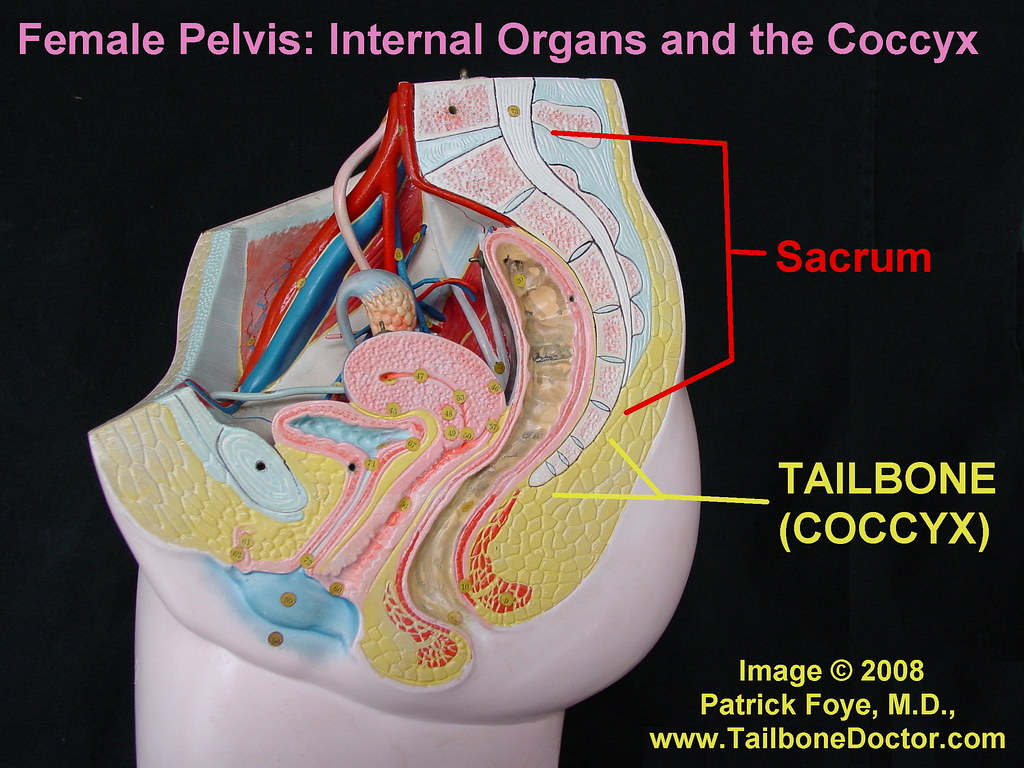
Anatomy of pelvic organs
Female Pelvic Floor Anatomy: The Pelvic Floor, Supporting Structures, and Pelvic Organs
Rev Urol. 2004; 6(Suppl 5): S2–S10.
Author information Copyright and License information Disclaimer
The development of novel, less invasive therapies for stress urinary incontinence in women requires a thorough knowledge of the relationship between the pathophysiology of incontinence and anatomy. This article provides a review of the anatomy of the pelvic floor and lower urinary tract. Also discussed is the hammock hypothesis, which describes urethral support within the pelvis and provides an explanation of the continence mechanism.
Key words: Pelvis, Pelvic diaphragm, Endopelvic fascia, Urogenital diaphragm, Urethra, Continence
The effective management of stress urinary incontinence (SUI) requires knowledge of the pathophysiologic mechanisms behind the disorder. Key to identifying these mechanisms and providing proper treatment to women with SUI is an understanding of the anatomy and function of the female pelvic floor and its supporting structures.
The maintenance of continence and prevention of pelvic organ prolapse rely on the support mechanisms of the pelvic floor. The bony pelvis consists of the 2 innominate bones, or hip bones, which are fused to the sacrum posteriorly and to each other anteriorly at the pubic symphysis. Each innominate bone is composed of the ilium, ischium, and pubis, which are connected by cartilage in youth but fused in the adult.1 The pelvis has 2 basins: the major (or greater) pelvis and the minor (or lesser) pelvis. The abdominal viscera occupy the major pelvis; the minor pelvis is the narrower continuation of the major pelvis inferiorly. The inferior pelvic outlet is closed by the pelvic floor.
The female pelvis () has a wider diameter and a more circular shape than that of the male. The wider inlet facilitates head engagement and parturition. The wider outlet predisposes to subsequent pelvic floor weakness. Numerous projections and contours provide attachment sites for ligaments, muscles, and fascial layers.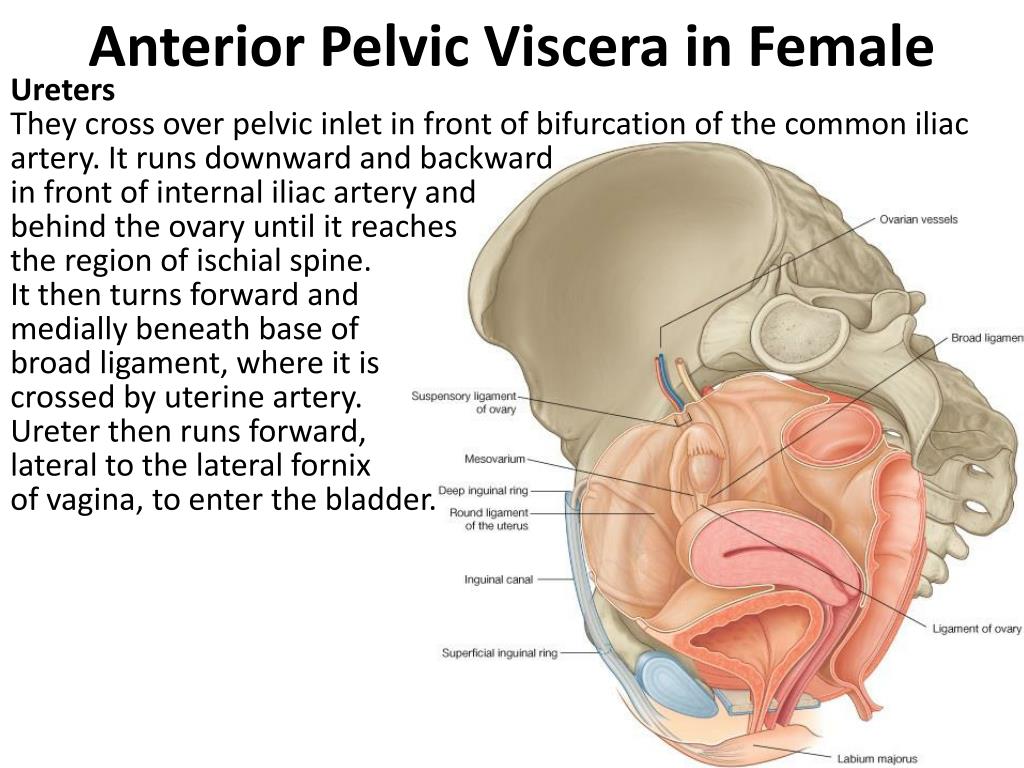 Of note is the thin and triangular sacrospinous ligament (), which extends from the ischial spines to the lateral margins of the sacrum and coccyx anteriorly to the sacrotuberous ligament. Its anterior surface is muscular and constitutes the coccygeus; the ligament is often regarded as the degenerate part of the muscle.1 The greater and lesser sciatic foramina are above and below the ligament.
Of note is the thin and triangular sacrospinous ligament (), which extends from the ischial spines to the lateral margins of the sacrum and coccyx anteriorly to the sacrotuberous ligament. Its anterior surface is muscular and constitutes the coccygeus; the ligament is often regarded as the degenerate part of the muscle.1 The greater and lesser sciatic foramina are above and below the ligament.
Open in a separate window
(A) The diameters of the female minor pelvis (superior aperture): A, sacroiliac joint; B, iliopubic eminence; C and D, middle of pelvic brim; E, sacral promontory; F, pubic symphysis. (B) The female pelvis from above: The sacrospinous ligament extends from the ischial spines to the lateral margins of the sacrum and coccyx anteriorly to the sacrotuberous ligament, which extends from the ischial tuberosity to the coccyx. The sciatic foramina are above and below the sacrospinous ligament and anterior to the sacrotuberous ligament. Reprinted, with permission, from Herschorn S, Carr LK.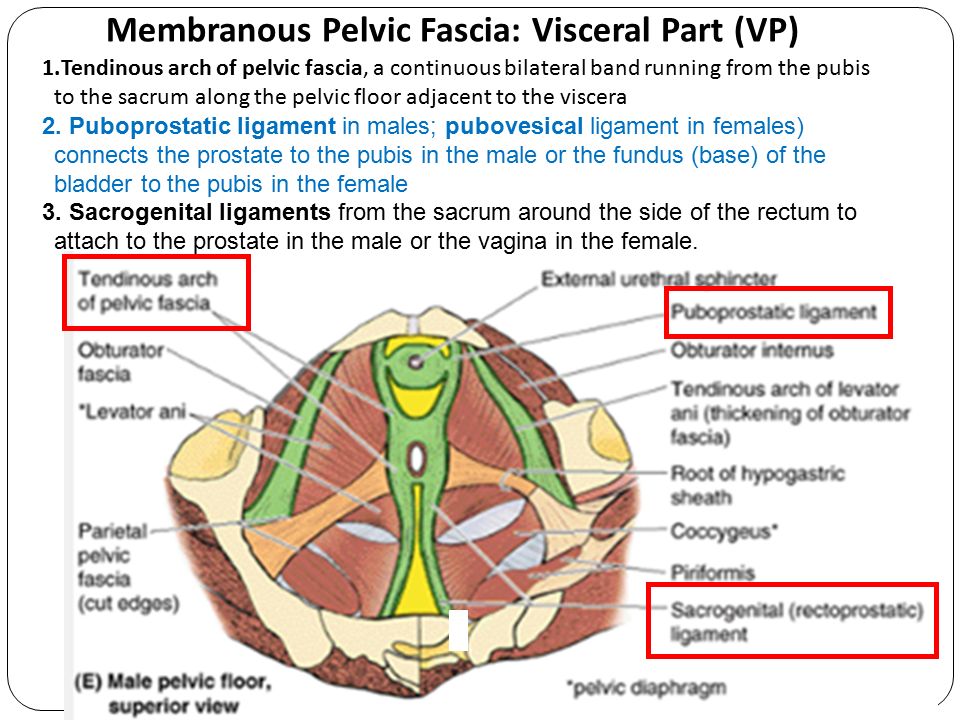 In: Campbell’s Urology. 2002:1092–1139.36
In: Campbell’s Urology. 2002:1092–1139.36
Pelvic Diaphragm
The levator ani and coccygeus muscles that are attached to the inner surface of the minor pelvis form the muscular floor of the pelvis. With their corresponding muscles from the opposite side, they form the pelvic diaphragm (). The levator ani is composed of 2 major muscles from medial to lateral: the pubococcygeus and iliococcygeus muscles.
The bulkier medial portion of the levator ani is the pubococcygeus muscle that arises from the back of the body of the pubis and anterior portion of the arcus tendineus. The arcus tendineus of the levator ani is a dense connective tissue structure that runs from the pubic ramus to the ischial spine and courses along the surface of the obturator internus muscle. The muscle passes back almost horizontally to behind the rectum. The inner border forms the margin of the levator (urogenital) hiatus, through which passes the urethra, vagina, and anorectum.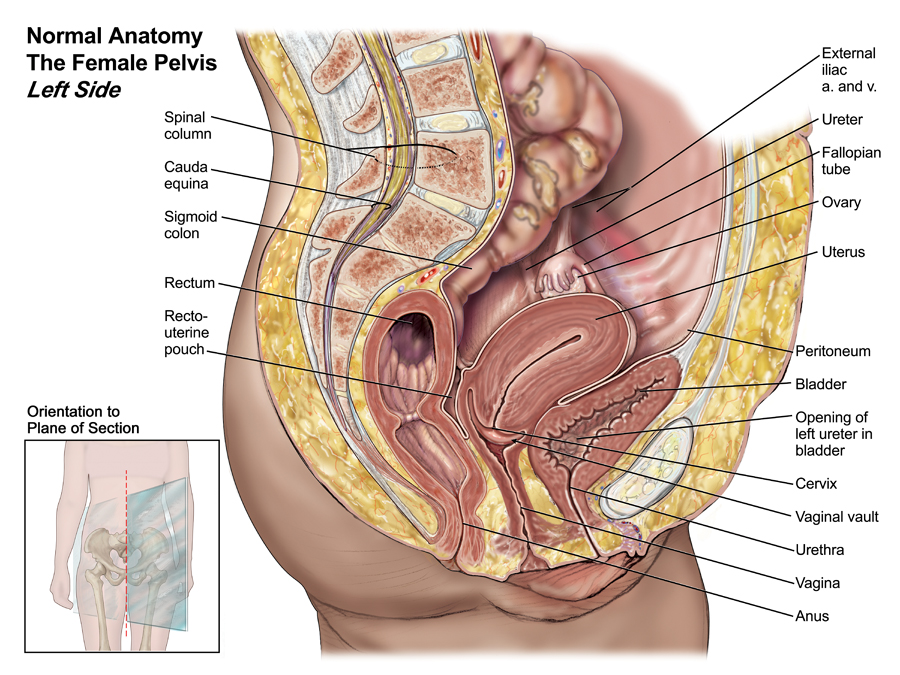
Various muscle subdivisions have been assigned to the medial portions of the pubococcygeus to reflect the attachments of the muscle to the urethra, vagina, anus, and rectum.2 These portions are referred to by some investigators as the pubourethralis, pubovaginalis, puboanalis, and puborectalis—or collectively as the pubovisceralis, because of their association and attachment to the midline viscera.3 The urethral portion forms part of the periurethral musculature, and the vaginal and anorectal portions insert into the vaginal walls, perineal body, and external anal sphincter muscle.4 The puborectalis portion passes behind the rectum and fuses with its counterpart from the opposite side to form a sling behind the anorectum. Other more posterior parts of the pubococcygeus attach to the coccyx.
The thin lateral part of the levator ani is the iliococcygeus muscle, which arises from the arcus tendineus of the levator ani to the ischial spine. Posteriorly it attaches to the last 2 segments of the coccyx. The fibers from both sides also fuse to form a raphe and contribute to the anococcygeal ligament. This median raphe between the anus and the coccyx is called the levator plate and is the shelf on which the pelvic organs rest. It is formed by the fusion of the iliococcygeus and the posterior fibers of the pubococcygeus muscles. When the body is in a standing position, the levator plate is horizontal and supports the rectum and upper two thirds of vagina above it. Weakness of the levator ani may loosen the sling behind the anorectum and cause the levator plate to sag.5 This opens the urogenital hiatus and predisposes to pelvic organ prolapse (). Women with prolapse have been shown to have an enlarged urogenital hiatus on clinical examination.6
The fibers from both sides also fuse to form a raphe and contribute to the anococcygeal ligament. This median raphe between the anus and the coccyx is called the levator plate and is the shelf on which the pelvic organs rest. It is formed by the fusion of the iliococcygeus and the posterior fibers of the pubococcygeus muscles. When the body is in a standing position, the levator plate is horizontal and supports the rectum and upper two thirds of vagina above it. Weakness of the levator ani may loosen the sling behind the anorectum and cause the levator plate to sag.5 This opens the urogenital hiatus and predisposes to pelvic organ prolapse (). Women with prolapse have been shown to have an enlarged urogenital hiatus on clinical examination.6
Open in a separate window
Pelvic floor support (midsagittal section of the pelvis): (A) normal tone in the levator ani with acute anorectal angle and horizontal levator plate; note the normal vaginal axis.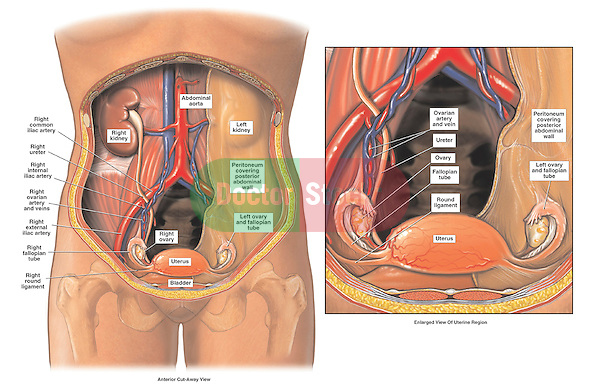 (B) With loss of tone in the levator ani, there is change in the vaginal axis, sagging of the levator plate, and enlargement of the urogenital hiatus. Reprinted, with permission, from Herschorn S, Carr LK. In: Campbell’s Urology. 2002:1092–1139.36
(B) With loss of tone in the levator ani, there is change in the vaginal axis, sagging of the levator plate, and enlargement of the urogenital hiatus. Reprinted, with permission, from Herschorn S, Carr LK. In: Campbell’s Urology. 2002:1092–1139.36
The coccygeus muscle that extends from the ischial spine to the coccyx and lower sacrum forms the posterior part of the pelvic diaphragm. It sits on the anterior surface of the sacrospinous ligament. Three-dimensional magnetic resonance imaging (MRI) of the pelvic diaphragm () shows its peripheral attachments and demonstrates the urogenital hiatus.7
Open in a separate window
Three-dimensional reconstructed magnetic resonance image of a 28-year-old healthy woman showing pelvic floor muscles and bones. The keyhole shape indicates normal separation of the vagina and rectum and intact perineal body. Reprinted from Fielding JR et al. AJR Am J Roentgenol. 2000; 174:657–660.7 Reprinted with permission from the American Journal of Roentgenology.
Direct innervation of the levator ani muscle on its cranial surface is primarily from the third and fourth sacral nerve roots via the pudendal nerve.8 The puborectalis may derive some if its innervation from a pudendal branch on the caudal side.2 Regarding the type of the striated muscle, it has been reported that the majority of the muscle fibers in the levator ani are slow-twitch fibers that maintain constant tone (type I),9 with an increased density of fast-twitch (type II) fibers distributed in the periurethral and perianal areas.10,11 This suggests that the normal levator ani maintains tone in the upright position to support the pelvic viscera. Furthermore, voluntary squeezing of the puborectalis may increase the tone to counter increased intra-abdominal pressure.
Urogenital Diaphragm (Perineal Membrane)
Another musculofascial structure, the urogenital diaphragm, is present over the anterior pelvic outlet below the pelvic diaphragm.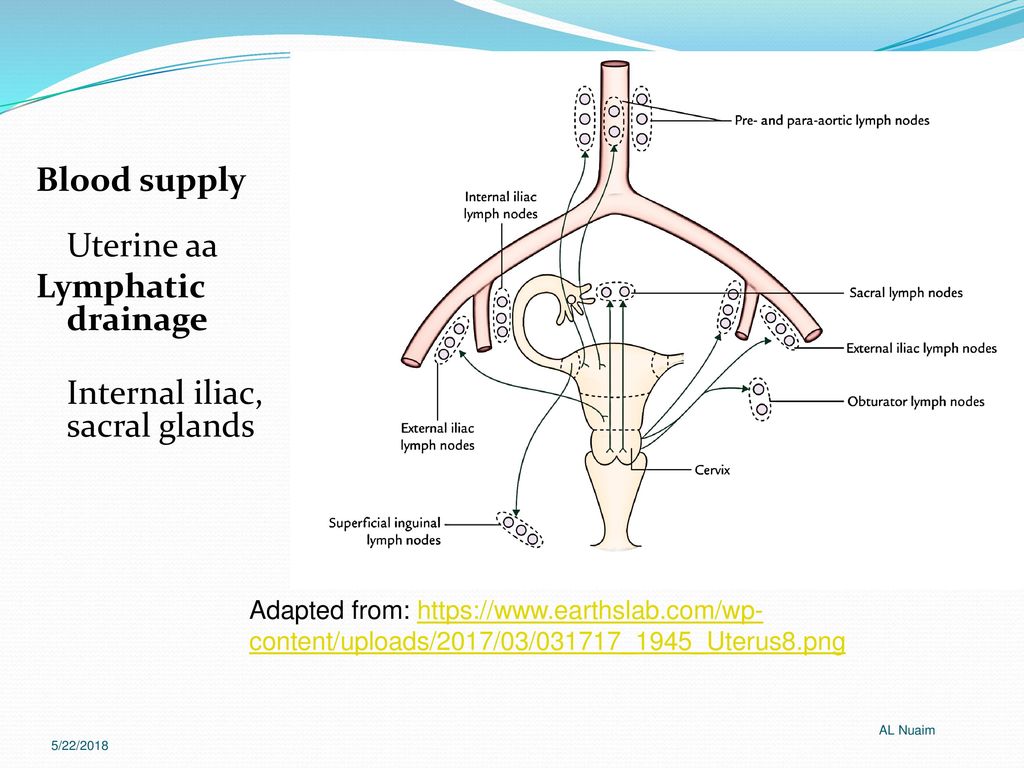 However, there is controversy over whether this structure contains a transverse sheet of muscle extending across the pubic arch (deep transverse perinei muscle) sandwiched between superior and inferior fascia12 or 3 contiguous striated muscles (compressor urethrae, sphincter urethrae, and urethra-vaginalis) and an inferior fascial layer called the perineal membrane ().4,13,14 Despite the controversy, MRI scans clearly depict the structure.2,12
However, there is controversy over whether this structure contains a transverse sheet of muscle extending across the pubic arch (deep transverse perinei muscle) sandwiched between superior and inferior fascia12 or 3 contiguous striated muscles (compressor urethrae, sphincter urethrae, and urethra-vaginalis) and an inferior fascial layer called the perineal membrane ().4,13,14 Despite the controversy, MRI scans clearly depict the structure.2,12
Open in a separate window
Muscles of the perineum: (A) On the subject’s right side, the membranous layer of the superficial fascia has been removed (note the cut edge). On the subject’s left side, the symphysis pubis, pubis, part of the ischiopubic ramus, superficial perineal muscles, and inferior fascia of the urogenital diaphragm have been removed to show the deep perineal muscles. (B) Deep perineal muscles are continuous with the sphincter urethrae.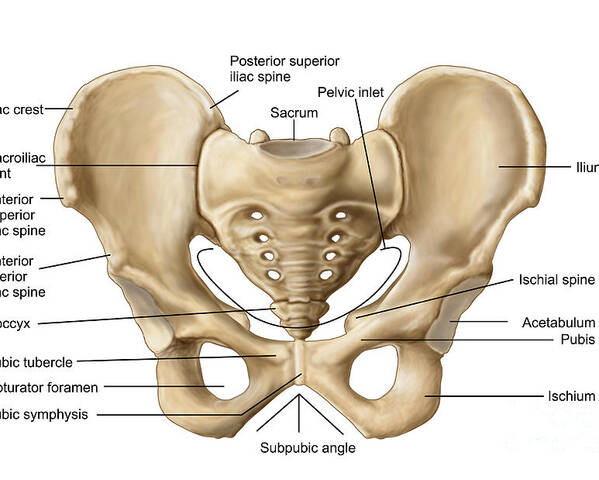 Reprinted, with permission, from Salmons S. In: Gray’s Anatomy. 1995:737–900.4
Reprinted, with permission, from Salmons S. In: Gray’s Anatomy. 1995:737–900.4
The more superficial ischiocavernosus and bulbocavernosus muscles, as well as the thin slips of the superficial transverse perinei,14 complete the inferior aspect of the urogenital diaphragm. The structure bridges the gap between the inferior pubic rami bilaterally and the perineal body. It closes the urogenital (levator) hiatus; supports and has a sphincter-like effect at the distal vagina; and, because it is attached to periurethral striated muscles, contributes to continence. It also provides structural support for the distal urethra. The posterior triangle around the anus does not have a corresponding diaphragm or membrane. The ischiorectal fossae are the spaces lateral to the anus below the pelvic diaphragm.
Perineal Body
The perineal body is a pyramidal fibromuscular structure in the midline between the anus and vagina with the rectovaginal septum at its cephalad apex. 4 Below this, muscles and their fascia converge and interlace through the structure. Attached to the perineal body are the rectum, vaginal slips from the pubococcygeus, perineal muscles, and the anal sphincter; it also contains smooth muscle, elastic fibers, and nerve endings. During childbirth, the perineal body distends and then recoils.12 It is an important part of the pelvic floor; just above it are the vagina and the uterus. Acquired weakness of the perineal body gives rise to elongation and predisposes to defects such as rectocele and enterocele.14,15 demonstrates the pelvic organs with the 2 major levels of muscular support: the upper muscular structure, with the pelvic diaphragm, and the lower muscular structure, with the perineal membrane anteriorly and anal sphincter posteriorly.
4 Below this, muscles and their fascia converge and interlace through the structure. Attached to the perineal body are the rectum, vaginal slips from the pubococcygeus, perineal muscles, and the anal sphincter; it also contains smooth muscle, elastic fibers, and nerve endings. During childbirth, the perineal body distends and then recoils.12 It is an important part of the pelvic floor; just above it are the vagina and the uterus. Acquired weakness of the perineal body gives rise to elongation and predisposes to defects such as rectocele and enterocele.14,15 demonstrates the pelvic organs with the 2 major levels of muscular support: the upper muscular structure, with the pelvic diaphragm, and the lower muscular structure, with the perineal membrane anteriorly and anal sphincter posteriorly.
Open in a separate window
The 2 major muscular supporting structures: the upper, with the pelvic diaphragm, and the lower, with the perineal membrane (urogenital diaphragm) anteriorly and anal sphincter posteriorly.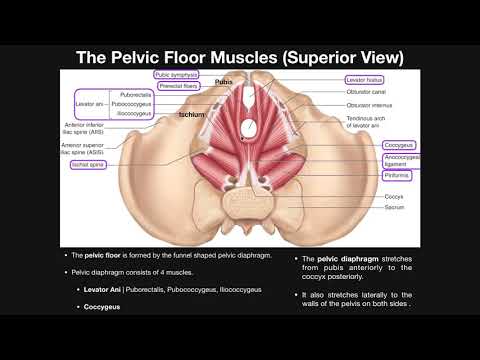 Reprinted, with permission, from Herschorn S, Carr LK. In: Campbell’s Urology. 2002:1092–1139.36
Reprinted, with permission, from Herschorn S, Carr LK. In: Campbell’s Urology. 2002:1092–1139.36
The bladder and urethra and the vagina and uterus are attached to the pelvic walls by a system of connective tissue that has been called the endopelvic fascia. This structure lies immediately beneath the peritoneum and is one continuous unit with various thickenings or condensations in specific areas. The endopelvic fascia is continuous with the visceral fascia, which provides a capsule containing the organs and allows displacements and changes in volume. The distinct regions of this structure are given individual names, specifically ligaments and fascia, with variable internal structure. Endopelvic fascia and ligaments are a mesh-like group of collagen fibers interlaced with elastin, smooth muscle cells, fibroblasts, and vascular structures. The structures that attach the uterus to the pelvic wall, the cardinal ligaments, derive strength from the supportive collagen forming the walls of arteries and veins.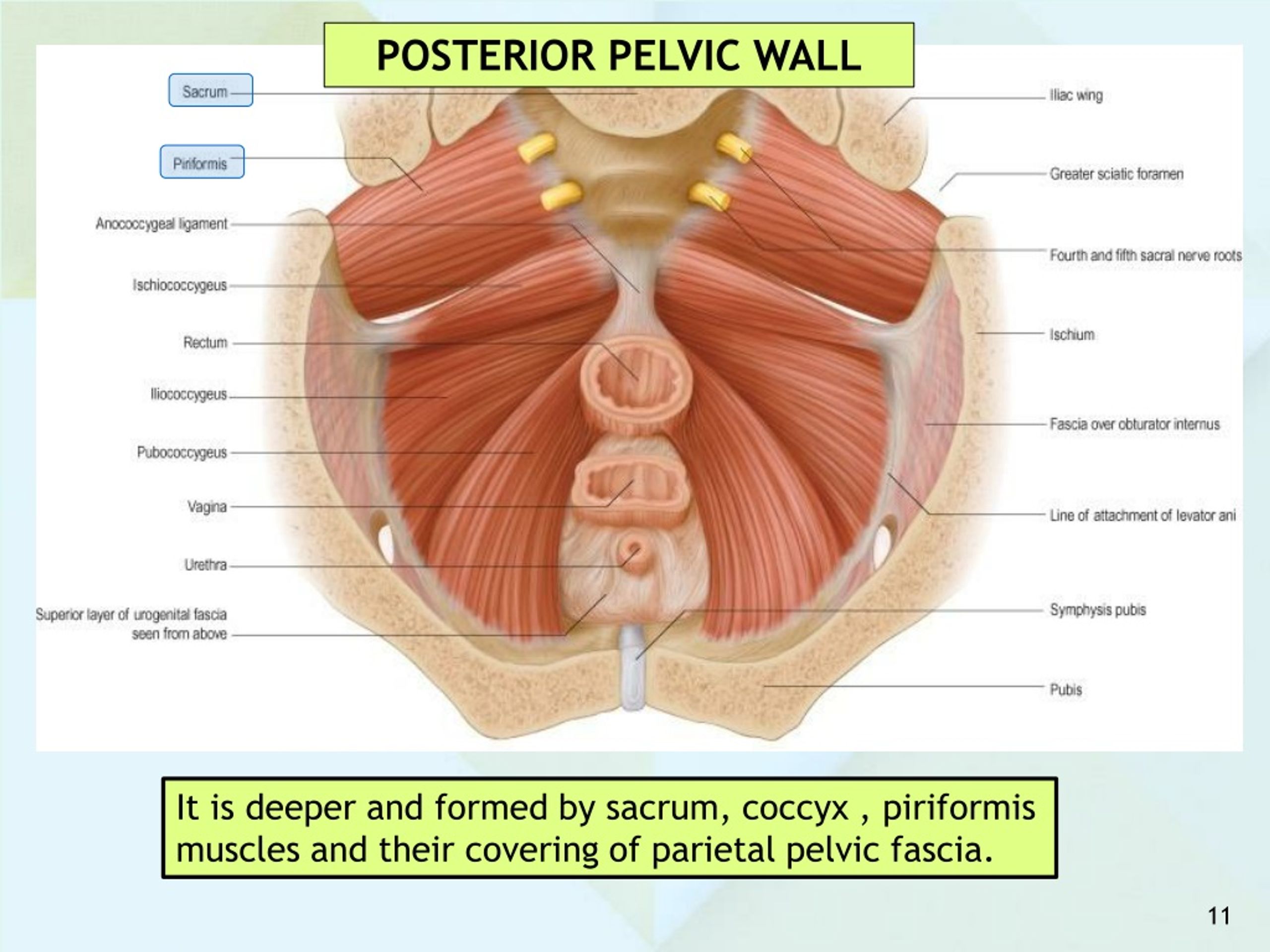 Other structures, such as the pelvic sidewall attachment of the endopelvic fascia (arcus tendineus of the pelvic fascia), are predominantly fibrous collagen.16
Other structures, such as the pelvic sidewall attachment of the endopelvic fascia (arcus tendineus of the pelvic fascia), are predominantly fibrous collagen.16
Anterior Supports
There is agreement among investigators that the connective tissue supports of the urethra, bladder, and vagina extend to the arcus tendineus of the pelvic fascia on the pelvic diaphragm.2,12,17,18 There is also agreement that a “hammock” of anterior vaginal wall tissue, bridging the gap medially in the urogenital hiatus, supports the vesical neck and urethra.17,19 There is controversy, however, focusing on the connective tissue structures that are associated with this hammock.
The pubourethral ligaments are connective tissue structures that extend from the urethra to the pubic bone. Various authors have described them as structures responsible for supporting the urethra and keeping the vesical neck closed.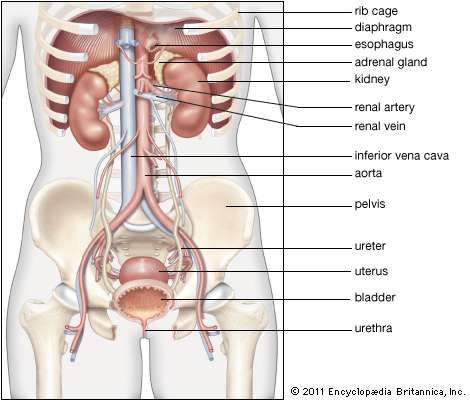 20–23 However, other authors have described connective tissue between the proximal urethra (and vesical neck) and the pubis as containing smooth muscle24 and cholinergic nerves25 that make the structures more suited to vesical neck opening during micturition than to urethral support. Because there is an attachment of the lower third of the urethra to the pubis, it is postulated that there are 2 separate structures—one for support at the mid- or distal urethra and one near the bladder neck that may open it during voiding. The distal support has been described as connective tissue that joins the vaginal wall and periurethral tissue to the arcus tendineus of the pelvic fascia and the levator muscles.24 Since the arcus tendineus inserts into the pubic bone, these are the ligaments that are palpable during a retropubic dissection. These ligaments are important for urethral support.23
20–23 However, other authors have described connective tissue between the proximal urethra (and vesical neck) and the pubis as containing smooth muscle24 and cholinergic nerves25 that make the structures more suited to vesical neck opening during micturition than to urethral support. Because there is an attachment of the lower third of the urethra to the pubis, it is postulated that there are 2 separate structures—one for support at the mid- or distal urethra and one near the bladder neck that may open it during voiding. The distal support has been described as connective tissue that joins the vaginal wall and periurethral tissue to the arcus tendineus of the pelvic fascia and the levator muscles.24 Since the arcus tendineus inserts into the pubic bone, these are the ligaments that are palpable during a retropubic dissection. These ligaments are important for urethral support.23
There is controversy regarding the amount of supportive tissue or fascia in the anterior vaginal wall.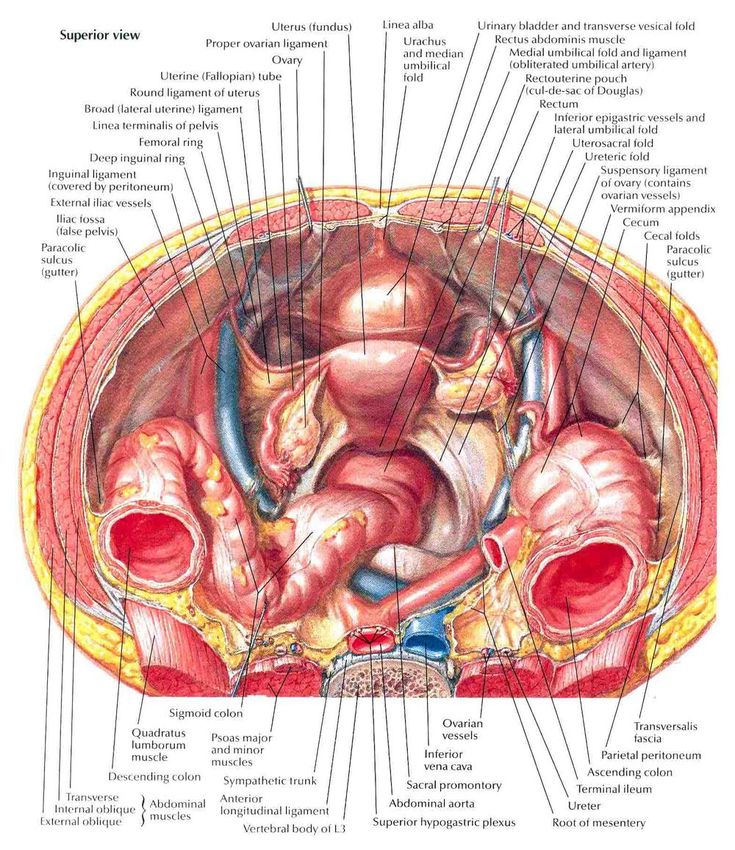 Although the wall is composed of mucosa, muscularis, and adventitia and abuts a similar arrangement in both the urethra and bladder, various authors have attributed to it a vaginal fascial layer. Weber and Walters18 cited many articles on both sides of the controversy and reported no specific fascial layer, whereas DeLancey17 demonstrated a fascial layer suburethrally on the anterior vaginal wall. With or without the suburethral fascial layer, the anterior vaginal wall supports the urethra by its lateral attachment to the levators (pubococcygeus) and to the endopelvic fascia from the arcus tendineus of the pelvic fascia ( and ). In essence, it is a double hammock. Paradoxically, the more advanced the prolapse, the more thickened and hypertrophied is the vaginal submucosal layer.18
Although the wall is composed of mucosa, muscularis, and adventitia and abuts a similar arrangement in both the urethra and bladder, various authors have attributed to it a vaginal fascial layer. Weber and Walters18 cited many articles on both sides of the controversy and reported no specific fascial layer, whereas DeLancey17 demonstrated a fascial layer suburethrally on the anterior vaginal wall. With or without the suburethral fascial layer, the anterior vaginal wall supports the urethra by its lateral attachment to the levators (pubococcygeus) and to the endopelvic fascia from the arcus tendineus of the pelvic fascia ( and ). In essence, it is a double hammock. Paradoxically, the more advanced the prolapse, the more thickened and hypertrophied is the vaginal submucosal layer.18
Open in a separate window
The hammock hypothesis: the anterior vaginal wall with its attachment to the arcus tendineus of the pelvic fascia forms a hammock under the urethra and bladder neck. Reprinted, with permission, from DeLancey JOL. Am J Obstet Gynecol. 1996;175:311–319.37
Reprinted, with permission, from DeLancey JOL. Am J Obstet Gynecol. 1996;175:311–319.37
Open in a separate window
Cross section of urethral supports below the bladder neck: The urethra is supported by a hammock of anterior vaginal wall suspended to the levators (pubococcygeus muscles) and the fascial attachments (FA) to the tendinous arch of the pelvic fascia. In essence, it is a “double hammock.” Reprinted, with permission, from Herschorn S, Carr LK. In: Campbell’s Urology. 2002:1092–1139.36
Urethropelvic ligaments from the suburethral fascia at the bladder neck and proximal urethra to the levators and arcus tendineus have been described and demonstrated on MRI scans.12 The existence of these ligaments as separate entities apart from the hammock of tissue supporting the proximal urethra has been disputed.26,27
At the level of the bladder base, there is little actual endopelvic fascia between the bladder and vaginal muscularis.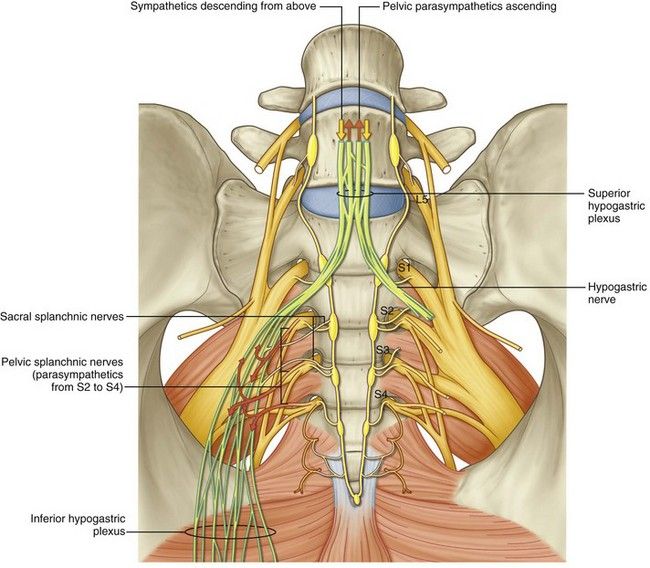 Here, the support comes from the lateral attachment of the vagina to the arcus tendineus of the pelvic fascia.17 The pubocervical fascia has been described as extending from the symphysis along the anterior vaginal wall to blend with the fascia that surrounds the cervix. It is continuous laterally with the pubococcygeus and also suspended to the arcus tendineus of the pelvic fascia. Its existence as a separate and discernable entity is also in dispute.18
Here, the support comes from the lateral attachment of the vagina to the arcus tendineus of the pelvic fascia.17 The pubocervical fascia has been described as extending from the symphysis along the anterior vaginal wall to blend with the fascia that surrounds the cervix. It is continuous laterally with the pubococcygeus and also suspended to the arcus tendineus of the pelvic fascia. Its existence as a separate and discernable entity is also in dispute.18
Middle Supports
The paracolpium and parametrium are the connective tissues surrounding the vagina and the uterus, respectively. In the midvagina, the paracolpium fuses with the pelvic wall and fascia laterally.28 The cardinal ligaments (also called the transverse cervical ligaments of Mackenrodt) extend from the lateral margins of the cervix and upper vagina to the lateral pelvic walls. They originate over a large area from the region of the greater sciatic foramen over the piriformis muscles, from the pelvic bones in the region of the sacroiliac joint, and from the lateral sacrum.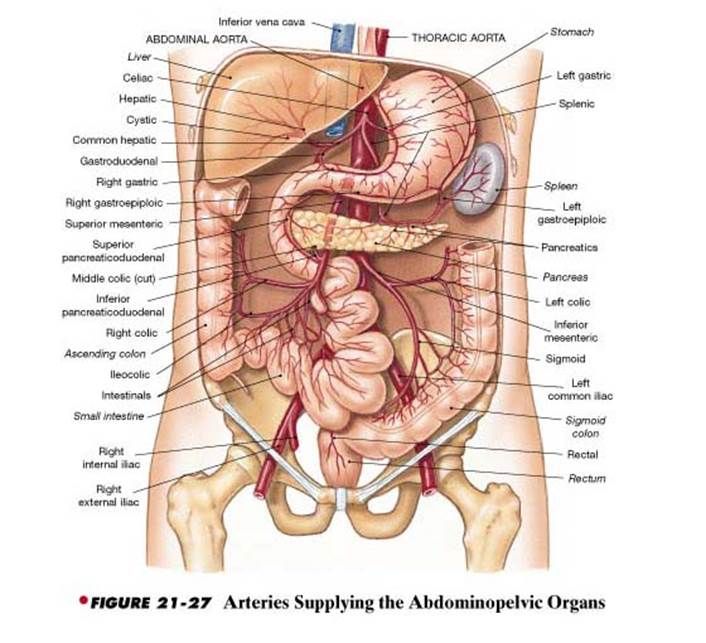 They are condensations of the lowermost parts of the broad ligaments. Laterally, the cardinal ligaments are continuous with the connective tissue surrounding the hypogastric vessels. Medially, they are continuous with the paracolpium and parametrium as well as the connective tissue in the anterior vaginal wall, the so-called pubocervical fascia.
They are condensations of the lowermost parts of the broad ligaments. Laterally, the cardinal ligaments are continuous with the connective tissue surrounding the hypogastric vessels. Medially, they are continuous with the paracolpium and parametrium as well as the connective tissue in the anterior vaginal wall, the so-called pubocervical fascia.
The uterosacral ligaments are attached to the cervix and upper vaginal fornices posterolaterally. Posteriorly, they attach to the pre-sacral fascia in front of the sacroiliac joint. The connective tissue of the uterosacral ligaments is continuous with that of the cardinals around the cervix. The cardinal and uterosacral ligaments hold the uterus and upper vagina in their proper place over the levator plate.29 This supportive structure is depicted in . According to Raz and colleagues,30 the cardinals and uterosacrals are not directly important for continence but do play a role in the support of the bladder base in the surgical correction of large cystoceles.
Open in a separate window
The cardinal and uterosacral ligaments provide support to the cervix and indirectly to the bladder base. The retropubic, vesicovaginal, and rectovaginal spaces are seen at the level of the cervix. Reprinted, with permission, from Raz S et al. In: Campbell’s Urology. 1998:1059–1094.30
Posterior Supports
The posterior vaginal wall, below the cardinals, is supported from the sides by the paracolpium, which is attached to the endopelvic fascia (referred to as rectovaginal fascia in this area) and pelvic diaphragm. The anterior and posterior fascial layers unite along the sides of the vagina (). According to DeLancey,28 the rectovaginal fascia is found mostly at the sides and is extremely thin in the midline of the vaginal wall. However, a posterior rectovaginal septum, consisting of fibromuscular elastic tissue, extending from the peritoneal reflection to the perineal body has been described.21 During fetal life, the peritoneal cavity extends to the cranial part of the perineal body, but it becomes obliterated in early life.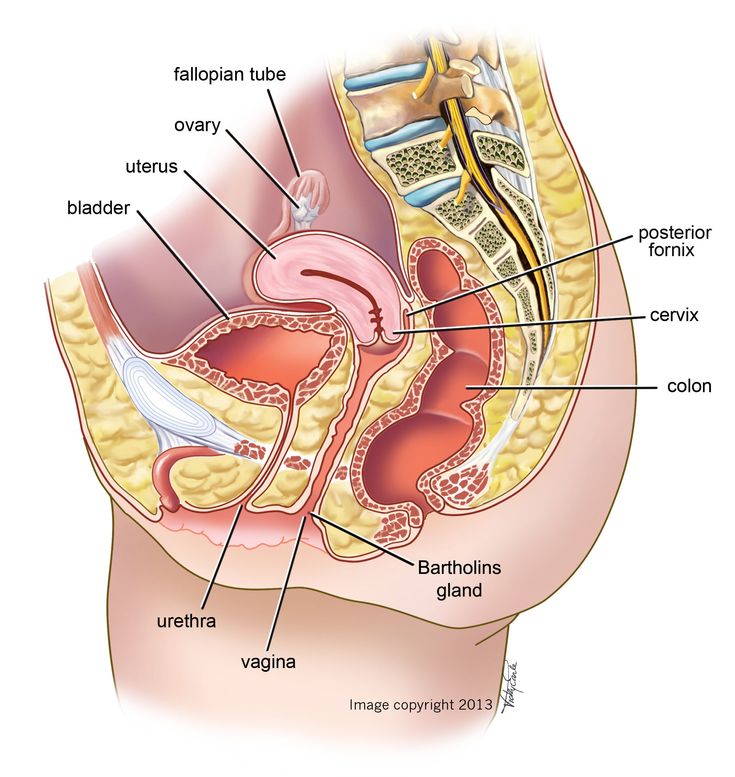 Its fused layers (Denonvillier’s fascia) probably become part of the rectovaginal septum adherent to the undersurface of the posterior vaginal wall. This fascia forms the anterior margin of another potential space, the rectovaginal space. The rectovaginal septum, if intact and normal, permits independent mobility of the rectal and vaginal walls.
Its fused layers (Denonvillier’s fascia) probably become part of the rectovaginal septum adherent to the undersurface of the posterior vaginal wall. This fascia forms the anterior margin of another potential space, the rectovaginal space. The rectovaginal septum, if intact and normal, permits independent mobility of the rectal and vaginal walls.
Open in a separate window
(A) Vagina and supportive structures drawn from dissection of a 56-year-old cadaver after hysterectomy: The bladder has been removed above the vesical neck. Paracolpium extends along the lateral wall of vagina. (B) In level I, paracolpium suspends vagina from the lateral pelvic walls. In level II, the vagina is attached to arcus tendineus of pelvic fascia and superior fascia of levator ani muscles. Reprinted, with permission, from DeLancey JOL. Am J Obstet Gynecol. 1992;166:1717–1728.28
In the distal vagina, 2 cm to 3 cm above the hymeneal ring, the vaginal wall is directly attached to surrounding structures without any intervening paracolpium.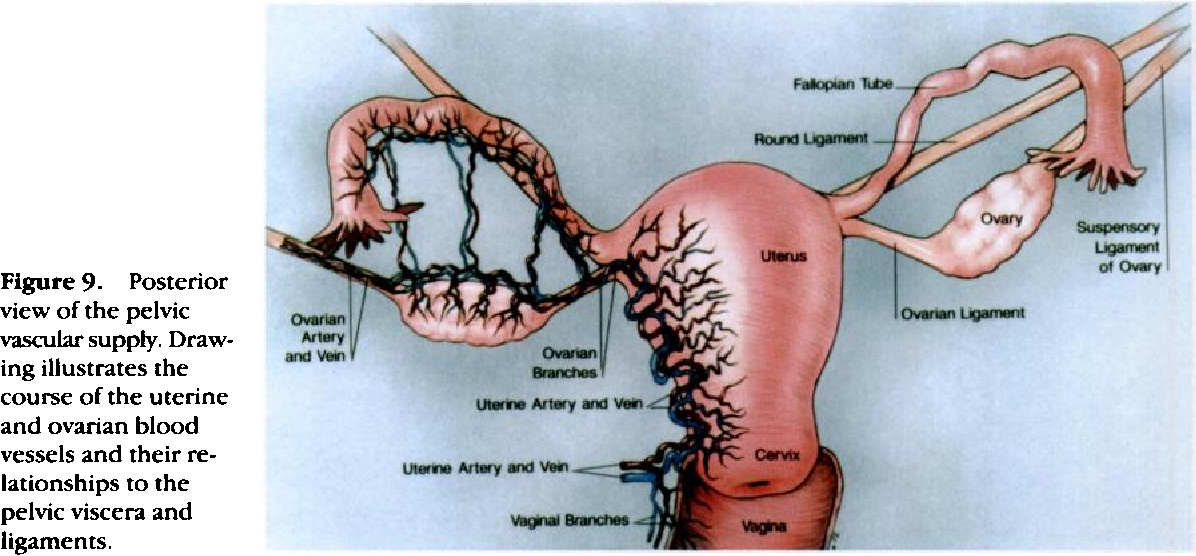 Anteriorly, the vagina fuses with the urethra and the connective tissue of the perineal membrane and muscles (urogenital diaphragm). Laterally, it blends with the levator ani muscles and, posteriorly, it fuses with the perineal body. The rectovaginal fascia is thickest in this region,31 and the vagina in this area has no mobility separate from its adjacent structures.28
Anteriorly, the vagina fuses with the urethra and the connective tissue of the perineal membrane and muscles (urogenital diaphragm). Laterally, it blends with the levator ani muscles and, posteriorly, it fuses with the perineal body. The rectovaginal fascia is thickest in this region,31 and the vagina in this area has no mobility separate from its adjacent structures.28
The fascial supports for the rectum, the lateral rectal ligaments, extend from the posterolateral pelvic side wall (level with the third sacral vertebra) to the rectum and surround the middle rectal arteries. Additional prerectal and pararectal fascial elements are frequently described.30
The urethra is a complex tubular structure extending below the bladder to the external meatus (). It has distinct muscular elements associated both within and without to permit its functioning for storage (continence) and voiding.
Open in a separate window
Urethral anatomy: the urethra has distinct muscular elements associated both within and without to permit its functioning for storage and voiding.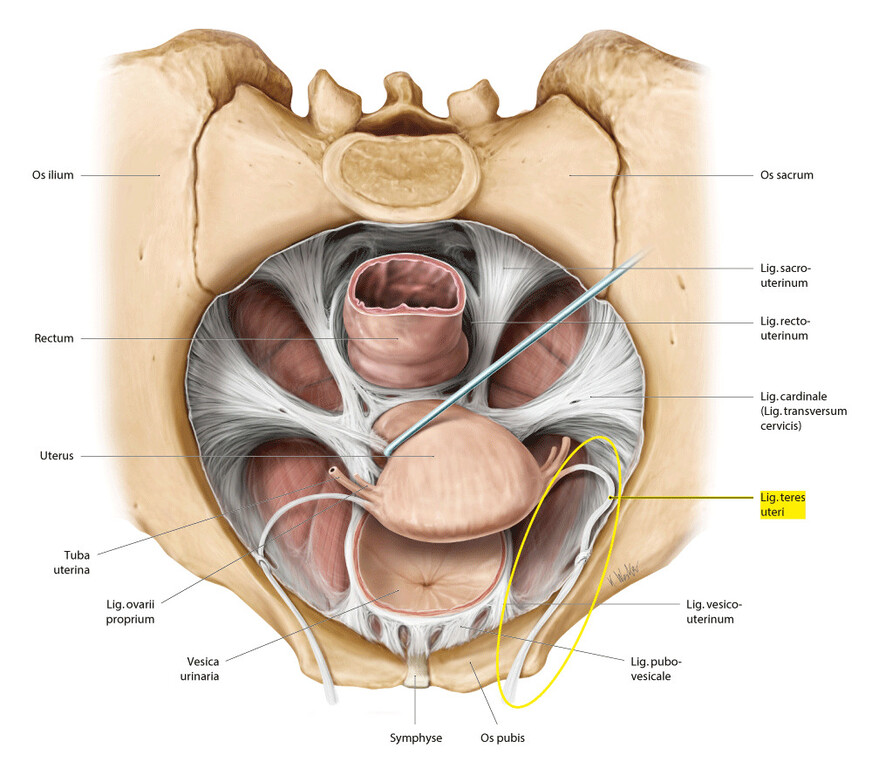 Reprinted, with permission, from Strohbehn K, DeLancey JOL. Oper Tech Gynecol Surg. 1997;2:5–16.38
Reprinted, with permission, from Strohbehn K, DeLancey JOL. Oper Tech Gynecol Surg. 1997;2:5–16.38
The smooth muscle of the urethra is contiguous with that of the trigone and detrusor.32 It has a prominent inner longitudinal and a thin outer circular layer. The layers lie inside the outer striated urogenital sphincter muscle and are present throughout the upper four fifths of the urethra. The configuration of the circular muscle implies a role in constricting the lumen, and the longitudinal muscle may aid in shortening the urethra during voiding.33
The outer layer of the urethra is formed by the muscle of the striated urogenital sphincter that is found in the middle three fifths of the length. In its upper two thirds, the sphincter-like fibers are circular. In the distal part, the fibers exit the urethra and surround the vaginal wall as the urethrovaginal sphincter or extend along the inferior pubic rami above the perineal membrane as the compressor urethra. 1 The muscle is composed mainly of slow-twitch fibers, well suited for maintaining constant tone.10 Voluntary muscle activation can also increase the constriction of the urethra when needed.
1 The muscle is composed mainly of slow-twitch fibers, well suited for maintaining constant tone.10 Voluntary muscle activation can also increase the constriction of the urethra when needed.
The urethral mucosa extends from the bladder transitional epithelium to the external meatus and is primarily nonkeratinizing squamous epithelium. It is derived from the urogenital sinus along with the lower vagina and vestibule. It is hormonally sensitive and undergoes changes with stimulation. 33 The hormonally sensitive submucosal tissue contains a rich and prominent vascular plexus. Several specialized types of arteriovenous anastomoses have been demonstrated, and it is thought that they provide a watertight closure of the mucosal surface with an increase in blood flow that may occur with an increase in pressure on abdominal vessels.32
In addition to the muscular and vascular tissue of the urethra, there is a considerable quantity of connective tissue interspersed within the muscle and submucosa.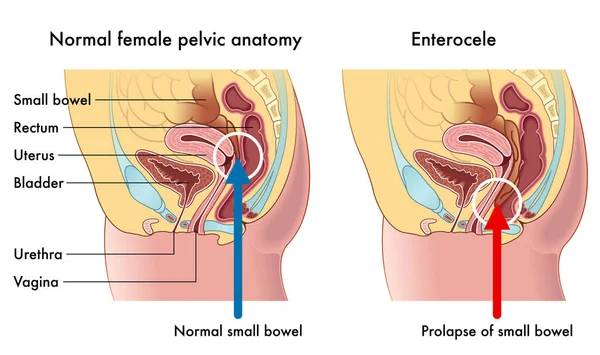 This tissue contains collagen and elastin fibers and is thought to add to urethral closure passively.33 Lastly, a series of glands are found in the submucosa, mainly along the vaginal surface of the urethra.34 They are most predominant in the middle and lower third of the urethra.
This tissue contains collagen and elastin fibers and is thought to add to urethral closure passively.33 Lastly, a series of glands are found in the submucosa, mainly along the vaginal surface of the urethra.34 They are most predominant in the middle and lower third of the urethra.
It is the admixture of the smooth and striated muscle, connective tissue, mucosa, and submucosa that accounts for a functional sphincter.35 A functional urethral sphincter has intact neural control and provides watertight apposition of the urethral lumen, compression of the wall around the lumen, and a means of compensating for abdominal pressure changes.
The “hammock hypothesis” () is a readily understood way to explain the continence mechanism. The requirements for continence include a quiescent bladder, functioning musculofascial supports, and a functional urethral sphincter mechanism. The fascial attachments connect the periurethral tissue and anterior vaginal wall to the arcus tendineus at the pelvic sidewall, whereas the muscular attachments connect the periurethral tissue to the medial border of the levator ani.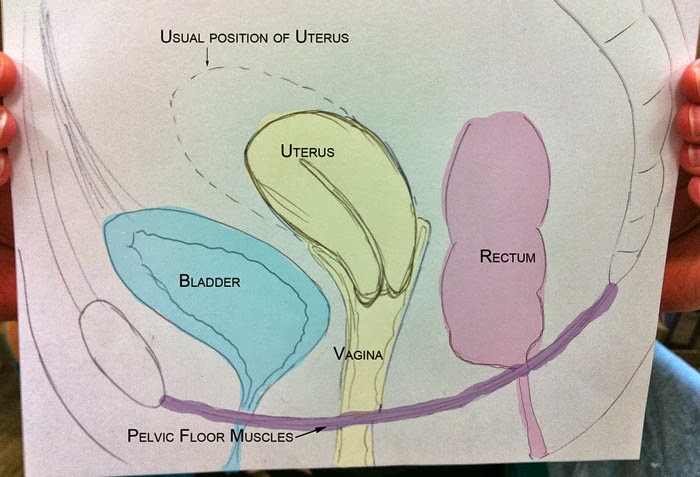 24 Urethral support is provided by a coordinated action of fascia and muscles acting as an integrated unit under neural control.33 This musculofascial support provides a hammock onto which the urethra is compressed during increases in intra-abdominal pressure. One can surmise that the urethral sphincter mechanism is operative and adds to the process. Furthermore, failure of one of the support components will not invariably produce stress incontinence, because of the compensatory effect of the other component. This may explain why some women with hypermobility have no incontinence. It may also explain why injectable agents can be used in women with hypermobility: the bulking agent may improve urethral sphincter function.
24 Urethral support is provided by a coordinated action of fascia and muscles acting as an integrated unit under neural control.33 This musculofascial support provides a hammock onto which the urethra is compressed during increases in intra-abdominal pressure. One can surmise that the urethral sphincter mechanism is operative and adds to the process. Furthermore, failure of one of the support components will not invariably produce stress incontinence, because of the compensatory effect of the other component. This may explain why some women with hypermobility have no incontinence. It may also explain why injectable agents can be used in women with hypermobility: the bulking agent may improve urethral sphincter function.
Open in a separate window
Lateral view of the pelvic floor with the urethra, vagina, and fascial tissues transected at the level of the vesical neck, drawn from 3-dimensional reconstruction indicating compression of the urethra by downward force (arrow) against the supportive tissues. Reprinted, with permission, from DeLancey JOL. Am J Obstet Gynecol. 1996;175:311–319.37
Increased knowledge of the anatomy of the pelvic floor and lower urinary tract has led to a better understanding of the pathophysiology of incontinence. This, in turn, has led to the development of newer and minimally invasive treatment options for stress incontinence. Although these treatment options are widely applicable, not all patients are candidates or will respond. It is in the setting of the complex patient with incontinence with or without pelvic organ prolapse that the clinician must call on his or her knowledge of anatomy to guide treatment decision making.
Main Points
The levator plate, the shelf on which the pelvic organs rest, is horizontal when the body is in a standing position and supports the rectum and upper two thirds of the vagina above it. Weakness of the levator ani may loosen the sling behind the anorectum and cause the levator plate to sag, opening the urogenital hiatus and allowing pelvic organ prolapse.
The urogenital diaphragm closes the levator hiatus, supports and has a sphincter-like effect at the distal vagina, provides structural support for the distal urethra, and contributes to continence in that it is attached to the periurethral striated muscles.
There is controversy regarding whether the anterior vaginal wall includes a suburethral fascial layer; regardless, the anterior vaginal wall provides support to the urethra by its lateral attachment to the levators and to the endopelvic fascia from the arcus tendineus of the pelvic fascia.
A combination of smooth and striated muscle, connective tissue, mucosa, and submucosa are necessary for a functional urethral sphincter, which provides watertight apposition of the urethral lumen, compression of the wall around the lumen, and a means of compensating for abdominal pressure changes.
The “hammock hypothesis” describes support of the urethra by a coordinated action of fascia and muscles, which provides a hammock onto which the urethra is compressed during increases in intra-abdominal pressure.
1. Soames RW, et al. Skeletal system. In: Williams PL, Bannister LH, Berry MM, et al., editors. Gray’s Anatomy. 38th ed. New York: Churchill Livingstone; 1995. pp. 425–736. [Google Scholar]
2. Strohbehn K. Normal pelvic floor anatomy. Obstet Gynecol Clin North Am. 1998;25:683–705. [PubMed] [Google Scholar]
3. Lawson JO. Pelvic anatomy I: pelvic floor muscles. Ann R Coll Surg Engl. 1974;54:244–252. [PMC free article] [PubMed] [Google Scholar]
4. Salmons S, et al. Muscle. In: Williams PL, Bannister LH, Berry MM, et al., editors. Gray’s Anatomy. 38th ed. New York: Churchill Livingstone; 1995. pp. 737–900. [Google Scholar]
5. Berglas B, Rubin IC. Study of the supportive structures of the uterus by levator myography. Surg Gynecol Obstet. 1953;97:677–692. [PubMed] [Google Scholar]
6. DeLancey JO, Hurd WW. Size of the urogenital hiatus in the levator ani muscles in normal women and women with pelvic organ prolapse. Obstet Gynecol. 1998;91:364–368.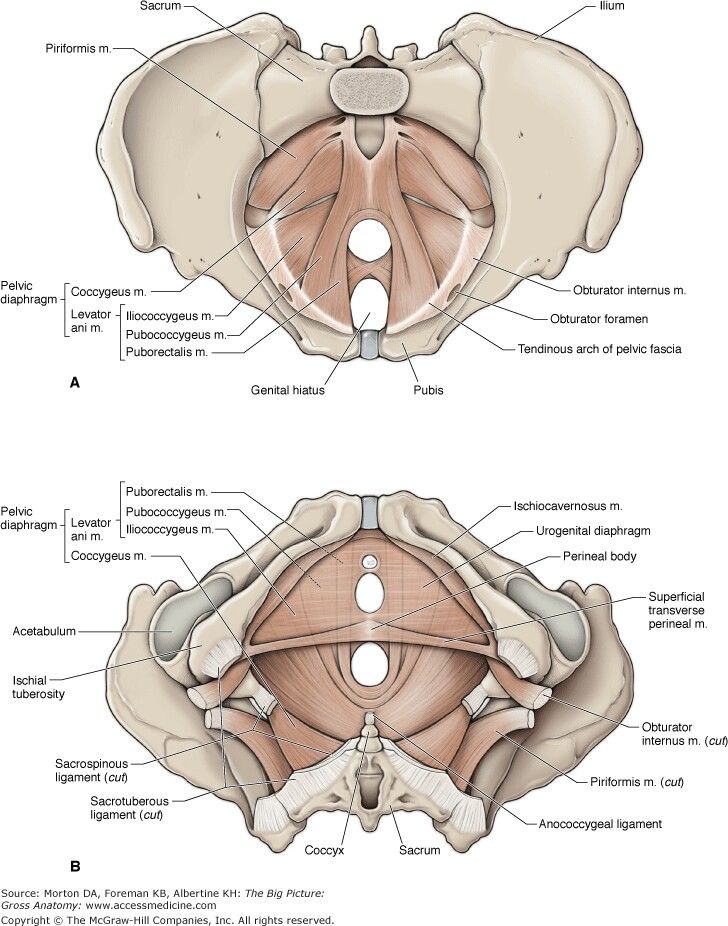 [PubMed] [Google Scholar]
[PubMed] [Google Scholar]
7. Fielding JR, Dumanli H, Schreyer AG, et al. MR-based three-dimensional modeling of the normal female pelvic floor in women: quantification of muscle mass. AJR Am J Roentgenol. 2000;174:657–660. [PubMed] [Google Scholar]
8. Gosling J, Alm P, Bartch G, et al. Gross anatomy of the lower urinary tract. In: Abrams P, Khoury S, Wein A, et al., editors. Incontinence: First International Consultation. Plymouth, UK: Health Publications Ltd; 1999. pp. 21–56. [Google Scholar]
9. Gilpin SA, Gosling JA, Smith AR, Warrell DW. The pathogenesis of genitourinary prolapse and stress urinary incontinence of urine: a histological and histochemical study. Br J Obstet Gynaecol. 1989;96:15–23. [PubMed] [Google Scholar]
10. Gosling JA, Dixon JS, Critchley HO, Thompson SA. A comparative study of the human external sphincter and periurethral levator ani muscles. Br J Urol. 1981;53:35–41. [PubMed] [Google Scholar]
11. Critchley HO, Dixon JS, Gosling JA. Comparative study of the periurethral and perianal parts of the human levator ani muscle. Urol Int. 1980;35:226–232. [PubMed] [Google Scholar]
Comparative study of the periurethral and perianal parts of the human levator ani muscle. Urol Int. 1980;35:226–232. [PubMed] [Google Scholar]
12. Klutke CG, Siegel CL. Functional female pelvic anatomy. Urol Clin North Am. 1995;22:487–498. [PubMed] [Google Scholar]
13. Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983;205:223–232. [PubMed] [Google Scholar]
14. DeLancey JO. Structural anatomy of the posterior pelvic compartment as it relates to rectocele. Am J Obstet Gynecol. 1999;180:815–823. [PubMed] [Google Scholar]
15. Nichols DH. Central compartment defects. In: Rock JA, Thompson JD, editors. Te Linde’s Operative Gynecology. 8th ed. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 1006–1030. [Google Scholar]
16. Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol. 1993;36:926–938. [PubMed] [Google Scholar]
17. DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–1723. [PubMed] [Google Scholar]
Am J Obstet Gynecol. 1994;170:1713–1723. [PubMed] [Google Scholar]
18. Weber AM, Walters MD. Anterior vaginal prolapse: review of anatomy and techniques of surgical repair. Obstet Gynecol. 1997;89:311–318. [PubMed] [Google Scholar]
19. Blaivas JG, Romanzi LJ, Heritz DM. Urinary incontinence: pathophysiology, evaluation, treatment overview, and nonsurgical management. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell’s Urology. 7th ed. Philadelphia: WB Saunders Company; 1998. pp. 1007–1043. [Google Scholar]
20. Krantz KE. The anatomy of the urethra and anterior vaginal wall. Am J Obstet Gynecol. 1951;62:374–386. [PubMed] [Google Scholar]
21. Milley PS, Nichols DH. A correlative investigation of the human rectovaginal septum. Anat Rec. 1969;163:433–451. [PubMed] [Google Scholar]
22. Zacharin RF. The suspensory mechanism of the female urethra. J Anat. 1963;97:423–427. [PMC free article] [PubMed] [Google Scholar]
23. Mostwin JL, Yang A, Sanders R, Genadry R.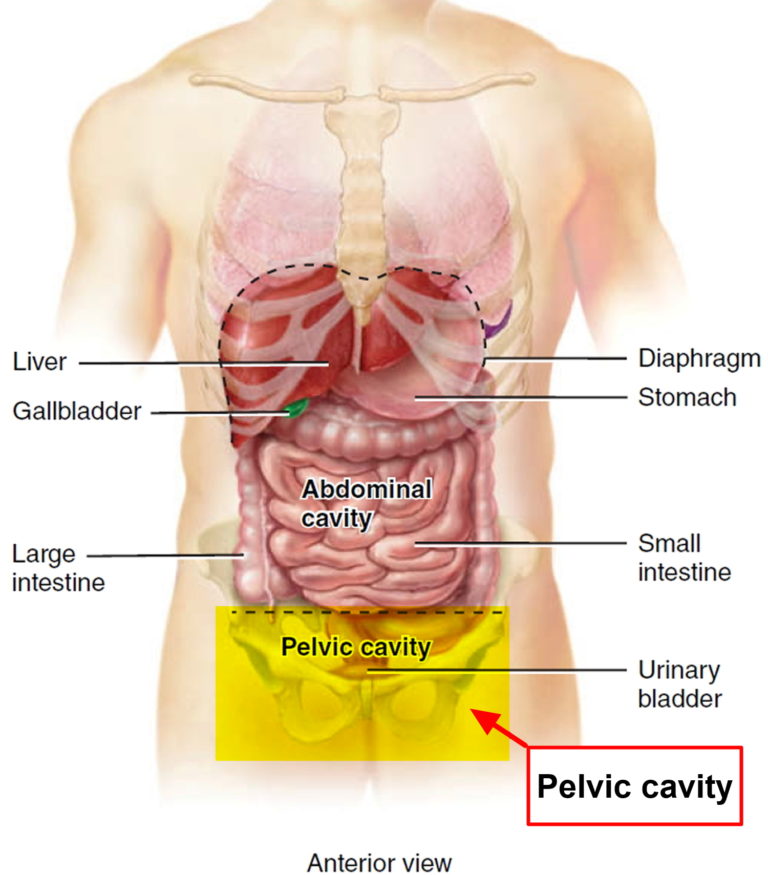 Radiography, sonography, and magnetic resonance imaging for stress incontinence: contributions, uses, and limitations. Urol Clin North Am. 1995;22:539–549. [PubMed] [Google Scholar]
Radiography, sonography, and magnetic resonance imaging for stress incontinence: contributions, uses, and limitations. Urol Clin North Am. 1995;22:539–549. [PubMed] [Google Scholar]
24. DeLancey JOL. Pubovesical ligament: a separate structure from the urethral supports (‘pubourethral ligaments’) Neurourol Urodyn. 1989;8:53–61. [Google Scholar]
25. Wilson PD, Dixon JS, Brown AD, Gosling JA. Posterior pubo-urethral ligaments in normal and genuine stress incontinent women. J Urol. 1983;130:802–805. [PubMed] [Google Scholar]
26. Kirschner-Hermanns R, Wein B, Niehaus S, et al. The contribution of magnetic resonance imaging of the pelvic floor to the understanding of urinary incontinence. Br J Urol. 1993;72:715–718. [PubMed] [Google Scholar]
27. Aronson MP, Bates SM, Jacoby AF, et al. Periurethral and paravaginal anatomy: an endovaginal magnetic resonance imaging study. Am J Obstet Gynecol. 1995;173:1702–1708. [PubMed] [Google Scholar]
28. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy.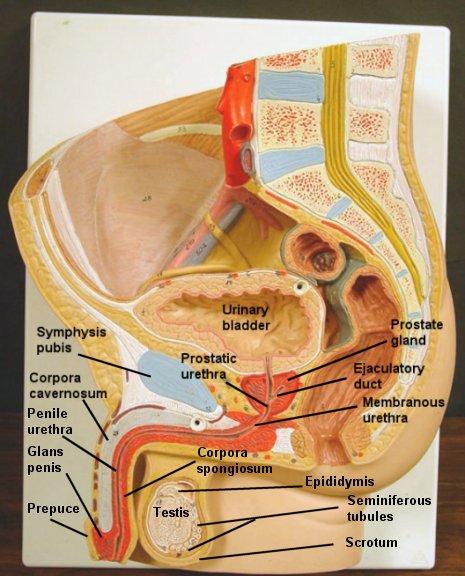 Am J Obstet Gynecol. 1992;166:1717–1728. [PubMed] [Google Scholar]
Am J Obstet Gynecol. 1992;166:1717–1728. [PubMed] [Google Scholar]
29. Thompson DJ. Surgical correction of defects in pelvic supports; pelvic organ prolapse. In: Rock JA, Thompson JD, editors. Te Linde’s Operative Gynecology. 8th ed. Philadelphia: Lippincott-Raven Publishers; 1997. pp. 951–979. [Google Scholar]
30. Raz S, Stothers L, Chopra A. Vaginal reconstructive surgery for incontinence and prolapse. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell’s Urology. 7th ed. Philadelphia: WB Saunders Company; 1998. pp. 1059–1094. [Google Scholar]
31. Reiffenstuhl G. Practical pelvic anatomy for the gynecologic surgeon. In: Nichols DH, editor. Gynecologic and Obstetric Surgery. St Louis: Mosby; 1993. pp. 26–71. [Google Scholar]
32. Huisman AB. Aspects on the anatomy of the female urethra with special relation to urinary incontinence. Contrib Gynecol Obstet. 1983;10:1–31. [PubMed] [Google Scholar]
33. DeLancey JO. Anatomy. In: Cardozo L, Staskin D, editors.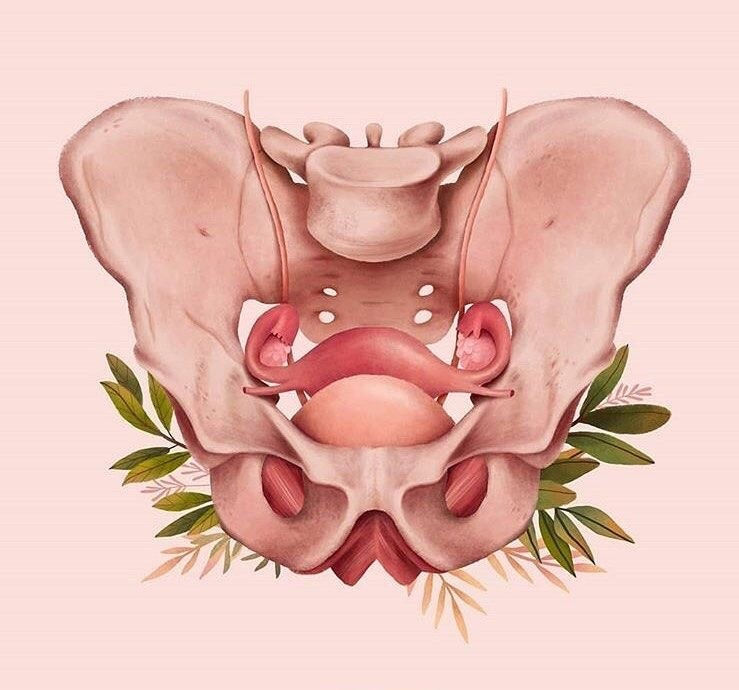 Textbook of Female Urology and Urogynaecology. 1st ed. London: Isis Medical Media; 2001. pp. 112–124. [Google Scholar]
Textbook of Female Urology and Urogynaecology. 1st ed. London: Isis Medical Media; 2001. pp. 112–124. [Google Scholar]
34. Huffman J. Detailed anatomy of the paraurethral ducts in the adult human female. Am J Obstet Gynecol. 1948;55:86–101. [PubMed] [Google Scholar]
35. Blaivas JG, Groutz A. Urinary incontinence: pathophysiology, evaluation, and management overview. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell’s Urology. 8th ed. Philadelphia: WB Saunders Company; 2002. pp. 1027–1052. [Google Scholar]
36. Herschorn S, Carr LK. Vaginal reconstructive surgery for sphincteric incontinence and prolapse. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell’s Urology. 8th ed. Philadelphia: WB Saunders Company; 2002. pp. 1092–1139. [Google Scholar]
37. DeLancey JOL. Stress urinary incontinence: where are we now, where should we go? Am J Obstet Gynecol. 1996;175:311–319. [PubMed] [Google Scholar]
38. Strohbehn K, DeLancey JOL. The anatomy of stress incontinence.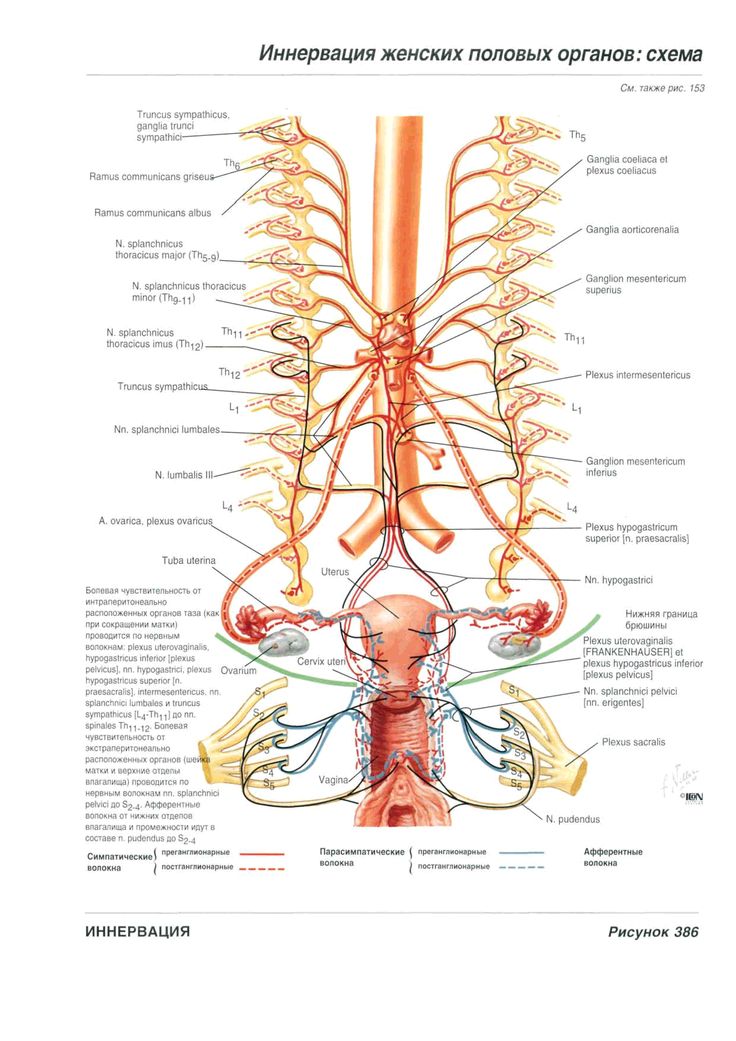 Oper Tech Gynecol Surg. 1997;2:5–16. [Google Scholar]
Oper Tech Gynecol Surg. 1997;2:5–16. [Google Scholar]
Anatomy, Abdomen and Pelvis, Female Pelvic Cavity - StatPearls
Introduction
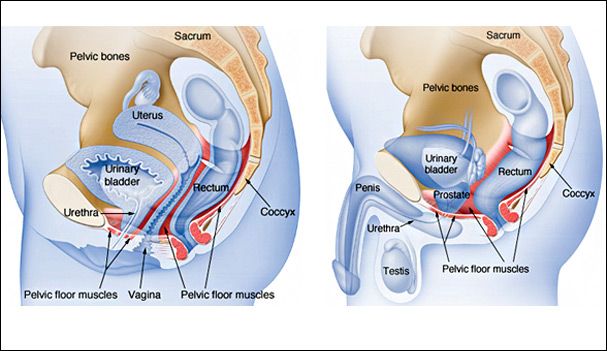
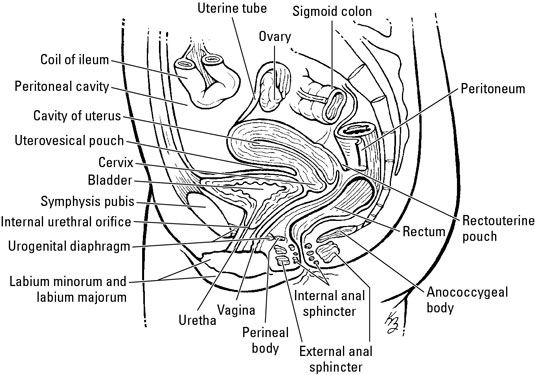
The pelvic cavity is a bowl-like structure that sits below the abdominal cavity. The true pelvis, or lesser pelvis, lies below the pelvic brim (Figure 1). This landmark begins at the level of the sacral promontory posteriorly and the pubic symphysis anteriorly. The space below contains the bladder, rectum, and part of the descending colon. In females, the pelvis also houses the uterus, fallopian tubes, and ovaries. Knowledge of anatomy unique to females is essential for all clinicians, especially those in the field of obstetrics and gynecology.
Structure and Function
The uterus sits in the center of the female pelvic cavity (Figure 2.) The most common position of the uterus in the pelvic cavity is anteverted and anteflexed.[1] "Version" refers to the angle between the cervix and the vagina. An anteverted uterus appears "tipped forward" in the pelvic cavity.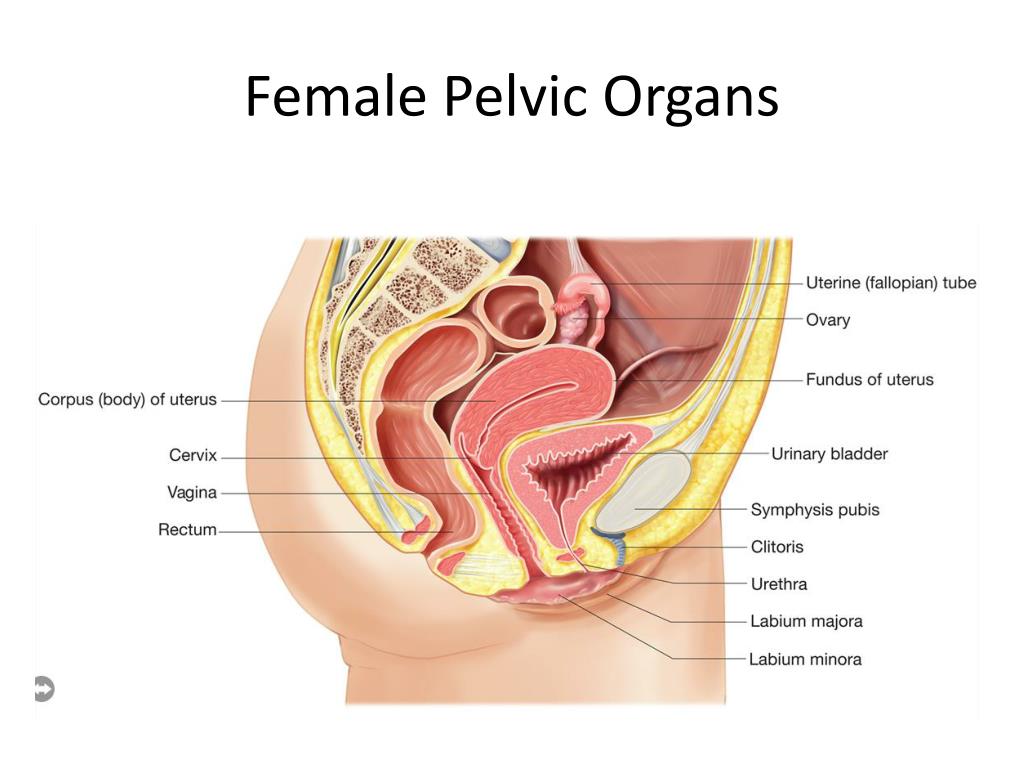 A retroverted uterus is "tipped backward." Retroversion is a normal variant but can lead to dyspareunia. Additionally, retroversion of a gravid uterus correlates with higher rates of vaginal bleeding and spontaneous abortion.[2]
A retroverted uterus is "tipped backward." Retroversion is a normal variant but can lead to dyspareunia. Additionally, retroversion of a gravid uterus correlates with higher rates of vaginal bleeding and spontaneous abortion.[2]
"Flexion" is the term for the angle between the cervix and uterine body. Anteflexed means the uterus is bent forward. Retroflexed means the uterus bends backward. Occasionally, retroflexion is seen after cesarean section and may be due to scar tissue that attaches the uterine body to the abdominal wall, causing the fundus to bend posteriorly.[3] However, the data supporting this theory is limited.
Anterior to the uterus is the bladder, with rectum located posteriorly. Between the uterus and the rectum is the recto-uterine space, also known as the posterior cul-de-sac. It is a potential space prone to fluid collection. Small amounts of physiologic fluid accumulate during ovulation and menses. Pathologic causes of fluid collection in the recto-uterine pouch include pelvic abscesses, drop metastasis from gastrointestinal malignancies, and endometriosis.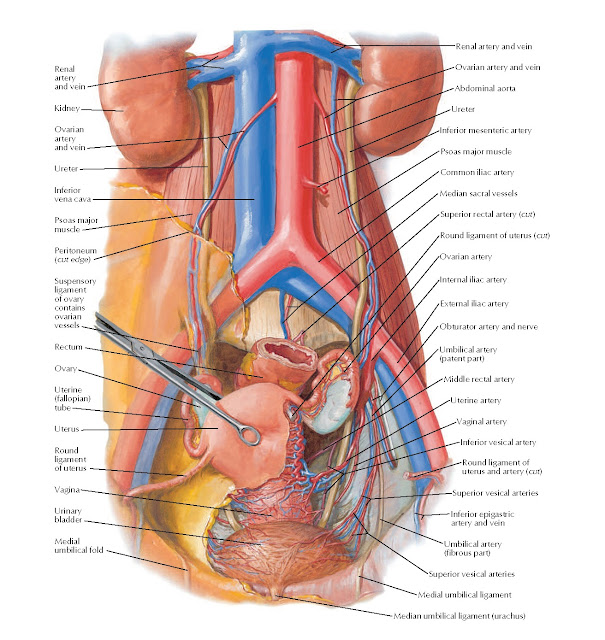 In certain situations, if fluid accumulation is severe, this space can be drained by performing a culdocentesis, which is accomplished by inserting a needle through the posterior fornix of the upper vagina to access the posterior cul-de-sac.
In certain situations, if fluid accumulation is severe, this space can be drained by performing a culdocentesis, which is accomplished by inserting a needle through the posterior fornix of the upper vagina to access the posterior cul-de-sac.
The posterior cul-de-sac communicates with the retroperitoneal space of the abdomen via the right and left epiploic gutters. The right gutter leads to the hepatorenal space, also known as Morrison's pouch. The right epiploic gutter also allows the spread of pelvic pathogens into the subphrenic space. Occasionally infection of the subphrenic space can occur, leading to adhesions on the capsule of the liver. This pathology is known as Fitz-Curtis-Hugh syndrome or gonococcal perihepatitis.
The left epiploic gutter leads to the splenorenal pouch. Due to the leftward position of the rectum, pelvic pathology is less likely to spread to the abdomen via the left epiploic communication.[4]
Embryology
The organs of the female reproductive tract each have a unique embryological origin. The exact embryological timeline in which these organs develop is still open to debate because most embryologic studies use animal models with different gestational ages. However, there is a consensus that the ovaries are the first to develop. They arise from the surface of the mesonephros at the gonadal ridge.[5] Later in development, the ovaries descend into the pelvis with guidance from the gubernaculum. The inferior aspect of the gubernaculum subsequently becomes the round ligament of the uterus and terminates at the labia majora.
The exact embryological timeline in which these organs develop is still open to debate because most embryologic studies use animal models with different gestational ages. However, there is a consensus that the ovaries are the first to develop. They arise from the surface of the mesonephros at the gonadal ridge.[5] Later in development, the ovaries descend into the pelvis with guidance from the gubernaculum. The inferior aspect of the gubernaculum subsequently becomes the round ligament of the uterus and terminates at the labia majora.
In females, the absence of a Y chromosome allows the uterus to form. It derives from the Müllerian ducts, also known as the paramesonephric ducts. These ducts fuse to form the uterus, fallopian tubes, and cervix. Some studies suggest that the paramesonephric ducts also give rise to the upper vagina while other studies indicate that the vagina exclusively derives from the urogenital sinus epithelium.[6] In males, a substance coded for on the Y chromosome, known as the anti-Mullerian hormone, prevents the formation of internal female reproductive organs.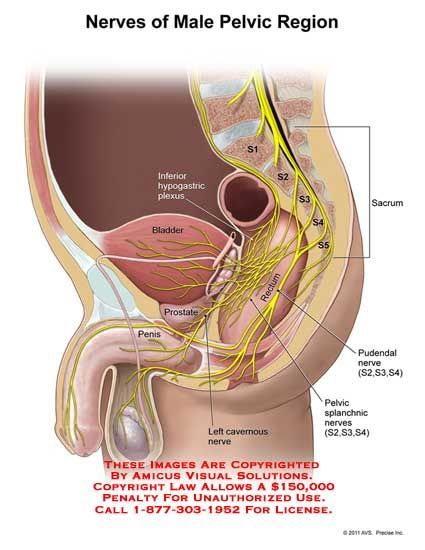
Blood Supply and Lymphatics
Arterial
The anterior branch of the internal iliac artery supplies most of the female reproductive organs. The uterine artery supplies the majority of the uterus (Figure 3). The lower uterine segment has a dual blood supply that includes branches of the vaginal artery. The ovaries are an exception because they receive blood from the ovarian arteries, which descend from the abdominal aorta.
The superior vesicle artery supplies the upper bladder. In females, the vaginal artery supplies the lower bladder. Both arteries are also branches of the anterior branch of the internal iliac artery.
The rectum receives vascular supply via three vessels. The superior rectal artery is the terminal branch of the inferior mesenteric artery. The middle rectal is a branch of the internal iliac artery. The inferior rectal is a branch of the pudendal artery.
Venous
The venous supply of pelvic organs follows the arterial supply. The uterine vein receives blood from the uterus and drains into the internal iliac vein. The ovarian veins receive blood from the ovaries. The right ovarian vein drains its contents directly into the inferior vena cava, while the left ovarian vein drainage is into the left renal vein. The increased length of the left ovarian vein makes it more susceptible to compression, especially during pregnancy.[7] Ovarian vein compression can lead to pelvic venous compression syndrome. The resulting pelvic vasculature congestion is a cause of chronic pelvic pain and may occur in non-pregnant patients as well.
The uterine vein receives blood from the uterus and drains into the internal iliac vein. The ovarian veins receive blood from the ovaries. The right ovarian vein drains its contents directly into the inferior vena cava, while the left ovarian vein drainage is into the left renal vein. The increased length of the left ovarian vein makes it more susceptible to compression, especially during pregnancy.[7] Ovarian vein compression can lead to pelvic venous compression syndrome. The resulting pelvic vasculature congestion is a cause of chronic pelvic pain and may occur in non-pregnant patients as well.
The left-sided venous supply is also unique because the left internal iliac artery travels from its right-sided origin at the inferior vena cava towards a leftward destination in the pelvis. The longer path makes the left internal iliac more prone to compression and may explain why venous thromboembolism in pregnancy most commonly occur in the left iliac and iliofemoral veins.[8] Additionally, the leftward position of the sigmoid colon causes a gravid uterus to tip toward the right, which is thought to increase the risk of iliac vessel compression further.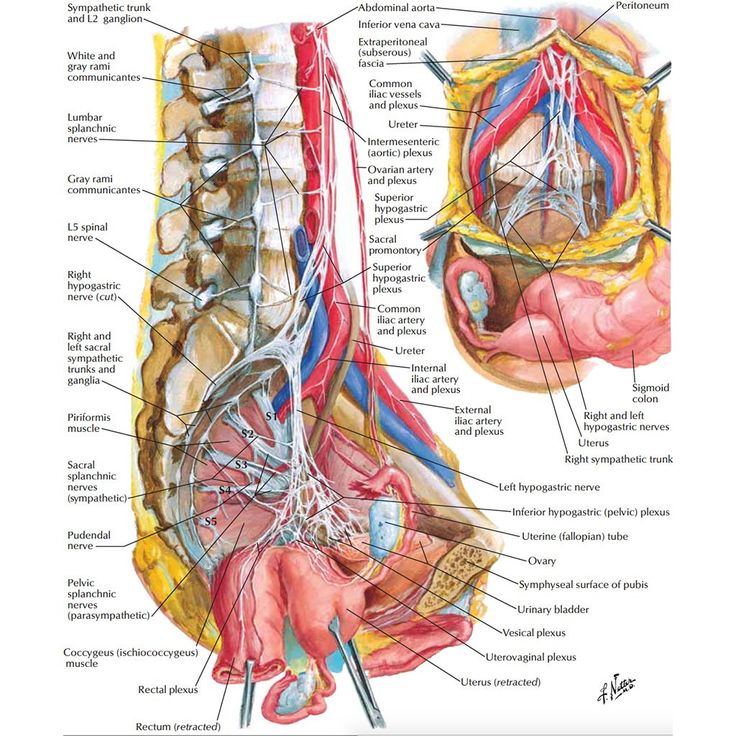
Lymphatics
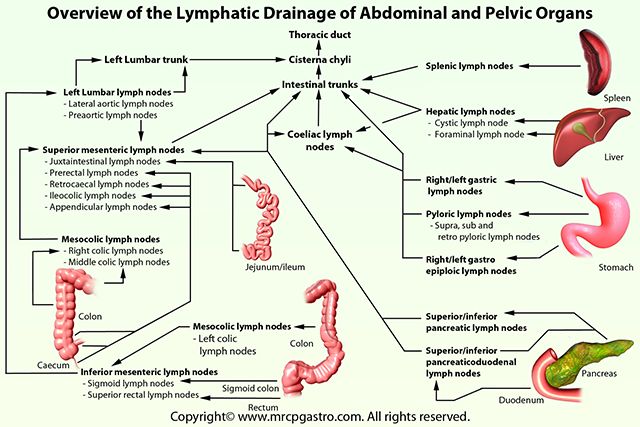
The lymphatic network of the pelvis is complex but is essential to understand when staging and treating gynecologic malignancies. Generally, the pelvic organs drain into the internal and external iliac lymph nodes (Figure 5).
The lymphatic drainage of the uterus is more complicated and remains somewhat ambiguous.[9] Some researchers suggest it may involve pelvic and para-aortic lymph nodes.[10] A more recent study from 2017 suggests that the uterus has two primary routes of lymphatic drainage, an upper pathway that drains to the external iliac and/or obturator lymph nodes and a lower pathway that drains to the internal iliac and/or presacral lymph nodes.[11]
The ovaries are an exception to the other female pelvic organs because they do not drain to pelvic lymph nodes. Their lymphatic drainage follows their blood supply; therefore they drain directly to the paraaortic lymph nodes.
Nerves
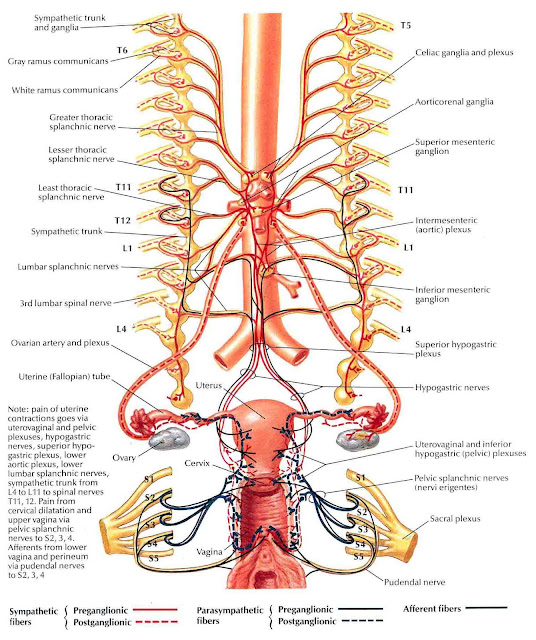
The female reproductive organs have both autonomic and sensory innervation. Beginning with the autonomic nervous system, sympathetic fibers exit at the level of T10 to L2 to form the superior hypogastric plexus, which divides into the left and right hypogastric nerve at approximately the level of the sacral promontory. The parasympathetic nerve fibers exit at the level of S2 to 4 and meet up with the sympathetic nervous system at the right and left hypogastric nerves. The right and left hypogastric nerves then migrate inferiorly to form the inferior hypogastric plexus. After this point, the nerve fibers follow blood vessels to their target organs. The inferior hypogastric plexus also receives sensory information from the uterus.
Beginning with the autonomic nervous system, sympathetic fibers exit at the level of T10 to L2 to form the superior hypogastric plexus, which divides into the left and right hypogastric nerve at approximately the level of the sacral promontory. The parasympathetic nerve fibers exit at the level of S2 to 4 and meet up with the sympathetic nervous system at the right and left hypogastric nerves. The right and left hypogastric nerves then migrate inferiorly to form the inferior hypogastric plexus. After this point, the nerve fibers follow blood vessels to their target organs. The inferior hypogastric plexus also receives sensory information from the uterus.
The ovarian nerve innervates the ovary. Although previously thought only to contain sensory fibers, recent animal studies suggest this nerve also carries autonomic fibers that may play a role in hormone secretion and constriction of ovarian vessels.[12][13][14] Recent studies suggest that the cervix and upper vagina also have autonomic innervation; however, the role of autonomic innervation in this region remains unclear.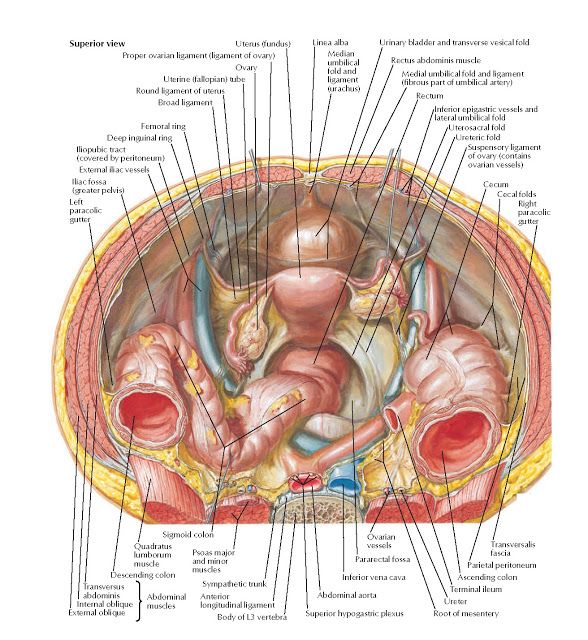 [15][16] Sensory innervation to the cervix and upper vagina has been more widely studied and receives supply by the pelvic splanchnic nerves. The pudendal nerve supplies the sensory innervation of the lower vagina. Pudendal nerve blocks can be used to provide local pain relief to laboring women by using the ischial spines as landmarks. Although once the treatment of choice for labor pain, it is no longer commonly used due to the widespread use of epidural anesthesia.[17]
[15][16] Sensory innervation to the cervix and upper vagina has been more widely studied and receives supply by the pelvic splanchnic nerves. The pudendal nerve supplies the sensory innervation of the lower vagina. Pudendal nerve blocks can be used to provide local pain relief to laboring women by using the ischial spines as landmarks. Although once the treatment of choice for labor pain, it is no longer commonly used due to the widespread use of epidural anesthesia.[17]
Muscles
The inferior border of the pelvic cavity is the pelvic diaphragm. It is made up of a group of muscles. From posterior to anterior, these muscles include:
Piriformis
Coccygeus
Iliococcygeus
Pubococcygeus
Puborectalis
The fibers of the iliococcygeus, pubococcygeus, and puborectalis make up the levator ani muscle. Because of its proximity to the vagina, the pubococcygeus and puborectalis are the most commonly injured muscles during vaginal deliveries.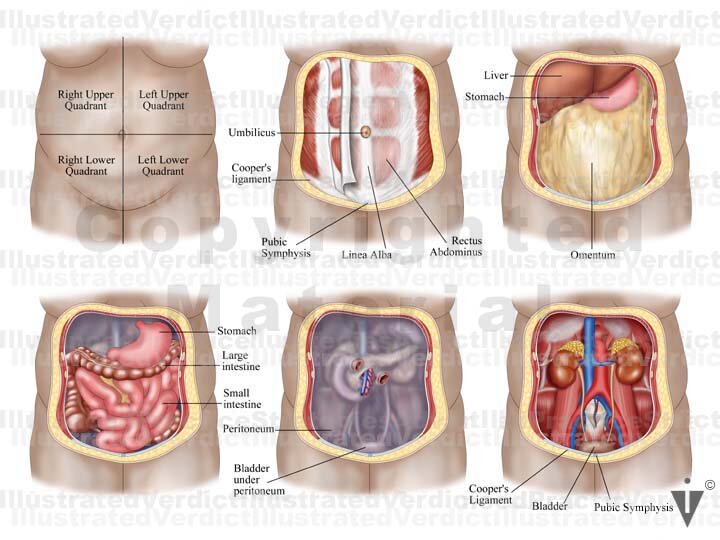 [18] The obturator internus muscles make up the peripheral borders of the pelvis but are not among the muscles of the pelvic floor.
[18] The obturator internus muscles make up the peripheral borders of the pelvis but are not among the muscles of the pelvic floor.
Ligaments
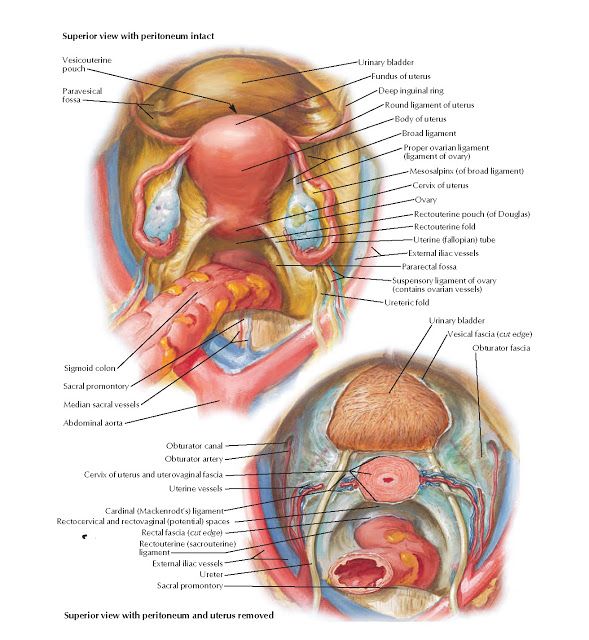
Three ligaments anchor the uterus. The uterosacral ligament supports the uterus posteriorly, and the pubocervical ligament anchors the uterus anteriorly. The transverse cervical ligament supports the uterus laterally. Unlike the anterior and posterior planes that contain the bladder and rectum respectively, the lateral plane lacks supporting structures other than the transverse cervical ligament. It is for this reason that this ligament also has the name of the "cardinal" ligament. The transverse ligament also differs from other supporting ligaments of the uterus because it is the only ligament that contains a vasculature structure, the uterine artery.
The ovary also has ligamentous support. The utero-ovarian ligament, also known as the ovarian ligament, extends from the ovary to the uterine body. The ovary is supported superiorly by the infundibulopelvic ligament, also known as the suspensory ligament of the ovary.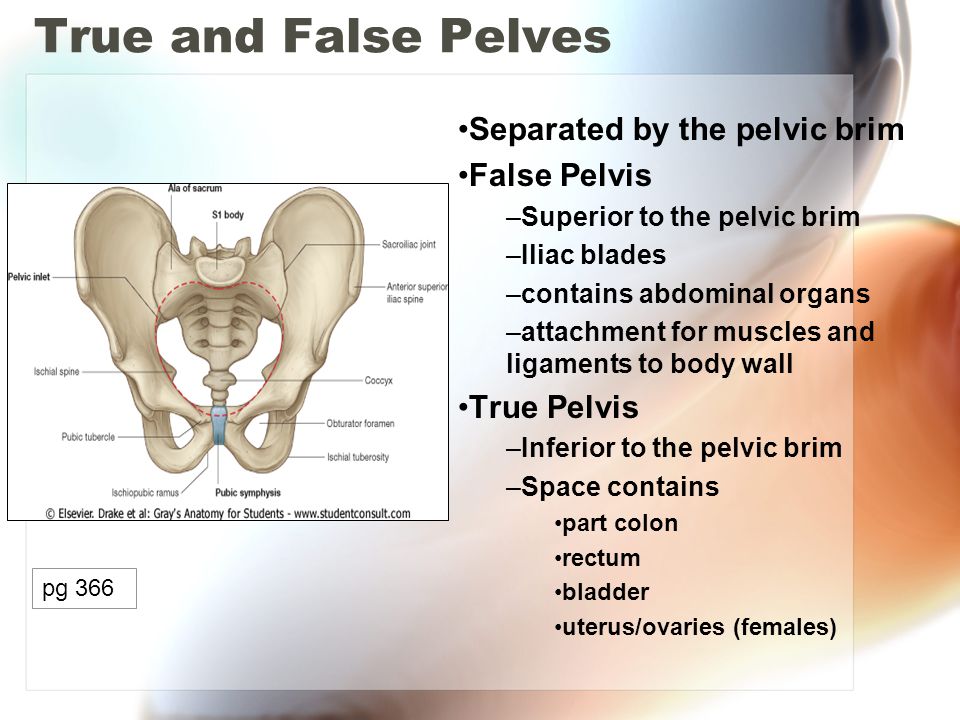 This ligament descends from the lateral aspect of the abdominal wall and contains the ovarian neurovascular bundle.
This ligament descends from the lateral aspect of the abdominal wall and contains the ovarian neurovascular bundle.
The broad ligament overlies the uterus, fallopian tubes, and ovaries. It is the inferior most extension of the parietal peritoneum and has three divisions based on location. The lateral most aspect of the broad ligament is the "mesovarium" and overlies the ovaries. The "mesosalpinx" covers the fallopian tubes. The largest portion of the broad ligament is the "mesometrium" and overlies the uterus.
Physiologic Variants
The uterine and ovarian arteries supply the majority of the female reproductive tract. The uterine artery, like many pelvic arteries, has a variable presentation. It most commonly arises from the anterior branch of the internal iliac artery and shares a common trunk with the obliterated umbilical artery.[19] One study that analyzed imaging from 218 patients reported this presentation in 80.7 % of cases.[20] The same study reported the uterine artery branched directly off of the internal iliac in 13.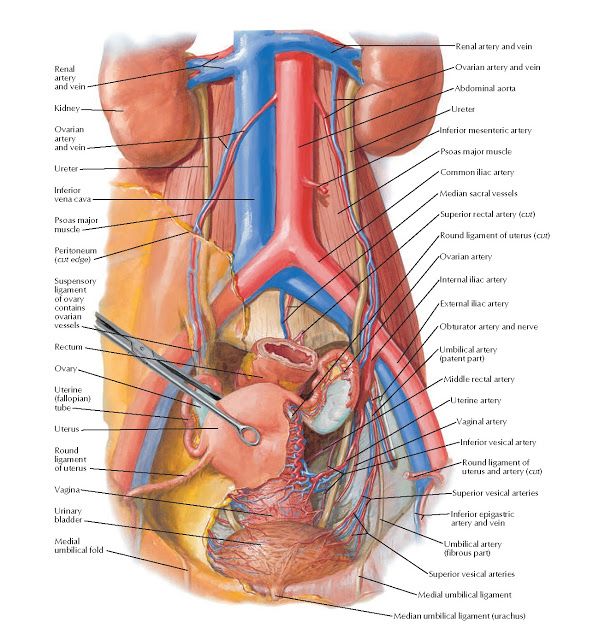 6% of cases, making this the most common variation. The second and third most common variations they found were direct branching from the superior gluteal artery and internal pudendal artery, respectively. These variations are necessary to be aware of during surgery as well as during uterine artery embolization.
6% of cases, making this the most common variation. The second and third most common variations they found were direct branching from the superior gluteal artery and internal pudendal artery, respectively. These variations are necessary to be aware of during surgery as well as during uterine artery embolization.
Ovarian artery variants are less common, but case reports exist that detail unique aberrations. One case report found an aberrant ovarian artery arising from the external iliac artery.[21] Another study reported an ovarian artery that branched directly from the common iliac artery.[22] These variations are thought to be due to abnormal descent of the ovaries into the pelvis during embryological development and may also correlate to other differences.[23]
Surgical Considerations
Several anatomical relationships are essential for surgeons to be aware of when operating in the female pelvis. For example, during a cesarean section, the bladder should always be identified to avoid iatrogenic cystotomy.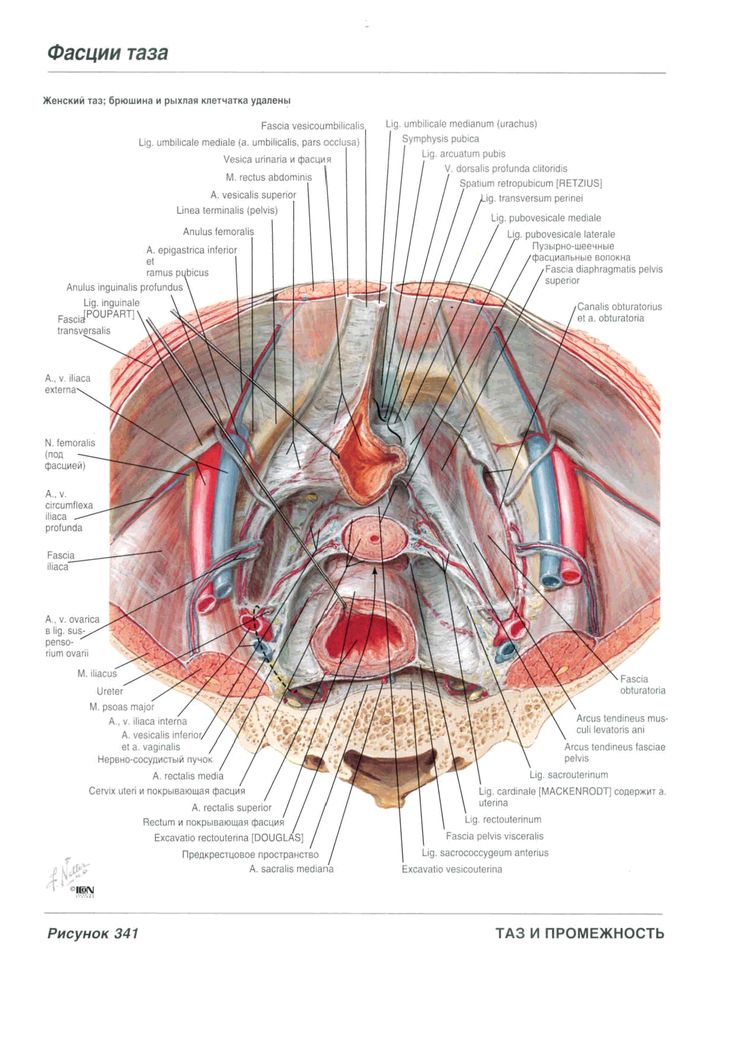 This cautionary measure is especially the case in patients that have had a prior cesarean section because scar tissue can cause the bladder to adhere to the anterior uterine wall.
This cautionary measure is especially the case in patients that have had a prior cesarean section because scar tissue can cause the bladder to adhere to the anterior uterine wall.
During any uterine surgery, identification of the uterine artery is vital. If cut, massive bleeding can ensue. During abdominal hysterectomies, the uterine artery is typically “skeletonized” so that its path along the uterus is clearly identifiable to the operating team.
If hemorrhage does occur, knowledge of pelvic vasculature becomes crucial. If the bleeding vessel cannot be clearly identified, the internal iliac should be clamped below the origin of the superior gluteal artery. This method is preferred so that necrosis of the gluteal muscle does not occur.
When operating deeper in the female pelvis, identification of the ureter is also crucial. There are three major locations where this structure may exist. Beginning superiorly, the ureters descend into the pelvis posterior to the infundibulopelvic ligaments. The ureters maintain this relationship until approximately the level of the iliac vessels, where they begin to travel more medially.
The ureters maintain this relationship until approximately the level of the iliac vessels, where they begin to travel more medially.
As the ureters continue to travel inferiorly, the next important landmark is the transverse cervical ligament. The ureters dive under this structure. Awareness of this relationship is of considerable importance when performing a hysterectomy. A popular pneumonic used by medical students is, "water under the bridge."
After passing under the transverse cervical ligament, the ureters continue to travel medially toward the bladder. Their insertion into the inferior aspect of the bladder is the third major location they should undergo positive identification intraoperatively. The insertion points are also visible from inside the bladder itself, during cystoscopy; this is sometimes performed after pelvic surgery if an injury to the bladder is suspected.
Injury to the bladder can also occur during pelvic surgery from the vaginal approach. When operating in this plane, injury to the rectum is also a possibility because at this level the rectum is directly posterior to the vagina (Figure 6.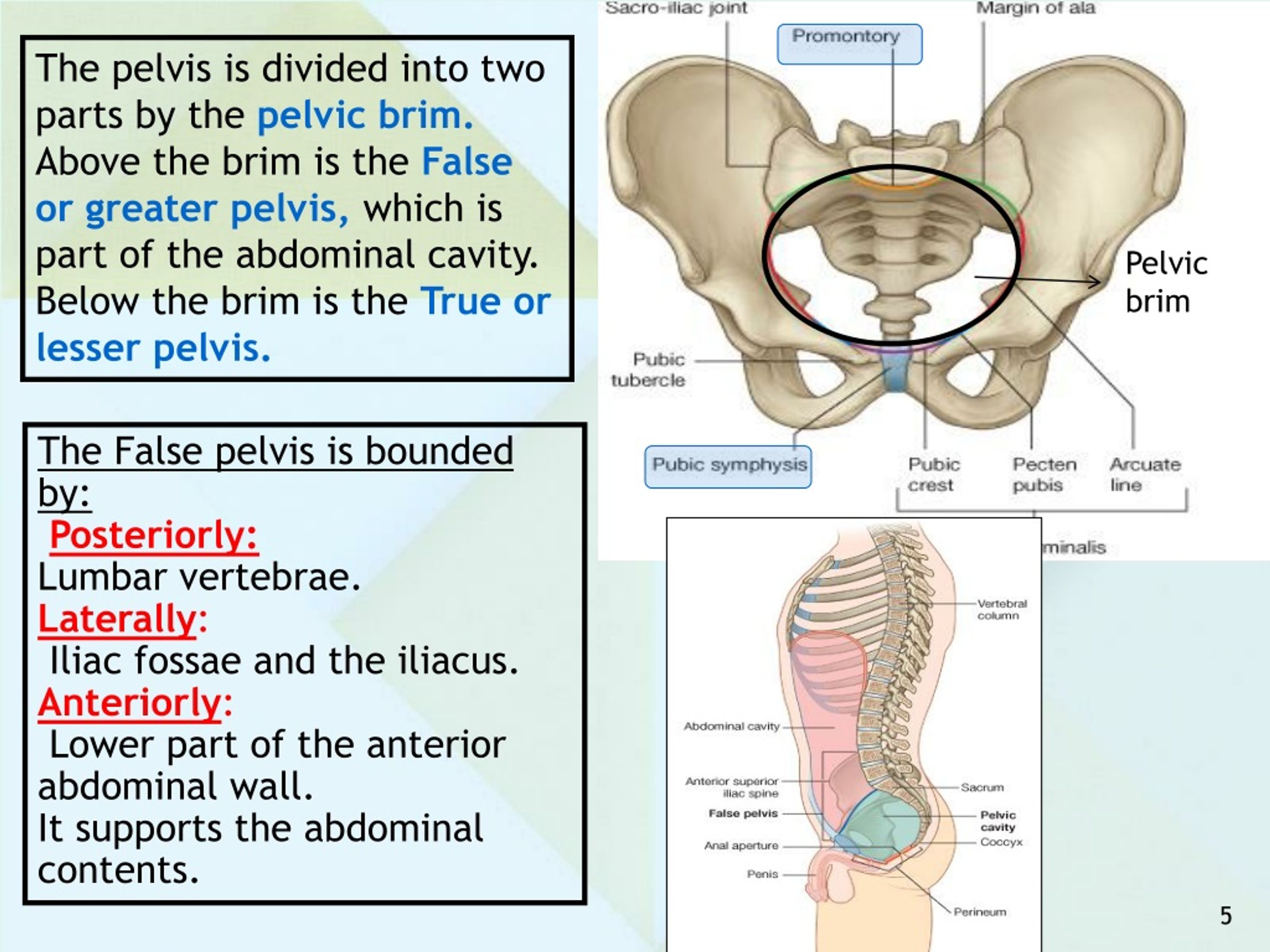 ) Avoiding bladder and rectal injury becomes increasingly difficult if the patient has pelvic organ prolapse, such as cystocele or rectocele.
) Avoiding bladder and rectal injury becomes increasingly difficult if the patient has pelvic organ prolapse, such as cystocele or rectocele.
When performing surgery on the fallopian tubes, such as during a tubal ligation or tubal anastomosis, the location of the round ligament should be determined. Due to the similarity in structure and location, the round ligament can be mistaken for a fallopian tube. Unlike the round ligaments, however, the fallopian tubes have fimbriae, a characteristic can be used to differentiate between these two structures intraoperatively.
Clinical Significance
Clinical correlates related to female pelvic anatomy can be summarized as follows:
The standard position of the uterus is anteverted and anteflexed. Abnormal positioning of the uterus is associated with pathology. Retroversion, for example, is a cause of dyspareunia. Additionally, retroversion of a gravid uterus is associated with higher rates of spontaneous abortion.
The posterior cul-de-sac, located between the uterus and the rectum, is a potential space prone to fluid collection.
 Physiologic fluid accumulates during menses and ovulation. If fluid collection is pathologic, this space can undergo drainage via culdocentesis.
Physiologic fluid accumulates during menses and ovulation. If fluid collection is pathologic, this space can undergo drainage via culdocentesis.The posterior cul-de-sac communicates with the abdomen via the left and right epiploic gutters, which allows the spread of pelvic pathogens into the abdominal cavity. Due to the leftward position of the rectum, infections usual take the path of the right epiploic gutter. The right epiploic gutter leads to potential spaces surrounding the liver. These include the hepatorenal space, also known as Morrison’s pouch, and the subphrenic space. Infection of the subphrenic space secondary to a gonococcal pelvic infection is termed Fitz-Curtis-Hugh syndrome or gonococcal perihepatitis.
The increased length of the left ovarian vein makes it more susceptible to compression, especially during pregnancy. Compression of this vessel sometimes leads to pelvic venous compression syndrome and is a cause of chronic pelvic pain in both pregnant and non-pregnant patients.
Venous thromboembolism in pregnancy is most commonly left-sided and occur in the iliofemoral vessels, which is thought to be because of pelvic vessel engorgement in combination with the increased path the left iliac vein takes across the pelvis. These factors make this vessel more susceptible to compression by a gravid uterus.
Understanding the lymphatic drainage of the female reproductive tract is important when tracking the spread of gynecologic malignancies. Generally, the female reproductive organs drain to the internal and external iliac lymph nodes. A notable exception is the ovaries, which drain to the paraaortic lymph nodes.
The pudendal nerve receives sensory innervation to the lower vagina. Historically, pudendal nerve blocks were used to alleviate labor pains. However, pudendal nerve blocks are no longer commonly practiced due to the wide-spread use of epidural anesthesia.
The muscles of the pelvic floor are susceptible to injury during vaginal deliveries.
 The most commonly injured muscles are the pubococcygeus and puborectalis muscles due to their proximity to the vagina.
The most commonly injured muscles are the pubococcygeus and puborectalis muscles due to their proximity to the vagina.The pelvic vasculature contains many physiologic variants that are important for surgeons to know. Interventional radiologists should also be knowledgeable of variants, particularly during uterine artery embolization to treat fibroids. The uterine artery most commonly arises from the anterior branch of the internal iliac and shares a trunk with the obliterated umbilical artery. The most common variation is direct branching from the internal iliac.
Review Questions
Access free multiple choice questions on this topic.
Comment on this article.
Figure
Compilation of 6 images detailing anatomy of female pelvic cavity. Contributed by Gray's Anatomy Plates (Public Domain)
References
- 1.
Roach MK, Andreotti RF. The Normal Female Pelvis. Clin Obstet Gynecol. 2017 Mar;60(1):3-10.
 [PubMed: 28005593]
[PubMed: 28005593]- 2.
Weekes AR, Atlay RD, Brown VA, Jordan EC, Murray SM. The retroverted gravid uterus and its effect on the outcome of pregnancy. Br Med J. 1976 Mar 13;1(6010):622-4. [PMC free article: PMC1639005] [PubMed: 1252851]
- 3.
Sanders RC, Parsons AK. Anteverted retroflexed uterus: a common consequence of cesarean delivery. AJR Am J Roentgenol. 2014 Jul;203(1):W117-24. [PubMed: 24951223]
- 4.
Turco G, Chiesa GM, de Manzoni G. [Echographic anatomy of the greater peritoneal cavity and its recesses]. Radiol Med. 1988 Jan-Feb;75(1-2):46-55. [PubMed: 3279472]
- 5.
Yao HH. The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol. 2005 Jan 31;230(1-2):87-93. [PMC free article: PMC4073593] [PubMed: 15664455]
- 6.
Robboy SJ, Kurita T, Baskin L, Cunha GR. New insights into human female reproductive tract development. Differentiation.
 2017 Sep-Oct;97:9-22. [PMC free article: PMC5712241] [PubMed: 28918284]
2017 Sep-Oct;97:9-22. [PMC free article: PMC5712241] [PubMed: 28918284]- 7.
Jeanneret C, Beier K, von Weymarn A, Traber J. Pelvic congestion syndrome and left renal compression syndrome - clinical features and therapeutic approaches. Vasa. 2016;45(4):275-82. [PubMed: 27428495]
- 8.
American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 196: Thromboembolism in Pregnancy. Obstet Gynecol. 2018 Jul;132(1):e1-e17. [PubMed: 29939938]
- 9.
Coleman RL, Frumovitz M, Levenback CF. Current perspectives on lymphatic mapping in carcinomas of the uterine corpus and cervix. J Natl Compr Canc Netw. 2006 May;4(5):471-8. [PubMed: 16687095]
- 10.
Burke TW, Levenback C, Tornos C, Morris M, Wharton JT, Gershenson DM. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: results of a pilot study.
 Gynecol Oncol. 1996 Aug;62(2):169-73. [PubMed: 8751545]
Gynecol Oncol. 1996 Aug;62(2):169-73. [PubMed: 8751545]- 11.
Geppert B, Lönnerfors C, Bollino M, Arechvo A, Persson J. A study on uterine lymphatic anatomy for standardization of pelvic sentinel lymph node detection in endometrial cancer. Gynecol Oncol. 2017 May;145(2):256-261. [PubMed: 28196672]
- 12.
Uchida S, Kagitani F. Autonomic nervous regulation of ovarian function by noxious somatic afferent stimulation. J Physiol Sci. 2015 Jan;65(1):1-9. [PMC free article: PMC4276811] [PubMed: 24966153]
- 13.
Pastelín CF, Rosas NH, Morales-Ledesma L, Linares R, Domínguez R, Morán C. Anatomical organization and neural pathways of the ovarian plexus nerve in rats. J Ovarian Res. 2017 Mar 14;10(1):18. [PMC free article: PMC5351206] [PubMed: 28292315]
- 14.
Cruz G, Fernandois D, Paredes AH. Ovarian function and reproductive senescence in the rat: role of ovarian sympathetic innervation. Reproduction. 2017 Feb;153(2):R59-R68.
 [PubMed: 27799628]
[PubMed: 27799628]- 15.
Mónica Brauer M, Smith PG. Estrogen and female reproductive tract innervation: cellular and molecular mechanisms of autonomic neuroplasticity. Auton Neurosci. 2015 Jan;187:1-17. [PMC free article: PMC4412365] [PubMed: 25530517]
- 16.
Mowa CN. Uterine Cervical Neurotransmission and Cervical Remodeling. Curr Protein Pept Sci. 2017;18(2):120-124. [PubMed: 27001061]
- 17.
Schrock SD, Harraway-Smith C. Labor analgesia. Am Fam Physician. 2012 Mar 01;85(5):447-54. [PubMed: 22534222]
- 18.
Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Womens Health (Lond). 2013 May;9(3):265-77; quiz 276-7. [PMC free article: PMC3877300] [PubMed: 23638782]
- 19.
Peters A, Stuparich MA, Mansuria SM, Lee TT. Anatomic vascular considerations in uterine artery ligation at its origin during laparoscopic hysterectomies. Am J Obstet Gynecol. 2016 Sep;215(3):393.e1-3. [PubMed: 27287682]
- 20.
Chantalat E, Merigot O, Chaynes P, Lauwers F, Delchier MC, Rimailho J. Radiological anatomical study of the origin of the uterine artery. Surg Radiol Anat. 2014 Dec;36(10):1093-9. [PubMed: 24052200]
- 21.
Kwon JH, Kim MD, Lee KH, Lee M, Lee MS, Won JY, Park SI, Lee DY. Aberrant ovarian collateral originating from external iliac artery during uterine artery embolization. Cardiovasc Intervent Radiol. 2013 Feb;36(1):269-71. [PubMed: 22565531]
- 22.
Kim WK, Yang SB, Goo DE, Kim YJ, Chang YW, Lee JM. Aberrant ovarian artery arising from the common iliac artery: case report. Korean J Radiol. 2013 Jan-Feb;14(1):91-3. [PMC free article: PMC3542308] [PubMed: 23323036]
- 23.
Rahman HA, Dong K, Yamadori T. Unique course of the ovarian artery associated with other variations. J Anat. 1993 Apr;182 ( Pt 2)(Pt 2):287-90. [PMC free article: PMC1259840] [PubMed: 8376204]
Pelvis / Female Anatomy
Pelvis
The small pelvis is the lower half of the pelvis of a woman, in which the uterus with appendages, the bladder and rectum, blood vessels and nerves are located. The organs of the small pelvis are fixed to the walls of the pelvis with the help of ligaments and fascia. For ease of understanding, this design can be compared to a suspension bridge.
The organs of the small pelvis are fixed to the walls of the pelvis with the help of ligaments and fascia. For ease of understanding, this design can be compared to a suspension bridge.
Front and back - bridge supports (pubis and sacrum). A bridge (vagina) is stretched between them. Ropes (bundles) stretch from the upper part of the supports to the bridge. From the back support (sacrum) - sacro-uterine ligaments. From the anterior support (pubis) - pubo-urethral ligaments.
The bridge (vagina) can be compared to the membrane of a trampoline, and the ligaments of the pelvis to its springs.
The bladder lies on the anterior wall of the vagina like in a hammock.
The main pelvic fascia is the pubocervical fascia. This is a connective tissue sheet between the anterior wall of the vagina and the bladder. This fascia is the hammock, trampoline, or suspension bridge. She fascia is stretched from front to back with the help of the described ligaments, and on the sides is attached to the walls of the pelvis.