Are vaccines safe during pregnancy
Vaccines During Pregnancy FAQs | Vaccine Safety
Moro PL, Zheteyeva Y, Lewis P, Shi J, Yue X, Museru OI, et al. Safety of quadrivalent human papillomavirus vaccine (Gardasil®) in pregnancy: Adverse events among non-manufacturer reports in the Vaccine Adverse Event Reporting System, 2006-2013. Vaccine. 2015 Jan 15; 33(4):519-22. Epub 2014 Dec 8.
Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway AL, Klein NP, Cheetham TC, et al. Receipt of pertussis vaccine during pregnancy across 7 Vaccine Safety Datalink sites. Prev Med. 2014 Oct; 67: 316-9. Epub 2014 Jun 18.
Naleway AL, Irving SA, Henninger ML, Li DK, Shifflett P, Ball S, Williams JL, Cragan J, Gee J, Thompson MG, Vaccine Safety Datalink and Pregnancy and Influenza Project. Safety of influenza vaccination during pregnancy: A review of subsequent maternal obstetric events and findings from two recent cohort studies. Vaccine. 2014 May 30; 32(26): 3122-3127. Epub 2014 Apr 14.
Naleway AL, Kurosky S, Henninger ML, Gold R, Nordin JD, Kharbanda EO, Irving S, Craig Cheetham T, Nakasato C, Glanz JM, Hambridge SJ, Davis RL, Klein NP, McCarthy NL, Weintraub E. Vaccinations given during pregnancy, 2002-2009: A descriptive study. Am J Prev Med. 2014 Feb; 46(2): 150-157.
Moro PL, Museru OI, Niu M, Lewis P, Broder K. Reports to the Vaccine Adverse Event Reporting System after hepatitis A and hepatitis AB vaccines in pregnant women. Am J Obstet Gynecol. 2014 Jun; 210(6): 561.e1-6. Epub 2013 Dec 27.
Moro PL, Museru OI, Broder K, Cragan J, Zheteyeva Y, Tepper N, Revzina N, Lewis P, Arana J, Barash F, Kissin D, Vellozzi C. Safety of influenza A (h2N1) 2009 live attenuated monovalent vaccine in pregnant women. Obstet Gynecol. 2013 Dec; 122(6): 1271-8.
Kharbanda EO, Vazquez-Benitez G, Lipkind H, Naleway A, Lee G, Nordin JD, Vaccine Safety Datalink Team. Inactivated influenza vaccine during pregnancy and risks for adverse obstetric events. Obstet Gynecol. 2013 Sep; 122(3): 659-667.
Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, Kharbanda EO, Irving S, Cheetham TC, McCarthy NL. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013 Jun 12; 31(27): 2898-2903. Epub 2013 Apr 30.
Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013 Jun 12; 31(27): 2898-2903. Epub 2013 Apr 30.
Henninger M, Naleway A, Crane B, Donahue J, Irving S. Predictors of seasonal influenza vaccination during pregnancy. Obstet Gynecol. 2013 Apr; 121(4): 741-749.
Zheteyeva Y, Moro PL, Yue X, Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: A review of the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2013 Jun; 208(6): 478.e1-6. Epub 2013 Feb 20.
Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, Cheetham TC, Jackson LA, Naleyway AL, GLanz JM, Nordin JD, Belongia EA, Vaccine Safety Datalink. Trivalent inactivated Influenza vaccine and spontaneous abortion. Obstet Gynecol. 2013 Jan; 121(1): 159-65.
Moro PL, Tepper NK, Grohskopf LA, Vellozzi C, Broder K. Safety of seasonal influenza and influenza A (h2N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines. 2012 Aug; 11(8): 911-21.
Expert Rev Vaccines. 2012 Aug; 11(8): 911-21.
Kharbanda EO, Vazquez-Benitez G, Shi WX, Lipkind H, Naleway A, Molitor B, Kuckler L, Olsen A, Nordin JD. Assessing the safety of influenza immunization during pregnancy: The Vaccine Safety Datalink. Am J Obstet Gynecol. 2012 Sep; 207(3 Suppl): S47-S51. Epub 2012 Jul 9.
Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, Kissin D, Lewis PW, Yue X, Haber P, Tokars JI, Vellozzi C, Broder KR. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012 Jul; 207(1):59.e1-7. Epub 2012 May 14.
Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, Barash F, Arana J, Brantley MD, Ding H, Singleton JA, Walton K, Haber P, Lewis P, Yue X, Destefano F, Vellozzi C. Adverse events following administration to pregnant women of influenza A (h2N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2011 Nov; 205(5): 473.e1-9. Epub 2011 Jun 21.
Am J Obstet Gynecol. 2011 Nov; 205(5): 473.e1-9. Epub 2011 Jun 21.
Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol. 2011 Feb; 204(2): 146.e1-7. Epub 2010 Oct 20.
France EK, Smith-Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, Shay D, Weintraub E, Fry AM, Black SB, Shinefield HR, Mullooly JP, Jackson LA. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med. 2006 Dec; 160(12): 1277-1283.
Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D, Vaccine Safety Datalink Workgroup. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004 Aug; 21(6):333-339.
Am J Perinatol. 2004 Aug; 21(6):333-339.
Vaccine Safety for Moms-to-Be | CDC
Pregnant women may safely receive inactivated vaccines (Tdap and flu), mRNA (Moderna and Pfizer), and viral vector vaccines (J&J).
Vaccines help protect pregnant people and babies against serious diseases
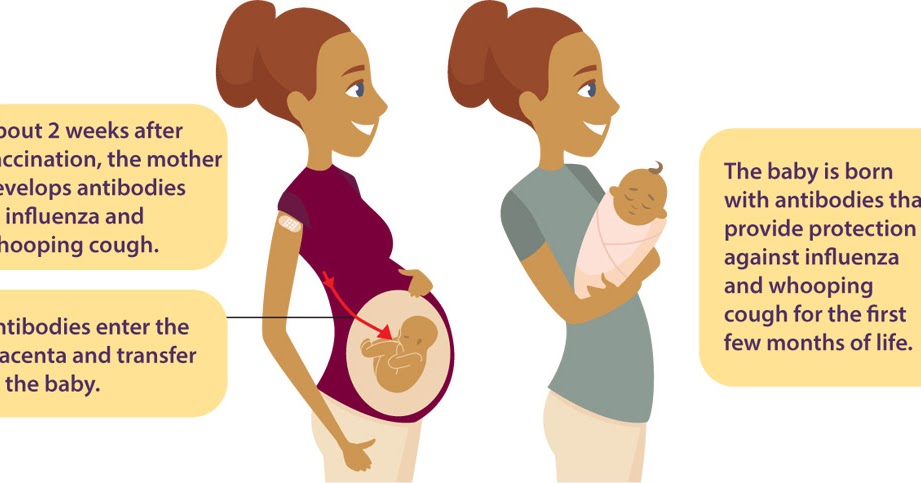
Pregnant people share everything with their babies. That means when a pregnant person gets vaccines, she isn’t just protecting herself— they are giving the baby some early protection too.
CDC has recommendations for the vaccines needed before, during, and after pregnancy. Currently, CDC routinely recommends Tdap and flu shots during pregnancy.
- Get the Tdap vaccine (to help protect against whooping cough), during pregnancy.
- The flu shot can be given before or during pregnancy, depending on whether or not it is flu season during a pregnancy.
- It is safe for pregnant people to receive vaccines right after giving birth, even while breastfeeding.
- Some vaccines, such as the measles, mumps, rubella (MMR) vaccine, should be given a month or more before pregnancy if a pregnant person didn’t get the vaccine as a child.
Live virus vaccines, such as the MMR and chickenpox, should not be given to pregnant people, but should be given to them before or after pregnancy, if indicated. Talk to your doctor about the MMR, Tdap, and flu vaccines before getting vaccinated. The COVID-19 vaccine is also recommended for pregnant people. The authorized and recommended COVID-19 vaccines for pregnant people are the mRNA Moderna and Pfizer-BioNTech vaccines, which contain no live virus, and the J&J/Janssen viral vector vaccine, meaning it uses a modified version of a different virus (the vector) to deliver important instructions to our cells. If you have any questions about these vaccines, talk to your doctor.
Vaccine safety before, during, and after pregnancy
It’s important to know that the Tdap and flu vaccines are safe for a pregnant person and their baby. Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
Likewise, the limited information collected for COVID-19 vaccines given to pregnant people have not identified any safety concerns for them or their babies.
- The Tdap and flu vaccines are inactivated vaccines, which means they are made by inactivating or killing the germ during the process of making the vaccine.
- Studies done on the Tdap vaccine have concluded that it is safe and effective for pregnant people and babies.
- Similarly, results from multiple studies on the flu shot continue to support the safety and effectiveness of the vaccine during pregnancy.
- There is limited information available about the safety of the COVID-19 vaccines for people who are pregnant; however, based on how these vaccines work in the body, experts believe they are unlikely to pose risk for pregnant people.
It is important to get MMR before becoming pregnant to reduce the risk of becoming infected with rubella which can pass on to the unborn child, causing Congenital Rubella Syndrome (CRS). CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
CRS can cause severe birth defects and neurodevelopmental problems. Even though MMR is a safe and effective vaccine, there is a theoretical risk to the baby. This is because it is a live vaccine, meaning it contains a weakened version of the living viruses.
- Live vaccines are generally not recommended during pregnancy.
- If a pregnant person did not get MMR as a child, she should get the vaccine before pregnancy.
All vaccines are held to the highest standards of safety—meaning they are carefully studied and monitored for side effects. Vaccines are like any medicine, which means they can have some side effects. However, most people who get vaccinated have no side effects or only mild side effects. CDC continually monitors vaccine safety, and the most common side effects seen are mild and go away quickly on their own (redness, swelling, and tenderness at the site where the shot was given. Other possible side effects associated with the COVID-19 vaccine are tiredness, headache, muscle pain, chills, fever, and nausea. ).
).
For more studies, the FDA also has a pregnancy exposure registry,external icon which is a study that collects health information from pregnant persons who take medicines or vaccines when they are pregnant.
How safe are COVID-19 vaccines for pregnant women and those planning to become pregnant? WHO responds
1. Can pregnant women be vaccinated against COVID-19?
Short answer: yes. Pregnant women can be vaccinated against COVID-19. Vaccines provide reliable protection against serious diseases caused by coronavirus. Pregnant women, if not already vaccinated, should have access to WHO-approved vaccines, as COVID-19 during pregnancy puts them at higher risk of serious illness and premature birth. nine0005
Growing evidence of the safety and efficacy of COVID-19 vaccination during pregnancy suggests that the benefits of vaccination during pregnancy outweigh the potential risks. Vaccination against COVID-19 before or during pregnancy is particularly important in settings with moderate or high risk of transmission in a particular community, and for women with an increased individual risk of infection or severe disease.
2. Like COVID-19affect pregnant women?
Multiple studies show that pregnant women with COVID-19 are more likely to develop severe illness than non-pregnant women. This means that pregnant women who are infected with the coronavirus are more likely to require hospitalization, intensive care, and invasive ventilation to help breathe easier. In addition, compared with healthy pregnant women, pregnant women with COVID-19 have an increased risk of preterm birth and babies requiring intensive care. They may also have an increased risk of stillbirth and maternal death. nine0005
Pregnant women of mature age (35 years and older), who are overweight, or who have conditions such as diabetes or hypertension may be at even higher risk of serious adverse health outcomes.
3. Are COVID-19 vaccines effective during pregnancy?
Research has found that COVID-19 vaccines are highly effective in preventing severe illness, hospitalizations, and deaths from COVID-19. Given experience with other vaccines during pregnancy, scientists expect all WHO-approved COVID-19 vaccines towill work equally effectively regardless of the presence or absence of pregnancy.
Given experience with other vaccines during pregnancy, scientists expect all WHO-approved COVID-19 vaccines towill work equally effectively regardless of the presence or absence of pregnancy.
In addition, studies have shown that pregnant women who are vaccinated against COVID-19 develop antibodies that pass into the cord blood of babies. This suggests that children may also be protected as a result of mothers being vaccinated.
4. What is known about the safety of COVID-19 vaccines during pregnancy?
Although pregnant women were not included in initial clinical trials of COVID-19 vaccines, the database of evidence of the safety of vaccination during pregnancy is constantly expanding.
In several countries where vaccination against COVID-19 is widely used during pregnancy, pregnant women are monitored closely. At the same time, they did not have any problems associated with the course of pregnancy.
Thus, as of February 2022, more than 198,000 pregnant women were under observation after vaccination in the USA.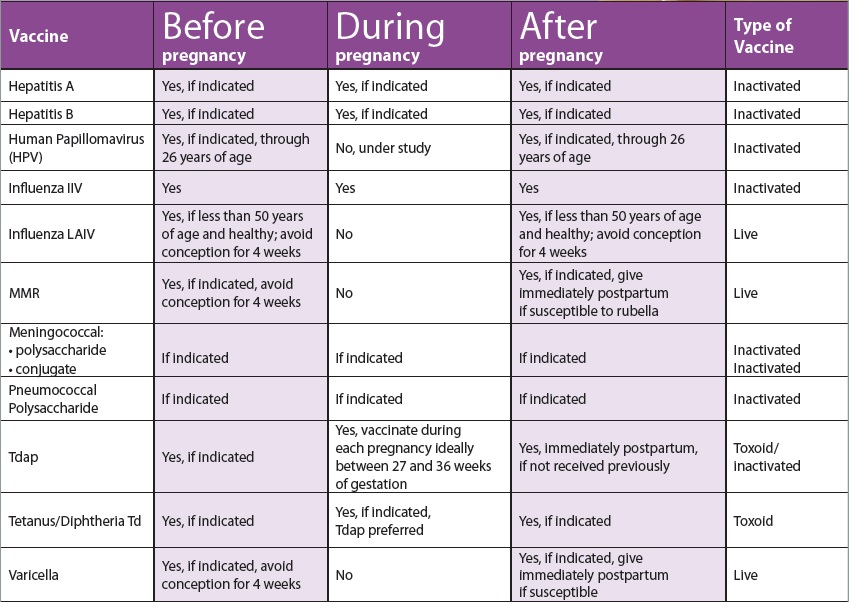 Most of them received Pfizer-BioNTech, BNT162b2 and Moderna mRNA-1273 vaccines. The study did not find any adverse outcomes associated with vaccination during pregnancy. nine0005
Most of them received Pfizer-BioNTech, BNT162b2 and Moderna mRNA-1273 vaccines. The study did not find any adverse outcomes associated with vaccination during pregnancy. nine0005
In the UK, as of February 2022, more than 100,000 pregnant women have been vaccinated against COVID-19. Most of them were vaccinated with mRNA vaccines; approximately 10 percent received the AstraZeneca AZD1222 vaccine. Data analysis revealed similar rates of birth outcome in both vaccinated and unvaccinated pregnant women.
All vaccines covered by WHO advance recommendations have been tested in animals. These studies did not demonstrate any harmful effects of vaccination in pregnant animals and their young. nine0005
None of the vaccines covered by the WHO interim recommendations contain the live virus that causes COVID-19. This means that vaccines are basically incapable of infecting pregnant women or their children.
5. Should women who are trying to conceive be vaccinated against COVID-19?
Yes. Pre-vaccination is an important tool to protect women and their children from COVID-19 during pregnancy. Women who are trying to get pregnant can get vaccinated against COVID-19. A growing body of data has not revealed any negative effect of vaccination on fertility or the ability to conceive. In vaccine clinical trials and in a large study of couples trying to conceive, pregnancy rates were similar for those who received COVID-19 vaccines and those who did not.
Pre-vaccination is an important tool to protect women and their children from COVID-19 during pregnancy. Women who are trying to get pregnant can get vaccinated against COVID-19. A growing body of data has not revealed any negative effect of vaccination on fertility or the ability to conceive. In vaccine clinical trials and in a large study of couples trying to conceive, pregnancy rates were similar for those who received COVID-19 vaccines and those who did not.
WHO does not recommend postponing or terminating pregnancy due to COVID-19 vaccination. According to experts, no pregnancy tests should be done before vaccination. nine0005
6. So, in summary, what do pregnant women and those who plan to get pregnant need to know about COVID-19 vaccinations?
Given the significant risks associated with COVID-19 during pregnancy, it is critical that pregnant women and those who are planning to become pregnant have access to WHO-approved COVID-19 vaccines as soon as possible to help protect their health and that of their children. Current evidence suggests that if pregnant women are not already vaccinated, the benefits of vaccination against COVID-19during pregnancy outweighs any potential risks.
Current evidence suggests that if pregnant women are not already vaccinated, the benefits of vaccination against COVID-19during pregnancy outweighs any potential risks.
Pregnant women and those who plan to become pregnant should be informed in a timely manner about the risks of contracting COVID-19 during pregnancy, the benefits of vaccination, and the factors that indicate the benefits of vaccination:
• Infection with COVID-19 during pregnancy can lead to consequences: Available evidence suggests that pregnant women with COVID-19 are at increased risk of severe illness, premature birth, and likely other adverse pregnancy outcomes such as stillbirth. nine0005
• COVID-19 vaccines are extremely effective, providing strong protection against serious illness and death due to coronavirus infection. Pregnant women appear to receive the same level of protection from vaccination as non-pregnant women.
• New data on the safety of vaccination during pregnancy are encouraging: to date, animal studies, observation of pregnant women who have received vaccines, and experience with vaccines with similar components have not revealed any safety problems during pregnancy. nine0005
nine0005
• Several factors make it particularly important to get vaccinated against COVID-19 before or during pregnancy: the risk of acquiring COVID-19 increases in settings where the risk of transmission is higher. Even in regions with a low risk of transmission, people may still be at high risk of infection, such as healthcare workers. Pregnant women aged 35 years and older who are overweight or have underlying medical conditions may be at even greater risk of serious consequences if they become infected with COVID-19.
Fomin's clinic - a network of multidisciplinary clinics
You probably know that in the modern world there is a fierce struggle. And this is not about military conflicts or political strife, but about clashes between supporters of vaccination and anti-vaccinationists who claim that vaccination is a real universal evil that causes irreparable consequences - pathologies and disorders. Nevertheless, pregnant women and newborns are the most vulnerable groups, which can be taken care of by timely vaccination. nine0005
nine0005
What diseases should a pregnant woman be vaccinated against?
Pregnancy is stressful for the body, and therefore it functions differently than before or after it. It is because of a weakened immune system that the risk of severe influenza in pregnant women is much higher than in non-pregnant women. Influenza can not only cause premature birth, but also harm the health of the unborn baby.
Influenza vaccination is strongly recommended by all world medical organizations (including the well-known World Health Organization). There are controversial opinions about vaccination in the first trimester, therefore, if it does not fall in autumn or winter - the most dangerous months for the active spread of the disease, it is better to get vaccinated in the II-III trimester. nine0005
Get vaccinated
When you get vaccinated against influenza, it first of all has a preventive effect directly for you - the expectant mother, but the whooping cough vaccine is about taking care of the baby's health. The woman who received the vaccine will pass antibodies to the child in utero - and this will save him from the disease in the first months of life, because for the newborn it can be fatal.
The woman who received the vaccine will pass antibodies to the child in utero - and this will save him from the disease in the first months of life, because for the newborn it can be fatal.
Whooping cough is difficult to recognize immediately, because it does not always manifest itself with a characteristic cough - at one moment breathing can simply stop, although there were no visible symptoms. For your baby's safety, you should get the whooping cough vaccine (along with diphtheria and tetanus) during every pregnancy - best done in the third trimester. nine0005
No, not dangerous.
There is a myth that you can get the flu after vaccination. It is impossible to catch the flu from a vaccine, since the vaccine does not contain the whole virus, but only its parts - antigens. Antibodies are produced against these antigens, our defending cells. And the next time our body encounters a virus, already real, our antibodies will actively destroy the viral particles.
Many people do not consider it necessary to get vaccinated because they think that the vaccine protects against only a few strains of influenza, and there are many. But every year the vaccine contains precisely the actual strains for this season and this territory. So mass vaccination forms a stable herd immunity. Immunity after vaccination lasts for a year, so the flu shot is annual. nine0005
But every year the vaccine contains precisely the actual strains for this season and this territory. So mass vaccination forms a stable herd immunity. Immunity after vaccination lasts for a year, so the flu shot is annual. nine0005
In some cases, pregnant women need additional vaccinations:
Vaccination against hepatitis B. The immunobiological preparation does not contain live or whole virus. According to medical research and statistics, this hepatitis shot is safe for a child. However, the vaccine is recommended for expectant mothers who are at risk of infection with this type of hepatitis (for example, if a loved one is infected). If the likelihood of the disease increases, then the pregnant woman is given emergency vaccination and a specific immunoglobulin is administered. nine0005
Vaccination against hepatitis A. Hepatitis A can be contracted through contact with objects that contain the virus, after drinking contaminated water or food. The immunobiological preparation contains an inactivated virus, and therefore the likelihood of its negative effect on the fetus is minimal. Vaccination is carried out if the risk of infection increases. This is possible if a pregnant woman enters a region unfavorable for hepatitis A or doctors suspect that contact with the virus has already taken place. In some cases, human normal immunoglobulin is given with the vaccine. nine0005
Vaccination is carried out if the risk of infection increases. This is possible if a pregnant woman enters a region unfavorable for hepatitis A or doctors suspect that contact with the virus has already taken place. In some cases, human normal immunoglobulin is given with the vaccine. nine0005
Rabies vaccination. Infection usually occurs after the bite of a sick animal. The rabies virus is quite strong and dangerous, with the development of the disease provokes death. It is for this reason that rabies vaccination is an urgent and necessary measure. The vaccine contains an inactivated virus, so the drug is considered safe for the fetus. If the bites and injuries are severe, then the victim is injected with a specific immunoglobulin.
Meningococcal vaccination may also be recommended by a doctor during pregnancy in the event of a meningococcal outbreak or if traveling to an area endemic for meningococcal disease. nine0005
The possibility of vaccinating a pregnant woman against pneumococcal infection may be considered by a doctor for women who have chronic respiratory diseases during the II-III trimesters of pregnancy.