao Y, Xie YJ, et al. The associations of high birth weight with blood pressure and hypertension in later life: a systematic review and meta-analysis. Hypertens Res. 2013;36:725–35. doi: 10.1038/hr.2013.33. [PubMed] [CrossRef] [Google Scholar]
The associations of high birth weight with blood pressure and hypertension in later life: a systematic review and meta-analysis. Hypertens Res. 2013;36:725–35. doi: 10.1038/hr.2013.33. [PubMed] [CrossRef] [Google Scholar]
58. Skilton MR, Siitonen N, Würtz P, Viikari JS, Juonala M, Seppälä I, et al. High birth weight is associated with obesity and increased carotid wall thickness in young adults: the cardiovascular risk in young Finns study. Arterioscler Thromb Vasc Biol. 2014;34:1064–8. doi: 10.1161/ATVBAHA.113.302934. [PubMed] [CrossRef] [Google Scholar]
59. Knop MR, Geng TT, Gorny AW, Ding R, Li C, Ley SH, et al. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta-analysis of 7 646 267 participants from 135 studies. J Am Heart Assoc. 2018;7:e008870. doi: 10.1161/JAHA.118.008870. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
60. Bouhours-Nouet N, Dufresne S, de Casson FB, Mathieu E, Douay O, Gatelais F, et al. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects? Diabetes Care. 2008;31:1031–6. doi: 10.2337/dc07-1647. [PubMed] [CrossRef] [Google Scholar]
High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects? Diabetes Care. 2008;31:1031–6. doi: 10.2337/dc07-1647. [PubMed] [CrossRef] [Google Scholar]
61. Evagelidou EN, Kiortsis DN, Bairaktari ET, Giapros VI, Cholevas VK, Tzallas CS, et al. Lipid profile, glucose homeostasis, blood pressure, and obesity-anthropometric markers in macrosomic offspring of nondiabetic mothers. Diabetes Care. 2006;29:1197–201. doi: 10.2337/dc05-2401. [PubMed] [CrossRef] [Google Scholar]
62. Schmelzle HR, Quang DN, Fusch G, Fusch C. Birth weight categorization according to gestational age does not reflect percentage body fat in term and preterm newborns. Eur J Pediatr. 2007;166:161–7. doi: 10.1007/s00431-006-0209-x. [PubMed] [CrossRef] [Google Scholar]
63. Chiavaroli V, Cutfield WS, Derraik JG, Pan Z, Ngo S, Sheppard A, et al. Infants born large-for-gestational-age display slower growth in early infancy, but no epigenetic changes at birth. Sci Rep. 2015;5:14540. doi: 10.1038/srep14540. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
Sci Rep. 2015;5:14540. doi: 10.1038/srep14540. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
64. Taal HR, Vd Heijden AJ, Steegers EA, Hofman A, Jaddoe VW. Small and large size for gestational age at birth, infant growth, and childhood overweight. Obesity (Silver Spring) 2013;21:1261–8. doi: 10.1002/oby.20116. [PubMed] [CrossRef] [Google Scholar]
65. Bueno AC, Espiñeira AR, Fernandes-Rosa FL, de Souza RM, de Castro M, Moreira AC, et al. Adiponectin: serum levels, promoter polymorphism, and associations with birth size and cardiometabolic outcome in young adults born large for gestational age. Eur J Endocrinol. 2010;162:53–60. doi: 10.1530/EJE-09-0697. [PubMed] [CrossRef] [Google Scholar]
66. Moschonis G, Grammatikaki E, Manios Y. Perinatal predictors of overweight at infancy and preschool childhood: the GENESIS study. Int J Obes (Lond) 2008;32:39–47. doi: 10.1038/sj.ijo.0803764. [PubMed] [CrossRef] [Google Scholar]
67. Vohr BR, McGarvey ST. Growth patterns of large-for-gestational-age and appropriate-for-gestational-age infants of gestational diabetic mothers and control mothers at age 1 year. Diabetes Care. 1997;20:1066–72. doi: 10.2337/diacare.20.7.1066. [PubMed] [CrossRef] [Google Scholar]
Diabetes Care. 1997;20:1066–72. doi: 10.2337/diacare.20.7.1066. [PubMed] [CrossRef] [Google Scholar]
68. Lurbe E, Garcia-Vicent C, Torro MI, Aguilar F, Redon J. Associations of birth weight and postnatal weight gain with cardiometabolic risk parameters at 5 years of age. Hypertension. 2014;63:1326–32. doi: 10.1161/HYPERTENSIONAHA.114.03137. [PubMed] [CrossRef] [Google Scholar]
Large for Gestational Age: Meaning and Complications
Written by Sarah Vallie
Reviewed by Dan Brennan, MD on July 24, 2022
In this Article
- What Does Large for Gestational Age Mean?
- Large for Gestational Age Causes
- Complications of LGA Babies
- What to Do If You’re Concerned
The average baby weighs about seven pounds when they’re born. Sometimes, a baby will be born a lot larger than expected. In that case, the baby may be labeled “large for gestational age.”
What Does Large for Gestational Age Mean?
If your provider tells you that your baby is large for gestational age (LGA), that means that your baby measures larger than the doctor would expect for how far along you are in your pregnancy or how far along you were when the baby was born.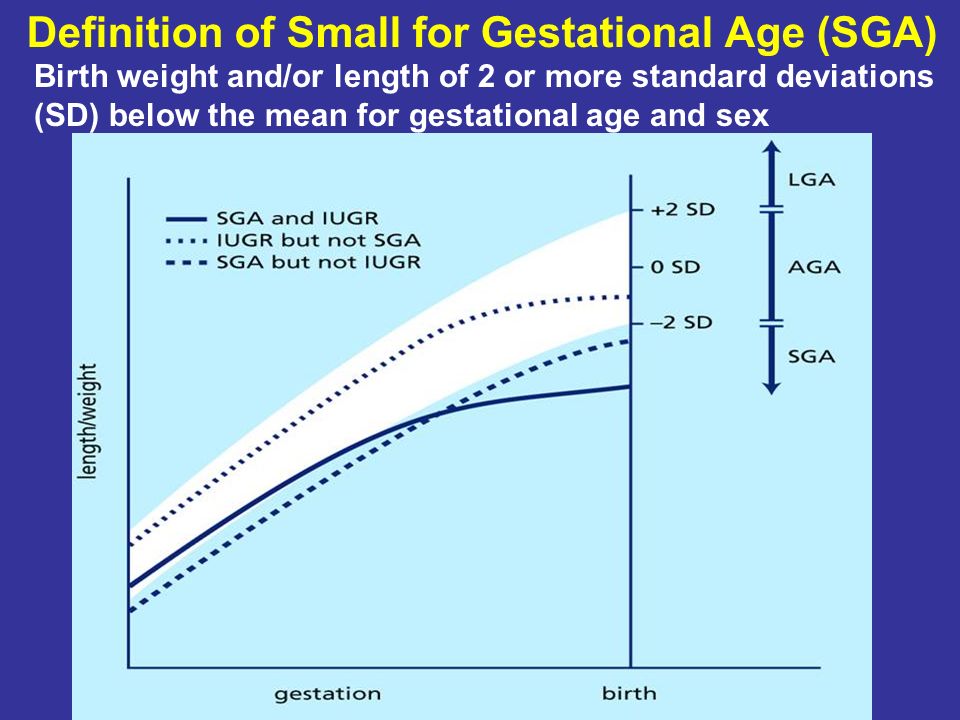
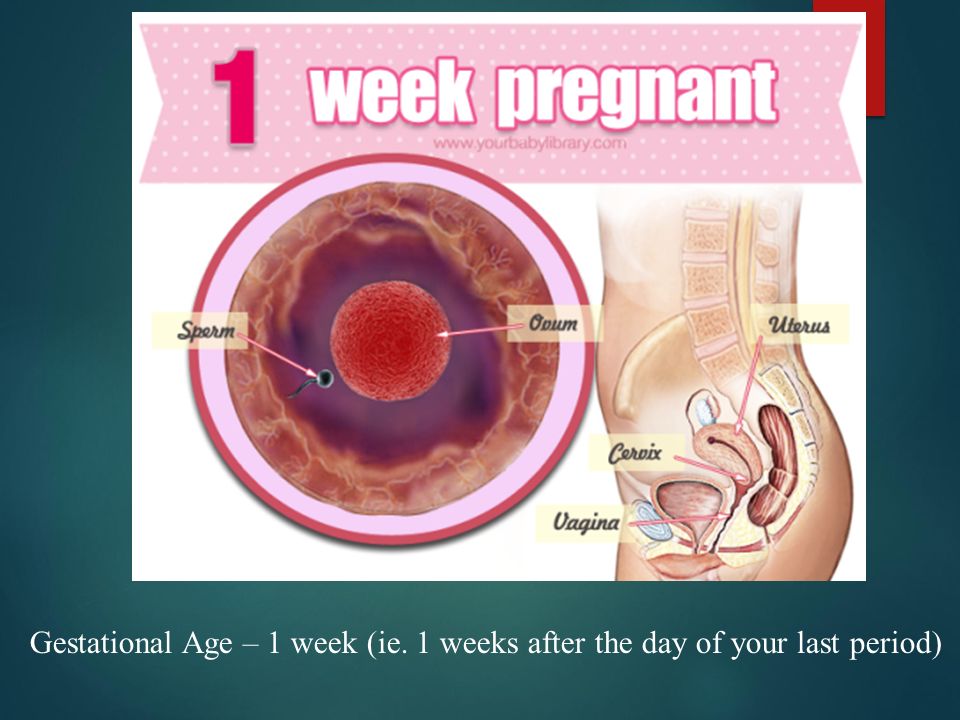
Gestational age is calculated by determining the first day of your last period. This means that at the time of ovulation, you may already be two weeks into your pregnancy. By the time someone misses their first period, they are already four weeks along. Therefore, even if you think you know the date of conception, your calculated due date may be different than your doctor’s.
If your periods are irregular, your doctor can also estimate gestational age with an ultrasound. The most accurate guesses for gestational age via ultrasound are from ultrasounds done sometime between the 8th and 18th weeks.
A fetus may be described as large for gestational age if they measure larger than their gestational age would usually indicate. A newborn baby is classified as large for gestational age if they are in the 90th percentile for their weight.
Large for Gestational Age Causes
A few factors can cause a fetus or baby to be large for gestational age, and some are more a cause for concern than others.
Incorrect estimated gestational age. The simplest, least concerning cause of a fetus or baby being LGA is that your doctor miscalculated gestational age. This can happen if your menstrual cycle is irregular, if you don’t remember when your last cycle was, if you take hormones or hormonal birth control, or if the doctor miscalculated from the ultrasound or the ultrasound was difficult to see.
Diabetes. Diabetes is the number-one cause of LGA babies. This includes gestational diabetes and those who had diabetes before pregnancy.
Gestational diabetes is diabetes that occurs in pregnancy and often goes away after pregnancy. Researchers don’t know exactly why some pregnancies include gestational diabetes, and some don’t. The best guess is that, as hormones change within your body, sometimes those changes cause the body to have a difficult time managing blood sugar.
Someone with diabetes before pregnancy also has a higher chance of having an LGA baby, especially if their diabetes is not well managed. A person with diabetes may pass their high blood sugar on to their fetus, whose body then, in turn, makes extra insulin to compensate. This excess insulin can lead to fast growth and fat deposits.
A person with diabetes may pass their high blood sugar on to their fetus, whose body then, in turn, makes extra insulin to compensate. This excess insulin can lead to fast growth and fat deposits.
Size of the Parents. Genetics may contribute to the fetus or baby’s size. Parents with a larger stature are more likely to have babies that are large as well.
Gaining too much weight during pregnancy can also contribute to an LGA baby. Some weight gain during pregnancy is expected, but how much weight you should safely gain will depend on many factors like your pre-pregnancy size and activity levels. Much of this weight gain comes from simply growing another human, but too much excess weight gain can result in a large baby.
Complications of LGA Babies
In some cases, complications may arise if your baby is large for gestational age. The severity of these complications depends on what caused the LGA and the baby’s size.
Delivery Complications.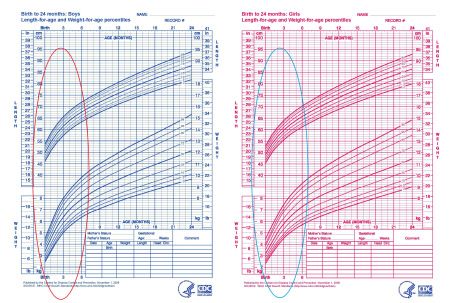 Sometimes, the LGA baby is too large to fit easily into the birth canal, which can cause delivery complications. These include:
Sometimes, the LGA baby is too large to fit easily into the birth canal, which can cause delivery complications. These include:
- Prolonged labor and delivery.Prolonged labor creates its own complications. A baby stuck in a prolonged labor might not get enough oxygen. They may also get an infection, get sepsis, or aspirate meconium, the infant’s first bowel movement.
- Difficult birth. A difficult birth isn’t just a long labor. A difficult birth can also include high-stress labor and delivery. It may also include the need for extra intervention, which could be the use of forceps, a vacuum extractor, or an episiotomy to get the baby out.
- Birth injuries. If the baby has difficulty getting through the birth canal, injuries like a broken collarbone or damaged arm nerves may occur. Other complications, like brain trauma or cerebral palsy, may occur if the baby doesn’t get adequate oxygen.
- Increased risk of cesarean delivery.
 While many pregnant patients have successful cesarean deliveries, an emergency cesarean can be incredibly scary. C-sections also have their own risks, such as an increased risk of heavy bleeding and blood clots. They may lead to a longer recovery time than a vaginal delivery.
While many pregnant patients have successful cesarean deliveries, an emergency cesarean can be incredibly scary. C-sections also have their own risks, such as an increased risk of heavy bleeding and blood clots. They may lead to a longer recovery time than a vaginal delivery.
Blood sugar complications. Why are LGA babies at risk for hypoglycemia? Hypoglycemia, or low blood sugar, occurs if the LGA is caused by gestational or poorly-controlled diabetes. While the baby is in utero, its body produces excess insulin to accommodate the high blood sugar it’s getting. After birth, the baby no longer has that supply of high sugar but still has high insulin. This can cause blood sugar levels to plummet, resulting in hypoglycemia.
Other complications for babies born to diabetic patients may include breathing difficulties, increased risk of obesity and type II diabetes later in life, and can even be stillborn if the diabetes is left untreated.
What to Do If You’re Concerned
If you have an LGA pregnancy or are concerned that your baby will be large, the best thing you can do is talk to your doctor. Together, the two of you can work out a plan for how you want your birth to go and what contingencies you may need to put in place.
Together, the two of you can work out a plan for how you want your birth to go and what contingencies you may need to put in place.
Use of various methods to detect large (for gestational age) fetus sizes to improve health outcomes
What is the problem (question)?
The baby (during fetal development) can sometimes grow to a size larger than expected and gain a high body weight by the time of birth. If overgrowth is suspected, the mother-to-be may require additional unscheduled antenatal visits [antenatal visits] and examinations to assess her health and the health of her developing baby.
Why is this important?
Examination can show if there are signs of any deterioration in the condition of the infant or the development of complications in the mother. The recommended frequency and combinations of types of examination (tests) vary in local protocols and guidelines. The examination may include counting fetal movements, assessing fetal heartbeats (cardiotocography), checking the mother's blood sugar or using ultrasound to determine fetal growth, Doppler ultrasound of the fetal blood vessels, and assessing fluid volume around the infant.
Large fetal (infant) size is associated with increased risk for both mother and infant, including increased risk of fetal death and stillbirth. At birth, such a baby has a higher risk of low oxygen levels, shoulder dystocia [difficulty in labor], nerve damage, bone fractures, low blood sugar, and admission to the neonatal intensive care unit. Maternal complications include prolonged labor, operative labor including caesarean section, perineal trauma, postpartum hemorrhage, and uterine ruptures.
Interventions that can slow accelerated fetal growth and improve maternal and child health outcomes include dietary advice, lifestyle changes, and blood glucose control and insulin therapy in women with diabetes or gestational diabetes.
What evidence did we find?
We searched for studies up to 10 August 2015, but did not find any randomized controlled trials investigating the effect of additional testing (examination) on health outcomes in pregnant women with post-infant overgrowth. 20 weeks pregnant.
20 weeks pregnant.
What does this mean?
Randomized controlled clinical trials are needed in this area to inform clinical practice when a large fetus/infant is detected during pregnancy, to assess whether additional testing (examination) or observation can improve the health of these women and their children. It is also important to identify any harm that may be associated with additional testing and follow-up. identifying women with suspected large fetuses may lead to unnecessary maternal anxiety through additional investigations and interventions, including induction of labor or caesarean section.
Translation notes:
Translation: Yudina Ekaterina Viktorovna. Editing: Ziganshina Lilia Evgenievna. Project coordination for translation into Russian: Cochrane Russia - Cochrane Russia (branch of the Northern Cochrane Center on the basis of Kazan Federal University). For questions regarding this transfer, please contact us at: [email protected]
LARGE FRUIT - Article I NEPLACEBO Evidence-Based Medicine Clinic
Site Search
Voronezh
Leninsky Prospect, 34
st. Begovaya, d. Kolyadina Anna Alexandrovna
Begovaya, d. Kolyadina Anna Alexandrovna
06/30/2021
Surely you have heard how after giving birth a large baby is called a “hero”? In fact, very often (although not always) the birth of a child over 4500 g indicates problems during pregnancy and / or carries risks directly during and after childbirth.
⠀
Gynecologist Anna Kolyadina about a fetus that is too large for her gestational age and, accordingly, a large newborn.
⠀
So, the diagnosis of fetal macrosomia is made if, during a full-term pregnancy, the child was born more than 4000-4500 g (in different countries, different upper limits), or more than 90-95 percentile for a given period.
⠀
Why is fetal macrosomia dangerous?
⠀
For the mother:
🔸Long labor
🔸Increased likelihood of operative vaginal delivery (vacuum extractor) or caesarean section
🔸Ruptures of the vagina, perineum of varying degrees
🔸Risk of postpartum hemorrhage
🔸In rare cases, rupture of the uterus
⠀
During childbirth for the fetus: manipulations).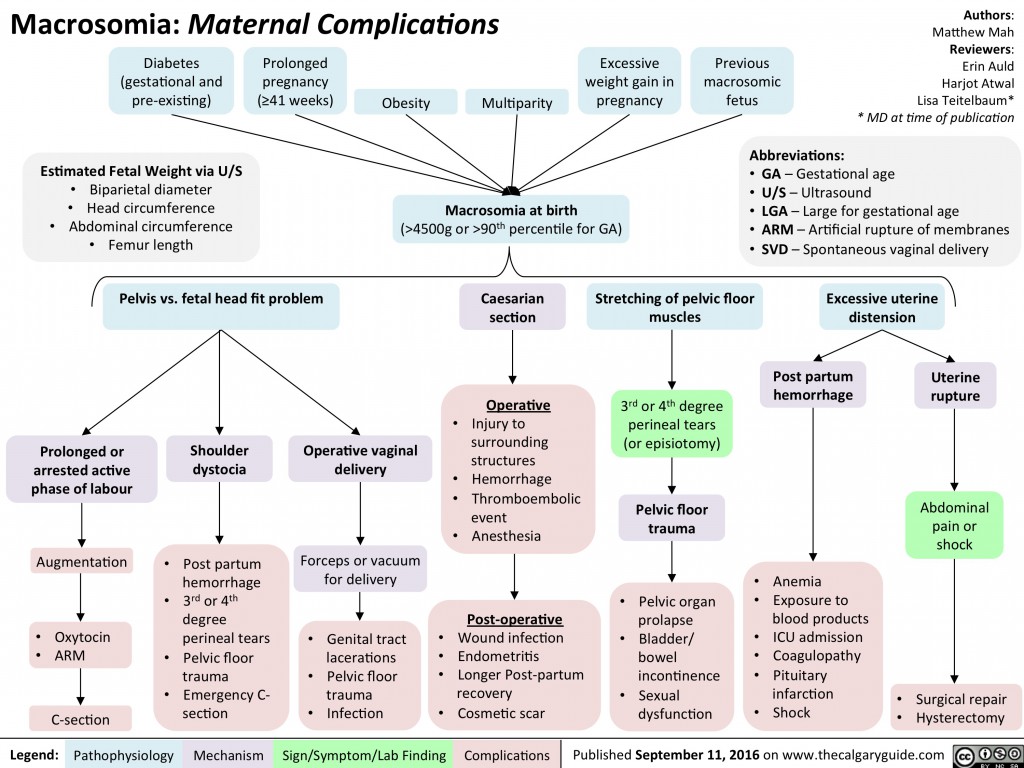 This situation increases the likelihood of trauma to the baby and mother during childbirth
This situation increases the likelihood of trauma to the baby and mother during childbirth
⠀
For newborns:
🔺 Hypoglycemia (low glucose levels)
🔺 Polycythemia (elevated hematocrit and hemoglobin, which can lead to increased blood viscosity)
🔺 Respiratory problems, risk of perinatal asphyxia
⠀
delayed consequences for children:
▪ ▪ The carbohydrate metabolism
▪ Metabolic syndrome
⠀
Risk factors that increase the likelihood of a large child:
oked
✔ Claus. a child over 4000 g in a previous pregnancy
✔ Mother's own birth weight over 4000 g
✔ Rarely - some hereditary syndromes
✔ Postterm pregnancy
✔ Maternal obesity
✔ Excessive weight gain during pregnancy (with a normal BMI and weight gain of more than 16 kg, the risk of having a fetus with macrosomia increases by 2.5 times) : many women who have given birth to large children do not have three main factors (obesity, diabetes and overweight)! Probably, the role of genetic and epigenetic factors is also great.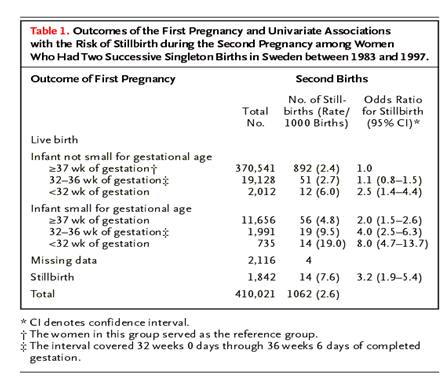
⠀
❓Why the fetus weighs more than it should
It is believed that the main mechanism for the development of fetal macrosomia is an increased level of glucose in a pregnant woman, which, in turn, leads to its increased level in the fetus. As a result, the level of insulin, IGF and growth hormone begins to “storage” fat by the fetus.
Other parameters associated with the risk of macrosomia include elevated triglycerides (⬆️risk) and HDL (⬇️risk) in the mother.
⠀
DIAGNOSTICS
⠀
During pregnancy, the large size of the fetus and large weight can be determined using ultrasound (the closer the ultrasound date to childbirth, the more accurate the weight forecast for the newborn)
⠀
During routine visits to the gynecologist, suspicion of a large the fetus can occur when it is palpated and measuring the height of the fundus of the uterus. But this method is quite subjective and imprecise.
⠀
PREVENTION
⠀
All of the above was not intended to scare the expectant mother, but to convey an important message: most macrosomia risk factors can be corrected!
⠀
In diabetes or gestational diabetes, prevention of fetal macrosomia is GLUCOSE CONTROL and maintenance of its target values.